Abstract
LPS given peripherally or into the brain induces a neuroinflammatory response. How peripheral LPS induces its effects on brain is not clear, but one mechanism is that LPS crosses the blood-brain barrier (BBB). Alternatively, LPS acts outside the BBB by stimulating afferent nerves, acting at circumventricular organs, and altering BBB permeabilities and functions. Here, we labeled LPS with radioactive iodine (I-LPS) and co-injected it with radioactively labeled albumin (I-Alb) which acted as a vascular space marker. Measurable amounts of I-LPS associated with the BBB, most reversibly bound to brain endothelia. Brain endothelia also sequestered small amounts of I-LPS and about 0.025% of an intravenously injected dose of I-LPS crossed the BBB to enter the CNS. Disruption of the BBB with repeated injections of LPS did not enhance I-LPS uptake. Based on dose-response curves in the literature of the amounts of LPS needed to stimulate brain neuroimmune events, it is unlikely that enough peripherally administered LPS enters the CNS to invoke those events except possibly at the highest doses used and for the most sensitive brain functions. I-LPS injected into the lateral ventricle of the brain entered the circulation with the reabsorption of cerebrospinal fluid (bulk flow) as previously described. In conclusion, brain uptake of circulating I-LPS is so low that most effects of peripherally administered LPS are likely mediated through LPS receptors located outside the BBB.
Keywords: lipopolysaccharide, blood-brain barrier, cytokines, neuroimmune, neuroinflammation, cerebrospinal fluid, brain endothelial cells, brain, central nervous system
Introduction
Lipopolysaccharide (LPS), or endotoxin, is the major component of the outer membrane leaflet of gram negative bacteria. The exact composition of LPS varies with bacterial species, but all consist of a hydrophilic polysaccharide domain covalently bound to a hydrophilic lipid component termed lipid A (Ulmer et al., 2002). Low levels of LPS are being discovered to circulate in the blood during an increasing number of conditions and diseases (Pussinen et al., 2004; Szeto et al., 2008; Brenchley et al., 2006; Zhang et al., 2009). These levels are thought to reflect bacterial translocation from the endogenous flora of the gastrointestinal tract, including the oral cavity. However, very high levels of LPS suggest infection, peritonitis, or sepsis.
The body has evolved to recognize LPS as a pathogen-associated molecular pattern, activating the innate immune system when significant levels of LPS are detected. The lipid A component with a molecular weight of less than 2000 Da is the main immunostimulatory component of LPS. The 55 kDa glycoprotein CD-14 and Toll-like receptor 4 are the major receptors binding LPS (Ulmer et al., 2002). Other proteins that can bind LPS (Ulmer et al., 2002) and that may participate in responses to LPS include Toll-like receptor 2, hsp90, TREM-1, decay accelerating factor (CD55), adhesion molecules of the β-integrin family, and the high-conductance calcium and voltage dependent potassium channel (MaxiK). Nod1 and Nod2 represent intracellular binding proteins capable of binding LPS, suggesting that sensing by the innate immune system may extend to intracellular infections (Girardin et al., 2001; Ulevitch et al., 2004).
Peripheral administration of LPS increases brain levels of interleukins, prostaglandins, nitric oxide, and other substances (Singh & Yiang, 2004; Larson & Dunn, 2001; Sugita et al., 2002). Injection of LPS directly into the CNS can induce a neuroimmune response similar to peripheral administration (Gottschall et al., 1992). In the study of Quan et al (Quan et al., 1994), central infusion of LPS first produced detectable IL-1 in the brain and later in the blood, whereas peripheral administration of LPS first produced detectable IL-1 in blood and later in the brain. With simultaneous administration, central LPS can oppose or even produce the opposite effect of peripheral LPS (Chen et al., 2000). These findings raise the question of how LPS on one side of the blood-brain barrier (BBB) is mediating changes on the other.
Peripheral LPS could induce CNS effects by crossing the BBB and directly activating cells within the CNS. This is how many authors have assumed that LPS works. Indeed, the CNS contains many cells that possess Toll-like 4 receptors (Chakravarty & Herkenham, 2005)or can directly respond to LPS, including brain endothelial cells (Reyes et al., 1999; Verma et al., 2006), microglia (Marzolo et al., 2000), and astrocytes (Chakravarty & Herkenham, 2005). However, work by Singh and Yiang suggests that LPS can associate with but does not cross the BBB (Singh & Yiang, 2004). LPS could act through indirect mechanisms (Watkins et al., 1995), such as vagal (Goehler et al., 1999) or other afferent nerve (Romeo et al., 2001) stimulation, release of substances from the periphery that can cross the BBB (Qin et al., 2007), enhancing interactions between the BBB and immune cells (Strosznajder et al., 1996), altering BBB permeability (Xaio et al., 2001), acting at circumventricular organs (Blatteis et al., 1983; Ulmer et al., 2002), or by inducing release of substances from the cells constituting the BBB (Quan et al., 2003; Verma et al., 2006). Here, we radioactively labeled LPS and examined with state-of-the-art methods the ability of LPS to cross the BBB.
Methods
Radioactive labeling and purification of LPS and albumin
LPS from Salmonella enterica (1 mg; Sigma Chemical Co, St. Louis, MO Cat # L-6511) was radioactively labeled according to the method of Ulevitch (Ulevitch, 1978). LPS was incubated with a 1 ml solution of methyl 4-hydroxybenzimidate HCl (MHBI; 9.4 mg/ml 50 mM borate buffer, pH 8.0) in a 37 °C water bath for 18 h. The MHBI-LPS mixture was added to a dialysis cassette (Slide-A-Lyzer; 10,000 Dalton cutoff and dialyzed × 2 in 500 ml saline for 2 h at 4 °C and then overnight in 500 ml saline to remove unreacted MHBI. The MHBI-LPS ligand was added to a glass test tube and 1 mCi of 125I and 50 µl of chloramine-T solution (2 mg/ml in distilled water) added. This mixture was incubated for 30 min at room temperature and the reaction halted by adding 0.2 mg of sodium metabisulfite in in 0.1 ml of distilled water. The radioactively labeled LPS (I-LPS) was purified on a column of G-50 Sephadex that was eluted in 1 ml aliquots with 0.05 M phosphate buffer (pH 7.4). I-LPS produced by this method does not differ from unlabeled LPS in terms of its biophysical properties, immunogenicity, lethality, thermogenic activity, or hypotensive effects (Ulevitch, 1978). Albumin was labeled with 131I by the chloramine-T method and the radioactive albumin (I-Alb) purified on a G-10 Sephadex column.
Clearance from blood and brain uptake
All animal work was done under protocols approved by our local IACUC at an ALAAAC approved facility. Male CD-1 mice aged 8–12 weeks from our in-house colony (VA-St. Louis) were anesthetized with ip urethane (4.0 g/kg) and the left jugular vein and right carotid artery exposed. A 0.2 ml volume of Ringer’s lactated solution (LR) with 1% bovine serum albumin (LR-BSA) containing 5×105 cpm of I-LPS was injected into the jugular vein. Blood was collected from the carotid artery and the whole brain removed 1–120 min after the injection into the jugular vein (n = 2/time). Blood was centrifuged at 5000 g for 10 min at 4 °C and the serum collected. The whole brain was cleaned of large vessels and weighed after discarding the pituitary and pineal gland. Levels of radioactivity in brain and serum were measured in a gamma counter and brain/serum ratios (µl/g) calculated. The cpm in a ml of serum was divided by the dose of injected I-LPS and multiplied by 100 to yield the percent of the intravenously injected dose present in a ml of arterial serum (%Inj/ml).
