Abstract
AIM: To summarize the clinical experience of laparoscopic hepatectomy at a single center.
METHODS: Between November 2003 and March 2009, 78 patients with hepatocellular carcinoma (n = 39), metastatic liver carcinoma (n = 10), and benign liver neoplasms (n = 29) underwent laparoscopic hepatectomy in our unit. A retrospective analysis was done on the clinical outcomes of the 78 patients.
RESULTS: The lesions were located in segments I (n = 3), II (n = 16), III (n = 24), IV (n = 11), V (n = 11), VI (n = 9), and VIII (n = 4). The lesion sizes ranged from 0.8 to 15 cm. The number of lesions was three (n = 4), two (n = 8) and one (n = 66) in the study cohort. The surgical procedures included left hemi-hepatectomy (n = 7), left lateral lobectomy (n = 14), segmentectomy (n = 11), local resection (n = 39), and resection of metastatic liver lesions during laparoscopic surgery for rectal cancer (n = 7). Laparoscopic liver resection was successful in all patients, with no conversion to open procedures. Only four patients received blood transfusion (400-800 mL). There were no perioperative complications, such as bleeding and biliary leakage. The liver function of all patients recovered within 1 wk, and no liver failure occurred.
CONCLUSION: Laparoscopic hepatectomy is a safe and feasible operation with minimal surgical trauma. It should be performed by a surgeon with sufficient experience in open hepatic resection and who is proficient in laparoscopy.
Keywords: Hepatectomy, Laparoscopy, Liver neoplasms
INTRODUCTION
Laparoscopic hepatectomy (LH) was first described in 1995 by Cuesta et al[1] and it remains an appealing concept: major surgery with a potential for bleeding, carried out using a minimally invasive approach. While the laparoscopic approach has been championed, notably by Cherqui et al[2] and Buell et al[3], it is carried out in only a few centers and has not become a widely accepted surgical technique. In general, laparoscopic liver resection offers some advantages over an open approach. There is less postoperative pain, early mobilization, minimal ileus, earlier resumption of oral intake and shorter hospital stay[4-7]. Initial minor hepatectomy for lesions located in superficial segments has been considered possible[8,9], with the development of laparoscopic instruments and parenchymal transection devices, combined with improved understanding of the vascular anatomy of the liver, it is now accepted widely that laparoscopy will be used increasingly in liver surgery[10].
Between November 2003 and March 2009, we performed total laparoscopic hepatectomy in 78 patients with liver neoplasms, the results of which were encouraging.
MATERIALS AND METHODS
Patients
Between November 2003 and March 2009, 78 LHs were performed in the Hepato-biliary Surgery Department at Sun Yat-sen Memorial Hospital of Sun Yat-sen University. Seventy-eight patients with liver neoplasms (50 men and 28 women), including primary liver carcinoma (n = 39), secondary liver carcinoma (n = 10), and benign liver neoplasm (n = 29). The mean age was 46.6 years (range, 20-82 years). Preoperative liver function was as follows: Child classification A (n = 52), B (n = 22) and C (n = 4). Evidence of cirrhosis, based on clinical and/or morphological abnormalities, was not considered an absolute contraindication in the absence of decompensated cirrhosis. There was no upper limit on neoplasm size, and LH was contraindicated if venous or biliary reconstruction was required.
