Abstract
The cerebellar peduncles are excellent candidates for composite indicators of regional degeneration in posterior fossa structures, as the peduncles show histopathological changes in degenerative ataxia. We postulate that magnetic resonance imaging will reveal evidence of disease specific peduncle degeneration through macro-structural (cross-sectional area) and microstructural (fractional anisotropy, mean diffusivity) measures. This study presents a “proof of principle” using orthogonal diffusion tensor imaging cross-sections of the cerebellar peduncles to distinguish categories of cerebellar disease.
Introduction
The cerebellar peduncles are excellent candidates for indicators of composite regional degeneration in posterior fossa structures, as the peduncles show histopathological changes in degenerative ataxia. We postulate that magnetic resonance imaging will show disease-specific degeneration of the cerebral peduncles using macrostructural (cross-sectional area) and microstructural [fractional anisotropy (FA), mean diffusivity (MD)] measures. This study presents a “proof of principle” using orthogonal diffusion tensor imaging (DTI) cross-sections of the cerebellar peduncles to distinguish types of cerebellar disease.
Methods
Patients
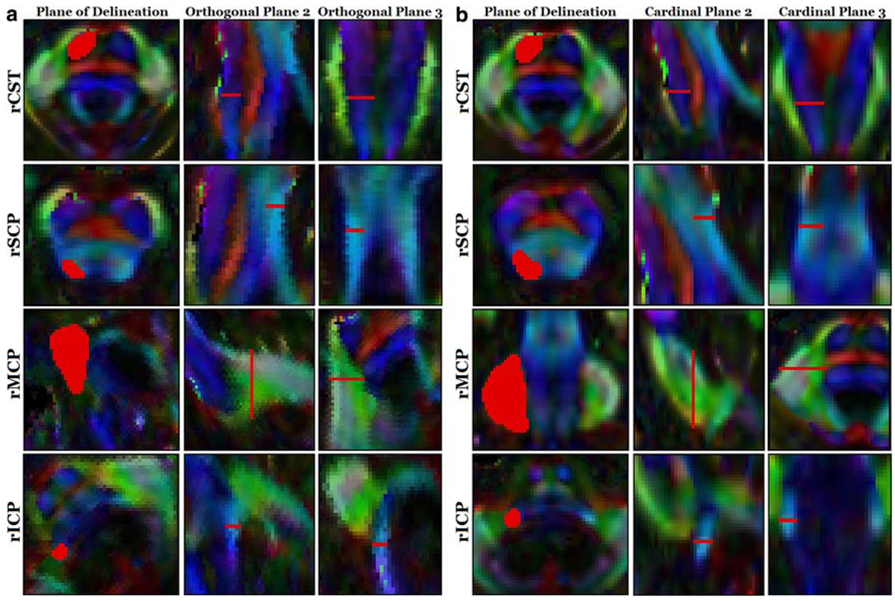
Twenty-eight patients with progressive ataxia were recruited prospectively through the Johns Hopkins neurology clinics, referrals from other neurologists, and patient support groups, such as the Chesapeake Chapter of the National Ataxia Foundation. Clinical diagnoses were verified through a directed history and physical assessment, and patients were separated into three groups for analysis. The first group included 17 patients who had a pure late-onset cerebellar ataxia (LOCA) with duration of symptoms greater than 4 years. Three individuals included in the LOCA group had hereditary forms of ataxia. The second group included six patients who had the diagnosis of spinocerebellar ataxia type 6 (SCA6). The third group included four patients who had a cerebellar-plus syndrome with autonomic dysfunction or other brainstem symptoms and were classified as having olivopontocerebellar atrophy (OPCA) [1]. One patient was excluded due to resolution of her cerebellar dysfunction. The patient group was compared with 15 normal controls. The anatomy of the posterior fossa anatomy was evaluated using DTI colormaps (Philips 3T Intera: multi slice EPI, SENSE = 2.0, 3 repetitions, No-Overplus-High diffusion encoding, 2.2 mm isotropic nominal resolution) (Fig. 1; Table 1).
Fig. 1.
Comparison of ViPAR versus legacy method of white matter tract delineation. a Delineations in orthogonal planes (ViPAR), b Delineations in cardinal planes (legacy method). All regions of interest are indicated in red. Measurements were performed on the superior (rSCP), middle (rMCP), and inferior (rICP) cerebellar peduncles of the right hemisphere, along with the right corticospinal tract (rCST)
Table 1.
