Abstract
A growing body of evidence indicates that subpopulations of cancer stem cells (CSC) drive and maintain many types of human malignancies. These findings have important implications for the development and evaluation of oncologic therapies and present opportunities for potential gains in patient outcome. The existence of CSC mandates careful analysis and comparison of normal tissue stem cells and CSC in order to identify differences between the two cell types. The development of CSC-targeted treatments will face a number of potential hurdles, including normal stem cell toxicity and the acquisition of treatment resistance, which must be considered in order to maximize the chance that such therapies will be successful.
INTRODUCTION
Recent insights into tumor biology, uncovered by applying the techniques and principles of normal stem cell biology to the study of cancer biology, indicate that in many cancers only a subset of malignant cells has the potential to proliferate indefinitely and therefore to give rise to macroscopic metastases or to cause tumor recurrence after treatment. It is this subset of cells, often called cancer stem cells (CSC), that needs to be targeted and eliminated in order to achieve cures, since the remaining cancer cells (called non-tumorigenic cells) are destined to stop dividing or to die. The CSC hypothesis suggests that agents targeting these cells should ultimately lead to improved outcomes for cancer patients.
PRINCIPLES OF NORMAL STEM CELL BIOLOGY
In order to fully appreciate the cancer stem cell hypothesis and its implications for therapy, it is critical to understand basic concepts of normal stem cell biology. Most human tissues are composed of rapidly dividing cells that continually die or are lost and that are replenished through tightly regulated mechanisms. In such tissues, a cellular hierarchy exists that starts with a relatively small number of stem cells, which give rise to daughter cells, called progenitor or transit amplifying cells, that have limited proliferative potential and that eventually differentiate into mature effector cells. These stem cells can also undergo a type of division in which they give rise to more stem cells by a process called self-renewal. Such self-renewal divisions maintain the stem cell pool for the life of an organism. Since many common human cancers arise in such hierarchical tissues, it is likely that a deeper understanding of the properties of the tissue stem cells will yield new insights into tumor biology.
Normal tissue stem cells are characterized by three critical properties. First, stem cells must be able to self-renew. Importantly, self-renewal and cellular proliferation are not synonymous, since in addition to cell division the former term encompasses both the differentiation and future mitotic potential of the daughter cells. Secondly, stem cells must give rise to daughter cells (i.e. progenitors) that have limited proliferative potential and are destined to differentiate. Through this process stem cells give rise to the mature effector cells that perform a given tissue’s biological functions. Finally, the number of stem cells in normal tissues must be under strict genetic control in order to prevent run-away expansion or exhaustion of a particular tissue.
A closer examination of the mammary gland, a tissue system involving adult stem cells that gives rise to a common human malignancy, will demonstrate these properties. Mammary glands are made up of tubuloalveolar structures formed by stratified epithelium that is composed of an inner layer of luminal epithelial cells and an outer layer of myoepithelial cells. The identification of the mammary stem cell rested on the development of an in vivo clonogenic assay that allowed transplantation of mammary epithelial glands and cells into recipient fat pads that were cleared of their endogenous epithelium1. Using this assay, it was initially demonstrated that transplantation of short segments of mammary ducts led to the formation of extensively arborized ductal networks in the recipient gland and that these result from clonal outgrowths1-3. More recently, several studies have used fluorescence activated cell sorting (FACS) to prospectively isolate so called mammary repopulating units (MRU), which are synonymous with mammary stem cells. Mammary epithelial structures were dissociated, the resulting cell suspension was stained with fluorescently labeled antibodies against surface proteins, and sub-populations of cells were purified based on their pattern of expression of these proteins. The various subpopulations were then injected into cleared mammary fat pads and assayed for their ability to generate mammary gland outgrowths. These experiments revealed that mammary glands contain a small subpopulation of cells that are able to give rise to entire epithelial outgrowths starting from a single cell4, 5. Such epithelial outgrowths can then be re-transplanted into new recipient fat pads for multiple generations, documenting that MRU are able to self-renew. Since most of the cells in the outgrowths are not stem cells but rather mature mammary epithelial cells and since these structures fill the fat pad but do not continue to proliferate uncontrollably, MRU display the three basic properties of normal tissue stem cells.