The half-time clearance rate from blood in units of min was calculated by plotting the log(%Inj/ml) against time. The inverse of the slope was multiplied by 0.301 to yield the half-time clearance. The initial volume of distribution (Vd) in units of ml was determined by multiplying the antilog of the Y intercept by 100.
Multiple-time regression analysis (Blasberg et al., 1983; Patlak et al., 1983) was used to calculate the blood to brain unidirectional influx rate (Ki). The brain/serum ratios were plotted against their respective exposure times (Expt). Expt was calculated from the formula:
where Cp is the level of radioactivity in serum and Cpt is the level of radioactivity in serum at time t. Expt corrects for the clearance of I-LPS from the blood. Ki with its error term is measured as the slope for the linear portion of the relation between the brain/serum ratios and Expt and the Y intercept of the linearity measures Vi, the distribution volume in brain at t = 0, so that the equation describing the linear portion of the relation between brain/serum ratios and Expt is:
In other mice, I-LPS and I-Alb were coinjected with or without 100 µg/mouse of unlabeled LPS included in the iv injection. Arterial blood from the abdominal aorta was collected 1, 2, or 5 min after the iv injection (n = 6/time). Immediately after collection of blood, half the mice in each group were immediately decapitated. In the other half, the vascular space of the brain was washed out by severing both jugular veins, opening the thorax, clamping the descending thoracic aorta, and infusing 20 ml of lactated Ringer's solution through the left ventricle of the heart in 60 sec. Brain and arterial serum was counted in a gamma counter and brain/serum ratios for I-LPS and I-Alb determined. The I-LPS values were corrected for vascular space by subtracting the I-Alb values to yield delta brain/serum ratios. Delta values, being corrected for vascular space, more accurately reflect the amount of I-LPS associated with the BBB than uncorrected values.
Capillary depletion and brain distribution
The capillary depletion method (Triguero et al., 1990) as modified for mice (Gutierrez et al., 1993) was performed to determine whether the I-LPS completely crossed the capillary wall of the BBB to enter the brain parenchyma. Anesthetized mice received an injection into the jugular vein of 0.2 ml of LR-BSA containing 7.5(105) cpm of I-LPS and of I-Alb. At either 10 min or 2h after the i.v. injection, the abdomen was opened and arterial blood was collected from the abdominal aorta. The thorax was then opened to expose the heart. The descending aorta was clamped, both jugular veins severed, and 20 ml of LR-BSA perfused over 1 min into the left ventricle of the heart. After that, the mouse was decapitated and the frontal cortex removed, weighed, and placed in an ice-cold glass homogenizer. The brain was homogenized (10 strokes) in 0.8 ml of physiological buffer (10 mM HEPES, 141 mM NaCL, 4 mM KCl, 2.8 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, and 10 mM d-glucose, pH 7.4). Dextran solution was added to the homogenate to a final concentration of 26%, and homogenized a second time (3 strokes). The homogenate was centrifuged at 5400 × g for 15 min at 4°C. The pellet containing the brain microvessels (capillary fraction) and the supernatant containing brain (parenchymal fraction) were carefully separated. Results were expressed as parenchyma/serum and capillary/serum ratios with both corrected for vascular space contamination by subtracting the respective ratios for I-Alb. The corrected values were also expressed as the percent of the injected dose of I-LPS (%Inj/g) present in the parenchyma fraction. This was calculated by dividing the µl/g in the parenchymal fraction at time t by 1000 to yield ml/g and then multiplying by the %Inj/ml at time t to yield %Inj/g.
Effect of innate immune system activation on brain uptake
Mice were treated with 3 injections of unlabeled LPS (100 µg/injection) by ip injection at 0, 6, and 24 h. At 28 h after the first injection of LPS, mice were anesthetized with urethane and given an iv injection into the right jugular vein of 0.2 ml of LR-BSA containing 5×(105) cpm of I-LPS and 5×(105) cpm of I-Alb. Ten min after the iv injection, blood was collected from the descending aorta and the vascular space of the brain was washed out by severing both jugular veins, opening the thorax, clamping the descending aorta, and perfusing 20 ml of LR through the left ventricle of the heart in one min. The levels of I-LPS and I-Alb remaining in the brain and in the arterial serum were determined and brain/serum ratios calculated.
Intracerebroventricular studies
Two month old male CD-1 mice from our in-house colony kept on a 12/12 hour light/dark cycle with food and water freely available were anesthetized on the day of study with 0.15 ml of 40% urethane. The scalp was removed and a hole made into the lateral ventricle of the brain 1.0 mm lateral and 1.0 mm posterior to the bregma with a 26 gauge needle with a tubing guard which limited the depth of penetration to 3.5 mm. Mice received 1.0 ul intracerebroventricular (icv) injections containing 5(103) cpm of I-LPS in lactated Ringer’s solution with 1% bovine serum albumin. Mice were decapitated at 2, 5, 10, and 20 minutes after injection, the whole brain removed, the pituitary and pineal glands discarded, and the level of residual radioactivity in the whole brain determined in a gamma counter. The level of radioactivity in whole brain at t=0 was determined in mice overdosed with anesthetic as previously described (Banks & Kastin, 1989). The mean of 3 mice was used at each time point and each time point was studied twice. The level of residual radioactivity was divided by the cpm injected and multiplied by 100 to yield %Inj/brain. The log(%Inj/brain) was regressed against time. Other mice received an icv injection of I-LPS ± 1 µg/mouse of unlabeled LPS. The brains of these mice were collected 10 min after the icv injection and results reported as %Inj/brain.
Brain perfusion
Mice were anesthetized with ip urethane (4.0 g/kg). The thorax was opened, the heart was exposed, both jugular veins severed, and the descending thoracic aorta clamped. A 26 gauge butterfly needle was inserted into the left ventricle of the heart and Zlokovic’s (Zlokovic et al., 1988) buffer containing I-LPS [(10)5 cpm/ml] but no BSA was infused at a rate of 2 ml/minute for 1–5 minutes (Shayo et al., 1996). The exact cpm infused was determined on a 1 ml aliquot of perfusion fluid. After perfusion, the brain was removed as above and brain/perfusion ratios calculated. The unidirectional influx rate (Ki in units of µl/g-min) was calculated by regressing the brain/perfusion ratio against perfusion time. In a second group of mice, washout of the vascular space of the brain was performed at the end of the perfusion by removing the perfusion needle and inserting a 21 gauge needle attached to a syringe with 20 ml of LR that was injected in 60 sec.
Characterization of radioactivity in blood
Mice were anesthetized and given 106 cpm of I-LPS iv. Arterial blood was collected 5 and 60 min after the iv injection. As a control (time 0), I-LPS was added to whole blood which was collected from a mouse not given radioactivity. Whole blood was centrifuged at 5,400 g for 15 min at 4 cC. The level of radioactivity in 1 µl of serum was determined and a known quantity of serum then added on a G-50 Sephedex column and eluted with 0.05 M phosphate buffer. The percent of radioactivity eluting in the fraction in which I-LPS elutes was determined. The eluted radioactivity was then added to a tube containing 500 µl of 1% BSA in LR, mixed, and 500 µl of 30% TCA added. This combination was then mixed and centrifuged. The percent of radioactivity precipitated was calculated by the following formula:
Octanol/Buffer Partition Coefficient
Lipophilicity was determined by mixing 105 cpm of I-LPS in 0.5 ml of octanol with 0.5 ml of a 0.25 M phosphate buffer solution (pH 7.5). This was mixed vigorously for 1 min, agitated for 10 min, and the two phases separated by centrifugation. Aliquots of 100 µl were taken from each phase and counted in a gamma counter. This experiment was performed with an n of 3 with each determination in duplicate. The partition coefficient was expressed as the log of the ratio of cpm(octanol phase) to cpm(PBS phase).
