Abstract
The present study assesses socio‐demographic and health service determinants of termination of breastfeeding within the first 2 years of life in India by analysing data from the nationally representative National Family Health Survey‐2 using Cox regression modelling techniques. While the likelihood of stopping breastfeeding increased with increasing household wealth status, it declined with increasing maternal age at childbirth. The likelihood of stopping breastfeeding was significantly higher among female children compared with male children, and the gender differential was attenuated by increasing maternal educational status. Overall, findings of the present study suggest that breastfeeding promotion programmes in India should focus on certain high‐risk mother–child pairs such as female infants, first‐born babies, babies born in the private sector and in urban areas, as well as mothers who are literate, have a higher wealth status, are aged less than 20 years and belong to Sikh or Christian communities. Qualitative studies to understand cultural factors or norms and causal pathways responsible for the association of identified factors and early termination of breastfeeding, especially household wealth status and maternal education, are also called for.
Keywords: breastfeeding, socio‐economic status, Cox regression, maternal education, religion, gender bias, India
Key messages
-
1
Probability of continuing breastfeeding till 2 years of age in the National Family Health Survey‐2 (NFHS‐2) dataset from India is 63.14%.
-
2
Determinants of termination of breastfeeding within the first 2 years of life in the NFHS‐2 dataset from India include: household characteristics (household wealth, religion, caste and place of residence); maternal characteristics (age at childbirth, and educational status); child characteristics (gender, and birth order); and health service factors (antenatal care, post‐natal care and place of delivery).
-
3
There is evidence of gender differential in termination of breastfeeding, with a significantly higher likelihood of stopping breastfeeding among female children compared with male children.
-
4
Increasing maternal educational status attenuates the gender differential.
Introduction
Guidelines issued by the World Health Organization (Pan American Health Organization 2002; World Health Organization 2002) and Government of India (Ministry of Women and Child Development, Food and Nutrition Board 2006), recommend that a child should have exclusive breastfeeding till 6 months of age. Further, they advise that complementary feeding should be initiated at 6 months of age, with continuation of frequent, on‐demand breastfeeding at least until 2 years of age. The recommendation for continued breastfeeding has its roots in the role breast milk plays in a child's nutrition, growth and development, even after 6 months of age. With its high‐fat content, breast milk is an important source of energy and an excellent source of essential fatty acids for the child. Its nutritional impact is most evident during periods of illness, when the child's appetite for breast milk is maintained but decreases for other foods. (Brown et al. 1990) In disadvantaged populations, continued frequent breastfeeding protects child health by reducing risk of morbidity and mortality. (Molbak et al. 1994; WHO 2000) Studies from developing countries show that a longer duration of breastfeeding is associated with greater linear growth of the child. (Onyango et al. 1999; Simondon Simondon et al. 2001). Recent studies also show that longer duration of breastfeeding is associated with lower risk of overweight (Harder et al. 2005) and type 1 diabetes (Sadauskaite‐Kuehne et al. 2004) in later life.
Studies from India and other countries show various factors to be associated with duration of breastfeeding. Maternal factors that have been shown to be associated with duration of breastfeeding include age at birth (Bautista 1997; Nath & Goswami 1997; Dubois & Girard 2003; Giashuddin & Kabir 2004), educational status (Grummer‐Strawn 1996; Bautista 1997; Nath & Goswami 1997; Dubois & Girard 2003; Giashuddin & Kabir 2003, 2004), infant feeding attitudes (Scott et al. 2006), occupation or work status (Nath & Goswami 1997; Scott et al. 2006), and smoking habits (Scott et al. 2006). Paternal factors include educational status (Grummer‐Strawn 1996; Giashuddin & Kabir 2004). Child‐related factors include gender (Nath & Goswami 1997), parity or birth order (Grummer‐Strawn 1996; Bautista 1997; Hajian‐Tilaki 2005), birth interval (Giashuddin & Kabir 2004), and time of initiation of breastfeeding after birth (Bautista 1997). Household‐level factors include socio‐economic status (SES) (Grummer‐Strawn 1996; Bautista 1997; Nath & Goswami 1997; Dubois & Girard 2003; Giashuddin & Kabir 2004), and place of residence, in terms of rural or urban (Grummer‐Strawn 1996; Giashuddin & Kabir 2003, 2004). Health service factors include provision of antenatal care (Bautista 1997; Giashuddin & Kabir 2003), place of delivery (Bautista 1997), and person assisting the delivery (Bautista 1997; Giashuddin & Kabir 2004). While studies assessing factors associated with continuation of breastfeeding during infancy and beyond have been conducted at the national level in developing countries like Timor‐Leste (Senarath et al. 2007), Dominican Republic (Bautista 1997), and Bangladesh (Giashuddin & Kabir 2003, 2004), there is no such study at the national level from India. Previous Indian studies are based on state‐level samples (Nath & Goswami 1997). In the present study, we assess factors associated with termination of breastfeeding within the first 2 years of life in India by analysing data from the nationally representative National Family Health Survey‐2 (NFHS‐2) conducted in 1998–1999.
Materials and methods
National Family Health Survey‐2 provides fertility, health and nutrition‐related information collected from a nationally representative cross‐sectional sample of 92 486 households from 26 major states in India. The survey response rate ranged from 89% to 100% across the 26 states. Data were collected using structured interviews with more than 90 000 ever‐married women aged 15–49 years, using a questionnaire designed specially for women, in 90% of the targeted households. In addition, household‐ and village‐level data were also collected using questionnaires administered to the household head and the village head respectively. (IIPS 2000) Data from the survey are available, free of cost from MEASURE‐DHS (Demographic and Health Surveys), the organization that conducts and disseminates information on DHS surveys worldwide. The children's dataset for all of India was used for the present analysis with permission from MEASURE‐DHS. This dataset, which has de‐identified data, pertains to children aged 0–35 months. It has information collected from mothers about the last two live births that they had in the 3 years prior to the survey. Clearance for the study was obtained from the Human Subjects Committee of the Harvard School of Public Health.
