Abstract
AIM: To investigate gut barrier damage and intestinal bacteria translocation in severe acute pancreatitis (SAP), a simple rat model of SAP was induced and studied.
METHODS: Pancreatitis was induced by uniformly distributed injection of 3.8% Na taurocholate (1 mL/kg) beneath the pancreatic capsule. Rats in the control group were injected with normal saline in the identical location.
RESULTS: Serum amylase, plasma endotoxin, intestinal permeability, and pancreatitis pathology scores were all markedly higher in the pancreatitis group than in the control group (P < 0.01). The bacterial infection rate was significantly higher in the SAP group than in the control group (P < 0.01), observed in parallel by both bacterial culture and real-time polymerase chain reaction. Acute damage of the pancreas was observed histologically in SAP rats, showing interstitial edema, leukocyte infiltration, acinar cell necrosis and hemorrhage. The microstructure of the intestinal mucosa of SAP rats appeared to be destroyed with loose, shortened microvilli and rupture of the intercellular junction, as shown by electron microscopy.
CONCLUSION: Significant gut barrier damage and intestinal bacterial translocation were definitely observed with few potential study confounders in this SAP rat model, suggesting that it may be an appropriate animal model for study of gut barrier damage and bacterial translocation in SAP.
Keywords: Acute pancreatitis, Bacterial translocation, Inflammation, Real-time polymerase chain reaction
INTRODUCTION
A close link has been demonstrated between intestinal bacteria translocation and infection in pancreatic necrosis, which is a main predictor of clinical outcome in patients with severe acute pancreatitis (SAP)[1]. Although a multitude of animal models[2-5] have been used to study the mechanism of bacterial translocation, its exact origin, route, and mechanism are still unclear. The main reason for this uncertainty is the lack of an “ideal” animal model of acute pancreatitis (AP), especially for the purpose of studying bacterial translocation.
Taurocholate-induced pancreatitis animal models are commonly used nowadays, especially the model induced by perfusion of taurocholate into the biliary or pancreatic duct, which is thought to most closely resemble clinical biliary pancreatitis. However these models have some shortcomings such as prolonged preparation time, difficult operation, high death rate, and potential infection of the pancreas when puncturing through the gut, which limit their further popularity in study of bacterial translocation in SAP.
To overcome these shortcomings, in this paper we induced a taurocholate pancreatitis rat model by uniformly distributed injection of taurocholate directly beneath the pancreatic capsule. Gut barrier damage and intestinal bacterial translocation were studied and the advantages of the model were characterized.
MATERIALS AND METHODS
Animals and animal procedures
Thirty two specific pathogen-free rats (half male and half female, obtained from the Experimental Animal Center of Guangdong Province, China), were randomly divided into 2 groups (16 rats in the experimental group, 16 in the control group). Average weight at the time of the experiment was 250 ± 20 g. Anesthesia was performed with intraperitoneal injection of 10% chloral hydrate 3 mL/kg.
The study and all procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University, China.
Model induction procedure
Pancreatitis group: rats were fasted for 12 h before the operation. A 10 mm median laparotomy was made and the pancreas was well exposed. The pancreatic capsule was gently lifted up, and 3.8% Na taurocholate 1 mL/kg was injected beneath the pancreatic capsule and distributed by making a bubble of taurocholate, about 2-3 mm in diameter at every injection point. Several minutes later, the pancreatic tissue would become dark purple, swell, and bleed locally. Then the abdominal organs were gently repositioned, and the peritoneal cavity was douched with about 50 mL of normal saline. Finally the abdominal wall was sutured.
In the control group, rats were injected similarly with normal saline 1 mL/kg in place of Na taurocholate.
Post-surgery breeding
After surgery, rats were housed in a dry and clean room at a temperature of 20-26°C, and 12 h later standard chow and water were freely available. Mortality was observed and recorded every day.
Determination of serum amylase and plasma endotoxin level
Serum amylase and plasma endotoxin were detected by routine clinical chemistry methods. Serum amylase was measured at 3 time points, namely 6, 24 and 48 h. On the 4th and 7th days, plasma endotoxins of every live rat were measured.