Statistics
Means are reported with the number of mice used (n) and the standard error of the mean (SEM). Student’s t-test was used for comparison of two groups. The p values were reported for relevant statistically significant differences. Regression lines were calculated by the least squares method with the Prism 5.0 program (GraphPad, Inc., San Diego, CA) and the slope (m) with its error term, the correlation coefficient (r), the number of points on which the line was based (n) and the p value reported. Regression lines were compared for statistical differences with the Prism 5.0 program, which first determines whether there are differences between slopes and, if not, whether there are differences between intercepts.
Results
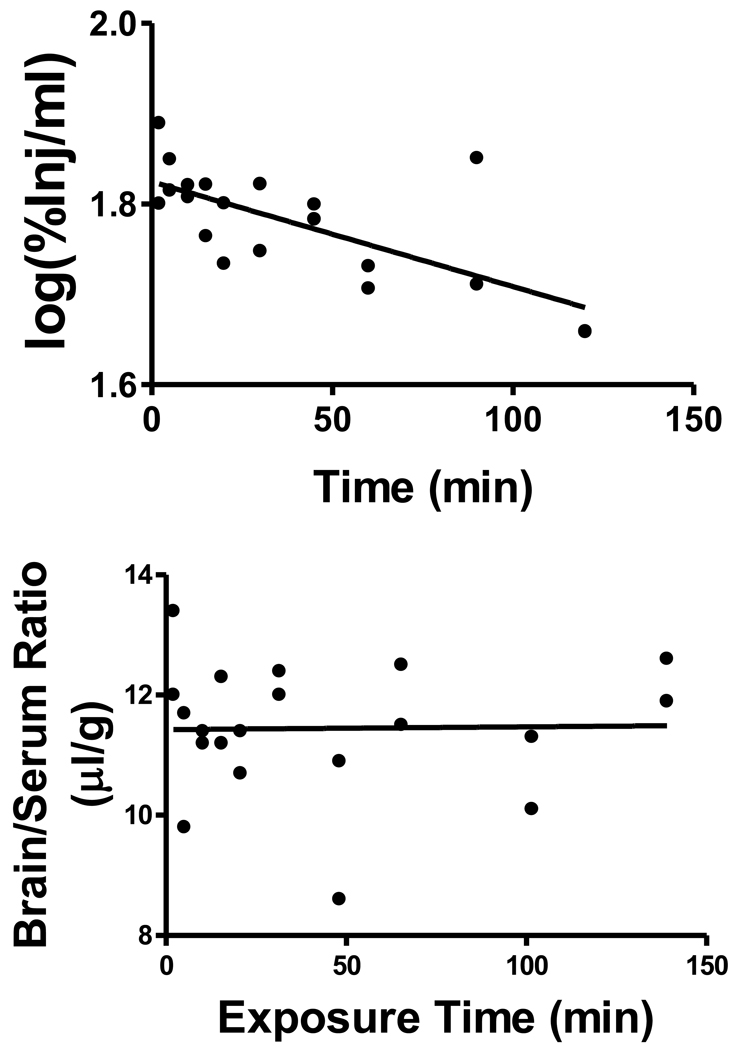
The relation between log(%Inj/ml) and time was statistically significant: r = 0.707, p<0.001, n = 20 (Figure 1, upper panel). The half-time clearance from blood was 259 min and the Vd was 1.50 ml. There was no relation between brain/serum ratios and exposure time indicating that there was no measurable transport over time across the BBB (Figure 1, lower panel). The mean brain/serum ratio was 11.3 ± 0.26 µl/g (n = 20). To determine whether an early phase of uptake occurred for I-LPS, mice were studied at 1 and 2 min after iv injection. The brain/serum ratio was 12.0 ± 0.67 µl (n = 4) at 1 min and 11.3 ± 0.54 µl (n = 4) at 2 min.
Figure 1.
Upper Panel: Clearance of I-LPS administered by intravenous injection. Results are expressed as the log of the level of radioactivity per ml of serum vs time. The relation was highly significant between log(serum cpm) and time (p<0.001) demonstrating clearance of I-LPS from blood; the half-time clearance was calculated to be 259 min with a volume of distribution of 1.5 ml. Lower Panel: Uptake by brain of I-LPS after its intravenous administration. There was no statistically significant correlation between brain/serum ratios and exposure time, indicting that there was no measurable BBB penetration of I-LPS by this method. The mean brain/serum ratio for I-LPS of 11.3 was in the upper range of the vascular space for brain.
I-LPS was stable in serum with 82% of the radioactivity in serum eluting in the region of I-LPS from the G50 column for the 0 min (control) sample, 74 % for the 5 min serum sample, and 82% for the 60 min sample. Acid precipitation was 99.6% at 0 min, 98.2% at 5 min, and 98.6% at 60 min. The octanol/buffer partition coefficient was (5.27 ± 0.34)10−2, giving a log value of (−)1.28.
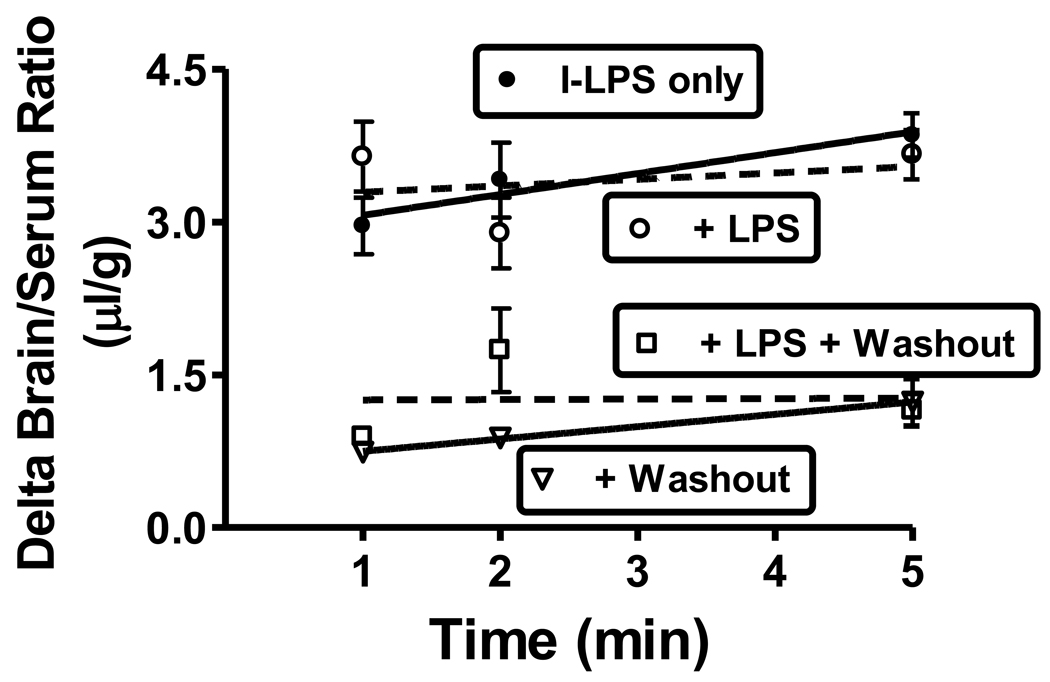
Inclusion of 100 µg of unlabeled LPS did not affect I-Alb uptake at 1, 2, or 5 min (n = 6/time point). The average for all time points combined for I-Alb was 10.3 ± 0.4 µl/g (I-LPS only), 10.1 ± 0.3 µl/g (+LPS), 0.92 ± 0.14 µl/g (+washout), and 0.87 ± 0.17 µl/g (+LPS + washout); n = 18/group (data not shown). The lack of effect of LPS on I-Alb brain/serum ratios shows that the acute injection of LPS had no effect on BBB integrity during the time studied. These results also show that washout removed over 90% of the vascular contents. Similarly, figure 2 shows that unlabeled LPS did not affect brain/serum ratios for I-LPS with or without washout, indicating no saturable component to the uptake of I-LPS. Delta values are shown for I-LPS (that is, values corrected for the albumin space) as these values better reflect I-LPS/BBB interactions. The delta brain/serum ratios in the absence of washout ranged between 3–4 µl/g showing that I-LPS uptake exceeded the vascular space by this amount. Washout reduced the delta brain/serum ratios to about 1 µl/g, indicating that about 2.5 µl/g was reversibly bound to the luminal surface of the capillaries.