Surgical procedure
All procedures were performed under general endotracheal anesthesia and after obtaining informed consent. All resections were performed with the patient in the supine position. The surgeon stood between the patient’s spread legs, the first assistant on the left side of the patient and the camera assistant on the right. The pneumoperitoneum was insufflated by umbilicus puncture and controlled electronically at a constant abdominal pressure of 12 mmHg. A 5-mm trocar placed at the umbilical port was used for abdominal exploration with a 30° laparoscope. The trocar insertion sites depended on the location of the hepatic lesion. The surgical technique was as follows. For mobilization of the liver, the falciform and round ligaments were divided by an endoscopic ultrasonic scalpel (LLC, Guaynabo, Puerto Rico). In the case of left hepatectomy, the left liver lobe was mobilized by dividing the left triangular, coronary, and hepato-gastric ligaments. In the case of right segmentectomy, the right coronary ligament was divided. It was not necessary to divide the ligament if the lesion was located at the edge of the liver. Laparoscopic ultrasonography (Esaote Biomedica, Genoa, Italy) was used for localization of the tumor and the supply vessels, demonstration of satellite nodules, and demarcation of an adequate tumor-free margin. To achieve afferent blood control in anatomical left liver resection, including left segmentectomy, left lateral lobectomy, and left hemi-hepatectomy, the portal pedicles were dissected outside the liver parenchyma, and the portal venous branch, hepatic arterial branch, and bile duct were separated, clipped and divided. When the branch was too large to apply clips, it was divided with an endoscopic linear stapler (Tyco Healthcare, Norwalk, CT, USA). In right liver resection, the supply vessels of the target segment were found by laparoscopic ultrasonography, and were clipped or divided with an endoscopic linear stapler extraparenchymally. To achieve efferent blood control, the inferior vena cava and left hepatic vein were dissected, and the trunk of the left hepatic vein was clipped with a 12-mm hemoclip, but was not divided before parenchymal division. Liver surface tissue transection was performed using an endoscopic ultrasonic scalpel, while deep parenchyma dissection was performed using LigaSure (Valleylab, Boulder, CO, USA); larger biliary and vascular radicals were clipped and divided. After careful hemostasis, fibrin glue sealant (FibinGluRAAS; Shanghai Raas Blood Products, China) was applied to the raw surface. The specimen was extracted using a plastic bag through an extending port site.
Assessment of operation
Surgical complications included biliary fistula, hemorrhage and incisional hernia. All patients underwent liver function tests and abdominal computed tomography (CT) before discharge. All patients underwent follow-up examination at 1-mo intervals after operation, and the follow-up examinations included clinical examination, liver function tests, and abdominal ultrasonography. No patient was lost at follow-up.
RESULTS
Operative results
Hepatic resection was performed laparoscopically in all 78 patients. There were no operative deaths and no conversions to laparotomy. The mean operation time was 165 min (range, 60-390 min). The mean blood loss was 288 mL (range, 10-1000 mL); only four patients received blood transfusion (400-800 mL). The mean size of the lesions was 6.2 cm (range, 0.8-15 cm) and the mean width of the specimen margins was 1.2 cm (range, 0.5-6 cm). The number of lesions was three (n = 4), two (n = 8), and one (n = 66) in the study cohort. Details of tumor sites are summarized in Table 1; Types of laparoscopic hepatectomy are given in Table 2; Details of histologic results are summarized in Table 3.
Table 1.
Tumor sites in 78 laparoscopic hepatectomy (LH) procedures
| n | |
| Segment I | 3 |
| Segment II | 16 |
| Segment III | 24 |
| Segment IV | 11 |
| Segment V | 11 |
| Segment VI | 9 |
| Segment VII | 4 |
| Total | 78 |
Table 2.
Types of LH n (%)
| n | |
| Anatomical resection | 32 (41) |
| Left hemi-hepatectomy | 7 |
| Left lateral hepatic lobectomy | 14 |
| Hepatic segmentectomy | 11 |
| Local resection | 39 (50) |
| Local liver resection for metastasis and colorectal laparoscopic resection | 7 (9) |
| Total | 78 |
Table 3.
Histological results in 78 LH procedures n (%)
| n | |
| Malignant tumor | 52 (67) |
| Hepatocellular carcinoma | 33 |
| Intrahepatic cholangiocarcinoma | 6 |
| Colorectal metastasis | 10 |
| Gastric carcinoma metastasis | 1 |
| Ovarian carcinoma metastasis | 1 |
| Kidney carcinoma metastasis | 1 |
| Benign tumor | 26 (33) |
| Haemangioma | 21 |
| Focal nodular hyperplasia | 3 |
| Granuloma | 1 |
| Adenoma | 1 |
| Total | 78 |
Postoperative recovery
There were no postoperative deaths or complications, such as bleeding and biliary leakage. Liver function returned to preoperative levels within 1 wk. Oral intake was begun on postoperative day 2. The mean postoperative hospital stay was 5.6 d (range, 2-10 d). The patients were discharged without narcotic analgesia. At the first postoperative month, all patients had resumed normal activities.
Follow-up
The mean follow-up was 30 mo (range, 2-60 mo). No port site metastasis was noted during follow-up in any patient who had hepatectomy for malignant lesions. Recurrence was detected in four patients with primary hepatocellular carcinoma and one with intrahepatic cholangiocarcinoma within 2 years. Two patients with primary hepatocellular carcinoma underwent a second LH for a recurrent lesion, and the other patients had transcatheter arterial chemoembolization and radiofrequency ablation (RFA). New isolated lesions in the liver were detected in two patients with rectal carcinoma within 1 year postoperatively, and were treated with RFA.