Determination of gut permeability by measurement of serum fluorescent isothiocyanate dextran (FITC-Dextran) in the superior mesenteric vein (SMV)
FITC-Dextran (Sigma), a polysaccharide macromolecule with a fluorescent marker, fluorescein isothiocyanate, cannot be digested and absorbed when in the normal alimentary tract, but can pass through the damaged gut barrier into capillaries of the gut, and finally into the blood of the SMV when intestinal permeability is increased[6-9]. The concentration of FITC-Dextran in the SMV depends on the dose of the drug and the severity of gut barrier damage. In this study, rats were perfused with 1 mL of 0.5% FITC-Dextran through a small tube placed in the stomach via the mouth, and 12 h later SMV blood samples were taken to measure the FITC-Dextran concentration as follows: blood was centrifuged at 1000 r/min, the supernatant was obtained, and absorbance was detected on a spectrofluorometer (Thermo Ltd., USA) at an excitation wave of 490 nm and transmission wave of 520 nm; the concentration of FITC-Dextran was finally calculated according to the standard curve of FITC-Dextran.
Bacterial culture for detection of infection rates and real-time polymerase chain reaction (PCR) to quantify the total number of translocated bacteria
One millilitre of blood from the inferior vena cava, 0.1 g mesenteric lymph node (MLN) tissue, 0.3 g pancreas tissue and 1 g liver tissue were taken from each rat under sterile conditions by laparotomy. Every sample had 1 mL saline added followed by homogenization by triturating, then uniform smearing on 4 brain heart solid culture medium (Hopebiotechnology Co. Ltd., Qingdao, China), 2 of which were placed in a 37°C thermostat, the other 2 were placed in an anaerobic jar, tightly closed and put in a 37°C thermostat. Results were observed and recorded 48 h later.
The same quantities of samples as above were taken for real-time PCR. Total bacterial genomic DNA was extracted by the DNeasy Blood & Tissue kit (Cat. 69506, Qiagen, USA) according to the manufacturer’s instructions. Li et al[10] confirmed the appropriateness and effectiveness of this kit for studying gut microbial ecology. The universal PCR primer sets for total bacteria (forward: TCCTACGGGAGGCAGCAGT; reverse: GGACTACCAGGGTATCTAATCCTGTT) used in this study were designed for a region in 16SrRNA and had been well demonstrated by other researchers to be uniformly successful in detecting a wide range of bacteria in samples of dentine[11] and feces[12]. Real-time PCR was performed with the ABI-Prism 7900 Sequence Detection System (Applied Biosystems, USA). The PCR reaction was performed in a total volume of 25 μL, containing SYBR Green Real-time PCR Master Mix (TOYOBO Inc., Japan) 12.5 μL, with 200 nmol/L of each of the forward and reverse primers and 2 ng of DNA for each reaction. The PCR reaction conditions for amplification of DNA were 95°C for 1 min and 40 cycles of 95°C for 20 s and 60°C for 1 min. Data analysis was performed using Sequence Detection Software supplied by Applied Biosystems.
Pancreatic pathology
The pancreas was cut, formalin-fixed and embedded in paraffin, and 4 μm sections were cut and stained with hematoxylin and eosin. The pancreatitis pathology score of every rat at both the 4th and 7th days were determined according to the severity of pancreatic damage by a pathologist using a double-blind method, according to the Schmidt Scoring Criteria[13] (Table 1).
Table 1.
Pancreatitis histopathology: Schmidt Scoring Criteria
|
Score |
||||
| 0 | 1 | 2 | 3 | |
| Interstitial edema | None | Interlobular | Lobule involved | Isolated island-like acinar cells |
| Leukocyte infiltration | None | < 20% | 20%-50% | > 50% |
| Acinar cell necrosis | None | < 5% | 5%-20% | > 20% |
| Hemorrhage | None | 1-2 points | 3-5 points | > 20% |
The Schmidt Scoring Criteria of pancreatitis pathology includes 4 aspects, including interstitial edema, inflammatory infiltration, parenchymal necrosis and hemorrhage, all of which were given scores of 0-3 according to the severity of the disease.
Intestinal mucosa electron microscopy
The 4th day, which was the mid-term course of the illness, was chosen to determine the microstructural changes in the intestinal mucosa. In order to sample from the same intestinal location of all rats, a 2 cm section of the distal ileum (next to the ileocecal valve) was taken and quickly placed in fixative solution, and sent to the Electron Microscopy Center of Sun Yat-sen University for electron microscopy examination.
Animal mortality
Daily observation of animal deaths was made for 7 d. The cumulative mortality rate was calculated daily.
Statistical analysis
Data were displayed as the mean ± SE. Statistical significance was calculated by one-way analysis of variance or the χ2 test, using the Sigma Plot Software Package (Systat Software Inc, Point Richmond, Ca, USA).