Figure 2.
Effect of inclusion of unlabeled LPS (100 µg/mouse) or washout of brain vascular space on brain/serum ratios of I-LPS 1, 2, and 5 min after the iv injection of I-LPS. Values are corrected for vascular space by including I-Alb in the iv injection and subtracting the brain/serum ratios for I-Alb from those for I-LPS. The lack of an effect for unlabeled LPS indicates no saturable component to I-LPS uptake. A decrease in brain/serum ratios for I-LPS with washout further to that seen with correction for the I-Alb space indicates a reversible binding of I-LPS to brain endothelial cells.
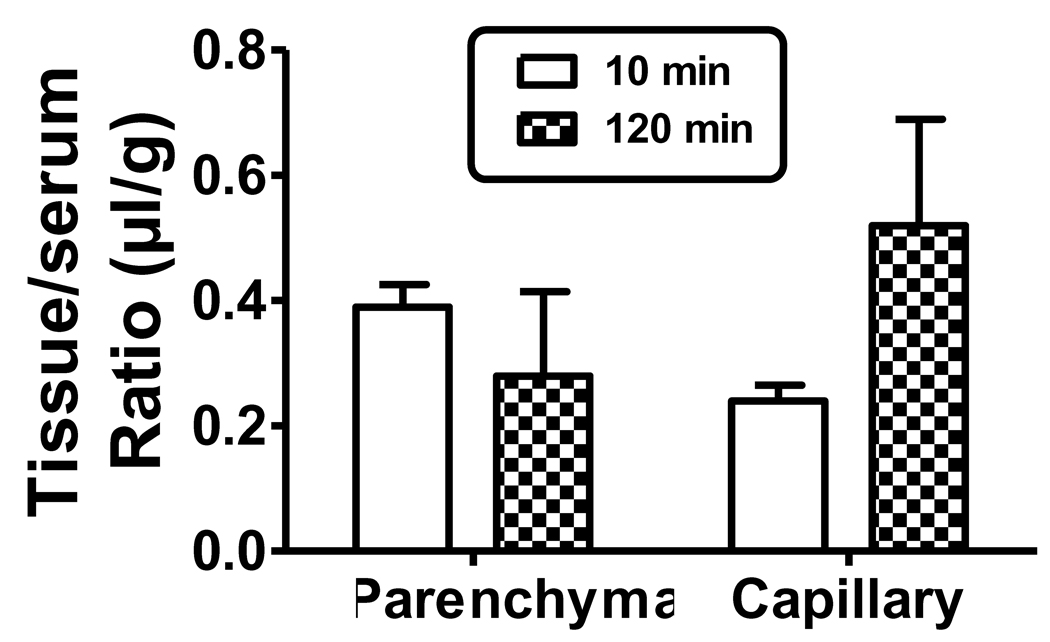
Capillary depletion was performed in mice given injections containing I-LPS and I-Alb. The washout of the vascular space reduced the parenchyma/serum ratio for I-Alb to about 0.4 µl/g at 10 min, consistent with other studies (Banks et al., 2000; Banks et al., 2002) showing that over 95% of the vascular contents of the CNS are cleared (I-Alb data not shown). The parenchyma/serum ratio for I-Alb at 120 min was about 0.76 µl/g. The difference of 0.36 µl/g between the 10 and 120 min values for the I-Alb ratio (0.76−0.40 = 0.36) likely represent leakage of I-Alb across the BBB. Dividing 0.36 µl/g by the lapsed time of 110 min gives a leakage rate for the BBB of 0.3(10−3) µl/g-min, a typical influx rate for I-Alb (Blasberg et al., 1983; Patlak et al., 1983). The capillary/serum ratio for I-Alb was about 0.01 and 0.02 µl/g at 10 min and 120 min respectively. The I-LPS values for both the parenchyma and capillary compartments were further corrected for vascular contamination or leakage by subtracting the I-Alb ratios. The corrected I-LPS ratios (figure 3) did not differ between 10 min and 120 for either parenchyma (0.39 µl/g and 0.28 µl/g, respectively) or capillary (0.24 µl/g and 0.520 µl/g, respectively) fractions. The percent of the injected dose per g of cortex was calculated for parenchyma using the values for serum of 65.0 %Inj/ml for 10 min and 48.5 %Inj/ml for 120 min as derived from the upper panel of figure 1. The calculated values for parenchyma was 0.023 %Inj/g at 10 min and 0.013 %Inj/g at 120 min. It could be argued that the value for leakage (0.36 µl/g) should be included in the calculation of the amount of I-LPS in the parenchyma at 120 min. In this case, the parenchyma/serum ratio for 120 min increases from 0.28 µl/g to 0.64 µl/g and the %Inj/g values increase from 0.013 to 0.031 %Inj/g.
Figure 3.
Capillary depletion demonstrates the relation of capillary sequestration and BBB penetration of I-LPS. The results show that about half of the I-LPS associated with brain had permeated the BBB and about half was sequestered by the capillary bed.