The children's dataset provides information on time to termination of breastfeeding in the form of months after birth for which the child was breastfed, ranging from 0 (meaning never breastfed) to 35 months. As guidelines recommend that breastfeeding be continued at least until 2 years of age (Pan American Health Organization 2002; World Health Organization 2002; Ministry of Women and Child Development, Food and Nutrition Board 2006), our window of observation for analysis was the first 2 years of life. Out of 33 026 children in the dataset, 1178 (3.57%) children were never breastfed, and information on duration of breastfeeding was missing for 203 (0.61%) children. We excluded these children from the analysis.
The dataset, based on retrospective data, had information on children who were aged 0–35 months. It included children aged less than 24 months who were being breastfed till the date of survey, children who had died at an age less than 24 months but were breastfed till age of death, and children who had been breastfed till 24 months of age and were either more than 24 months of age at the date of survey or had died at an age from 24 to 35 months. We censored follow‐up for such children at current age, age of death or 24 months, respectively, for the purpose of the analysis. We used the Kaplan‐Meier method to assess time to termination of breastfeeding and the Cox proportional hazard regression analysis to obtain hazard ratios for termination of breastfeeding by different socio‐demographic and health service factors. We assigned a value of 0.5 months as the time of termination of breastfeeding for children with duration of breastfeeding reported as less than 1 month.
The following factors were examined as potential determinants of termination of breastfeeding: religion (Hindu, Muslim, Christian, Sikh, others); caste [general, scheduled caste (SC), scheduled tribe (ST), other backward castes (OBC)]; place of residence (urban, rural); maternal age at childbirth, in years (<20, 20–29, 30–39, 40–49); maternal occupation (not working, non‐manual, agricultural, manual non‐agricultural); maternal educational status [categorization based on years of schooling: illiterate (0 years), up to primary(1–5 years), middle (6–8 years), secondary and above (9 years or more)]; paternal educational status [categorization based on years of schooling: illiterate (0 years), up to primary (1–5 years), middle (6–8 years), secondary and above (9 years or more)]; gender of the child (male, female); birth order (1st, 2nd, 3rd, 4th or higher); receipt of antenatal care (yes, no); receipt of health care by mother or child within 2 months of childbirth (yes, no); initiation of breastfeeding within 24 h of birth (yes, no); place of delivery (home, public sector, private sector); person conducting the delivery [no one, health professional (doctor, nurse or other health professional), traditional birth attendant, other (family member, friend, relative)]; and wealth status, measured through a standard of living index (SLI). Standard of living index has been defined in terms of living environment and material possessions, and is considered to be a reliable and valid measure of household material well‐being or wealth. (Filmer & Pritchett 2001) Each household is assigned a standard of living score based on a linear combination of scores for 19 different household characteristics, such as quality of the home, type of fuel used for cooking, and ownership of a bicycle or television, which are then weighted according to a factor analysis procedure and standardized with a mean of 0 and a standard deviation of 1. (Rutstein & Johnson 2004) The quintiles of these weighted scores are used to categorize SLI at the household level into five categories, Q1, Q2, Q3, Q4 and Q5, with households falling in Q1 being in the lowest quintile.
The data were analysed using Stata for Windows, Version 9 (StataCorp 2005). We obtained unadjusted Kaplan‐Meier curves for time to termination of breastfeeding (up to 2 years of age) for each factor of interest. Cox regression models require the assumption of the hazard rate among categories of a determinant to be proportional to time. To test this assumption, we obtained a ‘log‐log’ plot by plotting −ln[‐ln(Survival probabilities)] of categories of each factor against ln(months) and visually assessed for the plots of the categories to be approximately parallel to each other. Bivariate and multivariable Cox regression models were then applied to obtain unadjusted and adjusted quantitative estimates of the effect of each factor on the hazard of termination of breastfeeding.
The dataset we analysed was based on a multistage sampling design with the primary sampling unit (PSU) being villages in rural areas and wards in urban areas. This may have led to some degree of correlation between children in the same PSU, thereby influencing standard errors of the estimated hazard ratio. Therefore, 95% confidence intervals for the estimated hazard ratios in bivariate and multivariable analysis were obtained using robust standard error estimates, adjusting for clustering at the PSU level. Further, there were 7194 (22.7%) children in the dataset who belonged to the same mother, suggesting that there might be some degree of clustering at the level of the mother as well. As the software we were using allowed us to adjust for clustering for only one level at a time, we ran separate models to assess effect of clustering at the level of the mother, without adjusting for clustering at the higher level, i.e. PSU. A complete case approach was used to deal with missing data, and 491 (1.55%) children were excluded for the multivariable analysis. To assess the presence of effect measure modification, we introduced interaction terms in the multivariable model between the variables of interest, and then tested for the joint significance of the interaction terms.
