Abstract
Although the general role of the medial-temporal lobe (MTL) in episodic memory is well established, controversy surrounds the precise division of labor between distinct MTL subregions. The perirhinal cortex (PrC) has been hypothesized to support nonassociative item encoding that contributes to later familiarity, whereas the hippocampus supports associative encoding that selectively contributes to later recollection. However, because previous paradigms have predominantly used recollection of the item context as a measure of associative encoding, it remains unclear whether recollection of different kinds of episodic detail depends on the same or different MTL encoding operations. In our current functional magnetic resonance imaging study, we devised a subsequent memory paradigm that assessed successful item encoding in addition to the encoding of two distinct episodic details: an item–color and an item–context detail. Hippocampal encoding activation was selectively enhanced during trials leading to successful recovery of either an item–color or item–context association. Moreover, the magnitude of hippocampal activation correlated with the number, and not the kind, of associated details successfully bound, providing strong evidence for a role of the hippocampus in domain-general associative encoding. By contrast, PrC encoding activation correlated with both nonassociative item encoding as well as associative item–color binding, but not with item–context binding. This pattern suggests that the PrC contributions to memory encoding may be domain-specific and limited to the binding of items with presented item-related features. Critically, together with a separately conducted behavioral study, these data raise the possibility that PrC encoding operations—in conjunction with hippocampal mechanisms—contribute to later recollection of presented item details.
INTRODUCTION
Understanding how the operations of the medial-temporal lobe (MTL) enable us to remember our everyday episodes is a fundamental goal of cognitive neuroscience (Squire, Stark, & Clark, 2004; Schacter & Wagner, 1999; Zola-Morgan, Squire, & Ramus, 1994; Scoville & Milner, 1957). Importantly, everyday episodes are complex and contain various kinds of detail. For example, imagine strolling through a shopping mall with a friend. In front of a specific store, your friend suddenly stops and points to an array of shirts in various colors, saying that he thinks the blue one looks best. A while later, close to your friend’s birthday, you remember the incident, return to the mall, find the store, and purchase the shirt as a gift. This simple example illustrates that in order to accomplish this task, you would need to remember the specific shirt itself, the color your friend preferred, and the particular store where you previously saw it. These components of the initial event or episode can be thought of as an item (the shirt), an associated item–context detail (the particular store where you saw it), and an associated item–feature detail (the color of the shirt). A key question is how the MTL supports the associative binding of distinct elements to form a detailed episodic trace. Are different episodic details encoded by distinct patterns of MTL activation? If so, what is the precise nature of this division of labor?
Two MTL regions that have exhibited dissociable involvement in episodic encoding are the hippocampus and the adjacent perirhinal cortex (PrC). In conjunction with data from animal studies (for a review, see Brown & Aggleton, 2001) and neuropsychological studies (Holdstock, Mayes, Gong, Roberts, & Kapur, 2005), a number of recent functional magnetic resonance imaging (fMRI) studies in humans have found that hippocampal encoding activation correlates with later recovery of associated contextual details, whereas PrC encoding activation correlates with later item recognition and not episodic recollection (Dougal, Phelps, & Davachi, 2007; Kensinger & Schacter, 2006; Uncapher, Otten, & Rugg, 2006; Kirwan & Stark, 2004; Ranganath et al., 2004; Davachi, Mitchell, & Wagner, 2003; but see Gold et al., 2006). However, there is evidence that different episodic details may be represented in distinct MTL cortical regions. Namely, neuroanatomical studies in nonhuman primates have shown that the PrC receives the majority of its inputs from ventral unimodal areas largely dedicated to visual object processing (Eichenbaum, 2006; Suzuki & Amaral, 1994), suggesting that the PrC may play a domain-specific role in memory encoding and may selectively support object or item memory (Brown & Aggleton, 2001; Meunier, Bachevalier, Mishkin, & Murray, 1993).
How might domain specificity impact upon subsequent memory effects? In the majority of the previous fMRI studies cited above, subsequent recollection was measured using the recovery of only one associated episodic detail (but see Uncapher et al., 2006; Prince, Daselaar, & Cabeza, 2005), often the encoding task context. It follows then that the consistent correlation between PrC activation and item encoding, but not associative encoding, may have resulted from recollection being assessed through the recovery of information that is not represented or processed in the PrC (Davachi, 2006). In fact, the most recent proposed models of MTL functional organization explicitly consider that the MTL may contribute to associative encoding in a domain-specific manner (Eichenbaum, Yonelinas, & Ranganath, 2007; Mayes, Montaldi, & Migo, 2007; Davachi, 2006).
In the present experiment, we examine what will happen if, instead, we query memory for an episodic detail that is presented as a to-be-bound feature of the study item itself, as the color is a feature of the shirt in the example above. Will the PrC still only support simple item encoding, or will it also contribute to the associative encoding of this kind of item-related detail? Item–feature representations have been strongly linked to PrC function (Murray, Graham, & Gaffan, 2005; Bussey & Saksida, 2002; Eacott, Machin, & Gaffan, 2001; Murray & Bussey, 1999; Buckley & Gaffan, 1998), and memory decisions made on the basis of the conjunction of individual elements (i.e., objects) within a scene have been shown to depend on an intact PrC, even when the elements are not colocalized (Saksida, Bussey, Buckmaster, & Murray, 2007). Furthermore, in a previous imaging experiment, we found evidence that PrC encoding activation correlates with later recovery of a presented item–feature detail but not with free recall (Staresina & Davachi, 2006). However, as we did not directly compare recovery of an item–feature with recovery of another episodic detail, such as the encoding task context, it remains to be tested whether associative encoding mechanisms in the PrC are domain-specific in that they selectively support binding of item-related details.