RESULTS
Abnormal performance of rats
Pancreatitis group: Several minutes after injection of taurocholate beneath the pancreatic capsule, the pancreas was found to become swollen, dark purple, with partial subcapsular hemorrhage. The rats took more time to wake after surgery than control rats, and were tired on the first day with reduced activity, no appetite, and slow responses. Conditions became worst on the 2nd and 3rd days, when some rats were lethargic, had conjunctival congestion or petechiae, abdominal distention and hyperventilation and some rats died. However after the 4th day, the surviving rats would always recover gradually.
Control group: Rats awoke soon after operation, recovered in 6-8 h with normal characteristics and activity. 24 h later the rats performed as normal, and with no deaths.
Feasibility of the surgical procedure
Uniformly distributed injections beneath the pancreatic capsule were successfully achieved in all animals and required an average of 15 ± 2 min per animal when the technique was well mastered. The cumulative death rate at 7 d in the pancreatitis group was about 30%, with most occurring on the 3rd and 4th days (about 80% of all), while no deaths occurred in the control group.
There were 2 main points of note during the operation. First, a puncture too deep into the pancreatic parenchyma could injure the pancreatic veins and cause direct bleeding, but simply pressing with aseptic gauze for a moment would stop this. Second, if the syringe needle pierced through the pancreatic capsule, or taurocholate bubbles under the pancreatic capsule burst, the taurocholate could go into the abdominal cavity and cause biliary peritonitis. However, as a whole, the operation itself was simple and safe, causing few complications.
Rat autopsy
Autopsies were carried out on dead rats. In all cases, much yellow or bloody exudate with a slight smell would come out of the peritoneal cavity when the abdomen was opened. The intestine would expand to 2-3 times of normal with gas and fluid inside and with a thinner wall. The pancreas was swollen and dark colored with some local hemorrhagic spots. Yellow-white saponified spots were always found in the peripancreatic omentum and retroperitoneum. The lower part of the spleen was always dark black. A pancreatic or hepatic abscess could be observed if the rat died after the 4th day or later.
Serum level of amylase
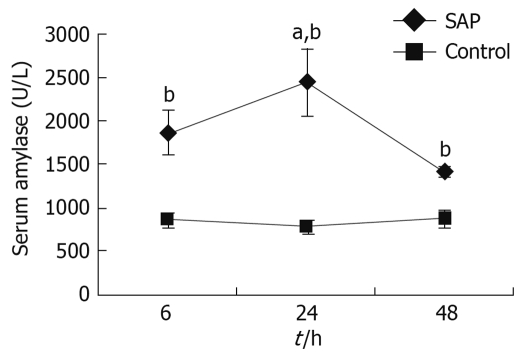
In pancreatitis rats, compared with the control group, serum amylase began increasing 6 h after the operation, and the level reached a peak at 24 h, remaining higher than the control group 48 h later (Figure 1).
Figure 1.
Amylase level in serum. Compared with control animals, serum amylase levels in severe acute pancreatitis (SAP) rats were higher at each time point (6, 24 and 48 h), bP < 0.01. At 24 h, the amylase level of the pancreatitis model reached a peak, and was higher than the other 2 time points, aP < 0.05.
Pancreatic histology
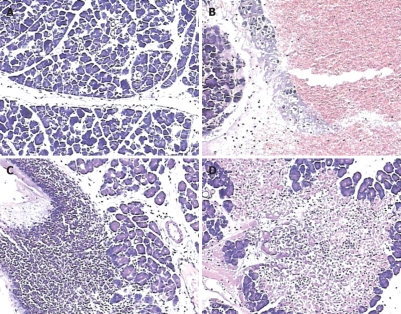
Microscopic pancreatic damage was pronounced in pancreatitis animals in the first 48 h and showed a progressive disease course; 6 h after pancreatitis induction, typical pathological features were edema and hemorrhage (Figure 2B), while apparent leukocyte infiltration was observed 24 h after operation (Figure 2C), and areas of acinar cell necrosis could be observed 48 h after operation (Figure 2D). The control animals were observed without such abnormalities, and had normal pancreatic gland lobules and cells. (Figure 2A)
Figure 2.
Pancreatic pathology (HE, × 100). A: Normal pancreatic histology; B: Pancreatic damage induced 6 h after pancreatitis induction. Typical pathological features were edema and hemorrhage; C: Apparent leukocyte infiltration was observed 24 h after surgery; D: Pancreatic pathology induced 48 h after surgery, showing a confluent area of acinar cell necrosis.
On both the 4th and 7th days, pancreatitis pathology scores according to the Schmidt criteria were observed to be significantly higher in the pancreatitis group than in the control group in all 4 single aspects, as well as the total score (P < 0.01, Table 2), showing that significant damage had occurred in the pancreas of SAP rats.
Table 2.