Results
The distribution of socio‐demographic and health service factors in the dataset for 31 645 children, obtained after excluding 1178 children who were never breastfed (3.6%) and 203 (0.6%) children for whom information on duration of breastfeeding was missing, is given in Table 1. The largest proportion of children by variable were Hindu (74.3%), belonged to general caste category (37.8%), resided in rural areas (74.2%), belonged to Q1 of the SLI (23.0%) and had fathers with an educational level of secondary school or higher (38.8%). Mothers for the majority of the children were aged between 20 and 30 years (63.7%), not working (69.3%), illiterate (51.4%), had received antenatal care during pregnancy (67.7%), and had not seen a healthcare professional for either their own health or the child's health within 2 months of childbirth (68.6%). Further, the largest proportion of children by variable were male (52.1%), had a birth order of 1 (28.8%), were delivered at home (65.1%) and were delivered by a health professional (43.9%). Out of the 31 645 children, a total of 6974 children (22.0%) had their breastfeeding terminated before 24 months of age, irrespective of duration of follow‐up being 24 months or not.
Table 1.
Distribution of the various socio‐demographic and health service factors in the dataset (n = 31 645)
| Variable | n (%) | Variable | n (%) |
|---|---|---|---|
| Religion | Gender of the child | ||
| Hindu | 23 517 (74.3) | Male | 16 496 (52.1) |
| Muslim | 4 637 (14.7) | Female | 15 149 (47.9) |
| Christian | 2 085 (6.6) | Birth order of the child | |
| Sikh | 631 (2.0) | 1st | 9 122 (28.8) |
| Other | 742 (2.3) | 2nd | 8 208 (25.9) |
| Missing | 33 (0.1) | 3rd | 5 565 (17.6) |
| Caste | 4th or higher | 8 750 (27.7) | |
| General | 11 962 (37.8) | Antenatal care received | |
| Scheduled caste | 5 828 (18.4) | Yes | 21 432 (67.7) |
| Scheduled tribe | 4 650 (14.7) | No | 10 211 (32.3) |
| Other backward castes | 8 944 (28.3) | Missing | 2 (0.01) |
| Missing | 261 (0.8) | Saw health professional within 2 months of delivery | |
| Place of residence | Yes | 9 919 (31.3) | |
| Rural | 23 467 (74.2) | No | 21 718 (68.6) |
| Urban | 8 178 (25.8) | Missing | 8 (0.03) |
| Maternal age at childbirth (years) | Breastfed within 24 h of birth | ||
| <20 | 6 850 (21.6) | Yes | 14 316 (45.2) |
| 20–29 | 20 172 (63.7) | No | 17 303 (54.7) |
| 30–39 | 4 322 (13.7) | Missing | 26 (0.1) |
| 40–49 | 301 (1.0) | Place of delivery | |
| Maternal occupation | Home | 20 590 (65.1) | |
| Not working | 21 924 (69.3) | Public sector | 6 003 (19.0) |
| Non manual | 951 (3.0) | Private sector | 4 982 (15.7) |
| Agricultural | 6 656 (21.0) | Missing | 70 (0.2) |
| Manual non‐agricultural | 2 068 (6.5) | Delivery assisted by | |
| Missing | 46 (0.2) | No one | 207 (0.7) |
| Maternal educational status | Health professional | 13 878 (43.9) | |
| Illiterate | 16 261 (51.4) | Traditional birth attendant | 10 908 (34.5) |
| Up to primary school | 4 916 (15.5) | Other | 6 646 (21.0) |
| Middle school | 4 009 (12.8) | Missing | 6 (0.02) |
| Secondary school and above | 6 444 (20.4) | Standard of living index (SLI)* | |
| Missing | 15 (0.1) | Q1 | 7 283 (23.0) |
| Paternal educational status | Q2 | 7 210 (22.8) | |
| Illiterate | 8 656 (27.4) | Q3 | 6 733 (21.3) |
| Up to primary school | 5 296 (16.7) | Q4 | 6 010 (19.0) |
| Middle school | 5 337 (16.9) | Q5 | 4 409 (13.9) |
| Secondary school and above | 12 270 (38.8) | ||
| Missing | 86 (0.3) | ||
Each household is assigned a standard of living score based on a linear combination of the scores for 19 different household characteristics, such as the quality of the home, the type of fuel used for cooking, and the ownership of a bicycle or television, which are then weighted according to a factor analysis procedure and standardized with a mean of 0 and a standard deviation of 1 (Rutstein & Johnson 2004). The quintiles of these weighted scores are used to categorize the SLI at the household level into five categories, Q1, Q2, Q3, Q4 and Q5, with the households falling in Q1 being in the lowest quintile.
Figure 1 shows the unadjusted survival curve for the continuation of breastfeeding during first 2 years of life. The probability of being breastfed at 24 months was 63.14%, with a large number of breastfeeding terminations being reported at 12 and 18 months of age. Unadjusted Kaplan‐Meier survival estimates for continuing breastfeeding during the first 2 years of life with respect to SLI, maternal age at childbirth and religion, the three determinants having the maximum independent effect on likelihood of stopping breastfeeding in the multivariable analysis, are shown in 2, 3, 4 respectively. It appears that the probability of being breastfed at any point during first 2 years of life is lowest for those in the highest SLI quintile (Q5), those having mothers aged less than 20 years, and those belonging to the Sikh religion, within each of these determinants. By visual assessment of ‘log‐log’ plots for each determinant, plots of categories appeared to be reasonably parallel to each other for most of the variables. There was a slight deviation from the assumption for the variables of religion, maternal age at childbirth and initiation of breastfeeding within 24 h of birth. Within the variables of religion and maternal age at childbirth, there appeared to be a deviation from the assumption for Christians and mother's aged 40–49 years, which crossed over their respective baseline categories. Thus, the interpretation of hazard ratios for initiation of breastfeeding within 24 h of birth, and for Christians and mother's aged 40–49 years with respect to their baseline category, should be done with caution.
Figure 1.