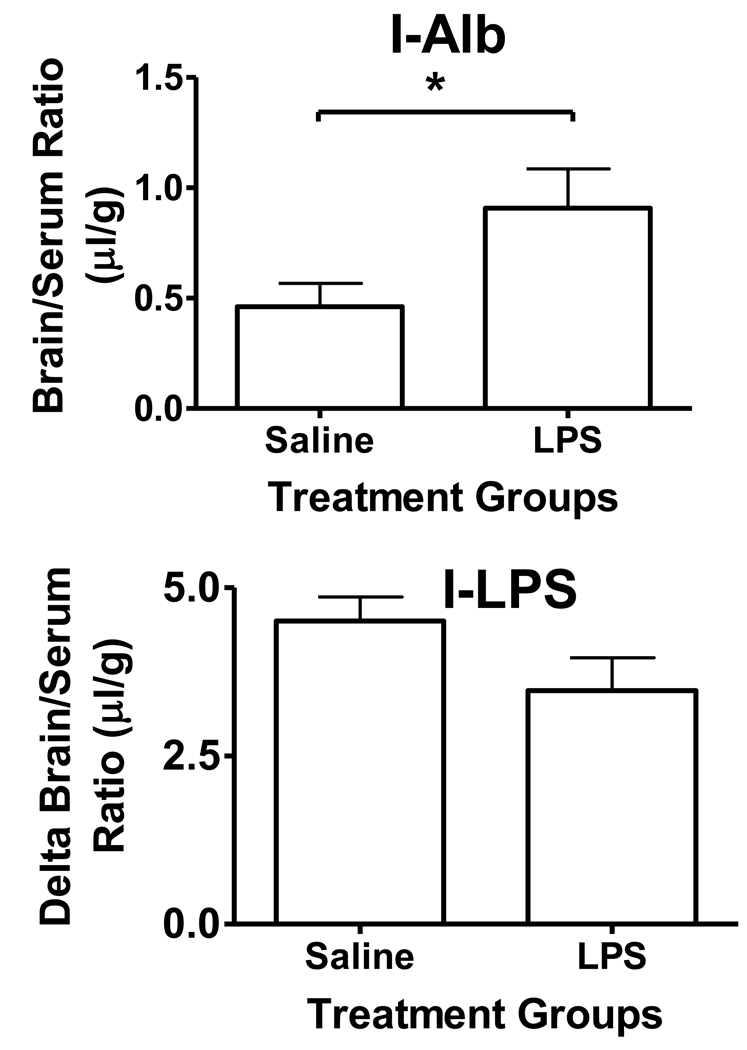
Treating mice with 3 injections of ip LPS increased the washout brain/serum ratio for I-Alb from 0.46 ± 0.10 µl/g (n = 9) to 0.91 ± 0.18 µl/g (n = 6), t = 2.33, df = 13, p<0.05, demonstrating a disruption of the BBB to I-Alb of 0.45 µl/g (Figure 4, upper panel). LPS treatment did not have a statistically significant effect on the brain/serum ratios for I-LPS with the LPS-treated group having arithmetically lower values (5.36 ± 0.52, n = 10 vs 4.90 ± 0.61, n = 7). The delta brain/serum ratios of I-LPS were also not statistically different in the LPS-treated group (3.47 ± 0.49, n = 6) having arithmetically lower values than the control group (4.50 ± 0.62, n = 9, Figure 4, lower panel).
Figure 4.
Effect of 3 injections of LPS over 24 h on the uptake of I-Alb and I-LPS by brain. Upper panel shows that LPS treatments increased I-Alb uptake (p<0.05), demonstrating BBB disruption. Lower panel shows I-LPS results corrected for the I-Alb space and shows that LPS treatment had no statistical effect.
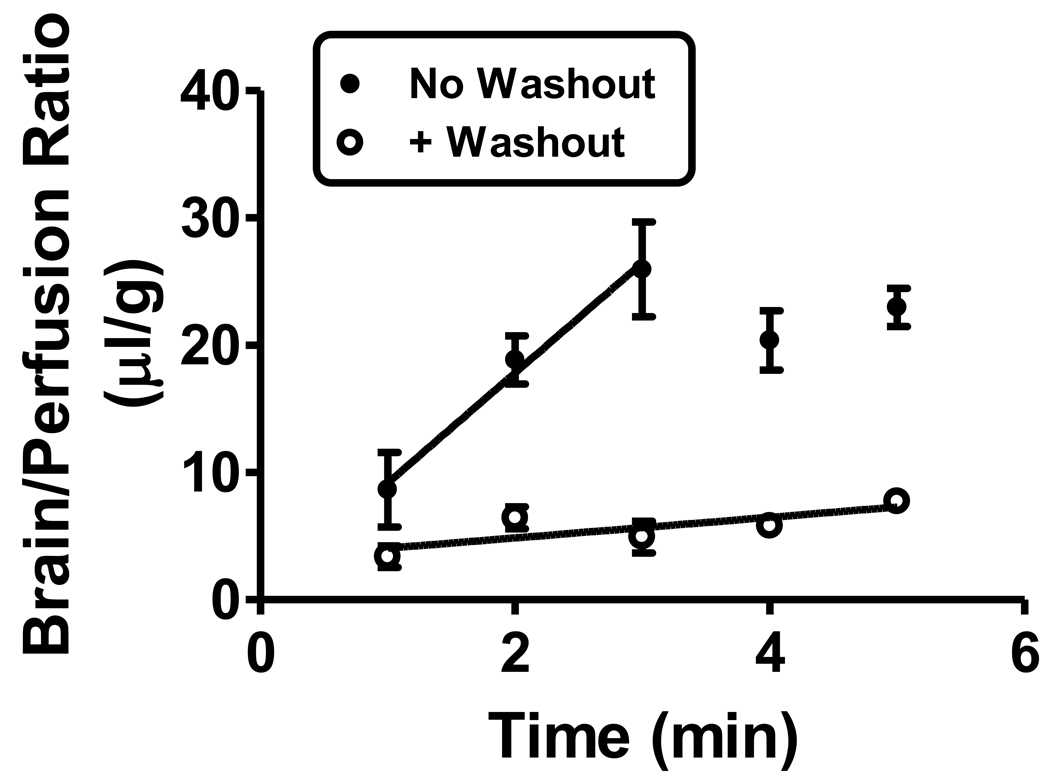
Brain perfusion (Figure 5) showed a linear uptake to 3 min with the unidirectional influx rate measured at 8.65 ± 1.96 µl/g-min (r = 0.858, n = 9, p<0.01). After this time, brain/perfusion ratios reached a plateau between 20–25 µl/g. Washout reduced the uptake rate to 0.809 ± 0.299 µl/g-min (r = 0.601, n = 15, p<0.05). The slopes of these two lines were statistically different: F(1,20) = 31.4, p<0.0001. The large decrease with washout is attributed to I-LPS that was reversibly binding to the luminal surface of the brain’s capillary bed.
Figure 5.
Brain perfusion followed with or without washout. Without washout, uptake of I-LPS was measured to be 8.65 ± 1.96 µl/g-min (n = 9); this compares to an unmeasurable uptake by brain after iv injection as shown in Figure 1, lower panel. This indicates that circulating factors retarded uptake. With vascular washout, this uptake was greatly reduced, demonstrating that most of the uptake is likely reversible binding to the luminal surface of brain endothelial cells.
