Abstract
Barrier epithelial cells and airway dendritic cells (DC) make up the first line of defence against inhaled substances like house dust mite (HDM) allergen and endotoxin. We hypothesized that these cells need to communicate to cause allergic disease. Using irradiated chimeric mice, we demonstrate that TLR4 expression on radioresistant lung structural cells is required and sufficient for DC activation in the lung and for priming of effector T helper responses to HDM. TLR4 triggering on structural cells caused production of the innate proallergic cytokines thymic stromal lymphopoietin, granulocyte-macrophage colony stimulating factor, interleukin-25 and IL-33. The absence of TLR4 on structural cells, but not on hematopoietic cells, abolished HDM driven allergic airway inflammation. Finally, inhalation of a TLR4 antagonist to target exposed epithelial cells suppressed the salient features of asthma including bronchial hyperreactivity. Our data identify an innate immune function of airway epithelial cells that drives allergic inflammation via activation of mucosal DCs.
Introduction
Aberrant innate and adaptive immune responses to allergens and environmental polutants lead to respiratory disease, including asthma and COPD1. Airway DCs continuously sample inhaled air for the presence of noxious substances via cellular processes that extend across the airway mucosal barrier2. By simultaneously expressing pattern recognition receptors such as the Toll like receptors (TLRs) to sense the presence of pathogens, and at the same time presenting pathogen-derived antigens to naïve T cells in the lymph nodes, DCs bridge innate and adaptive immunity. An emerging theme in the field of lung immunology is that structural cells of the airways like epithelial cells, endothelial cells, fibroblasts, and other stromal cells produce activating cytokines that determine the quantity and quality of the lung immune response3–8. For example, barrier epithelial cells make up the first line of defence against inhaled antigens and also express TLRs9,10, allowing them to sense the same types of stimuli that are recognized by innate immune cells. An important issue that remains to be elucidated is the precise contribution of epithelial TLR triggering to the DC-driven adaptive immune response. Here we address this issue by studying key aspects of airway DC biology following inhalation of the TLR4 ligand LPS, found predominantly in the wall of Gram-negative organisms, as well as following inhalation of HDM, a ubiquitous indoor allergen contaminated with LPS from colonizing bacteria and environmental pollution11. We generated radiation chimeric mice in which either radioresistant structural cells or radiosensitive hematopoietic cells (including DCs) were deficient in TLR4 expression and show that TLR4 expression on structural cells is necessary and sufficient for activation of immune responses by mucosal DCs and development of Th2 immunity and allergic inflammation to HDM allergen.
Results
TLR4 on radioresistant cells controls innate immunity to LPS
To determine the relative contribution of TLR4 signalling on lung structural cells versus hematopoietic cells in the innate immune response to inhaled LPS, we generated radiation-induced chimeric mice (Fig. 1a). In WT->WT mice, TLR4 expression was predominantly found on airway epithelial cells and alveolar macrophages, whereas in TLR4−/−->WT mice, expression was restricted to epithelial cells and in WT->TLR4−/−, expression was mainly found on alveolar macrophages (Fig. 1b). Twelve weeks after reconstitution, chimerism was confirmed by flow cytometry in lymph node (LN) B cells, DCs and autofluorescent alveolar macrophages (Fig. 1c).
Figure 1. Assesment of the reconstitution rate of chimeric animals.
(a) Various chimeric mice (coded as bone marrow donor genotype ->recipient genotype) were prepared. (b) The TLR4 expression in the airways of the diverse chimeric mice was assessed using immunostaining and studied by confocal imaging (purple). This image was merged with a bright field image. (c) The degree of chimerism was analyzed by determining the percentage of GFP+ B cells and DC in lymph node and alveolar macrophages of WTMHCIIgfp->WT mice (gray histogram). The background for GFP positivity was set on fluorescence intensity of mice receiving GFP-negative bone marrow; black histogram). These experiments were done 3 times.
First, we examined the cellular influx into the lung after an intratracheal (i.t.) administration of LPS. WT->WT animals exposed to LPS had increased numbers of Ly-6Ghi CD11b+ neutrophils (Fig. 2a) and Ly6Chi CD11b+ monocytes (Fig. 2b) in the lungs compared to mice given PBS but this response was markedly reduced in TLR4−/−->TLR4−/− animals. Expression of TLR4 on structural cells was crucial as WT->TLR4−/− animals failed to recuit neutrophils and monocytes. Neutrophil, but not monocyte, numbers were also mildly reduced in TLR4−/−-> WT mice. To determine the mechanism behind reduced cellular recruitment, we measured the lung levels of relevant chemokines. After the administration of LPS, WT->WT mice showed a substantial increase in the levels of several chemokines and growth factors for neutrophils (KC, Fig. 2c; G-CSF, supplementary Fig. 1 online) and monocytes/DCs (CCL-2 (Fig. 2d); CCL20 (supplementary Fig. 1 online)), compared to mice administered PBS. These responses were largely abolished in TLR4−/− ->TLR4−/− or WT->TLR4−/− chimeras given LPS, yet intact in TLR4−/−->WT chimeras.
Figure 2. TLR4 expression on radioresistant stromal cells is necessary and sufficient for recruitment of DCs to the lungs in response to LPS.