Pancreatitis pathology score
| Groups (n = 8) |
Score |
||||
| Interstitial edema | Leukocyte infiltration | Acinar cell necrosis | Hemorrhage | Total | |
| Control | |||||
| 4 d | 0.13 ± 0. 33 | 0.13 ± 0.33 | 0 | 0 | 0.25 ± 0.67 |
| 7 d | 0.13 ± 0.33 | 0 | 0 | 0 | 0.13 ± 0.33 |
| SAP | |||||
| 4 d | 1.5 ± 0.76a | 2.38 ± 0.74a | 1.75 ± 0.71a | 0.75 ± 1.17a | 6.38 ± 2.20a |
| 7 d | 1.25 ± 0.46b | 2.13 ± 0.99b | 1.5 ± 0.54b | 0.50 ± 1.07b | 5.38 ± 1.77b |
In this table, the pancreatitis pathology scores in severe acute pancreatitis (SAP) rats were significantly higher than in control rats in every single aspect as well as the total score, both on the 4th day (aP < 0.01) and on the 7th day (bP < 0.01).
Serum endotoxin
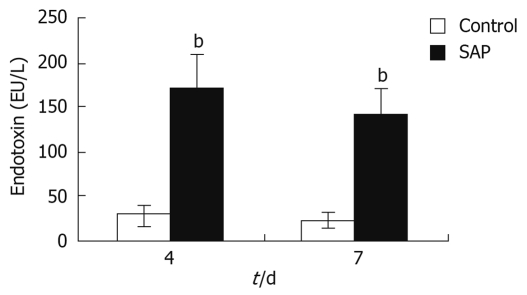
The serum endotoxin level was a sign of intestinal permeability and pancreatitis severity. The plasma endotoxin levels in the pancreatitis group were about 5-7 times higher than that in the control group on both the 4th and 7th days (P < 0.01), though the level had decreased almost a half on the 7th day compared to the 4th day (Figure 3).
Figure 3.
Endotoxin level in serum. On the 4th and 7th days, the serum endotoxin level was markedly higher in the pancreatitis group compared to the control group, bP < 0.01.
Intestinal permeability
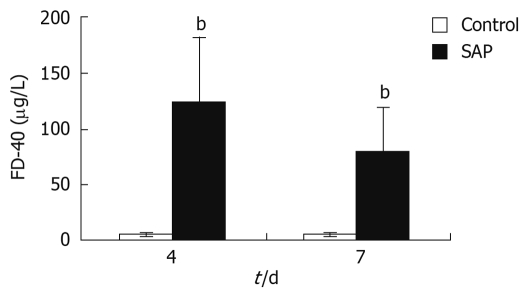
FITC-Dextran concentration in the SMV was a sign of intestinal permeability. In this study, FITC-Dextran levels in the SMV of pancreatitis rats were observed to be significantly higher than that of control rats on both the 4th and 7th days (P < 0.01, Figure 4), indicating higher intestinal permeability in the pancreatitis rats.
Figure 4.
FITC-Dextran (FD-40) levels of serum in the superior mesenteric vein (SMV). The serum level of FD-40 in SMV was significantly higher in the pancreatitis group than in the control group on the 4th and 7th days, bP < 0.01, though the level had decreased almost a half between days 4 and 7.
Ultrastructural changes of the iliac mucosa
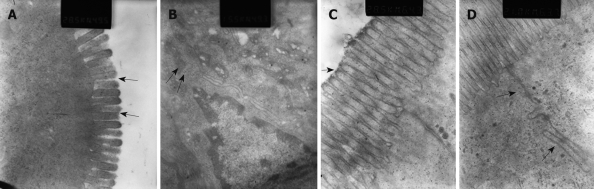
Microvilli of iliac mucosa in pancreatitis rats (Figure 5A) were shortened, about half the length of those of the control rats (Figure 5C), and with a loose structure, and some points had disappeared. Tight junctions and intermediate junctions of villous cells in SAP rats were unclear with discontinuities (Figure 5B), while intact and clear junctions between villous cells were seen in control rats (Figure 5D).
Figure 5.
Distal iliac mucosa electron microscopy. A: Changes of microvilli in a SAP rat, showing loose, shortened microvilli, with some points lost (arrows) (Original magnification, × 28.5 K); B: Changes of the intermediate junction in a SAP rat, which was unclear with breakage in some parts (arrows) (× 15.5 K); C (× 28.5 K) and D (× 21.0 K) are representative electron microscopy images of the iliac mucosa in control rats, showing long, tight microvilli (arrow) and intact intermediate junctions (arrows).