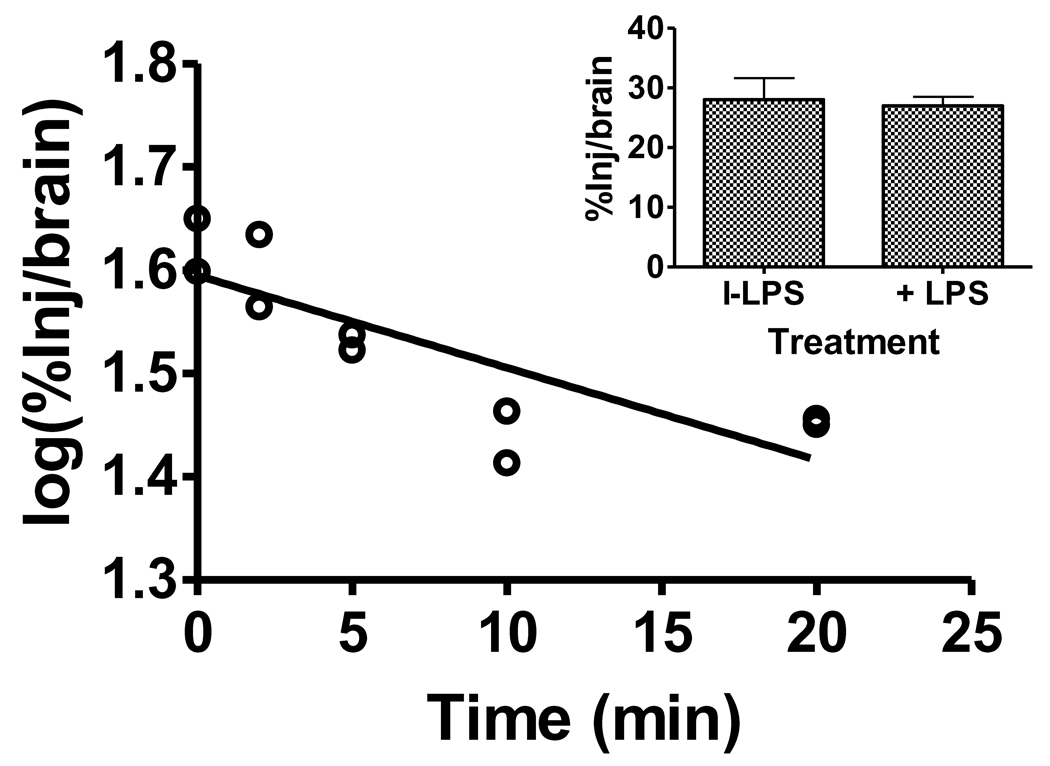
The rate of brain-to-blood clearance was measured after the injection of I-LPS into the lateral ventricle of the brain. A correlation between log(%Inj/brain) and time was statistically significant (r = 0.816, n = 10, p<0.005), demonstrating a measurable brain-to-blood efflux of I-LPS (Figure 6, main panel). The half-time clearance rate from brain was 33.7 min. Inclusion of 1 µg/mouse of unlabeled LPS did not produce inhibition in the efflux of I-LPS, indicating that the efflux is likely dependent on reabsorption of cerebrospinal fluid (Figure 6, inset, n = 6–8/group).
Figure 6.
Brain-to-blood efflux of I-LPS after intracerebroventricular injection. Main panel shows efflux rate of 33.7 min. Each point represents the mean of 3 mice. Inset shows that inclusion of unlabeled LPS did not alter efflux of I-LPS from brain (n = 6–8/group).
Discussion
These studies examined the ability of LPS labeled with radioactive iodine to cross the blood-brain barrier. We examined the ability of I-LPS to cross in both the blood-to-brain direction and in the brain-to-blood direction. We determined the influences of circulating factors, saturable processes, enzymatic degradation, BBB disruption through activation of the innate immune system, and capillary adhesion on the permeability process. In general, we found that I-LPS reversibly adhered to and was sequestered by brain endothelial cells with circulating factors greatly retarding BBB uptake. Very little I-LPS penetrated the brain, even when the BBB had been disrupted by repeated administration of LPS. However, enough LPS crosses in the blood-to-brain direction to possibly induce the most sensitive brain effects when the highest LPS doses are given. We also confirm that LPS crosses in the brain-to-blood direction, consistent with the previous suggestion that CNS LPS can be a source of blood LPS (Chen et al., 2000).
We first examined the clearance of I-LPS from blood. The amount of an intravenous dose entering the brain is a function of peripheral pharmacokinetics and the rate of passage across the BBB. We found that I-LPS distribution was confined to the vascular space and was very slowly cleared from the blood with a half-life of 259 min. Both the small Vd and long half-life would favorably influence uptake by brain. Both Sephadex chromatography and acid precipitation indicated that the recovered radioactivity represented I-LPS. This would also favor brain uptake. However, I-LPS was very water soluble as measured by the octanol/buffer partition coefficient and this would not favor passage across the BBB by nonsaturable processes.
Brain/serum ratios did not increase over time. This, along with a low brain/serum ratio that averaged 11.3 µl/g over the course of the study, indicated that little or no I-LPS crossed the BBB. However, the brain/serum ratio of 11.3 µl-g/min is in the upper range for vascular space markers. This raised the possibility that a small amount of I-LPS was interacting with or perhaps even crossing the BBB. To determine whether there was a predominating early phase uptake as can occur with robust brain-to-blood efflux systems, we examined uptake at 1 and 2 min after the iv injection of I-LPS. Although this study confirmed brain/serum ratios in the upper range of vascular markers, no evidence for an early phase penetration was found.
We further examined the early time points of 1, 2, and 5 min by including radioactively labeled albumin as the vascular marker. We included groups in which 100 µg of LPS was added to the iv injection and groups in which the vascular space of the brain was washed out. The purpose for adding the 100 µg of unlabeled LPS was not to activate the innate immune system as 5 min of exposure is too short a time for that, but to assess whether there was a saturable component to I-LPS uptake. Given our study above showing a Vd for I-LPS of about 1.5 ml, an iv injection of 100 µg would have produced serum levels of about 67 µg/ml. These studies revealed several interesting aspects about I-LPS interactions with the BBB. Delta values (I-LPS values corrected for the albumin space) are shown in figure 2. These delta values show that about 3 µl/g of the I-LPS space was unaccounted for by the I-Alb space. This clearly demonstrates that I-LPS is interacting with the BBB in some way. This interaction could be reversible binding to the luminal surface of brain endothelial cells, internalization/sequestration by the cells comprising the BBB, or permeation across the BBB. Washout of the vascular space greatly reduced the delta brain serum ratios from 3–4 µl/g to about 1 µl/g. This demonstrated that about 75% of the extra-albumin space represented I-LPS that was reversibly bound to the luminal surface of the capillary bed of the brain. Inclusion of 100 µg of unlabeled LPS had no affect on either the albumin or delta I-LPS space in either washout or no washout animals. This shows that acute LPS did not disrupt the BBB to albumin and that there was no saturable binding site for I-LPS that was demonstrable at this dose of LPS.
We then addressed the issue of whether the approximately one µl of I-LPS that is extravascular and not accounted for by reversible luminal binding was being sequestered by brain endothelial cells or was entering brain parenchyma. We investigated this by performing capillary depletion in mice that had both albumin used as a vascular marker and underwent washout. We found similar results at both10 min and 120 min after the iv injection of I-LPS. These results show that the 1 µl was about evenly divided between I-LPS sequestered by the brain endothelial cells and I-LPS entering the brain parenchyma space. The percent of the intravenous dose of I-LPS that was taken up by the parenchyma space was 0.026 %Inj/g at 10 min. The value at 120 min was 0.013 %Inj/g if corrected for the estimated leakage of the BBB and 0.031 %Inj/g if leakage was included. Using either calculation, the amount of I-LPS entering the brain parenchyma space is low, ranging somewhere between 0.013 and 0.031 %Inj/g over the 120 min period studied. For subsequent calculations below, we use the value of 0.025 %Inj/g as representative of the values during this time period.