Various chimeric mice (coded as donor genotype ->recipient genotype) were injected i.t with LPS or PBS. The recruitment of (a) BAL fluid neutrophils, (b) BAL fluid monocytes, (e) tracheal digest MHCII+CD11c+ dendritic cells and (f) MHCII+CD11c+CD11b+ DCs was analyzed 24h later. The tracheal counts have been corrected for 1.106 CD45neg structural cells to correct for differently sized explants. Production of KC chemokine for neutrophils (c) and CCL2 for monocytes and DCs (d) was measured in BAL fluids. *:p<0.05. This is a representative experiment out of 4. Four-six mice/group were used
As part of innate immunity, DCs are recruited to the lung. The number of MHCII-positive CD11chi DCs in the trachea (Fig. 2e) was strongly increased by LPS administration in WT->WT mice. In WT->TLR4−/− and TLR4−/−->TLR4−/− chimeras the response to LPS was substantially lower. In PBS-injected WT->WT mice, the majority of CD11chi DCs present in the trachea were CD11b− whereas in LPS-injected mice, there was accumulation of an inflammatory CD11bhi DC subset, an effect not seen in WT->TLR4−/−, and TLR4−/− ->TLR4−/− chimeras, yet maintained in TLR4−/− ->WT mice (Fig. 2f). As an additional control, we administered the TLR2 ligand peptidoglycan (PGN), and found that responses to this compound were not affected by TLR4 deficiency on stromal cells (Supplementary Fig. 1 online).
TLR4 on radioresistant cells determines dynamic DC behaviour
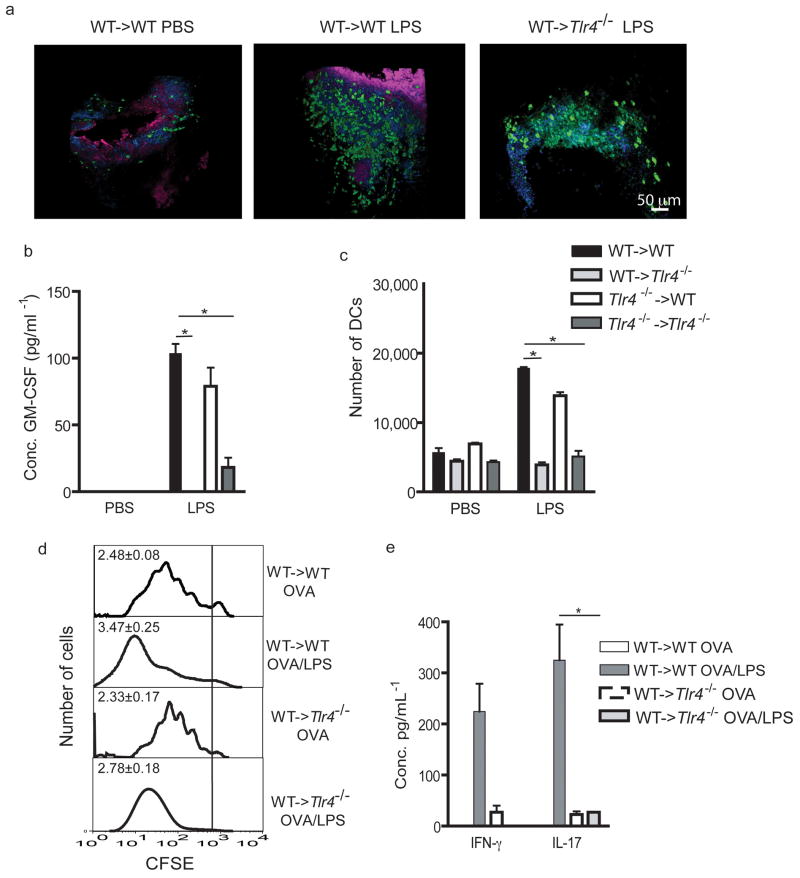
As part of their normal physiology, DCs residing in the periphery scan the environment for incoming antigen. We therefore evaluated, using two-photon dynamic imaging, the behavior of MHCII-GFP+ DCs in freshly isolated tracheal explants from chimeric mice exposed to LPS. To exclude the presence of contaminating MHCII+ B cells also found in the conducting airways of rodents, we generated chimeras using RAG-deficient donor MHCII-EGFP mice. As soon as 2h after in vivo administration of LPS to WT->WT chimeras, there was a massive recruitment of DCs to the trachea (Fig. 3a), an effect not seen in PBS treated or LPS-exposed WT->TLR4−/− animals. The few DCs in the trachea of WT->WT chimeras given PBS made only small lateral movements (Supplementary Movie 1 online), whereas after administration of LPS, DCs demonstrated an increased velocity and made rapid lateral movements (movements quantified in supplementary fig. 2 Online)
Figure 3. TLR4 expression on radioresistant stromal cells is necessary and sufficient for activation of mucosal DCs.
(a)WT->WT and WT->TLR4−/− chimeras were injected i.t with LPS or PBS. Tracheal explants were prepared 2hours later and analyzed by two-photon microscopy. Green DCs are located within red epithelial cells. Nuclei were counterstained in blue. (b) Levels of GM-CSF measured 24 h after LPS exposure. (c) Chimeric mice were injected i.t with fluorescent OVAAF647 together with LPS or PBS. The number of OVA+ migrating DCs was enumerated in the MLNs thirty-six hours later. (d) On day 0, WT->WT and WT->TLR4−/−- chimeras received an intravenous injection of CFSE-labelled OVA-specific naive OTII T cells, and were administered with OVA/LPS or OVA/PBS i.t on day 1. T cell proliferation was evaluated in the MLNs at day 5. Proliferation index for each group is shown in the upper right corner of the histograms. (e) Cytokine production by MLN cells 5 days after OVA/LPS administration. *:p<0.05. These panels show one representative out of 2–4 experiments. 4–6 mice/group were used.
Some DCs also migrated towards and away from the epithelium (Supplementary Movie 2 online). However, when LPS was administered to WT->TLR4−/− chimeras, the majority of DCs from the trachea showed the same sessile behaviour as DCs seen in PBS-injected mice (Supplementary Movie 3 online).