Bacterial culture results
The copies of translocated bacteria differed greatly among pancreatitis rats, sometimes by 103 times, so it was difficult to define the statistics on bacterial numbers by the bacterial culture method. Therefore we chose the infection rate as a variable to compare the bacterial translocation in the 2 groups by the agar plate culture method. Results showed that infection rates of all the 4 types of organ were higher in the pancreatitis group than in the control group (P < 0.01). When comparison was made among the infected rats of the 4 kinds of organs, blood of the inferior vena cava was found to have the lowest rate (P < 0.05, Table 3).
Table 3.
Infection determined by the bacterial culture method
| Groups (n = 8) |
Infected organs number |
||||||||||||||
|
Blood |
MLN |
Pancreas |
Liver |
Total |
|||||||||||
| Ae | Ana | T | Ae | Ana | T | Ae | Ana | T | Ae | Ana | T | Ae | Ana | T | |
| Control | |||||||||||||||
| 4 d | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 7 d | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAP | |||||||||||||||
| 4 d | 3 | 2 | 3 | 6 | 6 | 6 | 7 | 7 | 7 | 7 | 7 | 7 | 23 | 22 | 23a |
| 7 d | 3 | 3 | 3 | 5 | 6 | 6 | 7 | 8 | 8 | 5 | 5 | 5 | 20 | 22 | 22b |
| Total | 6 | 5 | 6c | 11 | 13 | 13 | 14 | 15 | 15 | 12 | 12 | 12 | 43 | 45 | 46 |
Infection rates of each organ and overall in the SAP group were higher than that in the control group, both on the 4th day (aP < 0.01) and 7th day (bP < 0.01); Compared with the other 3 types of organ, the infection rate of blood was lowest, aP < 0.05. MLN: Mesenteric lymph nodes; Ae: Aerobes; Ana: Anaerobes; T: Total.
Real-time PCR results of bacterial translocation
The infection rates detected by real-time PCR were mainly the same as that by bacterial culture. Infection rates by the culture method in MLN and pancreas were a little higher than that by real-time PCR method (MLN 12 to 10, pancreas 15 to 13), which may result from contamination during culture. An advantage of the real-time PCR method was its accuracy of bacterial enumeration. In this study, the results of bacterial number had been transformed by log10 for the purpose of better statistics. It was observed that the pancreas had the highest number of bacteria of the 4 kinds of organs (P < 0.05), which may relate to necrosis and hemorrhage in pancreas tissue as a good medium for bacteria breeding (Table 4).
Table 4.
Bacterial translocation detected by real-time polymerase chain reaction
|
Samples (n = 16) |
||||
| Blood | MLN | Pancreas | Liver | |
| Infected number in control group | 0 | 1 | 0 | 0 |
| Infected number in SAP group | 5a | 10a | 13a | 12a |
| Bacteria number (transformed by log10) | 5.43 ± 0.847 | 6.48 ± 1.56 | 7.70 ± 0.766b | 6.57 ± 1.44 |
P < 0.01, compared with the “Infected number” in the control group;
P < 0.05, compared with the “Bacteria number (transformed by log10)” in blood, MLN and liver.
DISCUSSION
Pancreatitis is a distressing disease clinically, especially SAP, which is associated with considerable mortality, as high as 30%-40%. The main features of this condition are pancreatic necrosis and associated sepsis, with both localized and systemic inflammatory response syndromes[1,14]. Infection of pancreatic necrosis is regarded to be a main predictor of outcome during SAP, and bacterial translocation of intestinal flora is considered to be the cause of pancreatic infection[15].
Since Bernard first introduced an AP model of rabbits by injection of bile and olive oil into the pancreatic duct 150 years ago, animal experiments have become an irreplaceable method to study the pathophysiology, diagnosis and treatment of AP which, compared with clinical trials, have many advantages such as accessible subjects, standardization of the degree of the lesion, practicability of invasive inspection, adequate tissue samples, practicability of prophylactic treatment.
Nowadays there are many kinds of experimental models used to study bacterial translocation in AP. An ideal model of AP should encompass several features including easy reproducibility, with the ability to vary the severity of AP in a standardized manner according to the experimental aim, and to mimic the morphology and pathophysiology of the human situation[16]. At present, several commonly used experimental models were developed to imitate the human biliary pancreatitis, including duodenal loop, biliopancreatic duct ligation, and biliopancreatic duct perfusion.
A duodenal loop in rats can lead to AP of varying severity[17]. However, block of the gastrointestinal tract leads to mucosal atrophy and functional changes to the mucosal barrier[18]. Obstruction of bile flow into the intestine was shown to reduce intestinal motility, causing small bowel bacterial overgrowth and increased bacterial translocation[19-21]. Furthermore, the reflux of duodenal contents, including bacteria, into the biliopancreatic duct will confound the bacterial infection of the pancreas. All these limit its popularity.