The capillary depletion studies were key to determining that a residual of I-LPS did enter the CNS. There are 3 potential sources of falsely elevated values for the parenchyma fraction and we took care to negate each of them. First is the possibility that I-LPS weakly adhering to the luminal surface of the endothelial cell would detach during processing and so present in the parenchyma fraction. We used brain washout of the vascular space, a method devised to counter this problem (Samii et al., 1994). Second is underestimation of the albumin space; here we used the combination of washout and radioactively labeled albumin to completely correct for the albumin space. Third is the acknowledgment that capillary depletion is an enrichment method with some capillary fragments remaining behind in the parenchyma. Any radioactivity associated with those capillaries would be erroneously mistaken as penetration into the parenchyma. However, the vascular marker gamma-glutamyl transpeptidase can be used as a measure of such contamination. As originally devised for rats (Triguero et al., 1990), capillary contamination is less than 5% and, as adapted by us to mice (Gutierrez et al., 1993), the contamination is less than 2%. As the values here for parenchyma exceeded this level of contamination, we can conclude that a small, but reliably measured amount of I-LPS enters the brain parenchyma space.
This raises the question of whether enough LPS enters the brain to active CNS receptors. The doses of LPS used in rodents vary greatly, most doses are given in kg, and most are given as intraperitoneal injections. In mice for example, most studies use doses between from 0.1–5 mg/kg (Alheim et al., 1997; Kozak et al., 1995) which, if an average weight of 25 g/mouse is assumed, gives a dose per mouse of 2.5 to 125 µg/mouse. It is unclear how much of an ip dose of LPS is absorbed into the blood stream, but for the purposes of the current calculations we will assume that iv and ip dosing are not different. To the extent that an ip dose is less than 100% bioavailable or produces lower blood levels than an iv injection, the following calculations will overestimate the amount of I-LPS entering the brain after an ip injection. An uptake of about 0.025 %Inj/g of brain of the injected dose is suggested by the capillary depletion studies above and would produce a range in brain of 0.6 to 31 ng of LPS per g of brain. Given an average brain weight for an adult mouse of 0.45 g, this would 0.3 to 14 ng/whole brain. The amount of LPS needed to induce an effect within the brain depends on the event being induced. For example, Johnson et al (Johnson et al., 1997) found icv LPS affected social exploration and weight loss at 100 ng/mouse, but not at 10 ng/mouse, whereas food motivated behavior, object recognition, and rearing were affected at 3 ng/mouse. Szczepanki et al (Szczepanki & Ringheim, 2003) found that MCP-1 levels in the cortex were stimulated by 30 ng/mouse icv but not by 3 ng/mouse, whereas levels of 300 ng were needed to stimulate IL-1β, 1 µg/mouse to stimulate IL-6, and 30 µg to stimulate TNF. Assuming similar blood levels after iv and ip injections, our results support the hypothesis that enough LPS enters the brain at the highest doses used in the literature to stimulate the most LPS-sensitive parameters of the brain. Our results would suggest that at the lower doses of ip LPS, the amount entering brain is below that needed to directly affect the brain. In these cases, peripherally administered LPS would not be affecting brain by acting through CNS receptors behind the BBB but through indirect mechanisms such as vagal stimulation, release of substances from the periphery that can cross the BBB, enhanced interactions with immune cells, action at circumventricular organs, and by inducing release of substances from brain endothelial cells.
A related question is whether LPS reaches the brain in those conditions in which LPS chronically circulates in blood. Recent work has shown that LPS is detectable in blood primarily because of bacterial translocation in several disease conditions including AIDS gastroenteropathy, peridontal disease, or strenuous exercise (Brenchley et al., 2006; Pussinen et al., 2004; Ng et al., 2008). Blood levels are usually in the low pg or ng/ml range (Pussinen et al., 2004; Ng et al., 2008). Our work here calculated pharmacokinetic parameters after acute administration of I-LPS and so we can only extrapolate to the chronic situation with caution. However, it is likely that given that capillary depletion showed that less than a µl/g was taken up into brain parenchyma, a low ng/ml level of LPS in blood will likely produce low-mid pg/g of LPS in brain. These levels are likely too low to stimulate brain receptors, even with chronic exposure.
We examined I-LPS by brain perfusion, a method in which the influence of circulating factors is eliminated. We found that I-LPS uptake for the first 3 min was relatively high at over 8 µl/g-min. This higher value with brain perfusion shows that the circulating factors exert a largely inhibitory effect on the association of I-LPS with the BBB. Brain perfusion followed with vascular washout decreased the uptake rate to about 1/10th of the non-washout brain perfusion uptake rate. This shows that most of the extra uptake by the BBB in the absence of circulating factors was confined to the reversible luminal binding which is readily removed with vascular washout.
We then determined the effect of BBB disruption induced by LPS on the entry of I-LPS into brain. The rationale for this study is that the innate immune system will likely be stimulated in most cases in which the BBB is significantly exposed to LPS. This experiment contrasted with the work in figure 2 which examined the immediate effect of LPS on BBB uptake of I-LPS and I-Alb. The longest exposure of the mouse to LPS in the figure 2 experiments was 5 min, a time too short to induce an immune reaction. However, this is the ideal experimental design to look for saturable uptake or binding of LPS. In figure 4, we studied mice that had received 3 injections of LPS over a 24 h period. This paradigm usually disrupts the BBB, enhances adsorptive transcytosis across the BBB, and induces some BBB transporters such as that for insulin (Banks et al., 1999; Xaio et al., 2001; Banks et al., 2008). Here, we found that the BBB was indeed disrupted to I-Alb. However, I-LPS uptake was not statistically enhanced and was even arithmetically lower than in control mice. The cause for this paradoxic decrease is not clear. Two possibilities are that activation of the innate immune system is accompanied by an increase in the blood-borne factors noted above to retard I-LPS association with the BBB and another is that brain-to-blood efflux of LPS is enhanced with inflammation. Our main finding, however, is that repeated exposure to LPS sufficient to disrupt the BBB does not increase I-LPS uptake by brain.
Finally, we examined the ability of I-LPS to cross the BBB in the brain-to-blood direction. The presence of a rapid efflux system would greatly alter the interpretation of many of the above studies. For example, a very robust efflux system can prevent accumulation by brain of a substance that otherwise can cross the BBB. However, we found efflux of I-LPS to be slow and non-saturable. This is consistent with I-LPS re-entering the blood stream with the reabsorption of CSF, termed bulk flow. These findings confirm those of Chen et al in the rat (Chen et al., 2000) who also found brain-to-blood passage of I-LPS at a rate suggestive of bulk flow and of Goralski et al (Goralski et al., 2005) who found 1–10 ng/ml of LPS in blood after the icv injection of 250 µg/mouse of LPS. Based on these papers, there are at least two ramifications of even this slow reabsorption of LPS. First, it provides a mechanism by which LPS produced by an infection in the CNS can enter the circulation, thus inducing an inflammatory response in the periphery. Second, in experiments in which a high dose of LPS is given into the CNS, enough LPS may enter the circulation to invoke an immune response. In fact, Chen et al found that about 2 h after injection, levels of LPS in blood were higher after icv injection than after iv injection. Because of efflux, central administration of LPS does not guarantee that resulting effects are centrally mediated.
In conclusion, very little of an intravenously administered dose of LPS interacts with the BBB. The majority, about 75%, of the LPS that does interact with the BBB is reversibly binding to the luminal surface of the BBB. Circulating factors greatly retard the interaction of LPS with the BBB and luminal binding is enhanced when those factors are removed. A small residual of LPS is sequestered by the brain endothelial cells and a small amount enters the brain parenchyma. The amount of LPS entering brain parenchyma is about 0.025% of an intravenously administered dose. Based on studies of the effects of icv dosing from the literature, this could be enough to produce the most sensitive effects in brain after the highest peripheral doses. However, our studies here would strongly suggest that most effects induced by the acute peripheral administration of LPS are not mediated through brain receptors.