TLR4 on radioresistant cells determines DC maturation
To determine to what extent structural cell-driven inflammation affects phenotypic maturation of DCs, we examined the expression of costimulatory molecules on airway DCs (Supplementary Table 1 online). After LPS instillation, airwayDCs from WT->WT and TLR4−/−->WT chimeras demonstrated more than threefold increased expression of CD86 and CD40 when compared with PBS controls, but this response was selectively abolished in WT->TLR4−/− and TLR4−/− ->TLR4−/− mice. The same conclusions were reached when we studied DCs from digested lung and mediastinal LN (MLN) DCs (data not shown). To determine the basis for the failure of phenotypic DC maturation in WT->TLR4−/− chimeras, we measured the lung levels of several DC-activating cytokines, including GM-CSF, IL-1β and TSLP. Although we could not detect any production of TSLP, the administration of LPS induced increased levels of GM-CSF (and IL-1β, data not shown) in the BAL of WT->WT and TLR4−/− ->WT chimeras (Fig. 3b), an effect not seen in WT->TLR4−/− and TLR4−/− ->TLR4−/− chimeras.
As part of their maturation, activated DC migrate to the draining MLNs8. Therefore, mice received fluorescently labeled ovalbumin (OVAAF647) intratracheally together with LPS or with PBS, and OVAAF647+ MHCIIhi CD11chi migrating DCs were enumerated in the MLNs 2. LPS administration into WT->WT chimeras strongly increased the number of OVA+ DCs to the MLNs compared to PBS (Fig. 3c), but this response was significantly reduced in WT->TLR4−/− and TLR4−/− ->TLR4−/− chimeras, yet was intact in TLR4−/− ->WT chimeras.
The phenotypic changes observed in PAMP-activated DCs usually – but not always -correlate with an increased capacity of the cells to stimulate antigen-driven T cell proliferation and differentiation12. To examine this issue in the present model system, chimeric mice received OVA-specific naïve CD4 T cells and were subsequently immunized by i.t. injection of OVA in combination with LPS (OVA/LPS) or control PBS (OVA/PBS). In WT->WT chimeras, OVA/PBS induced vigorous proliferation of naive CD4 T cells in the MLNs and this response was further increased by coinjection of LPS (Fig. 3d). Notably, T cell proliferation was reduced in WT->TLR4−/− chimeras injected with OVA/LPS compared with WT->WT mice, although not to the level seen in mice given OVA/PBS.
We next evaluated the levels of the prototypical Th1/Th2/Th17 cytokines (IFN-γ, IL-5 and IL-17) produced by MLN cells. Very low levels of IL-17 and IFN-γ were present in the supernatants of MLN cultures from OVA/PBS-injected animals (Fig. 3e). In WT->WT mice exposed to OVA/LPS, the levels of IL-17 and IFN-γ were increased compared to mice exposed to OVA/PBS, an effect not seen in WT->TLR4−/−chimeras. No IL-5 production was detected in any of the supernatants tested (data not shown). The unavailibility of TCR Tg T cells on a TLR4−/− background did not allow us to perform the opposite experiment (TLR4−/− -> WT)
TLR4 on radioresistant cells determines innate immunity to HDM
We next investigated the importance of TLR4 expression on structural cells in the innate response to complex and relevant allergens such as HDM extracts, also known to contain large amounts of endotoxin11,13. The HDM extract contained 1.05ng endotoxin/mg extract. HDM administration (100 μg) in the airways of WT->WT and TLR4−/−->WT mice resulted in an increase in monocytes (not shown) and DCs (Fig. 4a and b) in the airways and increase in CCL2 and CCL3 in the BAL fluid (Supplementary Fig. 3 online) compared with PBS administration, responses that were severely reduced in TLR4−/−->TLR4−/− and WT->TLR4−/− mice. There was no induction of KC nor G-CSF, and consequently airway neutrophilia did not develop (data not shown). Because the administration of HDM in the airways is known to induce Th2 responses in the lungs14–16, we evaluated the production cytokines known to promote eosinophilic inflammation17–19. The levels of GM-CSF, TSLP, IL-25, and IL-33 (Fig. 4c–f) were markedly increased in the BAL fluids of WT->WT animals exposed to a single administration of HDM compared with mice injected with PBS. For comparison, some mice were also injected with a low (100ng) and high (10μg) dose of endotoxin, previously shown to induce Th2 and Th1 immunity respectively 20. Whereas both doses of LPS and HDM were able to induce IL-33, production of TSLP and IL-25 was specific to HDM (Supplementary Fig. 4 online). This innate cytokine response to HDM was reduced in WT->TLR4−/− and TLR4−/−->TLR4−/− chimeras, yet maintained in TLR4−/−->WT mice, although there was a trend for reduced TSLP production in the latter mice.
Figure 4. TLR4 expression on airway structural cells is necessary and sufficient for an innate immune response to HDM allergen.