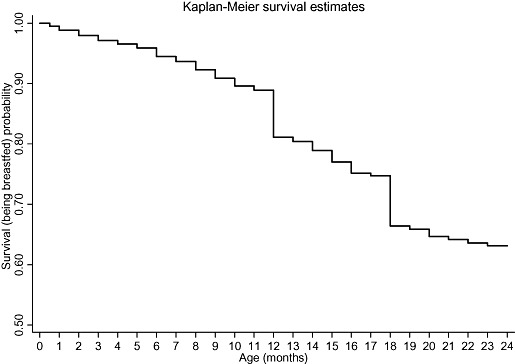
Unadjusted Kaplan‐Meier survival estimates for the probability of being breastfed, in the first 2 years of life, for those initiated on breastfeeding.
Figure 2.
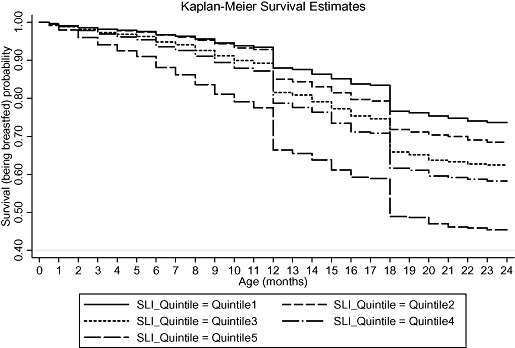
Unadjusted Kaplan‐Meier survival estimates for the probability of being breastfed by standard of living index (SLI), in the first 2 years of life, for those initiated on breastfeeding.
Figure 3.
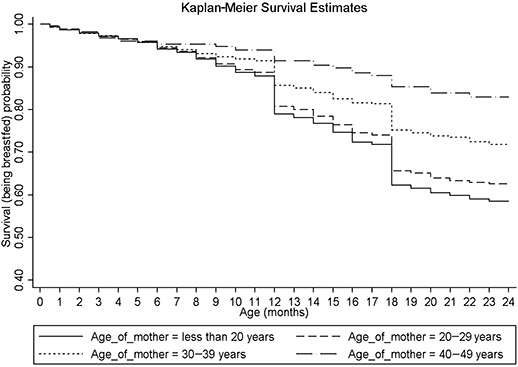
Unadjusted Kaplan‐Meier survival estimates for the probability of being breastfed by maternal age at childbirth, in the first 2 years of life, for those initiated on breastfeeding.
Figure 4.
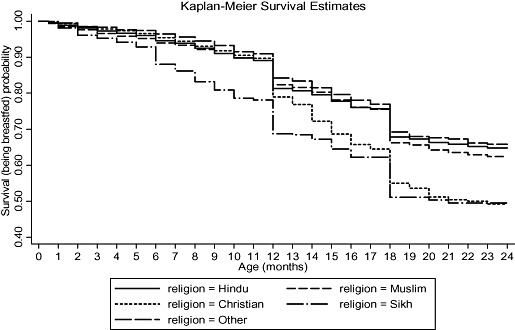
Unadjusted Kaplan‐Meier survival estimates for the probability of being breastfed by religion, in the first 2 years of life, for those initiated on breastfeeding.
The two sets of models, one adjusting for clustering at the PSU level and the other adjusting for clustering at the level of the mother, gave similar point estimates for bivariate and multivariable hazard ratios. The 95% confidence intervals were wider, however, in the models that adjusted for clustering at the PSU level. This is very likely because more than three‐fourth of the children (77.6%) in the dataset did not have any siblings in the study, thus reducing the mother‐induced clustering. Therefore, we are presenting the results based on models adjusting for clustering at the PSU level, which gave wider 95% confidence intervals.
Unadjusted and adjusted hazard ratios for stopping breastfeeding according to various socio‐demographic and health service factors are given in Table 2. Multivariable analysis revealed that, compared with Hindus, likelihood of stopping breastfeeding was higher for Muslims, Sikhs and Christians, being highest for the latter two. Likelihood of stopping breastfeeding for SCs and STs was similar to that of the general caste category, but higher for the OBCs. Male children and children residing in rural areas had a lower likelihood of stopping breastfeeding as compared with female children and children residing in urban areas respectively. There was an increase in likelihood of stopping breastfeeding with an increase in maternal educational status. In light of the literature (Bourne & Walker 1991; Borooah 2004) that purports a reduction in gender bias with increasing maternal educational status, we assessed the modification of the effect of gender on likelihood of stopping breastfeeding by maternal educational status. The test assessing the joint significance of the interaction terms between gender and maternal education status had a χ2 value of 7.67 at three degrees of freedom (P = 0.05), indicating presence of some degree of effect measure modification. The hazard ratios for stopping breastfeeding for males as compared with females were observed to be 0.81 (0.75, 0.87), 0.82 (0.73, 0.92), 0.84 (0.75, 0.95) and 0.94 (0.86, 1.03), when maternal education status was illiterate, up to primary school, middle school and secondary school and above respectively.
Table 2.
