Abstract
The nasofrontal suture links the nasal complex with the braincase and is subject to compressive strain during mastication and (theoretically) tensile strain during growth of nasal soft tissues. The suture’s ability to transmit compressive and tensile loads therefore affects both cranioskeletal stress distribution and growth. This study investigated the in vitro viscoelastic and failure properties of the nasofrontal suture in the pig, Sus scrofa. Suture specimens from two ages were tested in compression and tension and at fast and slow rates. In additional specimens, strain gauges were applied to the suture and nasal bone for strain measurement during testing. Relaxation testing demonstrated higher elastic moduli in tension than compression, regardless of test rate or pig age. In contrast, maximum elastic moduli from failure tests, as well as peak stresses, were significantly higher in compression than in tension. Sutures from older pigs tended to have higher elastic moduli and peak stresses, significantly so for tensile relaxation moduli. Strain gauge results showed that deformation at the suture was much greater than that of the nasal bone. These data demonstrate the viscoelasticity and deformability of the nasofrontal sutural ligament. The suture achieved maximal resistance to tensile deformation at low loads, corresponding with the low tensile loads likely to occur during growth of nasal soft tissues. In contrast, the maximal stiffness in compression at high loads indicates that the suture functions with a substantial safety factor during mastication.
Keywords: Mechanical testing, Compression, Tension, Facial suture
1. Introduction
Craniofacial sutures have a dual function in the cranioskeleton, contributing to growth at bone margins and absorbing the loads incurred by muscle activity and chewing. The nasofrontal suture of pigs (Fig. 1) is exceptional in its rapid growth rate, as well as its high magnitude compressive strains during chewing (Rafferty and Herring, 1999). It occupies the critical position between the braincase and snout. Although masticatory strain is strongly compressive, the nasofrontal suture may be strained in tension by the growth of underlying soft tissues (Copray, 1986; Moss, 1962; Sarnat, 1997). However, the mechanical properties of this suture, an essential consideration for understanding load transmission, are unknown.
Fig. 1.
Dorsal view of the pig skull showing the location of the nasofrontal suture.
Previous ex vivo studies of suture biomechanics have focused on cranial rather than facial sutures. These studies indicate that cranial sutures are zones of flexibility, but their properties are regionally specific and vary with age (Margulies and Thibault, 2000; McLaughlin et al., 2000; Sutton, 1993; Tanaka et al., 2000). Facial sutures generally fuse later in life than cranial sutures (Herring, 1972; Kokich, 1986), but in both, progressive ligament development and bone fortification (Pritchard et al., 1956) suggest that elastic moduli should increase with age.
The in vivo masticatory and growth loads placed on the facial sutures are strikingly different in rate. Pigs chew at 2–3 Hz and peak nasofrontal suture strains are high (Rafferty and Herring, 1999); thus the rate of compressive loading is high. In contrast, the interstitial growth of the nasal septum is expected to apply tensile force to the overlying nasofrontal suture at a low and relatively constant rate. Because sutures are viscoelastic (Tanaka et al., 2000), they should be stiffer at higher loading rates (Margulies and Thibault, 2000).
In order to evaluate the ability of the pig nasofrontal suture to transmit load in vivo, this study investigated its mechanical properties at two different ages, comparing viscoelastic responses to compression and tension at low and high rates of loading.
2. Materials and methods
Nasofrontal suture specimens were collected from farm pigs (breed and sex unknown) 3–6 weeks old (younger) and 5–6 months old (older); sample sizes are given in Tables 1 and 2. After dissection from fresh tissue, specimens were stored in 0.9% PBS or protease inhibitor solution at −20 °C. The protease inhibitor solution consisted of 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM EDTA and 1 mM benzamidine HCl in 0.1 M PBS. Specimens were approximately 65 mm in length and 5 mm in width with the suture in the center. To approximate the in vivo condition of the suture, the periosteum was left intact. The ends of compression test specimens were potted in dental acrylic and rehydrated overnight in the refrigerator. Specimens tested in tension were tested the same day as thawing. Overnight hydration was found to have no effect on tension test results.