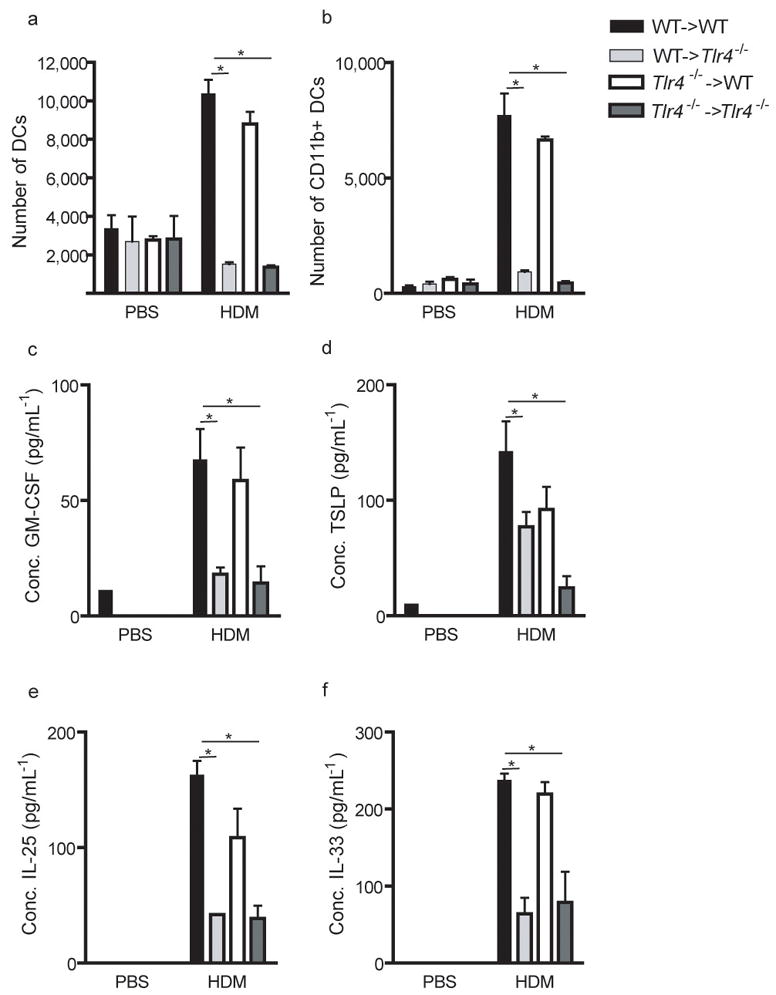
Chimeras were injected i.t with PBS or HDM allergen. The number of MHCII+CD11c+ (a) MHCII+CD11c+CD11b+ (b) DCs in tracheal digests was analyzed 24 hours later. The tracheal counts have been corrected for 1.106 CD45neg structural cells to correct for differently sized explants. (c) Production of GM-CSF, (D) TSLP, (E)IL-25 and (F) IL-33 was measured in BAL fluids. *:p<0.05. This is one representative experiment out of 3. 5–6 mice/group were used
TLR4 on radioresistant cells determines Th2 immunity to HDM
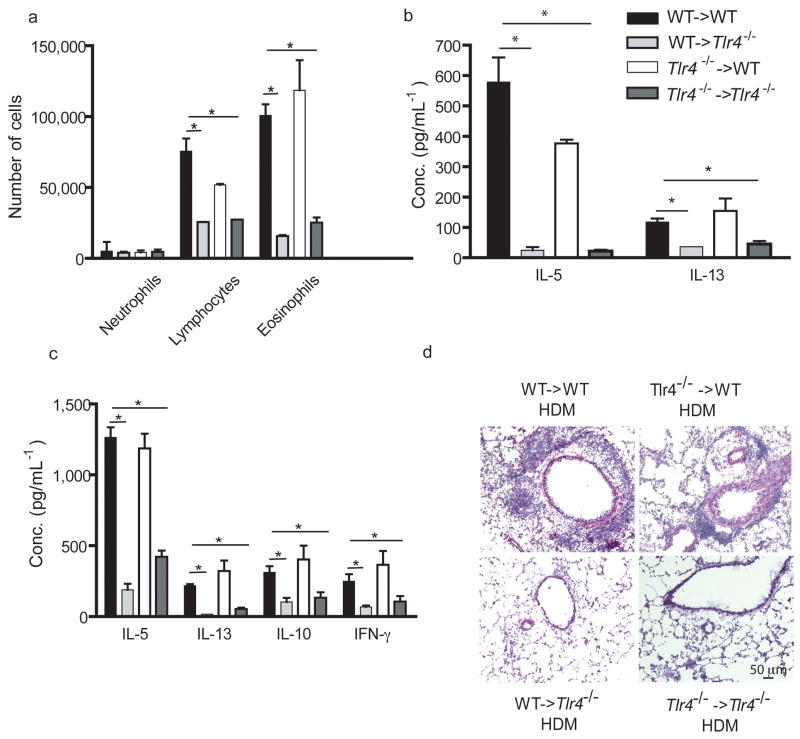
We next examined the influence of absence of TLR4 on structural versus hematopoietic cells on allergic inflammation induced by repeated i.t injection of HDM extracts14. WT->WT chimeric mice developed Th2-associated airway inflammation characterized by BAL fluid and bronchovascular lymphocytosis, eosinophilia, and goblet cell hyperlasia (Fig. 5a and d). These are features of a Th2 response, consistent with high level production of IL-5 and IL-13 in the BAL fluid (Fig. 5b) and in cultures of MLN lymphocytes restimulated with HDM in vitro (Fig. 5c). All these features of allergic inflammation were severly reduced in WT->TLR4−/− and TLR4−/−->TLR4−/− chimeras, yet maintained in TLR4−/−->WTmice.
Figure 5. TLR4 expression on airway structural cells is necessary and sufficient for house dust mite driven Th2 responses and allergic inflammation.
Chimeras were injected i.t with HDM allergen on days 0, 7, and 14. (a) BAL fluid was analyzed by flow cytometry 72h after challenge. (b) Cytokine measurements of BAL fluids. (c) Cytokine measurements of MLN cells restimulated in vitro for 5 days with 30mg/ml HDM. (d) Hematoxylin/eosin staining of lung sections. These experiments were performed 2 times with 4–6 mice/group. One representative experiment is shown
Inhalation of TLR4 antagonist reduces asthma features
Based on these data and on the fact that TLR4 was mainly expressed on exposed conducting airway epithelial cells (Fig. 1) we hypothesized that local intrapulmonary administration of a TLR4 antagonist might constitute a novel therapeutic strategy for allergic inflammation. Administration of an underacylated form of R. sphaeroides LPS (behaving as a functional TLR4 antagonist) at the time of HDM injections strongly reduced airway lymphocytosis and eosinophilia, down to the level of control non-sensitized mice (Fig. 6a), compared with vehicle-treated mice. This was accompanied by marked reduction in IL-5 and IL-13 production in the BAL fluid (Fig. 6b). On tissue histology, TLR4 treatment reduced the peri-bronchovascular infiltrates and goblet cell hyperplasia (Fig. 6c). Finally, TLR4 antagonist suppressed the invasively measured airway hyperresponsiveness to the bronchoconstrictor metacholine seen in actively sensitized mice to the level seen in control sham sensitized mice (Fig. 6d).