Furthermore, a separate but critical question regarding episodic memory formation is whether subsequent recollection can be graded (Wixted, 2007; Yonelinas, 2002). Although previous studies have shown that hippocampal activation correlates with the later recollection of some episodic detail, whether it will be modulated by the amount of successfully encoded episodic details remains unknown. In other words, will hippocampal activation increase when two associations are later available versus only one? Despite general agreement that the hippocampus is critical for associative encoding, a corresponding additive effect for the binding of multiple associations has not been shown so far. Modulation by the number, not the type of associated details successfully bound, would provide important evidence for a role of the hippocampus in domain-general associative encoding and would provide support for graded recollection accounts (Wixted, 2007).
The present experiment is designed to examine the precise mechanisms by which—within one given episode—an item and associated episodic details are successfully encoded by the MTL. To this end, we devised a novel episodic encoding scenario (Figure 1) that provides the opportunity for subjects to encode an item (a noun referring to a concrete object), a color associated with the item and the cognitive context in which the item was encountered. Based on a potential distinction between encoding processes and encoding domains, we first hypothesize that the hippocampus will not only support the binding of an item with both an associated color and an associated context (i.e., supports domain-general associative encoding), but it remains to be seen to what extent hippocampal encoding activation will scale with the amount of associations successfully bound within any given episode, thus, supporting the notion of graded recollection. Second, we hypothesize—given previous neuroimaging findings and animal work mentioned above—that PrC encoding activation will contribute to domain-specific encoding of both an item representation as well as an episodic detail related to that item (color), but, critically, less so to the associative encoding of the broader context in which an item was encountered (task context). Finally, corresponding to a single process account that attributes variations in memory outcome to varying engagement of one underlying mechanism (Dunn, 2004; Donaldson, 1996), MTL encoding mechanisms might operate at a more integrated level and might thus defy a clear-cut mapping of the hippocampus and the PrC to only one specific type of episodic encoding (e.g., domain-general vs. domain-specific; Gold et al., 2006; Squire et al., 2004; Zola-Morgan et al., 1994).
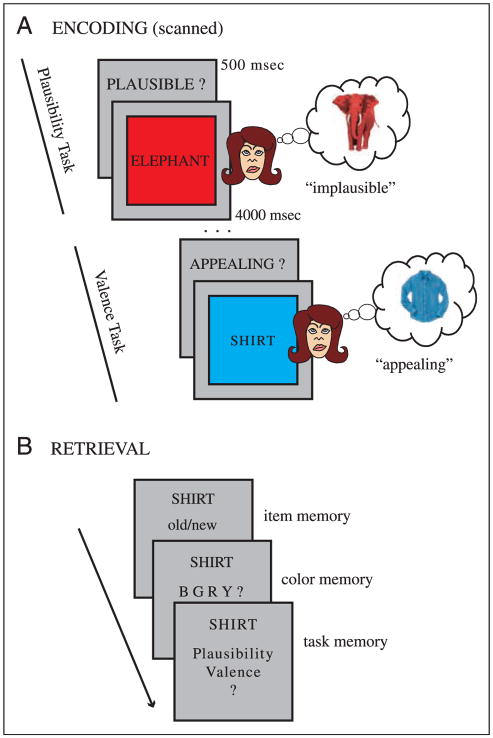
Figure 1.
Experimental design. (A) Encoding: Example trials from each of the two scanned encoding tasks (plausibility, valence). In both tasks, subjects were instructed to vividly imagine the referent of the noun in the color presented and to decide either whether this combination was plausible (plausibility task), or whether it was appealing (valence task). If subjects could not imagine the referent of the noun in the given color, a separate button was pressed and those trials were excluded from all analyses. (B) Three-step surprise recognition memory test (unscanned and self-paced), consisting of the assessment of item memory (old/new judgment), associated item-related detail (color memory), and associated item–context detail (task memory). Note that the task memory test was not contingent on the response on the color memory test and vice versa. Question mark responses were allowed to avoid forced-choice guesses.
METHODS
Subjects
Twelve female and 11 male right-handed native English speakers participated in the study. All subjects had normal or corrected-to-normal vision. Informed consent was obtained in a manner approved by the Institutional Review Board at New York University and subjects were paid for their participation. One male subject was excluded from all subsequent analyses for providing only one trial of successful item–context binding. Mean age of the remaining 22 subjects was 20 years (SD = 2).
Item Material
Seven hundred English nouns referring to concrete objects were obtained from the Medical Research Council Psycholinguistics database (www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). Words were three to eight letters long, with a Kucera–Francis written frequency of 10–100. Only words with concreteness and imageability ratings ranging from 400 to 700 (out of 700) were included. The item pool was counterbalanced so that across subjects, every word was presented with every possible color in both tasks during encoding and was part of both study words and test lures.
Behavioral Procedures
The encoding portion of the experiment was conducted in the fMRI scanner and consisted of five runs, each run containing 84 trials. The remaining 280 words from the item pool served as lures for the recognition test. Half of each run (42 sequential trials) was part of the plausibility task and the other half was part of the valence task (referred to as task blocks). The sequence of task blocks across the five runs was AB–BA–AB–BA–AB, with the assignment of the particular task to A and B being counterbalanced across subjects. Between two task blocks in a run, subjects were given 9 sec to rest while the instruction to prepare for the alternate task appeared on the screen. During both tasks, for a given trial (4.5 sec), subjects were presented with a noun (printed in black uppercase letters) that was superimposed on a color square (blue, green, red, or yellow; Figure 1A). Subjects were instructed to create a vivid mental image of the referent of the noun in the given color and to make one of two decisions based on the encoding task. In the plausibility task, subjects were asked to indicate whether it was plausible to encounter the imagined object/color combination in real life/nature or not. In the valence task, subjects were asked to indicate whether they thought the imagined object/color combination was aesthetically appealing or not. Note that both tasks put equal emphasis on incorporating the color feature into a vivid mental object representation and differed only in the cognitive set with which that word/color combination was processed. Five hundred milliseconds before the onset of each word/color trial, a brief cue reminded subjects which task to perform (“plausible?” or “appealing?”). Following cue offset, subjects were given 3 sec to conjure up a mental image of the word/color combination, after which the frame of the color square changed from black to white, prompting subjects to indicate their judgment within the remaining second (“plausible” or “implausible” in the plausibility task and “appealing” or “unappealing” in the valence task). Responses were given with a button box positioned under the subject’s left hand. Importantly, subjects were also instructed to press a separate button in case they were unable to create a vivid mental image of the given word/color combination that incorporates the referent of the word in the color presented. These trials were excluded from all subsequent analyses, as were trials for which responses were not given within the allotted time of 4.5 sec. Thus, for the trials analyzed in this study, it is fair to assume that subjects successfully imagined the referent of the noun in the given color (i.e., successfully turned the color into an object feature).