OBSERVATIONS SUGGESTING THE EXISTENCE OF CANCER STEM CELLS
Two key observations have long suggested that cancers may also be maintained by stem cells. Chief among these was the observation that tumor cells are heterogeneous in their capacity to give rise to tumors upon transplantation or to form colonies in culture assays. For in vivo tumor transplantation, this is reflected in the large numbers of tumor cells that must usually be transplanted to form a tumor. For primary human and mouse tumors, this number often resides in the thousands or millions6-8. Similarly, usually less than 1% of primary tumor cells cultured in vitro can give rise to colonies8-10. The other observation suggesting that cancers may contain stem cells is that tumors often recapitulate the histologic and molecular heterogeneity of the normal tissue from which they are derived. For example, many well- or moderately-differentiated squamous cell carcinomas form organized layers of tumor cells that are histologically similar to normal stratified squamous epithelia. Furthermore, analysis of differentiation markers can reveal similar patterns in tumors and normal tissues. This suggests that there may be a hierarchical relationship among tumor cells, with more immature cells giving rise to more mature-appearing cells, analogous to what is seen in normal tissues.
IDENTIFICATION OF CANCER STEM CELLS
The existence of CSC was first documented in acute myelogenous leukemia (AML). In the majority of patients with AML, leukemic stem cells reside in the CD34+CD38− subpopulation, which are the only these cells that can lead to engraftment of human leukemia in NOD/SCID mice. The more numerous leukemic blasts, generally displaying the CD34−CD38+ immunophenotype, are unable to transplant the disease11, 12. Soon after these discoveries, other investigators showed that CSC can be prospectively isolated from solid tumors. This was first accomplished for human breast cancers where tumorigenic activity was found to be highly enriched in cancer cells with the CD44+CD24−/lowLineage− immunophenotype. Transplantation of as few as 100 of these cells led to tumor formation in mice while tens of thousands of the remaining cancer cells did not13. Importantly, tumors arising from CD44+CD24−/lowLineage− cells contained the entire diversity of cancer cells found in the primary tumor, including cells with the CSC immunophenotype and a large population of cells with the non-tumorigenic cell immunophenotype. Re-transplantation of cells from first generation xenografts similarly led to tumors displaying the immunophenotypic diversity of the primary tumor. These data indicate that human breast cancers contain a hierarchy of cancer cells that originates from CD44+CD24−/lowLineage− CSC, which have the capacity to self-renew.
More recently, CSC-enriched populations have been isolated from many other human malignancies including those arising in the brain14, prostate15, colon16, 17, head and neck18, pancreas19, 20, and liver21. Thus, many human tumors appear to be maintained by subpopulations of CSC that are able to self-renew and to give rise to non-tumorigenic cells with limited proliferative potential.
CANCER STEM CELLS AND RESISTANCE TO CYTOTOXIC THERAPIES
A large number of the most frequently used therapies for cancer induce cell death by inducing DNA damage. These agents are also toxic to normal stem cells and their progeny. Fortunately, normal stem cells have evolved multiple mechanisms to protect themselves from toxins and genotoxic stress, which make them relatively resistant to cell killing with cytotoxic agents. This phenomenon can be observed clinically in patients treated with non-myeloablative cytotoxic regimens, since such therapies often result in drastic depletion of mature white and red blood cells as well as platelets. However, such drug-induced pancytopenia is transient, and several weeks later most patients completely recover. Such hematopoietic recovery would not be possible if hematopoietic stem cells were also eradicated by the drugs, suggesting that these cells are relatively resistant compared to most of their more mature counterparts. Similar organ recovery following chemotherapy can also be observed in the gastrointestinal tract.
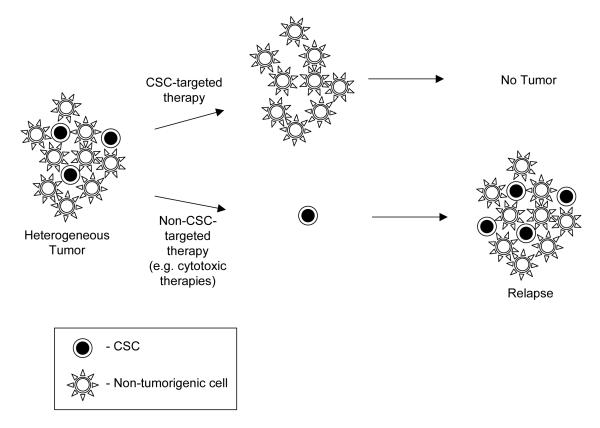
The apparent similarities between normal stem cells and CSC led to the hypothesis that CSC may be relatively resistant to common chemotherapeutic agents compared to their more differentiated non-tumorigenic counterparts22 and that improvements in patient outcomes might be achieved by directly targeting CSC (Figure 1). Mounting evidence suggests that this is the case. Recent studies have shown that “cancer stem cell-like cells” present in some established cell lines and in long-term xenografts appear to be relatively resistant to cytotoxic chemotherapeutics23-26. Since it remains to be determined how representative such “cancer stem cell-like” cells are of CSC in primary tumors, another group analyzed human CD44+ESA+ colon cancer stem cells in early passage xenografts and found that these were enriched in xenografts that had been treated with cyclophosphamide compared to untreated control tumors27. Enrichment was documented both as an increased percentage of cancer stem cells as measured by flow cytometry and as higher tumor forming capacity in limiting dilution assays. Recently, Chang and colleagues extended these observations to human patients. They measured the frequency of cancer stem cells in tumors of women receiving neoadjuvant chemotherapy for locally advanced breast cancer and found that cancer stem cells accumulated after treatment with cytotoxic chemotherapy, but not with certain biologically-targeted agents28. Thus, CSC in some tumors appear to be resistant to cytotoxic chemotherapies. As is discussed elsewhere in this issue, CSC in some tumors also appear to be resistant to radiotherapy. These findings suggest the overcoming CSC resistance mechanisms to cytotoxic therapies may result in higher cure rates.