DISCUSSION
Laparoscopic liver resection has been viewed with skepticism because of concerns regarding parenchymal transection, bleeding control, bile leakage and incomplete resection[3,11,12]. The present study suggests that LH may be safe and feasible in selected patients. The indication for laparoscopic liver resection should be selected with care because of the technical difficulty, and the size and location of the neoplasm must be evaluated before surgery. In patients with neoplasms located in the left liver lobe and the anterior and inferior liver segments (IVb, V and VI), LH has been shown to be safe. On the other hand, neoplasms located in the right lobe and the posterior and superior liver segments (I, IVa, VII and VIII) are technically more demanding and should be approached with caution[13,14]. In our cohort, most lesions (91%) were located in segment II, III, IV, V and VI, and no lesion located in segment VII was treated because of the difficulty of exposure. A neoplasm < 6 cm in size is thought to be preferred[15,16]. In fact, we believe the size of the neoplasm is not as important as its location for LH. We performed laparoscopic left hemi-hepatectomy in four patients with 10-15-cm neoplasms in the left liver lobe, but without invasion of the inferior vena cava and the root of the hepatic vein. No conversion to laparotomy in our cohort may have been, at least in part, because of the choice of indication before surgery.
One of the main concerns during hepatectomy is minimizing blood loss and avoidance of blood transfusion[17,18]. In our experience, laparoscopic surgery may provide better visualization of deep vascular structures and more precise and accurate surgery. To avoid injury to the hepatic veins, caution should be exercised during manipulation of secondary hilar structures, and the hepatic veins should be transected in the parenchyma using clips or an endoscopic linear stapler. Conversion to laparotomy is mandatory when a large venous injury occurs, even if initial laparoscopic control of the wound has been achieved[11]. In the early stage of LH, bleeding during parenchymal transection due to a lack of effective devices is another important cause of blood loss, but there are now numerous excellent devices for dividing the parenchyma, including an ultrasonic scalpel, microwave tissue coagulator, water jet dissector, LigaSure, Cavitron ultrasonic surgical aspirator, argon beam coagulator, Peng’s Multifunction Operative Dissector, and TissueLink[19-26]. Based on our experience, using an ultrasonic scalpel and LigaSure to transect the liver facilitates good hemostasis, clear anatomy, less effusion, and minor damage to liver function. The average blood loss was only 200 mL in our 78 cases. Another concern that occurs during LH when resecting a malignant neoplasm involves achieving a disease-free resection margin[27,28]. We believe a 1-cm free surgical margin can be obtained using laparoscopic ultrasonography. In our research and a European multicenter study, there was no evidence that the use of a laparoscopic technique increases the risk of local recurrence and port-site metastases[29,30]. LH carries the potential risk of gas embolism caused by a pneumoperitoneum during hepatic vein division[31]. Although this potential complication appears to be rare, we utilized several precautions to avoid embolic events, including careful dissection of the hepatic vein, a low-pressure pneumoperitoneum, and preventive obstruction of the left hepatic vein with a 12-mm hemoclip.
LH has the advantage of minimal scarring and fewer adhesions, thereby increasing the feasibility of repeat liver resection. We performed repeat laparoscopic liver resections in two patients for recurrent lesions, and the results were satisfactory. LH may very well become the gold standard for tumor control in patients with hepatocellular carcinoma awaiting liver transplantation[32]. The choice of an incision is a dilemma in the colorectal cancer patient complicated by liver metastasis. The present study has shown that simultaneous laparoscopic resection of colorectal cancer and synchronous liver metastases is feasible and safe. Indeed, we performed this operation successfully in seven patients with five or six ports, just one or two more than laparoscopic resection of colorectal cancer, and no complications occurred. LH also has an irreplaceable advantage in patients with poor liver function. We performed LH in four patients with cirrhosis and Child C liver function in whom liver resection via laparotomy carried a high risk of complications. They all recovered rapidly without development of ascites and jaundice. In one hepatocellular carcinoma patient with cirrhosis and hepatitis C infection, liver function did not improve following medical treatment, yet returned to normal within 5 d after local laparoscopic tumor resection. LH might improve the postoperative course of patients with cirrhosis for the following reasons: (1) preservation of the abdominal wall and the round ligament avoids interruption of collateral circulation; (2) less mobilization and manipulation of the liver reduces liver trauma; and (3) non-exposure of abdominal viscera restricts fluid requirements and decreases electrolytic and protein losses[33-35]. We suggest that local laparoscopic liver tumor resection in patients with cirrhosis not only treats the tumor, but also creates a damaged liver environment with minimal liver load, which provides ideal conditions for regeneration of remnant liver. In fact, with CT scanning, we confirmed that the volume of the liver increased after LH in these patients (Figures 1 and 2).