Table 1.
Nasofrontal suture specimen dimensions, mean±standard deviations (sample size)
| Tissue origin | Test type | Specimen dimensions |
|||||
|---|---|---|---|---|---|---|---|
| Length (mm) | Test length (mm) | Width (mm) | Thickness (mm) | X-sectional area (mm)2 | Suture width (μm) | ||
| Younger pigs | Fast (0.02 mm/s) | 342±75 (11) | |||||
| Compression (C) | 57±11 (11) | 34±7 (11) | 5±0.7 (11) | 7±2 (11) | 35±11 (11) | ||
| Tension (T) | 62±6 (8) | 32±6 (8) | 5±0.4 (8) | 9±3 (8) | 41±15 (8) | ||
| p-value, C vs. T | ns | ns | ns | ns | ns | ns | |
| Older pigs | Fast (0.02 mm/s) | 256±73 (5) | |||||
| Compression | 63±4 (4) | 33±5 (4) | 5±0.5 (4) | 9±5 (4) | 50±29 (4) | ||
| Tension | 63±6 (8) | 34±3 (8) | 5±0.9 (8) | 13±4 (8) | 67±22 (8) | ||
| p-value, C vs. T | ns | ns | ns | ns | ns | ns | |
| Slow (0.0002 mm/s) | |||||||
| Compression | 74±7 (15) | 36±6 (15) | 5±0.6 (15) | 14±3 (15) | 71±18 (15) | ||
| Tension | 64±5 (14) | 32±2 (14) | 5±0.6 (14) | 13±4 (14) | 64±19 (14) | ||
| p-value, C vs. T | 0.03 | ns | ns | ns | ns | ||
Table 2.
Mechanical properties of the nasofrontal suture at fast and slow test speeds, mean±standard deviations (sample size)
| Tissue origin | Test type | Elastic moduli from relaxation tests |
Failure tests |
p-value, elastic modulus, 0 s vs. max. modulus | |||
|---|---|---|---|---|---|---|---|
| Modulus at 0 s (MPa) | Modulus at 30 s (MPa) | Modulus at 2 min (MPa) | Maximum elastic modulus (MPa) | Peak stress (MPa) | |||
| Younger pigs | Fast (0.02 mm/s) | ||||||
| Compression (C) | 18±11 (11) | 16±9 (11) | 14±9 (11) | 68±32 (9) | 1.5±0.7 (11) | 0.001 | |
| Tension (T) | 38±18 (7) | 31±11 (8) | 27±10 (8) | 43±16 (7) | 0.7±0.2 (7) | ns | |
| p-value, C vs. T | 0.017 | 0.008 | 0.013 | 0.04 | 0.002 | ||
| Older pigs | Fast (0.02 mm/s) | ||||||
| Compression | 29±18 (4) | 25±17 (4) | 23±15 (4) | 115±45 (4) | 3.4±1.4 (4) | 0.03 | |
| Tension | 74±30 (8) | 49±19 (8) | 46±19 (8) | 70±33 (8) | 0.9±0.5 (4) | ns | |
| p-value, C vs. T | 0.009 | 0.06 | 0.05 | ns | 0.04 | ||
| Slow (0.0002 mm/s) | |||||||
| Compression | 18±8 (15) | 18±8 (11) | 19±8 (11) | 122±63 (13) | 2.8±1.4 (9) | 0.0001 | |
| Tension | 50±26 (12) | 35±25 (12) | 32±24 (12) | 46±30 (11) | 0.8±0.5 (7) | ns | |
| p-value, C vs. T | 0.003 | 0.05 | ns | 0.0008 | 0.002 | ||
| p-value, younger vs. older at 0.02 mm/s | |||||||
| Compression | ns | ns | ns | ns | ns | ||
| Tension | 0.02 | 0.04 | 0.02 | ns | ns | ||
Viscoelastic and failure testing were carried out in an MTS/Sintech 2 testing machine (Raleigh, NC, USA) with specimens suspended in a solution-filled plexiglass container at 22 °C. For most tests, the protease inhibitor solution was used; however, a few tests were performed in 0.9% PBS. ANOVA indicated that the solution was not a significant source of variation. Specimens were mounted in long-axis orientation between compression platens or pneumatic grips, depending on test type.