Figure 1. Implications of the CSC hypothesis for cancer treatment.
Conventional cytotoxic therapies can shrink tumors, but may preferentially spare some CSC. Since CSC are left behind, tumors will eventually regrow. However, CSC-targeted therapies could remove the self-renewing tumor cells and thus lead to tumor stabilization and likely eventual regression.
What are the mechanisms by which cancer stem cells resist chemotherapy- or radiotherapy-induced cell killing? One possible explanation involves cell cycle kinetics of CSC. Rapidly dividing cells have long been known to be more sensitive to cytotoxic therapies than quiescent cells. It has been shown that at least a subset of leukemia stem cells are quiescent and resistant to chemotherapy29. A similar phenomenon may exist in malignant epithelial stem cells, although this has not been conclusively documented. While a subset of skin stem cells are thought to be quiescent 30, at least a subset of normal intestinal stem cells divide daily31. Also, cell cycle profiles of human breast CSC and non-tumorigenic cells were found to be identical, arguing against large difference in cell cycle status between these populations13.
Other commonly cited mechanisms of CSC resistance to cytotoxic agents include the expression of multiple drug resistance transporters32-34, residing in hypoxic niches, enhanced DNA repair capacity, and the expression of specific drug detoxifying enzymes16, 27, 35. It is likely that CSC employ a combination of mechanisms that make them relatively resistant to cytotoxic therapies compared to their progeny. Furthermore, the mechanisms that predominate will likely vary between tumor types and even within tumors arising from the same organ. The CSC hypothesis implies that identification of the specific resistance mechanisms at work on these cells will allow rational design of chemo- and radio-sensitization protocols that could lead to higher tumor control and cure rates.
THERAPEUTIC TARGETING OF CANCER STEM CELLS
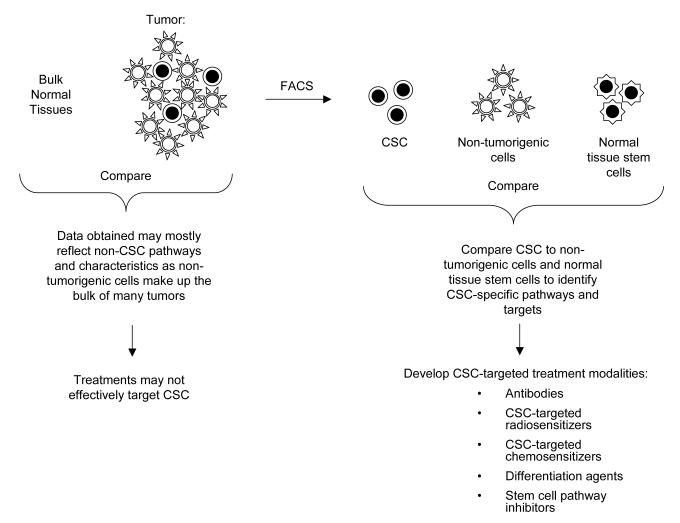
The CSC hypothesis predicts that the most effective clinical therapeutics would target survival, self-renewal or other critical pathways in CSC while causing minimal toxicity to normal cells. This suggests that research efforts must 1) identify the critical survival and self-renewal pathways that are active in CSC and 2) find ones that do not overlap with critical normal tissues and particularly their stem cells. The first goal is facilitated by the ability to separately isolate CSC and their non-tumorigenic counterparts. Since these two populations of cancer cells are closely related, they would be expected to share many similarities, including at the level of gene and protein expression. However, given the functional differences that exist between the two cell types, they also most contain specific differences, many of which are likely to be involved in important stem cell functions such as self-renewal. Precedent for this prediction comes from normal stem cell studies, where comparison of stem cells and their progenitors has allowed the identification of stem cell-specific gene expression programs in a variety of normal tissues5, 36-40. Importantly, comparisons of CSC and their non-tumorigenic counterparts may more rapidly identify critical self-renewal and survival genes and pathways than the more classical approach of comparing bulk tumor cells to normal tissues (Figure 2).