Encoding trials were intermixed with baseline trials of an active, sensorimotor task (Stark & Squire, 2001). Arrows that randomly pointed to the left or to the right for 1 sec were repeatedly presented for the length of a baseline trial, and subjects had to press the middle finger key if the arrow pointed to the left and the index finger key if it pointed to the right. The order of word/color trials and baseline trials was determined by using a sequencing program designed to maximize the efficiency of the event-related design (Dale, 1999). Conditions were jittered using variable duration (2.25–11.25 sec) baseline trials. Each encoding run was immediately followed by a 1-min distracter phase, after which subjects were given 3 min to freely recall verbally as many words from the previous run as possible. These data are beyond the current scope and will not be reported in this manuscript, but all the trials freely recalled were excluded from subsequent memory analyses to avoid any confounding effect of repeated exposure on subsequent recognition memory.
Approximately 30 min after the fMRI encoding session, subjects were given a surprise recognition memory test outside the scanner (Figure 1B). All the 420 previously presented words were presented together with 280 new words. First, subjects were prompted to indicate via button press whether they thought the item was old or new, that is, whether the word had been presented in the encoding session or not. A correct response for old items in this step was indicative of successful item encoding during the study phase, whereas an incorrect response was indicative of unsuccessful item encoding (forgetting). If the answer was “old,” the labels for the four encoding colors and a question mark appeared on the screen and subjects were prompted to indicate the color with which the word was associated at encoding or to press the question mark key if they did not remember the color. A correct response in this step was indicative of successful binding of the color feature (item–feature association) during the study phase. Finally, and irrespective of the answer for color memory, subjects were then asked to indicate in which of the two encoding tasks the item had been encountered. Answer options were: “plausibility,” “valence,” and, importantly, a question mark. A correct response in this step was indicative of successful binding of the current task context (item–context association) during the study phase. To account for the fact that chance correct (if not using the “don’t know” question mark response) performance is higher for task memory (50%) than for color memory (25%), confidence ratings (“high,” “low”) were additionally used for task memory. For both color and encoding task memory, the additional question mark was used to circumvent forced guesses about the answer. The recognition test was self-paced, and the mean duration across subjects was 52 min (SD = 13).
Assessment of whether successful encoding of one event detail (e.g., task memory) is greater when the other detail (e.g., color memory) is also successfully encoded (Uncapher et al., 2006) was done in two steps. First, the probability of correct color memory as a function of correct task memory was derived, for each subject, by calculating the probability of correct color memory, given that task memory was also correct [ pColor&Task/(pTaskOnly + pColor&Task)]. Second, the probability of correct color memory, given that task memory was incorrect [ pColorOnly/(pItemOnly + pColorOnly)] was calculated (see Uncapher et al., 2006). The same procedure was applied to derive the probability of correct task memory as a function of correct color memory. The effect of binding one event detail on the probability of binding the other detail was analyzed via repeated-measures analysis of variance (ANOVA).
fMRI Procedures and Analyses
Scanning was performed on a 3-T Siemens Allegra MRI system using a whole-head coil. Functional data were acquired using a gradient-echo, echo-planar pulse sequence (TR = 2.25 sec, TE = 30 msec, 40 slices oriented perpendicular to the hippocampal axis, 3 × 3 × 3 mm voxel size, 0.6 mm interslice gap, 256 volume acquisitions per run). High-resolution T1-weighted (MP-RAGE) images were collected for anatomical visualization. A vacuum pillow minimized head motion. Visual stimuli were projected onto a screen that was viewed through a mirror, and responses were collected with a magnet-compatible button box.
Data were analyzed using SPM2 (Wellcome Department of Cognitive Neurology, London). During preprocessing, images were corrected for differences in slice acquisition timing, followed by motion correction across all runs. Structural and functional data were spatially normalized to an echo-planar imaging template and voxels were spatially smoothed with a 6-mm full-width, half-maximum isotropic Gaussian kernel. Statistical analyses were performed using the general linear model implemented in SPM2.
Encoding trials were classified according to the following criteria: (i) items later forgotten (misses or M trials); (ii) items later recognized, without remembering the correct color or the correct encoding task (item-only recognition or IO trials); (iii) items later recognized, including memory for the correct color but not for the encoding task (item and color recognition or IC trials); (iv) items later recognized, including memory for the correct encoding task but not for the color (item and task recognition or IT trials); and finally (v) items later recognized, including memory for both the correct color and the correct encoding task (item and color and task recognition or ICT trials). Encoding trials were sorted according to these subsequent memory conditions (M, IO, IC, IT, and ICT trials) and modeled using a canonical hemodynamic response function and its temporal derivative. Additional regressors of no interest were created for invalid trials and for trials freely recalled, respectively. The five runs were concatenated and modeled as one continuous run in order to improve parameter estimability. Accordingly, mean signal per task block and drift per run were separately modeled as confounds. Parameter estimates (referred to as beta estimates) for each regressor of interest were derived for each subject and carried forward to second-level group analyses. We required a minimum of 10 trials for each condition to include a subject in the final group analysis. This was not an issue except for an insufficient number of IT trials in two subjects and of IO trials in one subject. Thus, these three subjects’ data were excluded from the fMRI analyses (remaining n = 19).