The duct ligation model, in which the common biliopancreatic duct is surgically clipped or tied at the sphincter of Oddi complex, could produce moderate pancreatitis with minimal acinar cell necrosis only in American opossum inducing SAP. There are several factors that limit its use. First, obstruction of bile flow into the intestine causes small bowel bacterial overgrowth and bacterial translocation[20]. Second, induction of jaundice would impair the immune system to an uncertain degree and make the research result inaccurate. Third, exclusion of pancreatic proteases in the gut lumen alters intestinal permeability, which does not truly occur in human pancreatitis[22,23].
The duct perfusion model, usually made by perfusion of taurocholate after puncturing the duodenum and cannulating the papilla of Vater, was the most popular model of AP, with etiology resembling human billiary pancreatitis and with the advantage of quick induction of AP and reproducibility of results. However several potential confounders exist in it for study of bacterial translocation. The introduction of duodenal bacteria, through the papilla of Vater into the biliopancreatic duct could potentially affect the result of pancreatic infection; duodenal puncture and intestinal handling during surgery may also potentially affect the mucosal barrier function[24]. A complicated surgical procedure and prolonged time are other shortcomings of it.
In 2002 Wu et al[20] first reported that pancreatic subcapsular injection of 3.8% Na taurocholate could establish an SAP animal model with multiple organ dysfunction in rats, but with a high mortality rate (90% in a week), which may be related to excessive use of 3.8% Na taurocholate (1 mL/rat) and of small rats (body weight about 100 g). This study used pancreatic subcapsular injection of 3.8% Na taurocholate (1 mL/kg) in rats of about 250 g body weight, and successfully established a model of AP. AP rats showed abnormal performance resembling human disease and the autopsy was in line with the performance of human SAP as well. The serum amylase level and pancreatic tissue pathology gave a clear diagnosis of AP. Changes of serum amylase levels in the AP group were similar to that in human disease, and increased significantly 6 h after induction, reaching a peak in 24 h and remaining at a higher level 48 h later. Pancreatitis pathology showed interstitial edema of the pancreas 6 h after model induction, with local hemorrhaging and slight inflammatory infiltration. Lesions developed progressively to moderate necrosis with obvious inflammatory infiltration 48 h later. The pancreatic pathology score on the 4th and 7th days showed a clear phenomenon of necrotic pancreatitis, including interstitial edema, leukocyte infiltration, acinar cell necrosis and hemorrhage. The scores of edema and hemorrhage in the pancreatitis group were less than that of necrosis and inflammatory infiltration, which may be related to edema absorption and hematoma organization after several days. Some other organs were involved, peripancreatic calcified plaques formed, with liver and spleen ischemia, intestinal expansion, peritoneal exudates, and even death. Severity reached a peak in the medium term of the disease (3rd and 4th days), with the highest mortality, and although some indices were still high on the 7th day, few deaths occurred after 7 d, which may be related to the adaptation of the body or a sign of recovery. The cumulative mortality rate in 7 d was moderate, about 30%.
Agar plate culture is nowadays the gold standard method for bacterial measurement, however it has some shortcomings such as prolonged time and inaccuracy that limit early detection of bacterial translocation in the clinic. Recently, a real-time PCR method has been demonstrated successfully in the fast and accurate detection of bacteria in samples of blood, feces, and dentine; however few results are available on its use to detect bacteria in parenchymal organs such as lymphoid tissue, spleen, etc. Our study confirmed its use in enumeration of bacteria in this AP model in samples of not only blood but also parenchymal organs. Infection rates of all the 4 types of organ in the SAP group were significantly higher than that of the control group with quite similar results detected by bacterial culture or real-time PCR. Infection rate and bacterial number of inferior vena cava blood in the SAP group were both much lower than that of the other organs, while the MLN had a similar infection rate and bacterial number to that of the liver, suggesting a lymphatic route of bacterial translocation may exist in this SAP model.
A damaged intestinal barrier is thought to be a key factor for bacterial translocation in SAP[25-28]. In this study, obvious gut barrier damage was observed, with the FITC-Dextran in the SMV and endotoxin levels both significantly increased in AP rats compared to control rats. Electron microscopy of the intestinal mucosa directly showed the damage in the mucosa cells, with microvillus atrophy and intercellular junction disruption.