Acknowledgments
Supported by VA Merit Review, NS05047, and AG029839.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheim K, Chai Z, Fantuzzi G, Hasanvan H, Malinowsky D, Di Santo E, Ghezzi P, Dinarello CA, Bartfai T. Hyperresponsive febrile reactions to interluekin (IL) 1α and IL-1β and altered brain cytokine mRNA and serum cytokine levels, in IL-1β-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:2681–2686. doi: 10.1073/pnas.94.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Dohgu S, Nakaoke R, Lynch JL, Fleegal-DeMotta MA, Erickson MA, Vo TQ. Nitric oxide isoenzymes regulate LPS-enhanced insulin transport across the blood-brain barrier. Endocrinology. 2008;149:1514–1523. doi: 10.1210/en.2007-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE. Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. Journal of Gerontology: Biological Science. 2000;55A:B601–B606. doi: 10.1093/gerona/55.12.b601. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Quantifying carrier-mediated transport of peptides from the brain to the blood. In: Conn PM, editor. Methods in Enzymology. vol 168. San Diego: Academic Press; 1989. pp. 652–660. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Brennan JM, Vallance KL. Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp. Neurol. 1999;156:165–171. doi: 10.1006/exnr.1998.7011. [DOI] [PubMed] [Google Scholar]
- Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Transport of amyloid β protein antibody across the blood-brain barrier in an animal model of Alzheimer's disease. Peptides. 2002;23:2223–2226. doi: 10.1016/s0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- Blasberg RG, Fenstermacher JD, Patlak CS. Transport of α-aminoisobutyric acid across brain capillary and cellular membranes. J. Cereb. Blood Flow Metab. 1983;3:8–32. doi: 10.1038/jcbfm.1983.2. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Bealer SL, Hunter WS, Llanos QJ, Ahokas RA, Mashburn TA., Jr. Suppression of fever after lesions of the anteroventral third ventricle in guinea pigs. Brain Res. Bull. 1983;11:519–526. doi: 10.1016/0361-9230(83)90124-7. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV injection. Nature Medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, McCuskey RS, Reichlin S. Blood interleukin-6 and tumor necrosis factor-α elevation after intracerebroventricular injection of Escherichia coli endotoxin in the rat is determined by two opposing factors: peripheral induction by LPS transferred from brain to blood and inhibition of peripheral response by a brain-mediated mechanism. Neuroimmunomodulation. 2000;8:59–69. doi: 10.1159/000026454. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Journal. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1 beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goralski KB, Abdulla D, Sinal CJ, Arsenault A, Renton KW. Toll-like receptor-4 regulation of hepatic Cyp3all metabolism in a mouse model of LPS-induced CNS inflammation. Am. J. Physiol. 2005;289:G434–G443. doi: 10.1152/ajpgi.00562.2004. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Komaki G, Arimura A. Increased circulating interleukin-1 and interleukin-6 after intracerebroventricular injection of lipopolysaccharide. Neuroendocrinology. 1992;56:935–938. doi: 10.1159/000126328. [DOI] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Gheusi G, Segreti S, Dantzer R, Kelley KW. C3H/HeJ mice are refractory to lippolysacchardie in the brain. Brain Res. 1997;752:219–226. doi: 10.1016/s0006-8993(96)01454-0. [DOI] [PubMed] [Google Scholar]
- Kozak W, Conn CA, Klir JJ, Wong GH, Kluger MJ. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiology. 1995;269:R23–R29. doi: 10.1152/ajpregu.1995.269.1.R23. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain, Behavior, and Immunity. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, von Bernhardi R, Bu G, Inestrosa NC. Expression of alpha(2)-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J Neurosci Res. 2000;60:401–411. doi: 10.1002/(SICI)1097-4547(20000501)60:3<401::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ng QY, Lee KW, Byrne C, Ho TF, Lim CL. Plasma endotoxin and immune responses during a 21-km road race under a warm and humid environment. Ann Acad Med Singapore. 2008;37:307–314. [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Pussinen Pj, Vilkuna-Rautiainen T, Alfthan G, Palosuo T, Jauhiainen M, Sundvall J, Vesanen M, Mattila K, Asikainen S. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24:2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. GLIA. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, He L, Lai W. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res. Bull. 2003;59:447–452. doi: 10.1016/s0361-9230(02)00951-6. [DOI] [PubMed] [Google Scholar]
- Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49:125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999;851:215–220. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- Romeo HE, Tio DL, Rahman SU, Chiappelli F, Taylor AN. The glossopharyngeal nerve as a novel pathway in immune-to-brain communication: relevance to neuroimmune surveillance of the oral cavity. J. Neuroimmunol. 2001;115:91–100. doi: 10.1016/s0165-5728(01)00270-3. [DOI] [PubMed] [Google Scholar]
- Samii A, Bickel U, Stroth U, Pardridge WM. Blood-brain barrier transport of neuropeptides: analysis with a metabolically stable dermorphin analogue. Am. J. Physiol. 1994;267:E124–E131. doi: 10.1152/ajpendo.1994.267.1.E124. [DOI] [PubMed] [Google Scholar]
- Shayo M, McLay RN, Kastin AJ, Banks WA. The putative blood-brain barrier transporter for the β-amyloid binding protein apolipoprotein J is saturated at physiological concentrations. Life Sci. 1996;60:L115–L118. doi: 10.1016/s0024-3205(96)00685-6. [DOI] [PubMed] [Google Scholar]
- Singh AK, Yiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Strosznajder J, Chalimoniuk M, Strosznajder RP, Albanese V, Alberghina M. Arachidonate transport through the blood-retina and blood-brain barrier of the rat during aging. Neurosci. Lett. 1996;209:145–148. doi: 10.1016/0304-3940(96)12624-0. [DOI] [PubMed] [Google Scholar]
- Sugita H, Kaneki M, Tokunaga E, Sugita M, Koike C, Yasuhara S, Tompkins RG, Martyn JA. Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am J Physiology. 2002;282:E386–E394. doi: 10.1152/ajpendo.00087.2001. [DOI] [PubMed] [Google Scholar]
- Szczepanki AM, Ringheim GE. IL-10 and glucocorticoids inhibit Aβ (1–42) and lipopolysaccharide-induced pro-inflammatroy cytokine and chemokine induction in the centeral nervous system. Journal of Alzheimer's Disease. 2003;5:105–117. doi: 10.3233/jad-2003-5205. [DOI] [PubMed] [Google Scholar]
- Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J. Neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978;15:157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ, Mathison JC, da Silva Correia J. Innate immune responses during infection. Vaccine. 2004;22 Suppl1:S25–S30. doi: 10.1016/j.vaccine.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Ulmer AJ, Rietschel ETh, Zahringer U, Heine H. Lipopolysaccharide: structure, bioactivity, receptors, and signal transduction. Trends in Glycoscience and Glycotechnology. 2002;14:53–68. [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain,Behavior, and Immunity. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, Narvaez A, Honrada R, Ruvalcaba D, McGrath MS. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2009;206:121–124. doi: 10.1016/j.jneuroim.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Lipovac MN, Begley DJ, Davson H, Rakic L. Slow penetration of thyrotropin-releasing hormone across the blood-brain barrier of an in situ perfused guinea pig brain. J. Neurochem. 1988;51:252–257. doi: 10.1111/j.1471-4159.1988.tb04864.x. [DOI] [PubMed] [Google Scholar]