Comparison of white matter tract characteristics by disease group
| Disease group | Measure | CST | ICP | MCP | SCP |
|---|---|---|---|---|---|
| LOCA | FA | 0.339 | 0.024* | 0.147 | 0.931 |
| MD | 0.825 | 0.685 | 0.152 | 0.779 | |
| CS | 0.028* | 0.796 | 0.098 | 0.517 | |
| OPCA | FA | 0.020* | 0.167 | 0.000** | 0.056 |
| MD | 0.002** | 0.829 | 0.000** | 0.042* | |
| CS | 0.000** | 0.181 | 0.000** | 0.752 | |
| SCA6 | FA | 0.081 | 0.777 | 0.128 | 0.013* |
| MD | 0.002** | 0.878 | 0.297 | 0.036* | |
| CS | 0.684 | 0.825 | 0.275 | 0.004** |
All values represent significance levels determined by Student’s two-tailed t-tests. Areas of concordance between FA, MD, and cross-sectional area changes are indicated in bold text. Although the MD of the CST in SCA6 is statistically significant, the difference is in an unexpected direction, and there is no corresponding change or trend in the FA or CS
LOCA Late-onset cerebellar ataxia, OPCA olivopontocerebellar atrophy, SCA6 spinocerebellar ataxia type 6, FA fractional anisotropy, MD mean diffusivity, CS cross-sectional area, CST corticospinal tract, ICP inferior cerebellar peduncle, MCP middle cerebellar peduncle, SCP superior cerebellar peduncle
P < 0.05 trend,
P < 0.0065 critical value after false discovery rate control
Region of interest analysis
Cross-sectional areas of four white matter tracts, including the superior cerebellar peduncle (SCP), middle cerebellar peduncle (MCP), inferior cerebellar peduncle (ICP), and the cortical spinal tract (CST), were manually delineated on DTI colormaps. For “orthogonal plane analysis,” cross sections were identified by manually rotating FA colormap volumes of the principal eigenvectors using ViPAR (Visualization, Paint, Alignment, and Rotation) [2], a custom module used with MIPAV (Medical Image Processing, Analysis, and Visualization, version 3.1.0) [3]. Regions of interest (ROIs) were delineated in the selected plane that was perpendicular to local fiber orientation at specific anatomical landmarks. Predictably, these orthogonal planes were always oblique to the cardinal planes (axial, sagittal, coronal). These orthogonal plane ROIs were used for calculations of cross-sectional area, FA, and MD. For “cardinal plane analysis,” which represents the conventional method for evaluation of the cerebellar peduncles, ROIs were delineated along axial, sagittal, or coronal planes in the ViPAR interface without rotation into the orthogonal plane.
Evaluation of methods
To assess intra- and inter-rater reliability, two blinded raters repeated three orthogonal measurements of ten ROIs in two controls and two ataxia patients. To assess the accuracy and differences in reliability between the orthogonal delineation and conventional delineation [4] along cardinal directions, a single blinded rater used each method to repeat three measurements of four ROIs in four subjects.
Analysis
We compared cross-sectional areas, FA, and MD of each white matter tract between each disease and control group using Student’s two-tailed t-tests. False discovery rate control was used to correct for multiple comparisons, using α = 0.05.
Results
Within the white matter tracts and the CST, there were no significant differences in measurements between raters using orthogonal delineation (mean 0.24 ± 3.1 mm2). Intra-rater reliability depended on ROI size, and varied by approximately 4.7% of the cross-sectional area. No subject, disease state, or ROI-related differences were observed in measures of variability. Areas delineated along the cardinal planes were consistently and significantly (P < 0.001) larger than with orthogonal delineation and showed substantial variability in the percentage differences between the various regions measured (mean 39 ± 23%) even when performed by a single rater.
The OPCA disease group showed abnormalities in the MCP and CST, including smaller cross-sectional area, lower FA, and/or higher MD as compared to controls. In the SCA6 group, the SCP had a smaller cross-sectional area, with a trend toward lower FA and higher MD values than in controls. The idiopathic cerebellar group did not show differences in size or integrity of the white matter tracts compared to controls. Post hoc removal of the three patients with inherited cerebellar disease did not change the results. There was no statistically significant correlation between peduncle characteristics and duration of disease.
Discussion
We identify diagnostic white matter signatures for OPCA and SCA6 that distinguish them from LOCA. CST degeneration in the OPCA group is consistent with the presence of upper motor neuron signs, while MCP degeneration suggests involvement of the pontine nuclei. These findings complement earlier evidence of decreased FA in the MCP in multiple-system atrophy (MSA) [5] by including the additional criteria of MD and cross-sectional area and distinguishing the CST. SCP degeneration in the SCA6 group contrasts with earlier findings of preserved dentate and SCP diameter in SCA6 [6], probably reflecting the difference in orthogonal versus cardinal delineations.