As described in the Introduction, this study was designed to assess the extent to which MTL regions show a pattern consistent with (1) domain-specific item/object encoding, including associative binding of a presented detail that describes the item (item–color binding), and (2) with domain-general associative encoding, including the binding of both item–color and item–context (encoding task) details. To this end, we reasoned that a region important in item encoding, including item–color binding, would show greater activation for IO trials compared to M trials, followed by a further increase for IC trials, corresponding to an increasingly enhanced item representation including an associated item-related detail. Importantly, no additional increase for ICT trials should be evident, as the additional memory for the encoding task is not an item–feature per se but instead represents associative memory for the context in which an item was encountered. Furthermore, a region important in item/item–color binding, but not domain-general associative binding, should also exhibit a drop in activation from IC trials to IT trials, as IC trials represent successful encoding of the item representation plus an associated item-related detail (color), whereas IT trials represent successful encoding of the item representation plus associated item–context information (encoding task).
On the other hand, a region important in domain-general associative encoding should contain the highest level of activation for ICT trials, where both item–color and item–context associations are successfully bound to the item representation. Furthermore, if this region is sensitive to the amount of information bound, trials for which only one associative detail is bound (i.e., both IC and IT trials) should result in reduced engagement compared to ICT trials, but still enhanced engagement relative to IO trials, where no associative detail is bound to the item representation. Note that a domain-general associative encoding model is agnostic to the level of activation during M trials because this trial type is characterized by the failure to successfully encode an item representation. Interestingly, previous studies have shown enhanced activation in the hippocampus for M trials compared to IO trials (Kensinger & Schacter, 2006; Kirwan & Stark, 2004; Davachi et al., 2003), whereas others showed the opposite pattern (Tendolkar et al., 2007; Gold et al., 2006). Thus, M trials were excluded from the predictions in the domain-general associative encoding model.
The correspondence of different MTL subregions with an item/item–color and a domain-general associative encoding pattern, respectively, was assessed via separate parametric analyses, which constitute an efficient and parsimonious statistical procedure to reveal, in one step, voxels that show a particular pattern of activation across several conditions (Buchel, Holmes, Rees, & Friston, 1998). More precisely, a parametric regressor was defined for both model predictions that represented the specific weighting (i.e., the parametric modulation) of the individual memory conditions. The weights for the memory conditions (parametric modulation at the first polynomial order) were [M = 1, IO = 2, IC = 3, IT = 2, and ICT = 3] for the item/item–color model and [IO = 1, IC = 2, IT = 2, and ICT = 3] for the domain-general associative model (Figure 2A). This analysis was conducted at the individual-subject fixed-effects level, yielding subject-specific estimates for the fit of the parametric regressor at each voxel. The subject-specific estimates of the parametric effect were then entered into a second-level random effects group analysis (one-sample t test). Because the rationale of this study was to assess the contributions of different MTL regions to episodic memory formation, we focused our analyses on the MTL. Statistical maps were thresholded at p < .001 (uncorrected), and a small volume correction (SVC; Worsley et al., 1996) was applied to the MTL bilaterally. The SVC mask comprised 5280 voxels total and is schematized in Figure S1 in the supplementary material. Clusters of at least five contiguous voxels exceeding the threshold of p < .05 after an SVC were considered reliable. Follow-up analyses on the resulting regions were conducted via pairwise comparisons of beta estimates for individual memory conditions (averaged across all significant voxels in a given cluster), with the statistical threshold set to p < .05 (two-tailed, unless otherwise noted). All voxel coordinates are reported in MNI space.
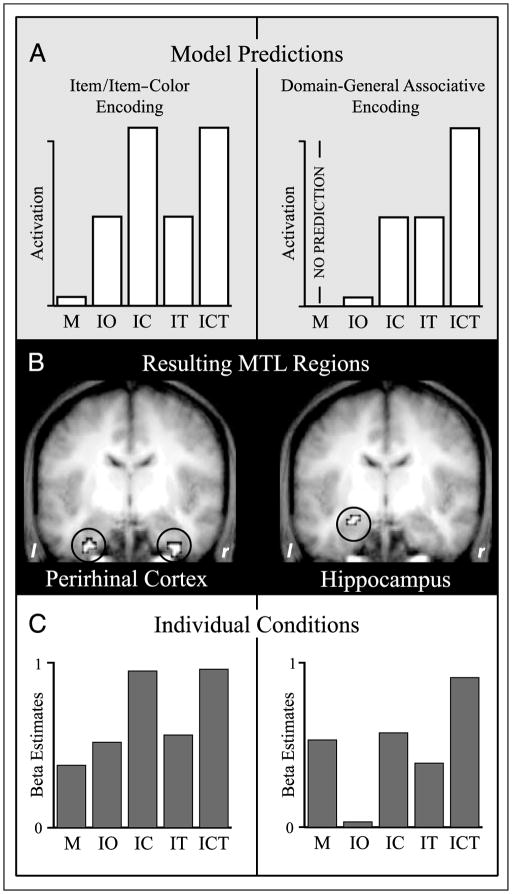
Figure 2.
Item/item–color encoding and domain-general associative encoding in the MTL. (A) Parametric model predictions for activation in regions supporting item/item–color (left) and domain-general associative (right) encoding. (B) MTL clusters resulting from the parametric analyses after small volume correction (p < .05). Left: The bilateral perirhinal cortex (y = −6), with the pattern of encoding activation corresponding to item/item–color encoding. Right: The left hippocampus (y = −6), with the pattern of encoding activation corresponding to domain-general associative encoding. Clusters are shown on the mean anatomical image across subjects. (C) Encoding activation (beta estimates) derived from a separate analysis that modeled each condition individually, shown for the perirhinal cortex (left; averaged across left and right hemisphere clusters) and the hippocampus (right). Activation for the individual subsequent memory conditions shows a strong match with the model predictions. M = misses (no item memory); IO = item only (item memory, no color or task memory); IC = item and color (item memory, color memory, no task memory); IT = item and task (item memory, task memory, no color memory); ICT = item and color and task (item memory, color memory, task memory).