Stress relaxation tests were conducted at test speeds of 0.02 mm/s (both ages) and 0.0002 mm/s (older pigs only, because of the limited number of young pigs). The higher rate was similar to in vivo chewing, and the lower rate was based on the rate of sutural growth (Herring and Mucci, 1991; Rafferty and Herring, 1999). Specimens were preloaded to 1 N, defined as 0% strain. Then loading was increased to a defined strain point (see below) and the strain was held for 5 min to achieve relaxation equilibrium. This test sequence was repeated for five different strain points with a rest period of 10 min between tests. The strain points were deliberately set low, 0.1% increments between 0.1% and 0.5%, to correspond with in vivo strain levels, averaging close to 0.2% (Rafferty and Herring, 1999). Because of variations in specimen size, measured strain points ranged from 0.1% to 0.9%. The order of defined strain points was randomized among specimens. Stress was estimated by dividing load by the cross-sectional area of the specimen at the location of the sutural ligament. Strain was calculated as deformation divided by the exposed specimen length (test length in Table 1), and thus represented a composite strain of both suture and bone. For compression tests, the test length was measured between compression platens, excluding acrylic pots, whereas for tension tests, the exposed specimen length was the distance between pneumatic grips. For each held strain point, the relaxed stress was recorded at 30 s and 2 min following the held strain point. Relaxed stresses and strains from each test were plotted and the slope of the linear region of these curves was defined as the relaxed elastic modulus for each time point. Specimen stiffness (elastic modulus prior to relaxation) was defined as the slope of the linear region of the stress/strain curve, and is here reported as the mean of five stress-relaxation tests. Following stress-relaxation tests, specimens were loaded until failure using the same rate. The highest stress achieved prior to specimen failure was defined as peak stress. Two-sample t-tests were used to compare compression and tension values at each test speed. ANOVA was used to determine whether relaxation time was a significant source of variation for elastic moduli measured at each rate.
In order to assess the distribution of strain across test specimens, an additional set of tests was carried out using strain gauges (EP-08-125BT-120, Measurements Group, Inc., Raleigh, NC, USA) to monitor the suture and nasal bone separately. Six specimens were tested, three in compression and three in tension. Width and thickness measurements were made at each gauge site and stress was estimated by dividing load by cross-sectional area. Specimens were loaded at 0.02 mm/s, as above. Simultaneous strain signals from the gauges were conditioned and amplified (2100 System, Measurements Group). Load cell data and amplified strains were converted to digital signals (MP100, Biopac Systems, Inc., Santa Barbara, CA, USA) using Acq-knowledge III (Biopac Systems, Inc.), and voltages were converted to strain. Data were analyzed as previously, except that during failure tests peak strain was recorded.
Specimens were prepared for histological processing following testing. The bony ends were reduced, followed by decalcification in formic acid, paraffin embedding and sagittal sectioning at 7 μm. Sections were stained with hematoxylin and eosin or picro-indigo carmine and examined blinded to test type for tissue damage. Suture width between bony interdigitations was measured on tissue sections using MetaVue (Molecular Devices Co., Sunnyvale, CA, USA) software. Histological slides of untested nasofrontal sutures served as controls.
3. Results
3.1. Specimen size
Specimen dimensions are reported in Table 1. Test length and width did not differ between younger and older pigs, but thickness was less in the younger pigs. Suture width was marginally greater in the younger pigs than in the older pigs (p = 0.06) and did not show significant differences between compressive and tensile tests.