Our approach provides several advantages over standard methodologies. First, the use of DTI colormaps allows us to distinguish the SCP, MCP, ICP, and CST from each other and the surrounding tissue. Second, the use of orthogonal delineations improves the reliability of cross-sectional measurements. Third, the aggregate involvement of a complex structure can be measured from a single cross-sectional measurement. Additionally, these changes are not correlated with duration of disease, but rather are present even in early stages of disease, thus further supporting the use of DTI in early diagnosis. Thus, we show that orthogonal DTI-based metrics of peduncle may be a simple, reliable and specific adjunct to the diagnosis of subtypes of cerebellar disease, such that SCA-subtypes and the progressive sporadic cerebellar-plus syndromes such as MSA could be distinguished from LOCA early in the course of the disease. The sensitivity and specificity of these characteristics should be prospectively tested in a longitudinal study.
Acknowledgments
This work was supported by the Arnold-Chiari Foundation, the Robin Zee Fund, the Dana Foundation Program for Brain and Immuno-Imaging, the National Organization for Rare Disorders, and the following NIH grants: 1K23EY015802, 5T32DC00023, R01EY01849, R01NS056307, and R21NS059830. Special acknowledgements to Andrew S. K. Liu, Katherine K. Loya, and Jennifer L. Cuzzocreo for their invaluable assistance.
Footnotes
Conflict of interest statement The authors have reported no conflicts of interest.
Contributor Information
Sarah H. Ying, Pathology 2-210, 600 N. Wolfe St, Baltimore, MD 21287, USA, sying@dizzy.med.jhu.edu
Bennett A. Landman, 324B Clark Hall, 3400 North Charles Street, Baltimore, MD 21218, USA
Shwetadwip Chowdhury, 201 Clark Hall, 3400 North Charles Street, Baltimore, MD 21218, USA.
Alexander H. Sinofsky, 50 E. 98th Street Apt 11L, New York, NY 10029, USA
Anna Gambini, 217 Traylor, 720 Rutland Avenue, Baltimore, MD 21205, USA.
Susumu Mori, 217 Traylor, 720 Rutland Avenue, Baltimore, MD 21205, USA.
David S. Zee, Pathology 2-210, 600 N. Wolfe St, Baltimore, MD 21287, USA
Jerry L. Prince, 201B Clark Hall, 3400 North Charles Street, Baltimore, MD 21218, USA
References
- 1.Berciano J, Boesch S, Perez-Ramos JM, Wenning GK. Olivopontocerebellar atrophy: toward a better nosological definition. Mov Disord. 2006;21:1607–1613. doi: 10.1002/mds.21052. [DOI] [PubMed] [Google Scholar]
- 2.Landman BA, Chowdhury S, Sinofsky AH, Liu ASK, Mori S, Zee DS, Prince JL, Ying SH. Delineation of cerebellar fiber tracts on anatomically aligned planes with ViPAR, a novel MRI visualization and manipulation tool. Organization for Human Brain Mapping, Florence. 2006 [Google Scholar]
- 3.McAuliffe MJ, Lalonde F, McGarry DP, Gandler W, Csaky K, Trus BL. Medical image processing, analysis and visualization in clinical research; Proceedings of the 14th IEEE symposium on computer-based medical systems (CBMS 2001); 2001. [Google Scholar]
- 4.Mandelli ML, De ST, Minati L, Bruzzone MG, Mariotti C, Fancellu R, Savoiardo M, Grisoli M. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. Am J Neuroradiol. 2007;28:1996–2000. doi: 10.3174/ajnr.A0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiga K, Yamada K, Yoshikawa K, Mizuno T, Nishimura T, Nakagawa M. Local tissue anisotropy decreases in cerebellopetal fibers and pyramidal tract in multiple system atrophy. J Neurol. 2005;252:589–596. doi: 10.1007/s00415-005-0708-0. [DOI] [PubMed] [Google Scholar]
- 6.Murata Y, Kawakami H, Yamaguchi S, Nishimura M, Kohriyama T, Ishizaki F, Matsuyama Z, Mimori Y, Nakamura S. Characteristic magnetic resonance imaging findings in spinocerebellar ataxia 6. Arch Neurol. 1998;55:1348–1352. doi: 10.1001/archneur.55.10.1348. [DOI] [PubMed] [Google Scholar]