This rat model requires an invasive laparotomy, with some inevitable potential confounding factors, such as anesthesia, trauma, stress and so on, but few other potential confounding factors existed in it. The surgical procedures were so simple that they could be finished in 15 min once the skills were mastered. No other organs except the pancreas were affected during the surgery, therefore avoiding some potential factors that could affect the measurement of bacterial translocation.
In conclusion, in this study we induced a simple taurocholate pancreatitis model in rats to study the situation of intestinal barrier damage and bacterial translocation in SAP. The results confirmed the situation, as observed clinically, that intestinal barrier damage and bacterial translocation exist in SAP with endotoxemia. However the exact pathophysiologic mechanism of bacterial translocation in SAP is still unknown and needs more intense research for the purpose of determining better preventative measures and treatment of SAP in the clinic.
COMMENTS
Background
Severe acute pancreatitis is associated with considerable mortality, as high as 30%-40%. Infection of pancreatic necrosis is regarded to be a main predictor of outcome during severe acute pancreatitis (SAP), and bacterial translocation of intestinal flora is considered to be the cause. Although a multitude of animal models have been used to study the mechanism of bacterial translocation, the exact origin, route, and mechanism of bacterial translocation causing infection of the necrotic pancreas are still unclear.
Research frontiers
There are now many kinds of experimental models used to study bacterial translocation in acute pancreatitis (AP). However, an “ideal” animal model of SAP was lacking, and should encompass several features including easy reproducibility, ability to vary the severity of AP in a standardized manner, and similar morphology and pathophysiology to that of the human situation.
Innovations and breakthroughs
A new simple model of SAP in the rat was induced by Na taurocholate, and results showed that significant gut barrier damage and intestinal bacterial translocation occurred in this SAP rat model, with few potential study confounders and with a shorter induction time compared to other commonly used animal models of SAP, suggesting that it may be an appropriate animal model for the study of gut barrier damage and bacterial translocation in SAP.
Applications
In future, this simple taurocholate-induced rat model may be used in the study of SAP, especially to determine the exact origin, route, and mechanism of bacterial translocation causing infection of the necrotic pancreas.
Terminology
Intestinal bacterial translocation: normally, intestinal bacteria cannot go through the gut barrier and into the blood or other organs. In the situation of SAP or other severe disease such as shock or major burns, the gut barrier would be damaged and the intestinal permeability increased, causing the intestinal bacteria to translocate from the gut to blood or even other distant organs and induce sepsis, septicemia or even death.
Peer review
This is a well performed research trying to induce a new simple rat model of SAP, and results definitely showed it was an appropriate animal model for study of bacterial translocation in SAP, with significant gut barrier damage and intestinal bacterial translocation observed but with few potential confounders.
Footnotes
Supported by The Natural Science Foundation of Guangdong Province, China, Grant No. 2007A07001680
Peer reviewer: Dr. Albert F Pull ter Gunne, Department of General Surgery, St. Elisabeth Hospital, Hilvarenbeekseweg 60, Tilburg 5022 GC, The Netherlands
S- Editor Li LF L- Editor Cant MR E- Editor Lin YP
References
- 1.Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433–438. doi: 10.1016/0016-5085(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 2.Gianotti L, Munda R, Alexander JW, Tchervenkov JI, Babcock GF. Bacterial translocation: a potential source for infection in acute pancreatitis. Pancreas. 1993;8:551–558. [PubMed] [Google Scholar]
- 3.Kazantsev GB, Hecht DW, Rao R, Fedorak IJ, Gattuso P, Thompson K, Djuricin G, Prinz RA. Plasmid labeling confirms bacterial translocation in pancreatitis. Am J Surg. 1994;167:201–206; discussion 206-207. doi: 10.1016/0002-9610(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 4.Medich DS, Lee TK, Melhem MF, Rowe MI, Schraut WH, Lee KK. Pathogenesis of pancreatic sepsis. Am J Surg. 1993;165:46–50; discussion 51-52. doi: 10.1016/s0002-9610(05)80403-9. [DOI] [PubMed] [Google Scholar]
- 5.Cicalese L, Sahai A, Sileri P, Rastellini C, Subbotin V, Ford H, Lee K. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127–1132. doi: 10.1023/a:1010786701289. [DOI] [PubMed] [Google Scholar]