Figure 2. Identifying CSC-specific therapeutic targets.
Isolation of CSC allows comparison of CSC to their non-tumorigenic counterparts and to normal tissue stem cells. This facilitates the development of CSC-specific therapies. FACS- fluorescent activated cell sorting.
One vexing dilemma in the nascent field of CSC-based therapeutics is the high similarity between CSC and normal tissue stem cells, which complicates the second aforementioned goal of CSC-directed therapy – avoiding normal stem cell toxicity. The functional similarity between cancer and normal stem cells suggests that many pathways or markers that are specifically overexpressed by CSC compared to non-tumorigenic cells will also be overexpressed in normal tissue stem cells compared to their more mature counterparts. If this is the case, many potential CSC-specific therapies may induce significant normal tissue stem cell toxicity, just like standard cytotoxic therapies. The solution to this problem is identifying CSC-specific targets that are not vital to normal tissue stem cells, or at least not to critical normal tissue stem cells. For this reason, studies comparing CSC expression profiles and functional properties with those of relevant normal stem cells will be critical.
TARGETING CSC VIA MONOCLONAL ANTIBODY-BASED THERAPIES
The surface markers used to isolate CSC and their non-tumorigenic counterparts currently represent the most conspicuous expression differences between the two cell types. While it remains unclear if these markers play critical roles in CSC biology, they serve as attractive targets for monoclonal antibody based therapies. For solid tumors, one of the most useful CSC markers is CD44, which enriches for tumorigenic cells in breast13, prostate15, colon16, 17, head and neck18, pancreas19, 20, and liver21 tumors. The role CD44 plays in CSC biology remains poorly defined. It is widely expressed and extensively alternatively spliced as well as glycosylated. Furthermore, it can bind a number of ligands, including hyaluronan, osteopontin, and fibronectin, and can participate in adhesion as well as signal transduction41. Whether or not CD44 is intimately involved in CSC biology, it clearly is a cell surface marker that could serve as a potential target for monoclonal antibody-based therapies. Work along these lines has been performed in acute myelogenous leukemias (AML), where the leukemia stem cells (LSC) usually express a CD34+CD38− phenotype. Jin and colleagues found that they often also express significant levels of CD44. Treatment of immunodeficient mice bearing human AML grafts resulted in dramatic reduction of leukemia burden. Secondary transplants of leukemic cells into new recipients readily transplanted the disease if donors with pre-treated with control antibody, but could not transplant the disease at the cell numbers tested in anti-CD44 treated animals. Mechanisms invoked to explain this effect included the induction of differentiation of LSC and the interference with proper niche homing42. However, given the relatively diverse tissues and cell types that express CD4443, it is unclear whether this particular antigen will be useful clinically. Nonetheless, these preclinical data suggest that the cell surface antigens used to isolate LSC and likely CSC could potentially serve as useful therapeutic targets.
One potential shortcoming of targeting CSC surface antigens is that many such antigens may also turn out to be overexpressed by normal tissue stem cells. Thus, the expression of any proposed therapeutic target must be analyzed on critical normal tissue stem cells. While a systematic comparison of CSC and normal stem cells remains technically challenging, a number of investigators have tested the effect of proposed CSC-specific therapies on normal tissue stem cells. For example, in the aforementioned study on CD44 as a therapeutic target on AML LSC, the investigators also treated immunocompromised mice engrafted with normal human cord blood with anti-CD44 and found that unlike for the leukemic samples, engraftment of cord blood was minimally affected42. This result suggests that anti-CD44 therapy may spare hematopoietic stem cells. Before this approach could be considered a potentially viable therapeutic strategy for leukemias or solid tumors, it will be important to assess its effect on other types of stem cells, especially those of epithelial tissues.
TARGETING CSC VIA STEM CELL-SPECIFIC PATHWAYS
The CSC hypothesis suggests that the most effective therapeutics would target survival or self-renewal pathways specific to CSC compared to their normal tissue counterparts. Such agents would kill or inactivate CSC while sparing normal stem cells. It is currently unclear how many such pathways will be identified. Clearly, careful molecular comparisons of CSC and normal tissue stem cells will be critical in making advances in this area.