3.2. Stress–strain curves
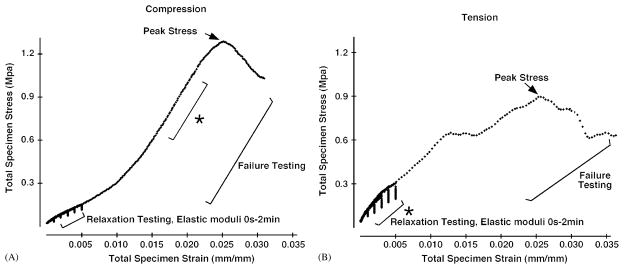
At both test speeds compressive failure testing produced stress/strain curves that were distinctively different from those occurring during tension (Figs. 2 and 3). In compression, failure curves were biphasic with an early linear region at low strain transitioning to a second linear region of higher slope. Peak stress was reached soon after the onset of the plastic region of the curve. During tensile tests, the failure curve showed a single linear region that began almost immediately following the onset of loading. The plastic region of the curve was more extensive, with peak stress often following a protracted period of deformation.
Fig. 2.
Stress–strain curves from relaxation and failure tests of nasofrontal sutures from younger pigs in compression (A) and tension (B). The asterisk designates the linear region of the curve corresponding with the maximum elastic modulus. In compression tests the relaxation elastic modulus at 0 s during relaxation testing is lower than the maximum elastic modulus occurring during failure testing. In tension tests, the elastic modulus at 0 s of relaxation testing is the maximum elastic modulus during failure tests.
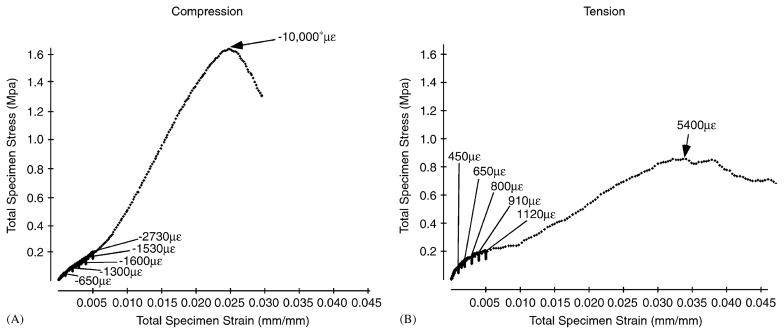
Fig. 3.
Stress–strain curves from relaxation and failure tests of nasofrontal sutures from older pigs in compression (A) and tension (B). The suture strains are given for relaxation tests at 0 s relaxation. The asterisk indicates that strain exceeded the gauge’s maximum. Sutural strain had similar ranges in compression and tension during relaxation testing; however, at peak stress sutural strain during compressive testing far exceeds that during tensile testing. Note that strain measured by strain gauges is less than the corresponding total specimen strain measured by the MTS machine. This consistent discrepancy may be due to the small area sampled by the gauge or to compliance in the testing set-up.
Relaxation tests performed at low strains resulted in a higher elastic modulus and relaxation moduli in tension than in compression (Fig. 2 and Table 2) for both rates and ages. Relaxation moduli tended to decrease with relaxation time, although significance was reached only for older sutures tested in fast tension (p = 0.05). Comparison within older pigs of slow and fast loading demonstrated no significant differences, although most values were slightly higher with faster loading (Table 2). Neither compressive nor tensile failure resulted in visible damage to the suture specimen.
In compression, the relaxation elastic modulus (0 s) was lower than the maximum elastic modulus measured during failure. In contrast, tensile relaxation elastic modulus (0 s) was not distinguishable from the maximum elastic modulus measured during failure (Table 2). Compression failure testing resulted in greater maximum elastic moduli and peak stress than in tensile tests (Table 2). These results were consistent across pig age and rate of testing.