Figure 6. Intrapulmonary delivery of a TLR4 antagonist reduces HDM driven inflammation and airway hyperresponisveness.
C57Bl/6 mice were exposed to HDM or to PBS (Control) on day 0. All the mice were then challenged with HDM admixed to a TLR4 antagonist or the vehicle on days 7 and 14. (a) Differential cell count of BAL fluid was determined by flow cytometry 72 h after challenge. (b) Cytokine measurements of BAL fluids (c) Hematoxylin staining of lung sections. (d) Lung function was determined by invasive measurement of airway resistance in response to increasing concentrations of metacholine. *, P < 0.05. These experiments were performed 2 times with 6 mice/group. One representative experiment is shown.
Discussion
Reis e Sousa’s group21 recently demonstrated that TLR4 stimulation of radioresistant structural cells of the spleen was neither required nor sufficient to induce functional splenic DC maturation in response to systemically administered LPS. In sharp contrast, our study shows that TLR4 triggering on lung structural cells by LPS and HDM is required and sufficient to activate many aspects of the functional behaviour of mucosal DCs in response to LPS inhalation. Of all the radioresistant structural cells, epithelial cells that line the airways are the most likely to mediate the effects of LPS, given their exposed position, their known9 and confirmed (Fig. 1) expression of TLR4 and their activation of TLR-dependent signalling cascades upon exposure to TLR ligands6,7,22–24.
The number of airway DCs increased following LPS and HDM exposure over the resting state, most likely due to production of DC-attractive chemokines by epithelial cells. The chemokine CCL20, produced by activated airway epithelial cells5,25 is known to attract CCR6+ human lung DCs and was found to be produced in a TLR4-dependent manner. Production of CCL20 also occurs when human bronchial epithelial cell lines are exposed to HDM, in a pathway requiring recognition of β-glucans but not endotoxin 26. Upregulation of the CCL2/CCR2 axis is a more credible explanation for the increase in DCs, as CCR2 positive Ly6chi monocytes are precursors to CD11b+ inflammatory DCs, and we observed a clear increase of these cells as well as the CCL2 ligand in the airways after LPS and HDM challenge27,28. During acute allergen challenge in the OVA model, CCR2 and not CCR6 was found to control inflammatory DC chemotaxis to the lung 27.
Another paradigmatic feature of resident and freshly recruited DCs is their potential to scan the environment for incoming antigen. We observed using live imaging of freshly explanted tracheal samples that LPS and HDM (not shown) inhalation induced a rapid scanning behaviour of DCs that depended on TLR4 expression by structural cells. Although we do not yet fully understand the contribution of these rapid lateral movements to uptake of airway luminal contents, we propose that they are necessary for DCs to find antigen efficiently in this anatomical site. How this chemokinetic behaviour is induced, and which stromal-derived factors are involved in this phenomenon, remain unclear but might involve the radioresistant unmyelinated nervous system that closely coincides with DC processes 29. Alternatively, the chemokines may adhere to glycoaminoglycans in the airway lumen and constitute a solid phase matrix that evokes the migratory response, similar to that proposed for chemokines bound to fibroblastic reticular cells in the LNs 30.
Once activated in the airways, DCs migrate in a CCR7-dependent way to the T cell area of draining MLNs 31 and our data using LPS or HDM show this behaviour critically depends on TLR4 expression on structural cells. Whether this reduced migration of DCs to MLNs in is due to the absence of signals released from activated stromal cells, or whether it is just a reflection of the immature state of airway DCs in these chimeras, still needs to be elucidated. DC activation as read out by CD86 and CD40 expression was clearly reduced in the absence of TLR4 triggering, most likely due to reduced production of DC-maturation cytokines like GM-CSF 32,33. Once migrating DCs arrive in the lymph node, they induce T cell activation as part of their maturation program 12. Although signals derived from TLR4 triggering on structural cells were not required for DC-driven antigen-specific T cell proliferation (Fig. 3d), they were crucial for induction of effector Th1 and Th17 responses to OVA mixed with LPS (Fig. 3e) and a Th2 response to HDM allergen (Fig. 5a,b). The fact that T cell proliferation of WT->TLR4−/− chimeras exposed to OVA and LPS was higher than that seen in those given OVA alone argues that in addition to signalling on structural cells, there is also direct recognition of LPS by airway DCs that might be crucial for inducing effector potential in OVA-specific T cells. Others have suggested that lung DCs directly react to OVA contaminated with LPS in an MyD88-dependent way 20,34. However, experiments in TLR4−/−->WT chimeras exposed to the real life antigen HDM revealed that TLR4 expression on stromal cells alone is sufficient to induce Th2 effector potential in T cells. The concerted effects of mediators derived from both DCs and stromal cells might be required in synergy to optimally induce effector T cell immunity to a harmless antigen like OVA, as opposed to HDM that contains enzymes known to activate epithelial cells and basophils 8.