While much work remains to be done, promising data exist which suggest that such CSC-specific pathways can be successfully targeted. Specifically, a number of pathways known to be important in oncogenesis and oncologic therapy have also been implicated in stem cell self-renewal. For example, HER2 overexpression is found in ~25% of human breast cancers. These tumors are characterized by aggressive growth and a high likelihood of metastasis. Over the past few years, targeting of HER2 using the monoclonal antibody trastuzumab or small molecule lapatinib have led to dramatic improvements in outcomes for this subset of breast cancers44, 45. Recent studies indicate that HER2 overexpression is closely linked to stem cell function. Overexpression of HER2 in normal human mammary epithelial cells leads to enhanced production of mammary outgrowths upon transplantation into immunocompromised mice46. Similarly, introduction of HER2 into mammary cell lines increases their tumorigenicity and the percentage of ALDH1 positive cells, which appears to be a CSC marker in some tumors16, 27, 35. Importantly, a recent study found that breast CSC accumulated after neoadjuvant treatment with cytotoxic chemotherapy, but appeared to be reduced after treatment with lapitinib28. These data suggest that HER2 overexpression is linked to breast CSC function and that the clinical effectiveness of therapies targeting this pathway may be due to toxicity against CSC.
Other potential CSC-related pathways that could serve as therapeutic targets include WNT5, 47, NOTCH48, Hedgehog49, BMI118, 39, 50, 51, PTEN52 and BMP53, 54. Another example is the pro-survival transcription factor NF-κB. Studies by Jordan and colleagues suggest that the drug parthenolide, which inhibits NF-κB and elevates intracellular reactive oxygen species, may be selectively toxic for human AML stem cells compared to normal hematopoietic stem cells55. Interestingly, NF-κB signaling also seems to be activated in breast CSC, at least as measured by gene expression profiling56. Future efforts to develop drugs targeting these pathways combined with analysis of their effects on CSC populations in human tumors holds significant promise for developing CSC-directed therapies.
THE ORIGINS OF CANCER STEM CELLS AND IMPLICATIONS FOR THERAPY
A common point of confusion surrounding the CSC hypothesis concerns the origin of CSC in relation to normal tissue stem cells. Currently there are two predominant models explaining the development of CSC. The first argues that normal tissue stem cells are the cell of origin. Proponents of this view usually point to the fact that normal tissue stem cells are thought to be the only long-lived populations in most rapidly renewing tissues and therefore represent the likely candidate for accumulation of mutations. The identification of leukemia stem cells bolstered this model, since in many patients with AML, the markers used to isolate the AML stem cell (CD34+CD38−) are shared by the normal hematopoietic stem cells.
The second model posits that cancer stem cells can also arise from progenitor cells that have newly acquired the ability to self-renew. Evidence for this model stems from a variety of sources. First, Fialkow and colleagues studied X-linked inactivation of glucose-6-phosphate dehydrogenase in female leukemia patients with heterozygous alleles of this enzyme and found that in chronic myeloid leukemia (CML) and AML the leukemic cells could sometimes give rise to mature cells of both the myelocytic and lymphocytic lineages. In the majority of patients they only gave rise to cells of the myelocytic lineage. This suggested that in many cases leukemia arises in cells with restricted differentiation capacity, which would favor a normal progenitor rather than the hematopoietic stem cell as the transformation precursor57-59. More recent studies of the progression of chronic phase CML to blast crisis by Jamieson and colleagues has shown that although leukemia stem cells expressing an immunophenotype similar to hematopoietic stem cells are the self-renewing cells in chronic phase CML, leukemic cells expressing a marker profile similar to that of more mature normal progenitor cells are the self-renewing cells in blast crisis CML60. Thus, in many cases progenitor cells appear to be the transformation precursors of CSC.
In tumors where CSC are derived from progenitor cells, one would expect these cells to partially retain expression profiles of these progenitors. A recent study utilizing a murine leukemia model supports this idea. Kristov and colleagues initiated leukemias by transducing committed murine granulocyte macrophage progenitors with the MLL–AF9 fusion protein. This led to the development of a leukemia with an identifiable leukemic stem cell subpopulation. Gene expression profiling revealed that the leukemic stem cells mostly expressed genes found in normal progenitors and not in hematopoietic stem cells. However, they also “re-activated” a small group of genes that are only expressed by hematopoietic stem cells and not by the normal progenitors61. Extrapolating to solid tumors, it is possible that CSC in at least some tumors arose from normal progenitor cells. Such CSC would then be expected to express many markers found in progenitor cells and not in normal stem cells. These markers could serve as ideal therapeutic targets, since the long term toxicity of eliminating both CSC and normal tissue progenitor cells should be readily reparable by the unaffected normal tissue stem cells.