Moduli and peak stress were typically higher in older pigs, but most comparisons were not statistically significant. During tensile testing, the moduli at 0 s–2 min from relaxation tests were the only significantly different biomechanical properties in younger vs. older pigs (Table 2).
3.3. Strain
Relaxation strain ranges were similar for tension and compression (Table 3 and Fig. 3). Sutural strain was always much higher than bone strain. This differential persisted during failure tests. Peak bone strain was usually lower during tensile than during compressive failure. Peak sutural strain was uniformly high during failure testing and exceeded measurable limits in two compressed samples.
Table 3.
Nasal bone and nasofrontal suture stresses and strains from strain gauge data
| Sample# | Relaxation tests |
Failure tests |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Nasal bone |
Suture |
Nasal bone |
Suture |
||||||
| Strain range (με) | Stress range (MPa) | Strain range (με) | Stress range (MPa) | Peak strain (με) | Stress at peak strain (MPa) | Peak strain (με) | Stress at peak strain (MPa) | ||
| Tension | 1 | 30–70 | 0.1–0.2 | 450–1120 | 0.1–0.2 | 260 | 1.0 | 5300 | 0.9 |
| 2 | 7–13 | 0.1–0.4 | 800–2360 | 0.2–0.4 | 320 | 1.2 | 9400 | 1.5 | |
| 3 | 143–420 | 0.2–0.4 | 410–1140 | 0.1–0.3 | 260 | 1.1 | 5400 | 0.8 | |
| Compression | 4 | −100 to −230 | 0.2–0.6 | −650 to –2730 | 0.1–0.2 | −2100 | 4.5 | −10,000a | 1.5 |
| 5 | −3 to −230 | 0.1–0.4 | −470 to –1760 | 0.1–0.5 | −1050 | 2.4 | −5700 | 2.6 | |
| 6 | −2 to −40 | 0.1–0.2 | −220 to –400 | 0.1–0.2 | −860 | 3.6 | −10,000a | 5.1 | |
Values exceeded the capacity of the gauge.
3.4. Histology
The nasofrontal suture is beveled with the nasal bone overlying the frontal bone (Herring, 1972). General structure was the same as in control slides (Rafferty and Herring, 1999). Interdigitations of cellular woven bone (Fig. 4) were separated by fiber bundles that were embedded in one bone surface and extended into the ligament space. The highly cellular ligament was organized and unidirectional near the bone but centrally the fibers were cruciate, appearing as a mat of fiber bundles in diverse orientations. In many but not all locations the fiber bundles were oriented to resist compression; however, at the tips of the bone spicules the fibers often were arranged to resist tension (Fig. 4A).
Fig. 4.
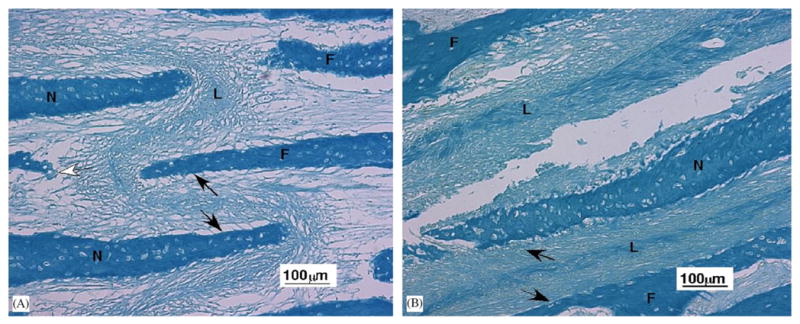
Light microscopy of the nasofrontal suture ligament (10 ×). (A) Portion of nasofrontal suture from an older pig tested in tension. The central sutural ligament (L) contains fibers oriented in multiple directions. Black arrows illustrate the fibers oriented to resistant compression and the white arrow fibers oriented to resist tension. Frontal bone (F), nasal bone (N). (B) Tear of the ligament in a nasofrontal suture from a younger pig that underwent compression testing. Black arrows illustrate compression resistant fibers. The central ligament (L) also contains fibers oriented to resist forces from other directions. Although the tension-tested ligament (A) appears stretched and the compression-tested ligament (B) appears compacted, this was not a consistent relationship. Although the bones are thicker in older than younger animals, the trabeculae forming the suture are about the same size.