We finally addressed whether absence of TLR4 from stromal cells would affect allergic responses to HDM. HDM extract contains endotoxin and its levels in house dust have been correlated with a modifying effect on allergic sensitization in children 13. Eisenbarth et al. proposed that low dose endotoxin promotes Th2 immunity, whereas hight dose promotes Th1 responses 20. Surprisingly, the degree of endotoxin contamination of HDM extract we could measure was in the subnanogram range, far below the dose previously used to promote Th2 responses to OVA 20. The innate cytokine immune response to HDM extract (as measured by GM-CSF, TSLP, IL25 and IL-33) was profoundly altered when TLR4 was absent on structural cells, suggesting an important role for this low level of contaminating endotoxin. yet also profoundly different from just giving low dose endotoxin. Recently, the relevant Der p 2 allergen was found to enhance the response of murine bronchial epithelial cells to endotoxin by acting as an MD2 like chaperone that promotes TLR4 signalling 11. It will be interesting to study whether this MD2-like effect of Der p 2 not just enhances but also alters the type of innate immune response induced by endotoxin in the airways, as it might explain the profound proallergic innate response to HDM.
A failure to produce these innate cytokines in TLR4−/− mice might explain how HDM allergy is avoided. GM-CSF promotes DC maturation and breaks inhalation tolerance 3. TSLP is produced by epithelial cells, mast cells, and basophils and activates DCs and mast cells 19,35–37. Interleukin-25 boosts Th2 cytokine production and is made by mast cells, basophils, eosinophils, and epithelial cells 36. Interleukin-33 boosts Th2-cytokine production and promotes goblet cell hyperplasia 18,38. The induction of these cytokines in BAL fluid strongly depended on structural TLR4 expression, most likely reflecting production by epithelial cells. Not surprisingly, when we exposed WT->TLR4−/− mice to repeated HDM aerosols, they failed to develop the salient features of allergic inflammation such as airway eosinophilia, goblet cell hyperplasia, and peribronchial and perivascular inflammation. Even more strikingly, we found that an in vivo TLR4 antagonist given via the airways was likewise able to inhibit these features, including bronchial hyperreactivity to metacholine, an IL-13 dependent response 39.
Although endotoxin has long been held responsible for determining the development and severity of asthma 13,20, our data show that these effects occur mainly through TLR4 triggering of lung structural cells, thus driving activation of the mucosal DC network.
Materials and methods
Mice
TLR4-deficient mice 40 were obtained fromThe Jackson Laboratory. MHC CII-EGFP knock-in mice 41 were provided by H. Ploegh (Harvard Medical School, Boston, MA). All other animals were on the C57BL/6 background, and handled according to NIH institutional guidelines and guidelines of Ghent University. OTII mice on a RAG−/− background were from Taconic.
Construction of bone marrow chimeras
3–5-wk-old TLR4-deficient or wild-type (WT) recipient mice were sublethally irradiated with 900 rads. On the same day, 5 × 104 bone marrow cells from MHCII-EGFP mice (WT for TLR4) or Tlr4−/− mice were harvested and infused via the tail vein, respectively. For the imaging experiments, MHCII-EGFP mice on a Rag−/−background (no B and T cells) were used as bone marrow donors. These mice were kept in isolators andprovided neomycin-containing water until use 10–12 wk later. To permit chimerism in the lung to be complete, we allowed 10–12 weeks of reconstitution time before experiments were started 42; the degree of chimerism of CD19+MHCII+B cells, CD11c+MHCII+DCs, and alveolar macrophages, known to be slowly repopulated following irradiation, was confirmed by measuring GFP positivity after 12 weeks of chimerism, or by immunostaining of TLR4 expression on cryosections of lungs, followed by confocal imaging.
Reagents
APC-labelled anti-CD11c antibodies, PE-labelled anti-CD11b and anti-CD86 antibodies, biotinylated anti-Ly6C antibodies, and 7-AAD were obtained from BD Biosciences. Ultra-pure preparations of LPS and PGN, as well as the TLR4 antagonist (R. sphaeroides ultrapure LPS) were obtained from Invivogen. Endotoxin-free OVA was obtained from Seikagaku (Japan). HDM extracts were obtained from Greer Laboratories. OVA alexa Fluor 647 was obtained from Invitrogen. Anti-TLR4 antibodies were obtained from Santa Cruz.
Intratracheal administration of reagents
Mice were anesthetized by gas anesthesia using isoflurane. Eighty microliters of PBS, LPS (10 μg or 100 ng/mouse), PGN (10 μg/mouse), HDM (100 μg/mouse) or TLR4 antagonist (1 μg/mouse) was administered intratracheally 43.
Immune analysis
Broncho-alveolar lavage (BAL) was performed by injecting 1 ml of PBS containing 0.01mM EDTA. Thoracic draining LNs, lungs, and trachea were collected, and single cell suspensions were prepared as reported 2. Cell suspensions were stained in PBS supplemented with 2 mM EDTA, 0.5% BSA, and 0.01% sodium azide. Monoclonal antibodies (conjugated to various fluorochromes or biotin) and fluorescence-labeled streptavidin were obtained from BD Biosciences. 2.4G2 (anti-FcγRIII/II) was used to block unspecific antibody binding. Single cell suspensions were stained with FITC anti I-Ad/I-Ed, PE-labeled anti-CD40, anti-CD80, anti-CD86, and APC-labeled anti-CD11c antibodies (all from BD Biosciences). Data were collected on a FACSCalibur (Becton Dickinson) and were analyzed withFlowJo software (Treestar, Inc.).
To measure cytokine levels, MLN cells were plated in round bottom 96-well plates (106 cells/ml) and restimulated with HDM extracts (30 μg/ml) for 5 d, followed by collection of the culture supernantant and assay by commercially available ELISA (R&D systems).
House dust mite-induced airway inflammation
Mice were exposed to D.pteronyssinus extracts (Greer Laboratories, 10.52 EU/mg endotoxin) via the intratracheal injection of HDM (100 μg) on days 0, 7 and 14 and were analyzed on day 17. BAL was performed and cells were analyzed by flow cytometry, as described 44. Lungs slides were stained with Periodic Acid Shiff. Lung function was measured using Flexivent invasive measurement of dynamic resistance, as described 45.