TREATMENT RESISTANCE AND CSC-DIRECTED THERAPIES
Clearly, the existence of CSC in solid tumors has immense implications for cancer therapy. The excitement generated by this avenue of research must be tempered by the likelihood that traditional mechanisms of drug and radiation resistance will likely continue to present challenges in the clinic. Since CSC appear to be present in many patients with solid tumors who are currently being treated with various cytotoxic and biologic agents, typical patterns of tumor response should give clues to mechanisms of resistance that will likely also plague CSC-directed therapies. One instructive example occurs when tumors are initially responsive to chemotherapy and/or biologically targeted agents but after an initial period of shrinkage develop subsequent regrowth even while still being exposed to the therapeutic agents. Based on the data presented above and elsewhere in this issue, it is likely that the initial shrinkage is due at least in part to the disproportionate death of non-tumorigenic cancer cells compared to CSC. This results in a tumor that is relatively enriched for CSC. The fact that such tumors often regrow even while still exposed to the agents they were initially sensitive to indicates that they have acquired resistance.
How can the acquisition of treatment resistance be explained in light of the CSC hypothesis? Two competing models fit the observations. First, the CSC hypothesis predicts that acquisition of resistance must have occurred in a CSC. Given that many tumors are genomically unstable, it is likely that a given tumor contains multiple subclones of CSC with different sets of mutations and genomic aberrations. As CSC are repeatedly exposed to a given therapy, a CSC clone that contains genetic alterations which make it resistant to the therapy is selected for, leading to relapse. In this model, the percentage of CSC in the resistant tumors would be roughly the same as in the original tumor. Along these lines, a recent study on colon CSC suggests that genomic instability within CSC can lead to new clones of CSC, which have a similar but nonidentical karyotype compared to the parental clone62. These CSC can give rise to differentiating progeny that also contain the new karyotype.
A second model explaining the development of resistance argues that as tumors progress, CSC acquire additional mutations which restrict their ability to give rise to differentiating progeny and which preferentially promote CSC self-renewal. Since CSC appear to be intrinsically resistant to many therapies, this would explain why tumors appear to become unresponsive to previously effective therapies. In this model, one would expect the recurrent tumors to contain higher frequencies of CSC than the initial tumor. Thus, the natural history of tumor response to current therapies suggests that intrinsic stem cell resistance pathways and acquisition of new mutations could both contribute to resistance.
OVERCOMING CSC TREAMENT RESISTANCE
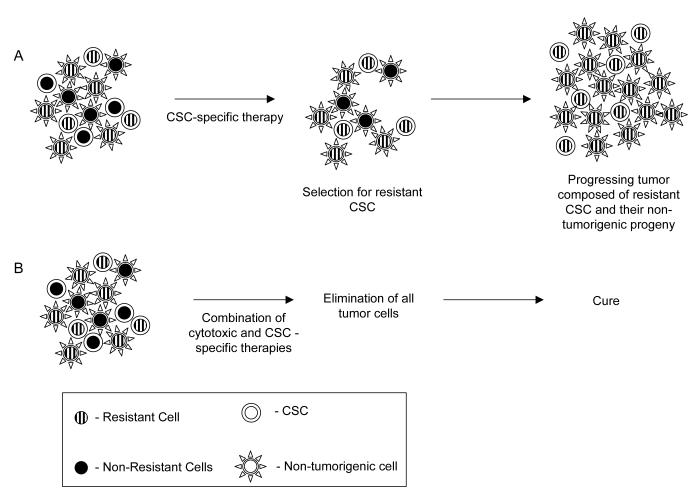
The clinical observations of treatment failure and recent data suggesting that genomic instability can result in CSC subclones indicate that dynamic development of treatment resistance will likely also plague CSC-directed therapies (Figure 3 A). Furthermore, microenvironmental characteristics such as tumor hypoxia and pharmacologic parameters such as drug penetration will also affect future CSC-specific therapies. At least initially, it is likely that the best approach to solving these problems will be the use of multimodality treatment paradigms. Much of the progress that has been made in recent decades in decreasing the morbidity and mortality of malignancies can be attributed to the use of combined modality treatment regimens such as chemoradiation, and combinations of multiple chemotherapeutics with targeted biologic agents. These time tested approaches will also likely be the best way to overcome the problem of selecting for CSC harboring resistance-promoting mutations. Particularly, we propose that combining cytotoxic therapies with new CSC-targeted agents may give the highest chance for increasing cure rates (Figure 3 B). In this approach, cytotoxic agents would “debulk” non-tumorigenic cells and cause modest levels of cell killing among CSC while the CSC-directed agents would aim to eliminate any surviving clonogens. For localized and oligometastatic disease, the combination of CSC-specific agents with ionizing radiation holds particular promise. It is likely the many CSC-targeted therapies will also adversely affect closely related normal tissue stem cells, and this poses potential problems for combining them with systemic chemotherapy. However, the combination of the anatomically restricted dose distributions achievable with modern radiotherapy techniques and carefully dosed CSC-targeted agents could allow relative sparing of normal tissue stem cells outside of the high dose region and could lead to a significant increase in the therapeutic index.