RESULTS
Behavioral Results
Out of all encoding trials, 7.23% (SD = 9.90) were excluded from subsequent analyses because subjects indicated they could not come up with an image that incorporated the referent of the noun with the color feature presented or because responses were not given within the allotted response time. For the remaining encoding trials, the proportions of “plausible” to “implausible” responses (plausibility task) and of “appealing” to “unappealing” responses (valence task) were balanced across subjects [both ts(21) < .91, p > .36]. Both encoding tasks produced high levels of subsequent item memory. 81.45% (SD = 12.98) of the study items from the plausibility task and 81.05% (SD = 12.20) of the study items from the valence task were later correctly recognized. Of the new test items, 84.71% (SD = 13.73) were correctly endorsed as new (correct rejection).
After the assessment of item memory, we separately queried memory for the associated color in which the item was imagined and the associated encoding task in which the item was encountered (Figure 1B). Importantly, for both of these source tests, a question mark response was provided to avoid forced guesses. For both source tests, subjects made use of this answer option to indicate they do not know the correct answer [18.82% (SD = 12.33) of the color test trials and 27.42% (SD = 17.49) of the task test trials]. Critically, of the trials in which subjects did make a source response, accuracy was high both for color memory [82.08% (SD = 11.10) correct] and for task memory [74.87% (SD = 9.68) correct], with accuracy being statistically greater for color memory [t(21) = 2.42, p < .05]. Consistent with a previous memory study that assessed the encoding of multiple sources within a given event (Uncapher et al., 2006), we found that the probability for successful encoding of one event detail was greater when the other detail was also successfully encoded (see Methods). A repeated-measures ANOVA with the factors detail (color, task) and accuracy of the other detail (correct, incorrect) revealed a significant main effect of accuracy of the other detail [F(1, 18) = 61.93, p < .001] as well as a significant main effect of detail [F(1, 18) = 37.60, p < .001] due to higher overall accuracy of color memory (see above). Importantly, despite the interesting overall mutual influence of binding the event details, our experimental protocol provided sufficient events in which one, but not the other, associative detail was successfully bound.
For task memory decisions, for which confidence ratings were recorded, 53.76% of the trials were given “high confidence” responses and the remaining 46.24% of the trials were given “low confidence” responses (SD = 21.02%). Critically, accuracy was above chance for both high [t(21) = 14.27, p < .001] and low confidence responses [t(21) = 7.35, p < .001]. Moreover, no statistical difference was seen in the PrC or the hippocampus [both ts(16) < 1.05, p > .30] between “high confidence” and “low confidence” ICT trials (the condition that had a sufficiently large number of trials to perform this analysis). Thus, to increase statistical power for the fMRI analyses, responses were collapsed across confidence ratings. Furthermore, because there were no differences in subsequent memory performance (item memory, color memory, task memory) between the two encoding tasks [all ts(21) < 1.22, p > .23], data from the plausibility and the valence condition were also collapsed.
fMRI Results
In order to statistically assess whether MTL regions show patterns of encoding activation in line with an item/item–color and a domain-general associative encoding pattern, respectively, parametric analyses were employed (described in the Methods and schematized in Figure 2A). The number of available trials per individual memory condition is shown in Table 1.
Table 1.
Mean Number (and Standard Deviation) of Trials per Memory Condition Available for fMRI Analyses
| Trial Type | Number of Trials |
|---|---|
| Miss (M) | 59 (42) |
| Item only (IO) | 64 (30) |
| Item and color (IC) | 61 (17) |
| Item and task (IT) | 31 (18) |
| Item and color and task (ICT) | 138 (56) |
We found that the only MTL regions to emerge from the item/item–color parametric analysis were the bilateral PrC (Figure 2B). In accordance with the anatomical demarcations of the PrC in humans (Insausti et al., 1998), the resulting clusters were located in the anterior portion of the collateral sulcus, ranging from y = 0 to y = −9 on the left and from y = 0 to y = −27 on the right. Because both the left and right PrC showed virtually identical patterns of activation in the subsequent analyses, data reported henceforth are collapsed across these regions.
Conversely, the only MTL region that emerged from the domain-general associative model was a region in the left hippocampus, with the resulting cluster ranging from y = −3 to y = −15 (Figure 2B). In both the PrC and hippocampal clusters, examination of the separately extracted beta estimates across the individual memory conditions was striking in their match to the model predictions (Figure 2C).
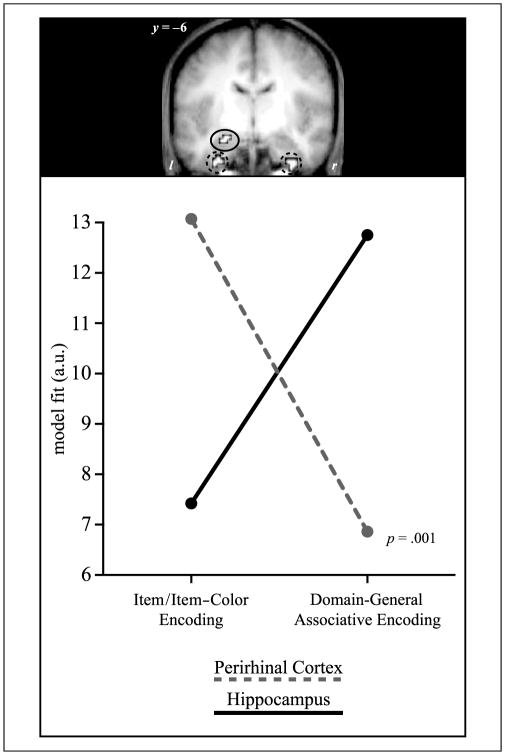
In order to formally assess a double dissociation of the model fits across the two MTL regions, subject-specific parameter estimates for both the item/item–color and domain-general associative model were compared across the PrC and the hippocampus with a repeated-measures ANOVA including the factors region (PrC, hippocampus) and encoding pattern (item/item–color, domain-general associative). In the absence of any main effects [both Fs(1, 18) < .06, p > .81], the interaction of Region × Encoding pattern was significant [F(1, 18) = 15.04, p = .001; see Figure 3].
Figure 3.