Preparation of explants
Mice were injected intratracheally with LPS or PBS 2 hrs before being euthanised by CO2 asphyxiation. The tracheal explant was stained with 100 μM SNARF (Invitrogen) for 10 sec. at 37°C in PBS. The trachea was then immobilized on the surface of a Petri dish using Vetbond epoxy glue (3M Animal Care Products) and a mechanical support, and the entire preparation submerged in 20 ml of phenol red free RPMI maintained at 37°C. During microscopy medium was superfused with a gas mixture of 95%O2/5% CO2.
Two-photon microscopy
Two-photon microscopy was conducted using a Bio-Rad Laboratories Radiance 2100MP system equipped with a Nikon 600FN upright microscope, 20× water immersion lens (N.A. 0.95; Olympus), and either a Mira 900 Sa:Ti femtosecond-pulsed laser driven by a 10-W Verdi pump laser (Coherent) tuned to 880 nm. The typical pixel size of the image field was 1.09 μm, and the x-y dimensions of the scan area were 560 × 560 μm. The typical optical z-step size was 2μm. Serial x-y images were collected over the entire z depth every 30–60 s, and the entire process was repeated for up to 60 min toobtain a four-dimensional dataset.
Image processing
Datasets were processed using edge-preserving filters for the green channel and a median filter for the red and blue channels (Bitplane, 4.2; Imaris) to denoise the images. The same software was used to colour balance the images, with all manipulations of colour and intensity applied equally to an entire image stack, and the resulting files used create two-dimensional maximum intensity projections for the image stack corresponding to each time segment. These projections were then combined using AdobeAfter Effects to generate video sequences.
Statistics
For all experiments, the difference between groups was calculated using the Mann-Whitney U test for unpaired data (GraphPad Prismversion 4.0; GraphPad Software). Differences were considered significant when P < 0.05.
Supplementary Material
Supplementary Figure 1: Recruitment of immune cells to the lungs in response to PGN is intact in WT->TLR4−/− chimeras. WT->WT and WT->TLR4−/− chimeras were injected i.t with LPS, PGN, or PBS. The recruitment of (A) neutrophils, (B) monocytes, (C) MHCII+CD11c+ dendritic cells and (D) MHCII+CD11c+CD11b+ DCs was analyzed. (E) Chemokine production in BAL fluids. *:p<0.05.
Supplementary Figure 2. Quantification of movements of MHCIIeGfp DCs in tracheal explants. Mice were treated in vivo with PBS or LPS and tracheal explants were made 2h later. Tracks of individual cells in the X-Y-Z space over time were calculated using Imaris 5.0 software package. This experiment was performed in WT->WT and WT->TLR4 chimeric mice. Bar histograms represent the average length of the displacement tracks.
Supplementary figure 3: TLR4 expression on airway structural cells is necessary and sufficient for chemokine production in the airways in response to HDM allergen. Chimeras were injected i.t with PBS or HDM allergen. Production of CCL2 (A), and CCL3 (B) was measured in BAL fluids 24 hours later. *:p<0.05. This is one representative experiment out of 3. 5–6 mice/group were used.
Supplementary Figure 4. Comparison of production of innate proallergic cytokines following administration of LPS or HDM. Mice received an intratracheal injection of 100 μg of HDM, 100 ng LPS or 10 μg of LPS. Thirty-six h later, the levels of TSLP, IL-25 and IL-33 were measured in the BAL fluid.
Supplementary movie 1: In situ behaviour of non-stimulated airway DCs. Video of a tracheal explant from a WT->WT mouse given PBS intratracheally. The trachea was explanted, stained with Hoechst (blue) and immobilized. Total time, 26 min. Playback speed, 120×. Bar, 70 μm.
Supplementary movie 2: In situ behaviour of LPS-activated airway DCs in WT->WT chimeric mice. Video of a tracheal explant from a WT->WT chimeric mouse given LPS intratracheally. The trachea was explanted, stained with Hoechst (blue) and immobilized. Total time, 26 min. Playback speed, 120×. Bar, 70 μm.
Supplementary movie 3: In situ behaviour of LPS-activated airway DCs in WT->TLR4−/− chimeric mice. Video of a tracheal explant from an MHCII-EGFP mouse given LPS intratracheally. The trachea was explanted, stained with Hoechst (blue) and immobilized. Total time, 26 min. Playback speed, 120×. Bar, 70 μm.
Supplementary table 1. Table summarizing the induction of MFI intensity of CD86 and CD40 expression on CD11c+ tracheal DC subsets 24h following in vivo exposure to LPS (fold induction over PBS exposure).
Acknowledgments
B.N.L is a recipient of an Odysseus grant of the Flemish Government. We wish to thank Dr. Tom Boterberg, Department of Radiotherapy for help with mouse irradiation, and S. De Prijck and M. Van Heerswinghel for help with experiments.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
H.H., M.A.W., F.P. performed and analyzed experiments; M.C. and R.N.G. were instrumental in setting up the live dual photon imaging; H.H. and B.N.L. wrote the manuscript; R.N.G. supervised the work at NIH and B.N.L. supervised the work at Ghent University.