Unadjusted and adjusted hazard ratios for the likelihood of stopping breastfeeding for various socio‐demographic and health service factors
| Variable | Unadjusted hazard ratio (95% CI)* | Adjusted hazard ratio (95% CI) † |
|---|---|---|
| Religion | ||
| Hindu | 1 | 1 |
| Muslim | 1.05 (0.97, 1.14) | 1.10 (1.01, 1.19) |
| Christian | 1.43 (1.30, 1.58) | 1.34 (1.20, 1.50) |
| Sikh | 1.72 (1.48, 1.99) | 1.33 (1.14, 1.54) |
| Other | 0.92 (0.76, 1.12) | 0.91 (0.77, 1.08) |
| Caste | ||
| General | 1 | 1 |
| Scheduled caste (SC) | 0.80 (0.74, 0.86) | 1.02 (0.95, 1.11) |
| Scheduled tribe (ST) | 0.86 (0.78, 0.94) | 1.09 (0.99, 1.20) |
| Other backward castes (OBC) | 0.96 (0.89, 1.02) | 1.13 (1.05, 1.20) |
| Place of residence | ||
| Urban | 1 | 1 |
| Rural | 0.59 (0.55, 0.62) | 0.81 (0.76, 0.87) |
| Maternal age at childbirth (years) | ||
| <20 | 1 | 1 |
| 20–29 | 0.90 (0.85, 0.95) | 0.93 (0.87, 0.99) |
| 30–39 | 0.65 (0.59, 0.71) | 0.78 (0.70, 0.87) |
| 40–49 | 0.39 (0.27, 0.54) | 0.48 (0.33, 0.69) |
| Maternal occupation | ||
| Not working | 1 | 1 |
| Non Manual | 1.34 (1.20, 1.50) | 1.08 (0.96, 1.22) |
| Agricultural | 0.72 (0.67, 0.77) | 1.00 (0.93, 1.07) |
| Manual Non Agricultural | 0.80 (0.72, 0.89) | 0.95 (0.85, 1.05) |
| Maternal educational status | ||
| Illiterate | 1 | 1 |
| Up to primary school | 1.39 (1.30, 1.50) | 1.16 (1.08, 1.25) |
| Middle school | 1.55 (1.44, 1.67) | 1.12 (1.03, 1.21) |
| Secondary school and above | 2.10 (1.97, 2.23) | 1.21 (1.11, 1.32) |
| Paternal educational status | ||
| Illiterate | 1 | 1 |
| Up to primary school | 1.12 (1.04, 1.22) | 0.96 (0.88, 1.04) |
| Middle school | 1.34 (1.24, 1.45) | 0.99 (0.91, 1.08) |
| Secondary school and above | 1.65 (1.54, 1.76) | 0.97 (0.89, 1.05) |
| Gender of the child | ||
| Female | 1 | 1 |
| Male | 0.86 (0.83, 0.90) | 0.85 (0.81, 0.89) |
| Birth order of the child | ||
| 1st | 1 | 1 |
| 2nd | 0.80 (0.76, 0.85) | 0.85 (0.80, 0.91) |
| 3rd | 0.60 (0.56, 0.65) | 0.71 (0.65, 0.77) |
| 4th or higher | 0.48 (0.45, 0.52) | 0.68 (0.62, 0.74) |
| Antenatal care received | ||
| No | 1 | 1 |
| Yes | 1.42 (1.34, 1.51) | 0.93 (0.87, 0.99) |
| Saw health professional within 2 months of delivery | ||
| No | 1 | 1 |
| Yes | 1.48 (1.40, 1.56) | 1.09 (1.03, 1.16) |
| Breastfed within 24 h of birth | ||
| No | 1 | 1 |
| Yes | 1.09 (1.03, 1.14) | 0.95 (0.90, 1.00) |
| Place of delivery | ||
| Home | 1 | 1 |
| Public sector | 1.39 (1.30, 1.48) | 0.95 (0.87, 1.05) |
| Private sector | 1.99 (1.86, 2.12) | 1.15 (1.05, 1.27) |
| Delivery assisted by | ||
| Health professional | 1 | 1 |
| Traditional birth attendant | 0.64 (0.60, 0.68) | 0.95 (0.87, 1.04) |
| Other | 0.59 (0.55, 0.64) | 0.96 (0.88, 1.06) |
| No one | 0.60 (0.46, 0.80) | 1.04 (0.77, 1.41) |
| Standard of living index ‡ | ||
| Q1 | 1 | 1 |
| Q2 | 1.22 (1.13, 1.33) | 1.14 (1.05, 1.24) |
| Q3 | 1.55 (1.43, 1.68) | 1.32 (1.21, 1.44) |
| Q4 | 1.80 (1.66, 1.96) | 1.36 (1.22, 1.50) |
| Q5 | 2.80 (2.57, 3.05) | 1.79 (1.60, 2.02) |
Adjusted for clustering at the Primary Sampling Unit (PSU) Level.
† Adjusted for clustering at the PSU Level and all other covariates.
Each household is assigned a standard of living score based on a linear combination of the scores for 19 different household characteristics, such as the quality of the home, the type of fuel used for cooking, and the ownership of a bicycle or television, which are then weighted according to a factor analysis procedure and standardized with a mean of 0 and a standard deviation of 1 (Rutstein & Johnson 2004). The quintiles of these weighted scores are used to categorize the standard of living index at the household level into five categories, Q1, Q2, Q3, Q4 and Q5, with the households falling in Q1 being in the lowest quintile.
While paternal educational status appeared to have a relationship similar to maternal educational status with the duration of breastfeeding in the bivariate analysis, it became non‐significant, once we controlled for other factors. A similar lack of association in the multivariable analysis was observed for maternal occupation. Increasing maternal age at childbirth and increasing birth order of the child were inversely associated with likelihood of stopping breastfeeding in the multivariable analysis. While, after adjusting for other factors, receipt of antenatal care reduced the likelihood of stopping breastfeeding at any given age during the first 2 years of life, the likelihood was higher if the mother visited a health professional for either her own health or the child's health within 2 months of childbirth. Whereas the likelihood was the same for children who were delivered at public facilities compared with those born at home, it was higher for babies delivered at private health facilities. There appeared to be no effect of time of initiation of breastfeeding after birth and person assisting the delivery on the likelihood of stopping breastfeeding. SLI showed a positive association with the likelihood of stopping breastfeeding, with an adjusted hazard ratio of 1.79 (1.60, 2.02) for the highest quintile compared with the lowest quintile of SLI.