Double dissociation between the contributions of the perirhinal cortex and the hippocampus to episodic encoding. The correspondence (expressed in parameter estimates for the model fits) of encoding activation in the perirhinal cortex (PrC) and the hippocampus is shown for the item/item–color and the domain-general associative model. Activation in the PrC (red; averaged across left and right hemisphere clusters) shows a high fit with the item/item–color model and a low fit with the domain-general associative model, whereas the hippocampus (blue) shows the opposite pattern. The interaction is significant with p = .001.
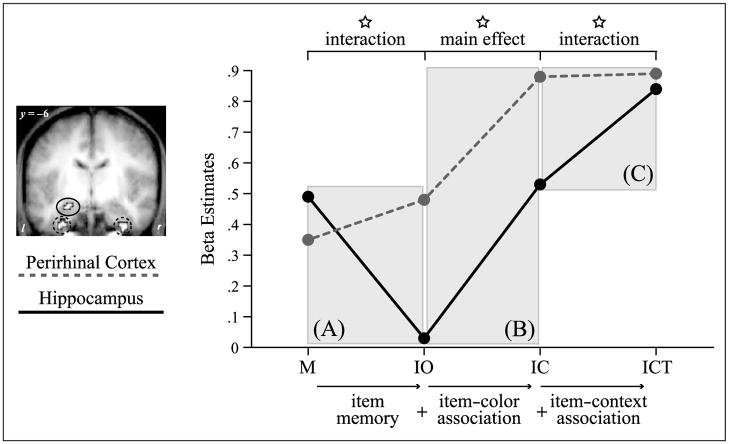
Importantly, this interaction between the PrC and the hippocampus was derived from comparing the observed encoding pattern in these regions with our a priori formulated models of item/item–color encoding and domain-general associative encoding. In order to examine the functional dissociation between the PrC and the hippocampus independent of all model predictions, we conducted pairwise comparisons between critical memory conditions that distinguish between item/item–color and domain-general associative encoding. The first comparison focused on critical condition comparisons relevant to item encoding. A repeated-measures ANOVA was performed on the beta estimates comparing M and IO trials, including the factors region (PrC, hippocampus) and memory (M trials, IO trials). In the absence of any main effects [both Fs(1, 18) < .99, p > .33], a significant interaction of Region × Memory was observed [F(1, 18) = 6.10, p < .05]. Specifically, greater activation was found in the PrC for IO trials compared to M trials, whereas the hippocampus showed the opposite pattern (Figure 4A). Using the same procedure, the second comparison focused on conditions critical in establishing a role in domain-general associative encoding. To this end, we first compared activation during ICT trials with that during IC trials, including the factors region (PrC, hippocampus) and memory (IC trials, ICT trials). Again, although no main effect was present [both Fs(1, 18) < 2.44, p > .14], we found a significant interaction of Region × Memory [F(1, 18) = 9.65, p < .01], due to an increase in activation from IC to ICT trials in the hippocampus but not in the PrC (Figure 4C).
Figure 4.
Division of labor between the perirhinal cortex and the hippocampus for different aspects in memory encoding. Encoding activation (beta estimates) is shown for the perirhinal cortex (red; averaged across left and right hemisphere clusters) and the hippocampus (blue) for critical subsequent memory conditions. (A) Interaction for the difference between M and IO, critical for establishing a role in nonassociative item encoding. (B) Main effect for the difference between IO and IC, with both regions showing enhanced activation for successful item–color associative encoding. (C) Interaction for the difference between IC and ICT, critical for establishing a role in domain-general associative encoding. Stars indicate statistical significance at p < .05. M = misses (no item memory); IO = item only (item memory, no color or task memory); IC = item and color (item memory, color memory, no task memory); ICT = item and color and task (item memory, color memory, task memory).
Finally, to ensure that the enhanced hippocampal activity during ICT trials was due to the number, and not the specific type of associative detail successfully encoded, we compared hippocampal activation during ICT trials to both IC trials and IT trials separately. Indeed, there was a significant difference in both comparisons [both ts(18) > 2.08, p < .03 (one-tailed, based on the directionality inherent in the preceding parametric model)], with no significant difference between IC and IT trials [t(18) = .61, p > .55 (two-tailed)]. This supports the notion that encoding activation in the hippocampus correlates with the amount of associative binding (two associative details vs. one) and is, therefore, not specific to item–color or item–context associations.
Taken together, these results demonstrate a clear functional dissociation between the roles of the PrC and the hippocampus in episodic memory formation. However, it deserves explicit mention that there is one point of overlap in the two patterns, namely, enhanced activation for IC trials compared to IO trials (Figure 4B). In a repeated-measures ANOVA on the beta estimates for IO trials and IC trials that included the factors region (PrC, hippocampus) and memory (IO trials, IC trials), the main effect of memory was significant [F(1, 18) = 5.80, p < .05] in the absence of an interaction [F(1, 18) = .30, p > .50]. This finding implies that certain types of memory that tax both item/object and associative encoding (IC trials in this paradigm) might rely conjointly on PrC and hippocampal encoding operations.
DISCUSSION
The current study was designed to elucidate the functional contribution of specific MTL subregions to episodic memory formation. First, consistent with previous reports, our data provide strong evidence for a dissociation between the PrC and the hippocampus in the service of successful episodic encoding (Figure 3). Importantly, however, our data extend current views of both hippocampal and PrC encoding mechanisms by revealing, for the first time, (1) a graded level of encoding activation in the hippocampus related to the amount of associative information successfully bound and (2) PrC encoding activation correlating with both subsequent item recognition as well as with associative recollection of a presented item feature, but not its context.
Domain-general Associative Encoding in the Hippocampus
Our current data reveal a pattern of activation in the hippocampus that increased with the amount of associative detail later remembered, regardless of the particular type (item–color or item–task association; Figure 2C). Although the role of the hippocampus in associative encoding has been well established in previous fMRI experiments (Dougal et al., 2007; Kensinger & Schacter, 2006; Staresina & Davachi, 2006; Uncapher et al., 2006; Prince et al., 2005; Jackson & Schacter, 2004; Kirwan & Stark, 2004; Ranganath et al., 2004; Davachi et al., 2003; Sperling et al., 2003), the current paradigm allowed assessment of two kinds of associative details from a single episode, and thus, allowed us to address questions of additivity. Critically, we found that an increased number of successfully encoded associations within any one episode is related to a stepwise increase in hippocampal encoding activation. Modulation by the number, not the type, of associative details is strong evidence for a role of the hippocampus in domain-general associative encoding.