There was no association of deformation and testing modality. Compressed specimens did not show bone-to-bone contact. Most sutures were compacted in some areas and attenuated in others. Control, as well as tested specimens, exhibited occasional detachment at the bone–ligament interface. Only tested specimens showed tears within the ligament (Fig. 4B).
4. Discussion
The nasofrontal suture demonstrated viscoelastic behavior during both compressive and tensile loading, similar to other collagen-rich tissues during stress-relaxation. Viscoelastic behavior is generally considered to result from complex interactions between various ligament components, such as collagen, water, surrounding protein, and ground substance (Woo et al., 1990, 2000). In the rabbit medial collateral ligament, a significant component of ligament relaxation was shown to be fluid flow; ligaments with higher water content demonstrated greater relaxation than those with lower water content (Chimich et al., 1992). Crosslinking has also been implicated in the response of collagen-rich tissues to load (Liao and Vesely, 2003; Thornton et al., 2000; Weiss et al., 2002). Decreased linkage between proteoglycans and collagen corresponded with greater stress-relaxation in chordae tendineae (Liao and Vesely, 2004). Both fluid flow and changes in collagen cross-linkage could account for the observed stress-relaxation of the nasofrontal suture. In addition, the fibers or ground substance could rearrange at a gross level during suture relaxation.
High sutural and low bone strain during testing indicate that stress/strain curves predominantly reflect deformation of the sutural ligament. The suture includes cells and glycosaminoglycans, as well as fibers, which are composed of collagens 1 and 3 (Opperman, 2000). Glycosaminoglycans fill space between fibers and cells (Persson, 1973), and these hydrophilic molecules are likely to bear load only in compression. In contrast, collagen resists tensile stress. The fibers of the rat interparietal suture visibly stretched under tension, even at low force levels (Miyawaki and Forbes, 1987). However, because the nasofrontal sutural ligament has fibers in multiple orientations, some portion of the ligament would be loaded in tension regardless of loading direction.
In contrast to expectation, changing the loading rate from 0.02 mm/s (similar to mastication) to 0.0002 mm/s (similar to growth) had little effect on the properties observed. Pig age also had surprisingly small effects. The only significant influence of pig age was that tensile relaxation moduli were lower in younger animals. This result may relate to the presumed higher growth rate of the nasal septum in younger animals, with the nasofrontal suture providing less resistance. We had expected a more general difference between ages. The small sample sizes for some tests may have hindered our ability to discern small differences. Morphological study of facial sutures suggests that density and thickness of collagen bundles should increase with age (Persson, 1973, 1995), as should the interlocking of serrated sutures (Kokich, 1976; Persson, 1973). However, these effects were not prominent in our histological comparisons, just as the sutural properties indicated only minor fortification with age.
Although we tested specimens in tension and compression, the curved and complex suture was probably predominantly under shear. Nevertheless, this is likely to occur in vivo as well. Differences in the response of the nasofrontal suture to compressive and tensile loading may correspond with the role of GAGs in absorbing compressive loads, and of collagen fibers in primarily resisting tension. During compression tests, the early slope of the stress/strain curve (modulus at 0 s) is likely to reflect the loading of GAGs and exodus of fluid (Table 2 and Fig. 2). With continued loading the compressive stress/strain curves developed a higher maximum elastic modulus, indicating resistance of collagen fibers within the sutural ligament. In contrast, the early slope of the tensile stress/strain curves (modulus at 0 s) was indistinguishable from the maximum elastic modulus observed during failure testing (Table 2 and Fig. 2). These data suggest that in tension the sutural ligament is loaded immediately and achieves its maximal resistance at low load levels. Thus, the greater stiffness of the suture in tension versus compression at low loads probably reflects the immediate resistance of the tension-adapted ligament fibers radiating from the endpoints of bony interdigitations. On the other hand, at higher loads the suture demonstrated greater stiffness and strength in compression versus tension. The biological basis for this increased stiffness in compression is likely due to the preponderance of sutural ligament fibers oriented to resist compression. Additive effects between GAGs and fibers and the resistance of nasal and frontal bones against one another could also be involved.