Figure 1.
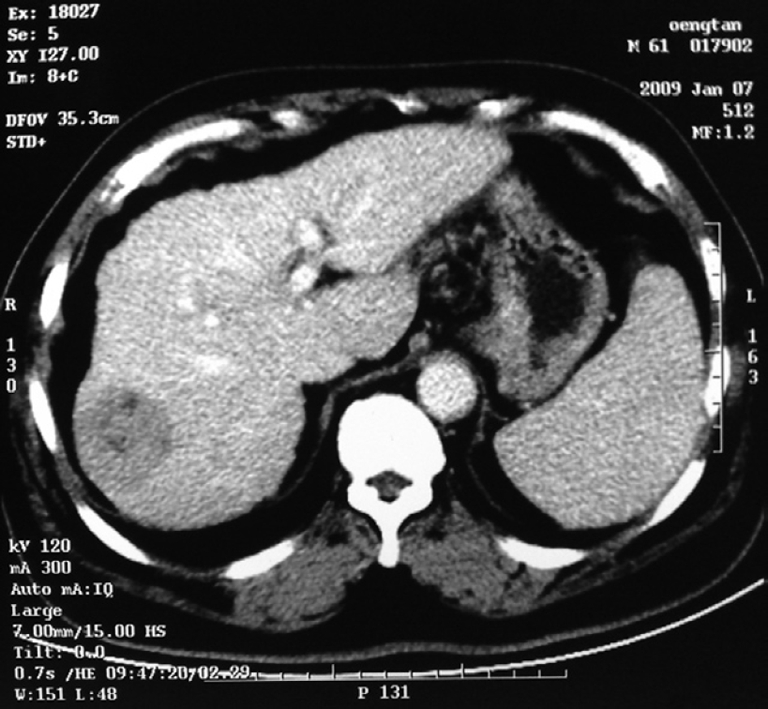
Computed tomography (CT) image before operation, which showed the lesion on the right lobe of the cirrhotic liver.
Figure 2.
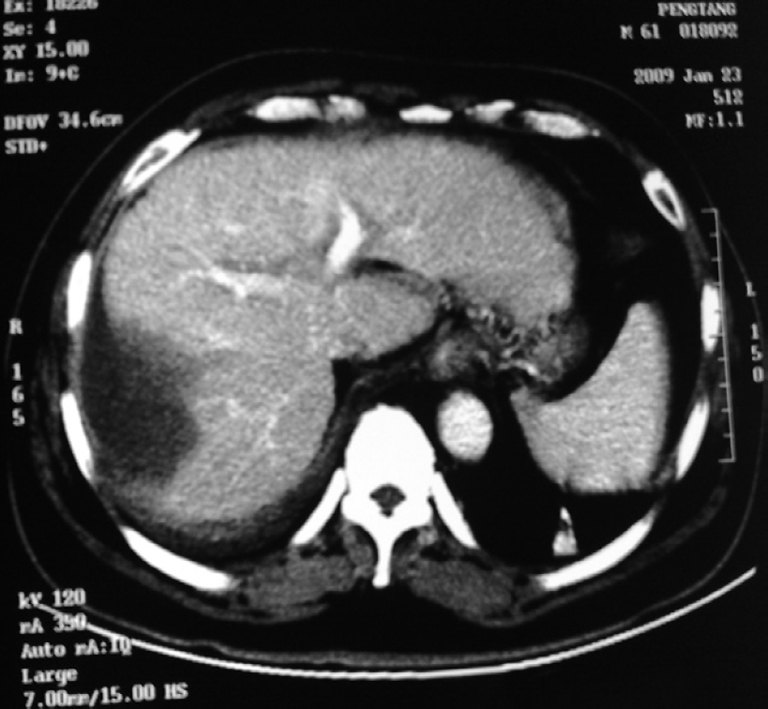
After laparoscopic hepatectomy (LH), the lesion disappeared and the volume of liver increased clearly.