Discussion
Our study provides the first comprehensive analysis of socio‐demographic and health service determinants of the termination of breastfeeding within first 2 years of life at the national level in India. Main strengths of this study are the use of a nationally representative dataset (the NFHS‐2), the use of Cox proportional hazards regression for modelling the likelihood of stopping breastfeeding, very high survey response rates and low rates of missing and excluded data.
Multivariable analysis indicated that with increasing household wealth status, as measured by the validated SLI, there was a progressive increase in likelihood of stopping breastfeeding. These results are consistent with previous reports of adverse impact of higher SES, measured by different constructs such as wealth, assets ownership, per capita income, education, and occupation of head of household or family members in different studies, on duration of breastfeeding in developing countries (Grummer‐Strawn 1996; Bautista 1997; Nath & Goswami 1997;Giashuddin & Kabir 2004). Some researchers have hypothesized that this negative association is due to greater affordability of foods other than breast milk by higher SES households (Nath & Goswami 1997; Giashuddin & Kabir 2004) and body image consciousness among mothers of high‐income groups (Giashuddin & Kabir 2004). However, further research is mandated to explore reasons for this association, especially qualitative research. Though it is encouraging that children in lower SLI quintiles are breastfed for a longer period, it is equally important to assess if the amount and composition of complementary foods that they consume are sufficient to meet their nutritional requirements.
In the present study, increasing maternal age at childbirth was associated with a progressive decrease in likelihood of stopping breastfeeding. Previous evidence on influence of increasing maternal age on duration of breastfeeding is mixed, with studies reporting either positive (Bautista 1997) or negative (Nath & Goswami 1997) or no influence (Giashuddin & Kabir 2004; Hajian‐Tilaki 2005). Lower likelihood of stopping breastfeeding among older mothers could reflect a change of child‐feeding practices over time, with persistence of healthier practices among older mothers. It could also be due to greater duration of exposure of older mothers to messages that promote breastfeeding. The finding calls for promotion of breastfeeding among young mothers, especially those aged less than 20 years, in addition to giving support to interventions to reduce teenage pregnancies.
We found that important disparities across religious categories, in terms of breastfeeding duration, persisted even after accounting for other factors like location, household wealth, maternal age and education. Muslims, Sikhs and Christians had a higher likelihood of stopping breastfeeding compared with Hindus. Sikhs and Christians, especially, had a 33–35% greater likelihood of stopping breastfeeding within the first 2 years of life. This might suggest that recommendations in religious scriptures or texts of some religions support early termination of breastfeeding. But, this does not appear to be the case. Religious texts across religions support not only breastfeeding, but also a long duration of breastfeeding. Some even recommend that a child should be breastfed for at least 2 years (Gatrad et al. 2005;Eidelman 2006; Laroia & Sharma 2006; Shaikh & Ahmed 2006). Thus, additional cultural factors or norms, other than religious recommendations, appear to play a role in the higher likelihood of stopping breastfeeding. The unadjusted Kaplan‐Meier Curve for religion (Fig. 4) suggests that there is a sudden decline in the probability of breastfeeding among Christians at 12 months of age. This could be due to a tendency among Christian mothers to centre at 12 months with regard to the recall of the duration of breastfeeding. But, as this decline is maintained further in the second year of life, even becoming nearly similar to Sikhs by 24 months, it might also indicate a role of cultural factors or norms that accelerate termination of breastfeeding after 1 year of birth among Christians. The possible role of cultural factors or norms in determining breastfeeding duration is also suggested by the difference in the likelihood of stopping breastfeeding among children belonging to different social groups or ‘castes.’ Children belonging to the OBC category had a 13% greater likelihood of stopping breastfeeding compared with children of the general caste category. Thus, all these findings suggest that cultural factors or norms play an important independent role in determining breastfeeding duration. Further research, especially qualitative, is required to elucidate the specific cultural factors or norms that may lead to earlier stopping of breastfeeding among Sikhs, Christians and OBCs.
In terms of location, our study corroborated a significant urban–rural differential in duration of breastfeeding as observed in studies in other countries, with longer duration of breastfeeding among children residing in rural areas (Grummer‐Strawn 1996; Giashuddin & Kabir 2003, 2004). Though likelihood of stopping breastfeeding did not depend on presence of a health professional attending the delivery, it was higher for babies delivered at private health facilities compared with those born at home. This finding could be due to residual or unmeasured confounding by wealth or income, whereby increasing wealth or income that is associated with increasing likelihood of stopping breastfeeding is being reflected here as a confounded association between place of delivery and time to termination of breastfeeding. A contributing reason for the above findings could also be that an urban setting and private healthcare institutions may be encouraging termination of breastfeeding by some mechanism such as bottle‐feeding (Winikoff 1989).