Comparison of nasofrontal suture strains measured in vitro and in vivo provides insight into the suture’s function during mastication and growth. During mastication, strains are compressive and corresponded in magnitude with those measured in vitro during relaxation testing (Rafferty and Herring, 1999), approximately −1600 με. In vitro nasofrontal suture strains were also disproportionately higher than those on the nasal bone, as observed in vivo (about −100 με). These data confirm that loads are largely absorbed at the suture, but are transmitted through bone with minimal deformation. Peak strains measured during compressive failure tests were higher than in vivo levels, indicating that during normal mastication the nasofrontal suture functions with a substantial safety factor. The high maximum stiffness and peak stress of the compressive failure curve demonstrate the suture’s resistance to high loads, however, should they occur. Relaxation testing in tension resulted in a similar range of sutural strains as in compression, yet in vivo tensile deformation of the suture through growth of the nasal septum is expected to be small. Interestingly, the maximum tensile stiffness also occurred during relaxation testing at low loads, indicating immediate resistance to growth pressures of the underlying nasal septum. Although the peak stresses occurring during failure testing were lower in tension than in compression, the safety factor might be equivalent, given the low level stresses that are likely to occur.
Acknowledgments
We thank Dr. Joan Sanders for animal tissues, Aaron Jacobson for help with testing, and Patricia Emry for histology. Supported by DE 08513 from NIH/NIDCR.
References
- Chimich D, Shrive N, Frank C, Marchuk L, Bray R. Water content alters viscoelastic behavior of the normal adolescent rabbit medial collateral ligament. Journal of Biomechanics. 1992;25:831–837. doi: 10.1016/0021-9290(92)90223-n. [DOI] [PubMed] [Google Scholar]
- Copray JCVM. Growth of the nasal septal cartilage of the rat in vitro. Journal of Anatomy. 1986;144:99–111. [PMC free article] [PubMed] [Google Scholar]
- Herring SW. Sutures—a tool in functional cranial analysis. Acta Anatomica. 1972;83:222–247. doi: 10.1159/000143860. [DOI] [PubMed] [Google Scholar]
- Herring SW, Mucci RJ. In vivo strain in cranial sutures: the zygomatic arch. Journal of Morphology. 1991;207:225–239. doi: 10.1002/jmor.1052070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokich VG. Age changes in the human frontozygomatic suture. American Journal of Orthodontic and Dentofacial Orthopedics. 1976;69:411–430. doi: 10.1016/0002-9416(76)90209-8. [DOI] [PubMed] [Google Scholar]
- Kokich VG. The biology of sutures. In: Cohen MM, editor. Craniosynostosis: Diagnosis, Evaluation and Management. Raven Press; New York: 1986. pp. 81–103. [Google Scholar]
- Liao J, Vesely I. A structural basis for the size-related mechanical properties of mitral valve chordae tendineae. Journal of Biomechanics. 2003;36:1125–1133. doi: 10.1016/s0021-9290(03)00109-x. [DOI] [PubMed] [Google Scholar]
- Liao J, Vesely I. Relationship between collagen fibrils, glycosaminoglycans, and stress relaxation in mitral valve chordae tendineae. Annals of Biomedical Engineering. 2004;32 (7):977–983. doi: 10.1023/b:abme.0000032460.97278.e9. [DOI] [PubMed] [Google Scholar]
- Margulies SS, Thibault KL. Infant skull and suture properties: measurements and implications for mechanisms of pediatric brain injury. Journal of Biomechanical Engineering. 2000;122:364–371. doi: 10.1115/1.1287160. [DOI] [PubMed] [Google Scholar]