As it is conceivable that an increasing number of associative details remembered reflects different levels of recollection, this graded hippocampal activation provides some support for the notion that recollection may be a continuous phenomenon (Wixted, 2007). Moreover, the finding of item–color associations being supported by the hippocampus is interesting in light of current debates emerging from studies of patients with selective hippocampal damage, where reports of impaired item memory (e.g., Stark, Bayley, & Squire, 2002) stand in conflict to reports of relatively spared item memory (e.g., Yonelinas et al., 2002). Given our data, one speculative explanation for this discrepancy might be related to the extent to which item recognition, in various paradigms, can benefit from the recovery of item–feature (e.g., item–color) associations. That is, the encoding of study items which provide a relatively rich set of item-level features or contain overlapping features (e.g., faces or houses; Stark et al., 2002) might benefit disproportionally from additional hippocampal engagement compared to the encoding of perhaps less complex study items, such as words (Yonelinas et al., 2002), so that patients with hippocampal damage would be expected to show worse item memory in paradigms of the earlier sort. It follows then that MTL cortex may be sufficient to support the encoding of featurally impoverished items or items from stimulus sets where there is minimal featural overlap (words alone tend to have nonoverlapping meaning), a notion further supported by our finding that the PrC, but not the hippocampus, shows enhanced activation for items later recognized compared to misses (Figure 4A).
Domain-specific Item and Associative Encoding in the PrC
First, our current results replicate the recent finding that PrC activation is modulated by later memory for an item–color association (Staresina & Davachi, 2006). Second, the current study extends this finding by showing that PrC activation does not, within the same encoding event, correlate with binding of an item–context association (in this paradigm, memory for the specific encoding task in which the item was encountered) (Figures 2C and 4). Given that the color, but not the encoding task, was made an object feature by virtue of the study paradigm (“Imagine the referent of the word in the color presented”), these data may suggest that associative encoding effects in the PrC are—as opposed to those seen in the hippocampus—specific to item-level representations, namely, item–feature associations. This pattern might imply that the PrC encoding operations are not specific to nonassociative rather than associative encoding, but to whether or not the to-be-encoded information lies within the item/object domain.
Although evidence from the animal literature has suggested that the PrC is critical for paired associate learning and encoding of object/feature conjunctions (Naya, Yoshida, & Miyashita, 2001; Buckley & Gaffan, 1998), typically, in those studies, the associations are learned across numerous repetitions, and thus, it is left open to what extent the PrC can support episodic associative encoding. In our current paradigm, task demands were such that item–color associations were formed between an imagined object and one of four possible color features during a single exposure, providing evidence for a pattern of activation consistent with rapid, single-trial associative encoding in the human PrC. Converging support for a role of the human MTL cortex in item-level associative encoding comes from a recent fMRI study where subjects studied scenes shaded in one of two colors (red or green) and in one of three possible hues (light, medium, or dark) (Tendolkar et al., 2007). The authors report parametrically enhanced encoding activation in rhinal and posterior parahippocampal (PhC) cortices correlating with subsequent color memory. Interestingly, in the present study, unlike the Tendolkar et al. (2007) findings, no evidence of activation in the PhC related to successful memory formation emerged, potentially due to the absence of scene stimuli (Epstein & Kanwisher, 1998) in our current paradigm. However, both our study and that of Tendolkar et al. provide support for the notion that the MTL cortex, albeit different subregions along its longitudinal axis, may, in concert with the hippocampus, support domain-specific associative item–feature encoding. This notion is in line with recent assertions that regions along the parahippocampal gyrus, including the PrC and the PhC, may contribute to domain-specific encoding, including associative encoding of item-level episodic details (Mayes et al., 2007; Davachi, 2006).
Of course, there are other possible interpretations of our findings. First, both the item and the color, but not the task information, were perceptually available during each study episode. By contrast, information required to perform the task was presumably generated and maintained internally. Hence, our results could be interpreted as reflecting a role for the PrC in perceptual item and associative encoding, but not in conceptual encoding. Although previous work has suggested that the PrC represents both perceptual and conceptual item information (Taylor, Moss, Stamatakis, & Tyler, 2006; O’Kane, Insler, & Wagner, 2005), this is an intriguing possibility that will require further work as the present conditions fully confound perceptual details with our item–feature condition.
In sum, we show that although PrC activation correlates with successful later item recognition and associative item–color binding, it does not correlate with creating item–context associations. Instead, consistent with current frameworks on how the MTL supports episodic encoding (Eichenbaum et al., 2007; Mayes et al., 2007; Davachi, 2006), enhanced activation for additional item–context binding was only evident in the hippocampus (Figure 4C).
Conjoint Involvement of the PrC and the Hippocampus for Associative Item–Color Binding
One remaining question is how PrC encoding operations relate to the subsequent sense of familiarity as opposed to recollection (Diana, Yonelinas, & Ranganath, 2007; Eichenbaum et al., 2007; Yonelinas, 2002; Brown & Aggleton, 2001; Jacoby, 1991; Mandler, 1980). Although it has been shown that unitization of disparate components can strengthen the sense of familiarity for an item (Quamme, Yonelinas, & Norman, 2007; Yonelinas, Kroll, Dobbins, & Soltani, 1999), it seems unlikely that memory for an item–color association is governed entirely by familiarity-based recognition in our current paradigm. First, the associated color was retrieved from among four alternative choices (the color names, not reinstantiations of the color square), where subjects were not forced to guess but had the option to indicate they do not remember the color. In this way, our testing protocol is more stringent than common two-alternative forced-choice source paradigms in assessing recollection. Second, in order to empirically test this notion, we conducted a separate behavioral study where subjects explicitly evaluated their word/color memory with regard to their subjective state of familiarity and recollection (see Supplementary Material) and found that for the majority of trials (63%), memory for the correct color association was, indeed, accompanied by subjective feelings of recollection. Taken together with our imaging results, these data open the possibility that, in addition to supporting later item familiarity, PrC encoding processes may also contribute to later recollection, at least under circumstances where the criterial information that triggers recollection lies within the item domain.