Male children had a lower likelihood of stopping breastfeeding compared with female children. This confirms the gender differential in favour of male children previously observed in relation to health outcomes, including duration of breastfeeding in India. (Nath & Goswami 1997; Khanna et al. 2003; Borooah 2004) Breastfeeding beyond 6 months of age is associated with a decrease in childhood morbidity and mortality. (Molbak et al. 1994; WHO 2000) The earlier termination of breastfeeding among female children thus may be a contributing factor to ‘mortality inequality’ (Sen 2001), wherein there is a higher mortality rate among females, and to the ‘missing women’ phenomenon, a term used for the low female–male ratio (Dreze & Sen 1995). It was interesting to note that the gender differential in likelihood of stopping breastfeeding was attenuated by increasing maternal educational status, becoming similar when the mother had an educational status of secondary school or higher. This finding, along with previous reports on the effect of maternal education on reducing gender bias (Bourne & Walker 1991; Borooah 2004), lends support to the case for promotion of education for women in India. However, similar to other reports from developing countries including India (Grummer‐Strawn 1996; Bautista 1997; Nath & Goswami 1997; El‐Gilany 2003; Giashuddin & Kabir 2004; Senarath et al. 2007), we observed that higher levels of maternal education were associated with a greater likelihood of stopping breastfeeding. This negative association is hypothesized to be due to earlier introduction of supplementary feeding among more educated mothers, leading to quicker termination of breastfeeding (Winikoff 1989; Bautista 1997). Thus, interestingly, it appears that increasing maternal education favours early termination of breastfeeding, but at the same time serves to reduce the gender differential in termination of breastfeeding. Although paternal educational status appeared to have a similar relationship as maternal educational status with the termination of breastfeeding in bivariate analysis, it became non‐significant once we controlled for other factors.
The finding of a lower likelihood of stopping breastfeeding among children whose mothers had received antenatal care after adjusting for other socio‐demographic and health service factors, was encouraging and is supportive of the benefits of antenatal care. This positive association could be due to communication of importance of breastfeeding by healthcare providers to mothers during antenatal visits. However, contrary to expectation, in the multivariable analysis, likelihood of stopping breastfeeding at any age was higher if the mother visited a health professional for either her own health or the child's health within 2 months of childbirth. This surprising finding could be explained by mother–child pairs visiting health professionals in the post‐natal period being sicker and more likely to stop breastfeeding as a result.
In accordance with previous studies, birth order of the child was inversely associated with likelihood of stopping breastfeeding. (Grummer‐Strawn 1996;Bautista 1997; Hajian‐Tilaki 2005) Interestingly, whether or not the child had been breastfed within 24 h of birth did not appear to have any effect on the termination of breastfeeding, though a positive association for the same has been reported before. (Bautista 1997)
Our findings are limited by the cross‐sectional and retrospective nature of the NFHS‐2 survey. Information on breastfeeding duration was accordingly based on recall by mothers, which introduces a potential for bias. However, we examined data collected from mothers pertaining only to the last two live births in the 3 years prior to the survey, which limits recall‐based errors as the recall period is relatively short. Furthermore, studies have affirmed validity and reliability of maternal recall of information on breastfeeding, especially recall of duration of breastfeeding if the recall period is limited to the previous 3 years. (Li et al. 2005) Nonetheless, the tendency of recalled ages to centre at half or whole years led to the Kaplan‐Meier curves to have major drops at 12 and 18 months.
Overall, findings of the present study suggest that breastfeeding promotion programmes in India should focus on certain high‐risk mother–child pairs such as female infants and first‐born babies as well as mothers with a higher wealth status, those aged less than 20 years and those who belong to Muslim, Sikh and Christian communities. Breastfeeding programmes should lay more focus on urban areas and on maternity facilities in the private sector. Qualitative studies to understand cultural factors or norms that have a bearing on the termination of breastfeeding in various religious or social communities are required. Also, qualitative research is needed to elucidate causal pathways that are responsible for the association of identified factors, especially household wealth status and maternal education, with early termination of breastfeeding. Strategies to increase education among women, for instance, through women's support groups, should also incorporate aspects of health education regarding ideal childcare and breastfeeding practices in order to address poor breastfeeding performance among more educated women. (Senarath et al. 2007) Further, promotion of education for women is critical in reducing the gender differential observed in likelihood of stopping breastfeeding, which in turn may influence childhood mortality, especially among female children in India. Critically, as overall probability of continuing breastfeeding till 2 years of age in the NFHS‐2 dataset is low (63.14%), a sustained effort to discourage premature discontinuation of breastfeeding across all population subgroups and providers is needed. It might also be informative to look at factors associated with non‐initiation of breastfeeding that was present for 3.57% children in the dataset.
Acknowledgements
We acknowledge the support of Macro International (http://www.measuredhs.com) for providing us access to the 1998–1999 Indian NFHS data. S V Subramanian is supported by the National Institutes of Health Career Development Award (NHLBI 1 K25 HL081275). We declare no conflict of interest.