- McLaughlin E, Zhang Y, Pashley D, Borke J, Yu J. The load-displacement characteristics of neonatal rat cranial sutures. Cleft Palate-Craniofacial Journal. 2000;37 (6):590–595. doi: 10.1597/1545-1569_2000_037_0590_tldcon_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Miyawaki S, Forbes DP. The morphologic and biochemical effects of tensile force application to the interparietal suture of the Sprague-Dawley rat. American Journal of Orthodontic and Dentofacial Orthopedics. 1987;92:123–133. doi: 10.1016/0889-5406(87)90367-2. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix. In: Kraus BS, Riedel RA, editors. Vistas in Orthodontics. Lea and Febiger; Philadelphia: 1962. pp. 85–98. [Google Scholar]
- Opperman LA. Cranial sutures as intramembranous bone growth sites. Developmental Dynamics. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Persson M. Structure and growth of facial sutures. Odontologisk Revy. 1973;24 (Suppl 26):1–146. [Google Scholar]
- Persson M. The role of sutures in normal and abnormal craniofacial growth. Acta Odontologica Scandinavica. 1995;53:152–161. doi: 10.3109/00016359509005965. [DOI] [PubMed] [Google Scholar]
- Pritchard JJ, Scott JH, Girgis FG. The structure and development of cranial and facial sutures. Journal of Anatomy. 1956;90:73–86. [PMC free article] [PubMed] [Google Scholar]
- Rafferty K, Herring SW. Craniofacial sutures: morphology, growth, and in vivo masticatory strains. Journal of Morphology. 1999;242:167–179. doi: 10.1002/(SICI)1097-4687(199911)242:2<167::AID-JMOR8>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat BG. A retrospective of personal craniodental research and clinical practice. Plastic and Reconstructive Surgery. 1997;100:132–153. doi: 10.1097/00006534-199707000-00024. [DOI] [PubMed] [Google Scholar]
- Sutton G. MSE Thesis. University of Washington; Seattle: 1993. The use of a non-contact video method to compare the compressive properties of bone and ligament in the zygomatic arch of pigs; p. 62. [Google Scholar]
- Tanaka E, Miyawaki Y, del Pozo K, Tanne K. Changes in the biomechanical properties of the rat interparietal suture incident to continuous tensile force application. Archives of Oral Biology. 2000;45:1059–1064. doi: 10.1016/s0003-9969(00)00082-0. [DOI] [PubMed] [Google Scholar]
- Thornton GM, Leask GP, Shrive NG, Frank CB. Early medial collateral ligament scars have inferior creep behaviour. Journal of Orthopaedic Research. 2000;18:238–246. doi: 10.1002/jor.1100180211. [DOI] [PubMed] [Google Scholar]
- Weiss JA, Gardiner JC, Bonifasi-Lista C. Ligament material behavior is nonlinear, viscoelastic and rate-independent under shear loading. Journal of Biomechanics. 2002;35 (7):943–950. doi: 10.1016/s0021-9290(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Woo SLY, Peterson RH, Ohland KJ, Sites TJ, Danto MI. The effects of strain rate on the properties of the medial collateral ligament in skeletally immature and mature rabbits: a biomechanical and histological study. Journal of Orthopaedic Research. 1990;8:712–721. doi: 10.1002/jor.1100080513. [DOI] [PubMed] [Google Scholar]
- Woo SL, Debski RE, Zeminski J, Abramowitch SD, Saw SS, Fenwick JA. Injury and repair of ligaments and tendons. Annual Review of Biomedical Engineering. 2000;2 (1):83–118. doi: 10.1146/annurev.bioeng.2.1.83. [DOI] [PubMed] [Google Scholar]