Lastly, we advocate LH as a complex procedure that requires experience and different skills from those of open surgery because of the 2D representation of the operative site, limited tactile feedback, and the need for eye-hand coordination skills. The surgeons in our study all had sufficient experience with open hepatic resection and proficient skills in laparoscopic procedures, and also had the ability to manage the various complications of liver resection.
COMMENTS
Background
With the development of laparoscopic instruments and parenchymal transection devices, combined with the improved understanding of the vascular anatomy of the liver, it is now accepted widely that laparoscopy will be used increasingly in liver surgery.
Research frontiers
The underlying intent of laparoscopic surgery for liver neoplasms is to provide curative resection while minimizing complications. In this study, the authors demonstrated that laparoscopic hepatectomy (LH) could be safe and feasible, with minimal trauma.
Innovations and breakthroughs
This manuscript described the retrospective evaluation of a significant cohort of patients who underwent LH at a single center. The good clinical outcome depended on the reasonable choice of indication, suitable parenchymal transection devices, and sufficient experience in hepatic resection and laparoscopy.
Applications
LH could be performed in most patients with liver neoplasms, particularly in patients with cirrhosis and those with colorectal cancer and synchronous liver metastases.
Terminology
Liver resection is always considered a major operation, with blood loss and lengthy hospitalization. LH could benefit patients in need of liver resection, with better cosmetic results, less postoperative pain and shorter hospital stay.
Peer review
Interesting report about a single centre experience with 78 laparoscopic liver resections of different kind for different indications in different liver diseases.
Footnotes
Peer reviewers: Silvio Nadalin, MD, PhD, Director of Transplant Programm, Department of General, Visceral and Transplant Surgery, University Hospital Tuebingen, Hoppe Seyler Str 3, 72076 Tuebingen, Germany; Dr. Olivier Detry, Department of Abdominal Surgery and Transplantation, University of Liège, CHU Sart Tilman B35, B-4000 Liège, Belgium
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Cuesta MA, Meijer S, Paul MA, de Brauw LM. Limited laparoscopic liver resection of benign tumors guided by laparoscopic ultrasonography: report of two cases. Surg Laparosc Endosc. 1995;5:396–401. [PubMed] [Google Scholar]
- 2.Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, Rotman N, Fagniez PL. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, Rudich SM, Woodle ES. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804–811. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Laurence JM, Lam VW, Langcake ME, Hollands MJ, Crawford MD, Pleass HC. Laparoscopic hepatectomy, a systematic review. ANZ J Surg. 2007;77:948–953. doi: 10.1111/j.1445-2197.2007.04288.x. [DOI] [PubMed] [Google Scholar]
- 5.Rau HG, Buttler E, Meyer G, Schardey HM, Schildberg FW. Laparoscopic liver resection compared with conventional partial hepatectomy--a prospective analysis. Hepatogastroenterology. 1998;45:2333–2338. [PubMed] [Google Scholar]
- 6.Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, Tanaka S, Adachi E, Sugimachi K. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541–544. doi: 10.1007/s004640080099. [DOI] [PubMed] [Google Scholar]
- 7.Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg. 2002;9:242–248. doi: 10.1007/s005340200026. [DOI] [PubMed] [Google Scholar]
- 8.Fong Y, Jarnagin W, Conlon KC, DeMatteo R, Dougherty E, Blumgart LH. Hand-assisted laparoscopic liver resection: lessons from an initial experience. Arch Surg. 2000;135:854–859. doi: 10.1001/archsurg.135.7.854. [DOI] [PubMed] [Google Scholar]
- 9.Katkhouda N, Hurwitz M, Gugenheim J, Mavor E, Mason RJ, Waldrep DJ, Rivera RT, Chandra M, Campos GM, Offerman S, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg. 1999;229:460–466. doi: 10.1097/00000658-199904000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherqui D. Laparoscopic liver resection. Br J Surg. 2003;90:644–646. doi: 10.1002/bjs.4197. [DOI] [PubMed] [Google Scholar]
- 11.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 12.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914–1918. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 13.Mouiel J, Katkhouda N, Gugenheim J, Fabiani P. Possibilities of laparoscopic liver resection. J Hepatobiliary Pancreat Surg. 2000;7:1–8. doi: 10.1007/s005340050146. [DOI] [PubMed] [Google Scholar]