References
- 1.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 2.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stampfli MR, et al. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest. 1998;102:1704–1714. doi: 10.1172/JCI4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 5.Sha Q, et al. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 6.Guillot L, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 7.Skerrett SJ, et al. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 8.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 9.Saito T, et al. Expression of toll-like receptor 2 and 4 in lipopolysaccharide-induced lung injury in mouse. Cell Tissue Res. 2005;321:75–88. doi: 10.1007/s00441-005-1113-9. [DOI] [PubMed] [Google Scholar]
- 10.Berndt A, et al. Elevated amount of Toll-like receptor 4 mRNA in bronchial epithelial cells is associated with airway inflammation in horses with recurrent airway obstruction. Am J Physiol Lung Cell Mol Physiol. 2007;292:L936–943. doi: 10.1152/ajplung.00394.2006. [DOI] [PubMed] [Google Scholar]
- 11.Trompette A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 13.Braun-Fahrlander C, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 14.Lewkowich IP, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattouh R, et al. House dust mite facilitates ovalbumin-specific allergic sensitization and airway inflammation. Am J Respir Crit Care Med. 2005;172:314–321. doi: 10.1164/rccm.200502-198OC. [DOI] [PubMed] [Google Scholar]
- 16.Cates EC, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 17.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo Y, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 19.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 20.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolte MA, Leibundgut-Landmann S, Joffre O, Sousa CR. Dendritic cell quiescence during systemic inflammation driven by LPS stimulation of radioresistant cells in vivo. J Exp Med. 2007;204:1487–1501. doi: 10.1084/jem.20070325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 23.Noulin N, et al. Both hematopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol. 2005;175:6861–6869. doi: 10.4049/jimmunol.175.10.6861. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz E, et al. Genes other than TLR4 are involved in the response to inhaled LPS. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1106–1114. doi: 10.1152/ajplung.2001.281.5.L1106. [DOI] [PubMed] [Google Scholar]
- 25.Pichavant M, et al. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J Allergy Clin Immunol. 2005;115:771–778. doi: 10.1016/j.jaci.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 26.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robays LJ, et al. Chemokine receptor CCR2 but not CCR5 or CCR6 mediates the increase in pulmonary dendritic cells during allergic airway inflammation. J Immunol. 2007;178:5305–5311. doi: 10.4049/jimmunol.178.8.5305. [DOI] [PubMed] [Google Scholar]
- 28.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets wih distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 29.Veres TZ, et al. Spatial Interactions Between Dendritic Cells and Sensory Nerves in Allergic Airway Inflammation. Am J Respir Cell Mol Biol. 2007 doi: 10.1165/rcmb.2007-0087OC. [DOI] [PubMed] [Google Scholar]
- 30.Bajenoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammad H, Lambrecht BN. Lung dendritic cell migration. Adv Immunol. 2007;93:265–278. doi: 10.1016/S0065-2776(06)93007-7. [DOI] [PubMed] [Google Scholar]
- 32.Stumbles PA, et al. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993;177:1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piggott DA, et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angkasekwinai P, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Wills-Karp M, et al. Interleukin-13: Central Mediator of Allergic Asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 40.Beutler B, Poltorak A. The sole gateway to endotoxin response: how LPS was identified as Tlr4, and its role in innate immunity. Drug Metab Dispos. 2001;29:474–478. [PubMed] [Google Scholar]
- 41.Boes M, et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 42.Matute-Bello G, et al. Optimal timing to repopulation of resident alveolar macrophages with donor cells following total body irradiation and bone marrow transplantation in mice. J Immunol Methods. 2004;292:25–34. doi: 10.1016/j.jim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–4097. [PubMed] [Google Scholar]
- 44.Van Rijt LS, et al. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods. 2004;288:111–121. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Hammad H, et al. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J Exp Med. 2007;204:357–367. doi: 10.1084/jem.20061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Recruitment of immune cells to the lungs in response to PGN is intact in WT->TLR4−/− chimeras. WT->WT and WT->TLR4−/− chimeras were injected i.t with LPS, PGN, or PBS. The recruitment of (A) neutrophils, (B) monocytes, (C) MHCII+CD11c+ dendritic cells and (D) MHCII+CD11c+CD11b+ DCs was analyzed. (E) Chemokine production in BAL fluids. *:p<0.05.
Supplementary Figure 2. Quantification of movements of MHCIIeGfp DCs in tracheal explants. Mice were treated in vivo with PBS or LPS and tracheal explants were made 2h later. Tracks of individual cells in the X-Y-Z space over time were calculated using Imaris 5.0 software package. This experiment was performed in WT->WT and WT->TLR4 chimeric mice. Bar histograms represent the average length of the displacement tracks.
Supplementary figure 3: TLR4 expression on airway structural cells is necessary and sufficient for chemokine production in the airways in response to HDM allergen. Chimeras were injected i.t with PBS or HDM allergen. Production of CCL2 (A), and CCL3 (B) was measured in BAL fluids 24 hours later. *:p<0.05. This is one representative experiment out of 3. 5–6 mice/group were used.
Supplementary Figure 4. Comparison of production of innate proallergic cytokines following administration of LPS or HDM. Mice received an intratracheal injection of 100 μg of HDM, 100 ng LPS or 10 μg of LPS. Thirty-six h later, the levels of TSLP, IL-25 and IL-33 were measured in the BAL fluid.
Supplementary movie 1: In situ behaviour of non-stimulated airway DCs. Video of a tracheal explant from a WT->WT mouse given PBS intratracheally. The trachea was explanted, stained with Hoechst (blue) and immobilized. Total time, 26 min. Playback speed, 120×. Bar, 70 μm.
Supplementary movie 2: In situ behaviour of LPS-activated airway DCs in WT->WT chimeric mice. Video of a tracheal explant from a WT->WT chimeric mouse given LPS intratracheally. The trachea was explanted, stained with Hoechst (blue) and immobilized. Total time, 26 min. Playback speed, 120×. Bar, 70 μm.
Supplementary movie 3: In situ behaviour of LPS-activated airway DCs in WT->TLR4−/− chimeric mice. Video of a tracheal explant from an MHCII-EGFP mouse given LPS intratracheally. The trachea was explanted, stained with Hoechst (blue) and immobilized. Total time, 26 min. Playback speed, 120×. Bar, 70 μm.
Supplementary table 1. Table summarizing the induction of MFI intensity of CD86 and CD40 expression on CD11c+ tracheal DC subsets 24h following in vivo exposure to LPS (fold induction over PBS exposure).