References
- Bautista L.E. (1997) [Factors associated with the initiation of breast feeding by women in the Dominican Republic. Revista Panamericana de Salud Publica 1, 200–207. [PubMed] [Google Scholar]
- Borooah V.K. (2004) Gender bias among children in India in their diet and immunisation against disease. Social Science and Medicine 58, 1719–1731. [DOI] [PubMed] [Google Scholar]
- Bourne K.L. & Walker G.M. (1991) The differential effect of mother's education mortality of boys and girls in India. Population Studies 45, 203–219. [Google Scholar]
- Brown K.H., Stallings R.Y., De Kanashiro H.C., Lopez de Romana G. & Black R.E. (1990) Effects of common illnesses on infants’ energy intakes from breast milk and other foods during longitudinal community‐based studies in Huascar (Lima), Peru. American Journal of Clinical Nutrition 52, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Dreze J. & Sen A.K. (1995) Economic Development and Social Opportunity, 1st edn. Oxford University Press: New Delhi. [Google Scholar]
- Dubois L. & Girard M. (2003) Social inequalities in infant feeding during the first year of life. The Longitudinal Study of Child Development in Quebec (LSCDQ 1998–2002). Public Health Nutrition 6, 773–783. [DOI] [PubMed] [Google Scholar]
- Eidelman A.I. (2006) The Talmud and human lactation: the cultural basis for increased frequency and duration of breastfeeding among Orthodox Jewish women. Breastfeeding Medicine 1, 36–40. [DOI] [PubMed] [Google Scholar]
- El‐Gilany A.H. (2003) Breastfeeding indicators in Dakahlia governorate. Eastern Mediterranean Health Journal 9, 961–973. [PubMed] [Google Scholar]
- Filmer D. & Pritchett L.H. (2001) Estimating wealth effects without expenditure data – or tears: an application to educational enrollments in states of India. Demography 38, 115–132. [DOI] [PubMed] [Google Scholar]
- Gatrad R., Jhutti‐Johal J., Gill P.S. & Sheikh A. (2005) Sikh birth customs. Archives of Disease in Childhood 90, 560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giashuddin M.S. & Kabir M. (2003) Breastfeeding duration in Bangladesh and factors associated with it. Indian Journal of Community Medicine 28, 34–38. [Google Scholar]
- Giashuddin M.S. & Kabir M. (2004) Duration of breast‐feeding in Bangladesh. Indian Journal of Medical Research 119, 267–272. [PubMed] [Google Scholar]
- Grummer‐Strawn L.M. (1996) The effect of changes in population characteristics on breastfeeding trends in fifteen developing countries. International Journal of Epidemiology 25, 94–102. [DOI] [PubMed] [Google Scholar]
- Hajian‐Tilaki K.O. (2005) Factors associated with the pattern of breastfeeding in the north of Iran. Annals of Human Biology 32, 702–713. [DOI] [PubMed] [Google Scholar]
- Harder T., Bergmann R., Kallischnigg G. & Plagemann A. (2005) Duration of breastfeeding and risk of overweight: a meta‐analysis. American Journal of Epidemiology 162, 397–403. [DOI] [PubMed] [Google Scholar]
- International Institute of Population Sciences (IIPS) (2000) National Family Health Survey 1998–99. International Institute of Population Sciences: Mumbai. [Google Scholar]
- Khanna R., Kumar A., Vaghela J.F., Sreenivas V. & Puliyel J.M. (2003) Community based retrospective study of sex in infant mortality in India. British Medical Journal 327, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroia N. & Sharma D. (2006) The religious and cultural bases for breastfeeding practices among the Hindus. Breastfeeding Medicine 1, 94–98. [DOI] [PubMed] [Google Scholar]
- Li R., Scanlon K.S. & Serdula M.K. (2005) The validity and reliability of maternal recall of breastfeeding practice. Nutrition Reviews 63, 103–110. [DOI] [PubMed] [Google Scholar]
- Ministry of Women and Child Development, Food and Nutrition Board (2006) National Guidelines on Infant and Young Child Feeding. Government of India: Delhi. [Google Scholar]
- Molbak K., Gottschau A., Aaby P., Hojlyng N., Ingholt L. & Silva A.P. (1994) Prolonged breast feeding, diarrhoeal disease, and survival of children in Guinea‐Bissau. British Medical Journal 308, 1403–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath D.C. & Goswami G. (1997) Determinants of breast‐feeding patterns in an urban society of India. Human Biology 69, 557–573. [PubMed] [Google Scholar]
- Onyango A.W., Esrey S.A. & Kramer M.S. (1999) Continued breastfeeding and child growth in the second year of life: a prospective cohort study in western Kenya. Lancet 354, 2041–2045. [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization (2002) Guiding Principles for Complementary Feeding of the Breastfed Child. Pan American Health Organization, World Health Organization: Washington, DC. [Google Scholar]
- Rutstein S.O. & Johnson K. (2004) The DHS Wealth Index. ORC Macro: Calverton, MD. [Google Scholar]
- Sadauskaite‐Kuehne V., Ludvigsson J., Padaiga Z., Jasinskiene E. & Samuelsson U. (2004) Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes/Metabolism Research and Reviews 20, 150–157. [DOI] [PubMed] [Google Scholar]
- Scott J.A., Binns C.W., Oddy W.H. & Graham K.I. (2006) Predictors of breastfeeding duration: evidence from a cohort study. Pediatrics 117, e646–e655. [DOI] [PubMed] [Google Scholar]
- Sen A.K. (2001) Many faces of gender inequality Frontline 18, [Online]. The Hindu Group: Chennai. Available online: http://www.hinduonnet.com/fline/fl1822/18220040.htm[Accessed on August 3, 2007]. [Google Scholar]
- Senarath U., Dibley M.J. & Agho K.E. (2007) Breastfeeding practices and associated factors among children under 24 months of age in Timor‐Leste. European Journal of Clinical Nutrition 61, 387–397. [DOI] [PubMed] [Google Scholar]
- Shaikh U. & Ahmed O. (2006) Islam and infant feeding. Breastfeeding Medicine 1, 164–167. [DOI] [PubMed] [Google Scholar]
- Simondon K.B., Simondon F., Costes R., Delaunay V. & Diallo A. (2001) Breast‐feeding is associated with improved growth in length, but not weight, in rural Senegalese toddlers. American Journal of Clinical Nutrition 73, 959–967. [DOI] [PubMed] [Google Scholar]
- StataCorp (2005) Stata Statistical Software: Release 9. StataCorp LP: College Station, TX. [Google Scholar]
- WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality (2000) Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet 355, 451–455. [PubMed] [Google Scholar]
- Winikoff B. & Laukaran V.H. (1989) Breast feeding and bottle feeding controversies in the developing world: evidence from a study in four countries. Social Science and Medicine 29, 859–868. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2002) Report of the Global Consultation Convened Jointly by the Department of Child and Adolescent Health and Development and the Department of Nutrition for Health and Development Geneva, 10–13 December 2001 and Summary of Guiding Principles for Complementary Feeding of the Breastfed Child. World Health Organization: Geneva. [Google Scholar]