- 14.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236–242. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 15.Takagi S, Kaneko H, Ishii T, Tamura A, Yamazaki K, Yoshino M, Tsuchiya M, Joubara N, Otuka Y, Shiba T. Laparoscopic hepatectomy for extrahepatic growing tumor. Surgical strategy based on extrahepatic growing index. Surg Endosc. 2002;16:1573–1578. doi: 10.1007/s00464-001-9205-4. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–194. doi: 10.1016/j.amjsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740–748; discussion 748-749. [PubMed] [Google Scholar]
- 18.Makuuchi M, Takayama T, Gunvén P, Kosuge T, Yamazaki S, Hasegawa H. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989;13:644–648. doi: 10.1007/BF01658893. [DOI] [PubMed] [Google Scholar]
- 19.Navarra G, Spalding D, Zacharoulis D, Nicholls J, Kirby S, Costa I, Habib N. Bloodless hepatectomy technique. HPB (Oxford) 2002;4:95–97. doi: 10.1080/136518202760378470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldrighetti L, Pulitanò C, Arru M, Catena M, Guzzetti E, Casati M, Ferla G. Ultrasonic-mediated laparoscopic liver transection. Am J Surg. 2008;195:270–272. doi: 10.1016/j.amjsurg.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Satoi S, Kamiyama Y, Matsui Y, Kitade H, Kaibori M, Yamamoto H, Yanagimoto H, Takai S, Kwon AH. Clinical outcome of 214 liver resections using microwave tissue coagulation. Hepatogastroenterology. 2005;52:1180–1185. [PubMed] [Google Scholar]
- 22.Kjossev KT, Losanoff JE. Surgery for deeply located hydatid cysts of the liver: a simple alternative. HPB Surg. 2000;11:307–310. doi: 10.1155/2000/36518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda M, Hasegawa K, Sano K, Imamura H, Beck Y, Sugawara Y, Kokudo N, Makuuchi M. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Ann Surg. 2009;250:199–203. doi: 10.1097/SLA.0b013e3181a334f9. [DOI] [PubMed] [Google Scholar]
- 24.Eiriksson K, Fors D, Rubertsson S, Arvidsson D. Laparoscopic left lobe liver resection in a porcine model: a study of the efficacy and safety of different surgical techniques. Surg Endosc. 2009;23:1038–1042. doi: 10.1007/s00464-008-0115-6. [DOI] [PubMed] [Google Scholar]
- 25.Peng SY, Li JT, Mou YP, Liu YB, Wu YL, Fang HQ, Cao LP, Chen L, Cai XJ, Peng CH. Different approaches to caudate lobectomy with "curettage and aspiration" technique using a special instrument PMOD: a report of 76 cases. World J Gastroenterol. 2003;9:2169–2173. doi: 10.3748/wjg.v9.i10.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko H, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Yamazaki K. Application of devices for safe laparoscopic hepatectomy. HPB (Oxford) 2008;10:219–224. doi: 10.1080/13651820802166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 28.Shirabe K, Takenaka K, Gion T, Fujiwara Y, Shimada M, Yanaga K, Maeda T, Kajiyama K, Sugimachi K. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. [PubMed] [Google Scholar]
- 29.Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, Etienne J, Marescaux J, Mutter D, van Krunckelsven L, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236:90–97. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763–769; discussion 769. doi: 10.1001/archsurg.138.7.763. [DOI] [PubMed] [Google Scholar]
- 31.Takagi S. Hepatic and portal vein blood flow during carbon dioxide pneumoperitoneum for laparoscopic hepatectomy. Surg Endosc. 1998;12:427–431. doi: 10.1007/s004649900696. [DOI] [PubMed] [Google Scholar]
- 32.Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885–892; discussion 892-893. doi: 10.1097/01.sla.0000098621.74851.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdel-Atty MY, Farges O, Jagot P, Belghiti J. Laparoscopy extends the indications for liver resection in patients with cirrhosis. Br J Surg. 1999;86:1397–1400. doi: 10.1046/j.1365-2168.1999.01283.x. [DOI] [PubMed] [Google Scholar]
- 34.Takenaka K, Kanematsu T, Fukuzawa K, Sugimachi K. Can hepatic failure after surgery for hepatocellular carcinoma in cirrhotic patients be prevented? World J Surg. 1990;14:123–127. doi: 10.1007/BF01670561. [DOI] [PubMed] [Google Scholar]
- 35.Wu CC, Hwang CR, Liu TJ, P'eng FK. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver. Br J Surg. 1996;83:121–124. doi: 10.1002/bjs.1800830139. [DOI] [PubMed] [Google Scholar]