Importantly, our data demonstrate that both the PrC and the hippocampus showed enhanced activation during successful item–color binding (Figure 4B), suggesting that these regions may work together to bind these episodic elements. However, despite the observation that both the PrC and the hippocampus participate in successful item–color binding, it should be noted that fMRI data cannot ascertain necessity or causality. This is true not only due to the inherently correlational nature of fMRI data but also to the relatively poor temporal resolution. For instance, it is possible that PrC engagement may, in fact, precede hippocampal activation by bringing on-line the semantic concepts of both the current item and the color (O’Kane et al., 2005) or by maintaining the separate components, whereas the binding of these elements is effectively accomplished by the hippocampus (Cohen et al., 1999; Cohen & Eichenbaum, 1993). This is consistent with the notion that although the hippocampus is necessary for binding to occur, it does so with the use of representations supported by MTL cortical regions. In this scenario, both an intact hippocampus and an intact PrC are necessary to support item–color binding: the hippocampus to support the conjunctive binding and the PrC to maintain and represent the to-be-bound representations. Consistent with the idea of a functional interdependence, it has been shown that an intact PrC is necessary for multitrial paired-associate learning (Buckley & Gaffan, 1998; Higuchi & Miyashita, 1996) and for successful encoding of feature conjunctions (Eacott et al., 2001; Buckley & Gaffan, 1998). Furthermore, increased neural coupling between the rhinal cortex and the hippocampus has been found during successful compared to unsuccessful episodic encoding with human intracranial electroencephalographic recordings (Fell et al., 2001), and data from work with monkeys point to an interdependence of the PrC and the hippocampus for particular associative memories. Specifically, using an “object-in-place” task, it has been shown that combined lesions to the PrC and the hippocampus result in a severe impairment in the performance of this task compared to a mild impairment after lesions to each region alone (Gaffan & Parker, 1996). Thus, despite differential functional imaging patterns correlating with item/item–color and domain-general associative encoding, it is plausible that mnemonic representations that are both item-level and associative require concurrent participation of the PrC and the hippocampus.
References
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus–response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998;36:535–546. doi: 10.1016/s0028-3932(97)00120-6. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: A connectionist model of effects of lesions in perirhinal cortex. European Journal of Neuroscience. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum HE. Memory, amnesia, and the hippocampal system. Cambridge: MIT Press; 1993. [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences; U.S.A. 2003. pp. 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Donaldson W. The role of decision processes in remembering and knowing. Memory & Cognition. 1996;24:523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- Dougal S, Phelps EA, Davachi L. The role of medial temporal lobe in item recognition and source memory for emotional stimuli. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:233–242. doi: 10.3758/cabn.7.3.233. [DOI] [PubMed] [Google Scholar]
- Dunn JC. Remember–know: A matter of confidence. Psychological Review. 2004;111:524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Machin PE, Gaffan EA. Elemental and configural visual discrimination learning following lesions to perirhinal cortex in the rat. Behavioural Brain Research. 2001;124:55–70. doi: 10.1016/s0166-4328(01)00234-0. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Remembering: Functional organization of the declarative memory system. Current Biology. 2006;16:R643–R645. doi: 10.1016/j.cub.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, et al. Human memory formation is accompanied by rhinal–hippocampal coupling and decoupling. Nature Neuroscience. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Parker A. Interaction of perirhinal cortex with the fornix–fimbria: Memory for objects and “object-in-place” memory. Journal of Neuroscience. 1996;16:5864–5869. doi: 10.1523/JNEUROSCI.16-18-05864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewer JB, Stark CE, et al. Item memory, source memory, and the medial temporal lobe: Concordant findings from fMRI and memory-impaired patients. Proceedings of the National Academy of Sciences; U.S.A. 2006. pp. 9351–9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Miyashita Y. Formation of mnemonic neuronal responses to visual paired associates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proceedings of the National Academy of Sciences; U.S.A. 1996. pp. 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR, American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Jackson O, III, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face–name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Sciences. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Graham KS, Gaffan D. Perirhinal cortex and its neighbours in the medial temporal lobe: Contributions to memory and perception. Quarterly Journal of Experimental Psychology B. 2005;58:378–396. doi: 10.1080/02724990544000077. [DOI] [PubMed] [Google Scholar]
- Naya Y, Yoshida M, Miyashita Y. Backward spreading of memory-retrieval signal in the primate temporal cortex. Science. 2001;291:661–664. doi: 10.1126/science.291.5504.661. [DOI] [PubMed] [Google Scholar]
- O’Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Norman KA. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ, Buckmaster CA, Murray EA. Impairment and facilitation of transverse patterning after lesions of the perirhinal cortex and hippocampus, respectively. Cerebral Cortex. 2007;17:108–115. doi: 10.1093/cercor/bhj128. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, et al. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Bayley PJ, Squire LR. Recognition memory for single items and for associations is similarly impaired following damage to the hippocampal region. Learning and Memory. 2002;9:238–242. doi: 10.1101/lm.51802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences; U.S.A. 2001. pp. 12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences; U.S.A. 2006. pp. 8239–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Anke BD, van Eijndhoven P, et al. Probing the neural correlates of associative memory formation: A parametrically analyzed event-related functional MRI study. Brain Research. 2007;1142:159–168. doi: 10.1016/j.brainres.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: An fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychological Review. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins IG, Soltani M. Recognition memory for faces: When familiarity supports associative recognition judgments. Psychonomic Bulletin & Review. 1999;6:654–661. doi: 10.3758/bf03212975. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]