- 6.Zhang LY, Wang ZG, Zhu PF, Qin HJ. Gut barrier function disturbance posterior to hemorrhagic shock resuscitation in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:767–770. [Google Scholar]
- 7.Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol. 2002;92:1750–1761; discussion 1749. doi: 10.1152/japplphysiol.00787.2001. [DOI] [PubMed] [Google Scholar]
- 8.Fihn BM, Jodal M. Permeability of the proximal and distal rat colon crypt and surface epithelium to hydrophilic molecules. Pflugers Arch. 2001;441:656–662. doi: 10.1007/s004240000440. [DOI] [PubMed] [Google Scholar]
- 9.Nejdfors P, Ekelund M, Jeppsson B, Weström BR. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: species- and region-related differences. Scand J Gastroenterol. 2000;35:501–507. doi: 10.1080/003655200750023769. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Gong J, Cottrill M, Yu H, de Lange C, Burton J, Topp E. Evaluation of QIAamp DNA Stool Mini Kit for ecological studies of gut microbiota. J Microbiol Methods. 2003;54:13–20. doi: 10.1016/s0167-7012(02)00260-9. [DOI] [PubMed] [Google Scholar]
- 11.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 12.Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2005;71:2318–2324. doi: 10.1128/AEM.71.5.2318-2324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu T, Shiratori K, Sawada T, Kobayashi M, Hayashi N, Saotome H, Keith JC. Recombinant human interleukin-11 decreases severity of acute necrotizing pancreatitis in mice. Pancreas. 2000;21:134–140. doi: 10.1097/00006676-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Banks PA. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 1997;92:377–386. [PubMed] [Google Scholar]
- 15.Beger HG, Rau B, Isenmann R, Schwarz M, Gansauge F, Poch B. Antibiotic prophylaxis in severe acute pancreatitis. Pancreatology. 2005;5:10–19. doi: 10.1159/000084485. [DOI] [PubMed] [Google Scholar]
- 16.Hue Su K, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson AP, Foulis AK, Imrie CW. Histology and bacteriology of closed duodenal loop models of experimental acute pancreatitis in the rat. Digestion. 1986;34:15–21. doi: 10.1159/000199305. [DOI] [PubMed] [Google Scholar]
- 18.Habold C, Chevalier C, Dunel-Erb S, Foltzer-Jourdainne C, Le Maho Y, Lignot JH. Effects of fasting and refeeding on jejunal morphology and cellular activity in rats in relation to depletion of body stores. Scand J Gastroenterol. 2004;39:531–539. doi: 10.1080/00365520410004514. [DOI] [PubMed] [Google Scholar]
- 19.Deitch EA, Sittig K, Li M, Berg R, Specian RD. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79–84. doi: 10.1016/s0002-9610(05)80610-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang DS, Jin DY, Wu ZH, Yang YM. A model of acute hemorrhagic-necrotizing pancreatitis in rats. Shanghai Shiyan Dongwu Kexue. 2002;22:23–26. [Google Scholar]
- 21.Ogata Y, Nishi M, Nakayama H, Kuwahara T, Ohnishi Y, Tashiro S. Role of bile in intestinal barrier function and its inhibitory effect on bacterial translocation in obstructive jaundice in rats. J Surg Res. 2003;115:18–23. doi: 10.1016/s0022-4804(03)00308-1. [DOI] [PubMed] [Google Scholar]
- 22.Cohen DB, Magnotti LJ, Lu Q, Xu DZ, Berezina TL, Zaets SB, Alvarez C, Machiedo G, Deitch EA. Pancreatic duct ligation reduces lung injury following trauma and hemorrhagic shock. Ann Surg. 2004;240:885–891. doi: 10.1097/01.sla.0000143809.44221.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 24.van Minnen LP, Blom M, Timmerman HM, Visser MR, Gooszen HG, Akkermans LM. The use of animal models to study bacterial translocation during acute pancreatitis. J Gastrointest Surg. 2007;11:682–689. doi: 10.1007/s11605-007-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Gong Z, Wu K, Wang B, Yuang Y. Gastrointestinal dysmotility in patients with acute pancreatitis. J Gastroenterol Hepatol. 2003;18:57–62. doi: 10.1046/j.1440-1746.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuoka H, Schmid-Schönbein GW. Mechanisms for blockade of in vivo activator production in the ischemic intestine and multi-organ failure. Shock. 2000;14:522–527. doi: 10.1097/00024382-200014050-00005. [DOI] [PubMed] [Google Scholar]
- 27.McClave SA, Ritchie CS. Artificial nutrition in pancreatic disease: what lessons have we learned from the literature? Clin Nutr. 2000;19:1–6. doi: 10.1054/clnu.1999.0071. [DOI] [PubMed] [Google Scholar]
- 28.Samel S, Lanig S, Lux A, Keese M, Gretz N, Nichterlein T, Sturm J, Löhr M, Post S. The gut origin of bacterial pancreatic infection during acute experimental pancreatitis in rats. Pancreatology. 2002;2:449–455. doi: 10.1159/000064714. [DOI] [PubMed] [Google Scholar]