Figure 3. Overcoming resistance to CSC-directed therapies.
Genomic instability within CSC likely results in CSC subclones, some of which may harbor genetic changes that make them partially resistant to a given CSC-specific therapy. Therefore, treatment with a CSC-specific therapeutic may fail due to selection for resistant CSC. However, multimodality approaches which combine conventional cytotoxic agents and CSC-specific therapies may overcome this problem. While CSC may be partially resistant to either modality, their combined effects could lead to the elimination of all CSC. This approach has the additional benefit of rapidly debulking tumors due to the effect of cytotoxic therapies on non-tumorigenic cells.
EVALUATING TREATMENT EFFICACY
The CSC hypothesis may explain an important paradox in oncologic drug development: tumor response rates and cure rates are often not linked63-65. In the past, drugs have usually been selected in Phase I and II trials for their ability to induce grossly measurable responses. These responses may mostly reflect elimination of the often more numerous non-tumorigenic cancer cells and thus obscure the fact that CSC are being preferentially spared. These observations suggest that there is a critical need for new methods of evaluating treatment efficacy, particularly in Phase I and II trials where local control rates and overall survival cannot be reliably assessed due to lack of a control group. Work in this area is still in its infancy, although a combination of approaches will likely be needed. First, preclinical assays using early passage xenografts and specially optimized culture systems need to be developed to allow rapid assessment of CSC viability27, 48. Second, molecular and histopathologic techniques must be identified which allow reliable measurements of CSC frequency to be made in tissue sections66-68. Finally, new molecular imaging approaches must be developed that allow in vivo measurements of stem cell frequency over time, without exposing patients to repeated biopsies that are not feasible for many tumor sites69. For the last two approaches, it will be critical to compare results from the new techniques to CSC frequency as determined using in vivo limiting dilution assays. Only with such tools will it be possible to rapidly develop and test potential CSC-directed therapies and therefore increase the likelihood that a Phase 3 trial testing such a therapy will be positive.
CONCLUSION
The growing body of evidence indicating that CSC drive and maintain many types of human malignancies has important implications for the development and evaluation of oncologic therapies. The CSC hypothesis suggests that identifying similarities and differences between CSC, non-tumorigenic cells, normal stem cells, and normal differentiated cells will allow the rational development of CSC-targeted agents with relatively low risks of normal tissue toxicity. Assessing the efficacy of such treatments will require the advent of new approaches to assessing CSC frequency and viability within tumors. Furthermore, classic mechanisms of treatment resistance, such as clonal selection and the tumor microenvironment, will continue to present obstacles for achieving cure. It is therefore likely that combinations of cytotoxic, biologic, and CSC-directed therapies will ultimately allow the largest possible improvements in patient outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial interest statement: M.F.C. is a member of the paid advisory board of Oncomed Pharmaceuticals, Inc., and owns stock options in the company.
REFERENCES
- 1.Deome KB, Faulkin LJ, Jr., Bern HA, et al. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–20. [PubMed] [Google Scholar]
- 2.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–30. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 3.Tsai YC, Lu Y, Nichols PW, et al. Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res. 1996;56:402–4. [PubMed] [Google Scholar]
- 4.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 5.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 6.Bruce WR, Van Der Gaag H. A Quantitative Assay for the Number of Murine Lymphoma Cells Capable of Proliferation in Vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt HB, Blake ER, Walder AS. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976;33:241–59. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill RP, Milas L. The proportion of stem cells in murine tumors. Int J Radiat Oncol Biol Phys. 1989;16:513–8. doi: 10.1016/0360-3016(89)90353-2. [DOI] [PubMed] [Google Scholar]
- 9.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–3. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 10.West CM, Davidson SE, Hunter RD. Evaluation of surviving fraction at 2 Gy as a potential prognostic factor for the radiotherapy of carcinoma of the cervix. Int J Radiat Biol. 1989;56:761–5. doi: 10.1080/09553008914552011. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 12.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 15.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 16.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien C,A, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2006 doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 18.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0610117104. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 20.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–66. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 23.Dangles-Marie V, Pocard M, Richon S, et al. Establishment of human colon cancer cell lines from fresh tumors versus xenografts: comparison of success rate and cell line features. Cancer Res. 2007;67:398–407. doi: 10.1158/0008-5472.CAN-06-0594. [DOI] [PubMed] [Google Scholar]
- 24.Ho MM, Ng AV, Lam S, et al. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 25.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 26.Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–21. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 30.Kobielak K, Stokes N, de la Cruz J, et al. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 32.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 34.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–7. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 35.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Easterday MC, Dougherty JD, Jackson RL, et al. Neural progenitor genes. Germinal zone expression and analysis of genetic overlap in stem cell populations. Dev Biol. 2003;264:309–22. doi: 10.1016/j.ydbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Forsberg EC, Prohaska SS, Katzman S, et al. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park IK, He Y, Lin F, et al. Differential gene expression profiling of adult murine hematopoietic stem cells. Blood. 2002;99:488–98. doi: 10.1182/blood.v99.2.488. [DOI] [PubMed] [Google Scholar]
- 39.Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–5. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 40.Park I-K, Klug CA, Li K, et al. Molecular Cloning and Characterization of a Novel Regulator of G-protein Signaling from Mouse Hematopoietic Stem Cells. J. Biol. Chem. 2001;276:915–923. doi: 10.1074/jbc.M005947200. [DOI] [PubMed] [Google Scholar]
- 41.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 42.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–74. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 43.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–31. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 44.Cameron DA, Stein S. Drug Insight: intracellular inhibitors of HER2--clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol. 2008;5:512–20. doi: 10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
- 45.Dinh P, de Azambuja E, Cardoso F, et al. Facts and controversies in the use of trastuzumab in the adjuvant setting. Nat Clin Pract Oncol. 2008 doi: 10.1038/ncponc1219. [DOI] [PubMed] [Google Scholar]
- 46.Korkaya H, Paulson A, Iovino F, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho R, Wang X, Diehn M, et al. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364–71. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- 48.Dontu G, Jackson KW, McNicholas E, et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dovey JS, Zacharek SJ, Kim CF, et al. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci U S A. 2008;105:11857–62. doi: 10.1073/pnas.0803574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung C, Lingbeek M, Shakhova O, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428:337–41. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–82. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 53.Lee J, Son MJ, Woolard K, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 55.Guzman ML, Rossi RM, Karnischky L, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–9. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–26. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 57.Fialkow PJ. Use of genetic markers to study cellular origin and development of tumors in human females. Adv Cancer Res. 1972;15:191–226. doi: 10.1016/s0065-230x(08)60375-9. [DOI] [PubMed] [Google Scholar]
- 58.Fialkow PJ. Stem cell origin of human myeloid blood cell neoplasms. Verh Dtsch Ges Pathol. 1990;74:43–7. [PubMed] [Google Scholar]
- 59.Fialkow PJ, Singer JW, Adamson JW, et al. Acute nonlymphocytic leukemia: heterogeneity of stem cell origin. Blood. 1981;57:1068–73. [PubMed] [Google Scholar]
- 60.Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 61.Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–22. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 62.Odoux C, Fohrer H, Hoppo T, et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68:6932–41. doi: 10.1158/0008-5472.CAN-07-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abratt RP, Brune D, Dimopoulos MA, et al. Randomised phase III study of intravenous vinorelbine plus hormone therapy versus hormone therapy alone in hormone-refractory prostate cancer. Ann Oncol. 2004;15:1613–21. doi: 10.1093/annonc/mdh429. [DOI] [PubMed] [Google Scholar]
- 64.Huff CA, Matsui W, Smith BD, et al. The paradox of response and survival in cancer therapeutics. Blood. 2006;107:431–4. doi: 10.1182/blood-2005-06-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lima CM Rocha, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–83. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 66.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–21. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 67.Honeth G, Bendahl PO, Ringner M, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horwitz KB, Dye WW, Harrell JC, et al. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A. 2008;105:5774–9. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hart LS, El-Deiry WS. Invincible, but not invisible: imaging approaches toward in vivo detection of cancer stem cells. J Clin Oncol. 2008;26:2901–10. doi: 10.1200/JCO.2008.16.9573. [DOI] [PubMed] [Google Scholar]