Abstract
It has been known for some time that thrombopoietin acts on megakaryocytic progenitor cells to stimulate platelet production. It has recently been discovered that it also stimulates the self-renewal and expansion of normal murine and human hematopoietic stem cells (HSCs) by acting on its cognate receptor, the product of the c-MPL proto-oncogene. c-MPL may also play an important role in the development of human myeloproliferative disorders, essential thrombocythemia, myelofibrosis, and polycythemia vera, cooperating with the dysregulated Janus kinase JAK2 V617F.
Keywords: thrombopoietin, stem cell, myeloproliferative disorders, c-MPL, JAK2, HOX
INTRODUCTION
Thrombopoietin was cloned nearly 15 years ago and was soon discovered to be the primary regulator of thrombopoiesis.1 Recently, two thrombopoietin mimics were approved by the US Food and Drug Administration for use in patients with severe refractory immune thrombocytopenic purpura. These agents act to massively stimulate platelet production, allowing marrow compensation for ongoing antibody-mediated platelet destruction and/or inhibition of megakaryocyte development in such patients and normalizing the platelet counts in the majority of patients given an adequate therapeutic dose.2,3 However, thrombopoietin is more than a thrombopoietic agent. Soon after its discovery, a number of research groups determined that in addition to acting on megakaryocytic progenitor cells, thrombopoietin also stimulates the self-renewal and expansion of normal murine and human hematopoietic stem cells (HSCs) by acting on its cognate receptor, the product of the c-MPL proto-oncogene. In fact, using genetic elimination strategies, thrombopoietin/c-MPL is one of only two cytokine-receptor systems (the other is stem cell factor (SCF)/c-KIT) found to affect the HSC in a nonredundant manner. Moreover, a number of lines of evidence suggest that c-MPL plays an important role in the development of human myeloproliferative disorders, cooperating with the dysregulated Janus kinase JAK2 V617F.
THROMBOPOIETIN IN NORMAL HSCs
The evidence for a role for thrombopoietin in normal HSC growth is based on single cell culture studies and genetic loss-of-function studies. We and others have shown that thrombopoietin alone can stimulate the survival of pure murine HSCs in culture and works in synergy with SCF to stimulate the expansion of these cells.4,5 Genetic elimination of thrombopoietin or its receptor in mice leads to an 8-fold reduction in the number of HSCs in mice,6 and genetic elimination of thrombopoietin in mice receiving a stem cell transplant from normal murine donors demonstrates a 17-fold reduced expansion of the transplanted HSCs when assessed in secondary transplant experiments.7 These effects on HSCs are very likely responsible for the favorable effects of the hormone on not only platelet recovery following myelosuppressive therapy, but also on leukocyte and erythrocyte recovery in mice and nonhuman primates.8,9
The intracellular signals responsible for the favorable effects of thrombopoietin on hematopoiesis have been studied using a number of biochemical strategies. Inhibition of protein phosphorylation eliminates all effects of the hormone on c-MPL receptor-bearing cells. Soon after it was discovered, the origin of this effect was traced to the effects of the hormone on activation of the Janus kinases JAK2 and TYK2 in engineered cell lines.10 However, using specific JAK kinase knock-out cell lines it was demonstrated that JAK2 and not TYK2 is essential for initiating thrombopoietin signaling.11 Amongst the targets of JAK2 kinase are tyrosine residues of the intracellular domain of the c-MPL receptor, and once SH2 domain-containing proteins bind to the phosphotyrosine residues so generated, JAK2 phosphorylates these molecules, including STAT3 and STAT5, SHC, GRB2, GAB1, SHIP, and SHP1 and SHP2. These molecules ultimately lead to the activation of phosphoinositol-3-kinase (PI3K), the mitogen-activated protein kinases (MAPKs) p44/p42, ERK1/2, and p38 MAPK, as well as several isoforms of protein kinase (PK) C. The physiological functions of these signaling pathways have also been explored using pathway-specific inhibitors in cell lines, primary murine megakaryocytes, and human platelets. Such studies have demonstrated that PI3K promotes both cell survival and entry into cell cycle,12 p44/p42 MAPK promotes cell proliferation and polyploidy in mature megakaryocytes,13 and STAT3 and STAT5 support cell survival by inducing the antiapoptotic molecule BCL-XL.14
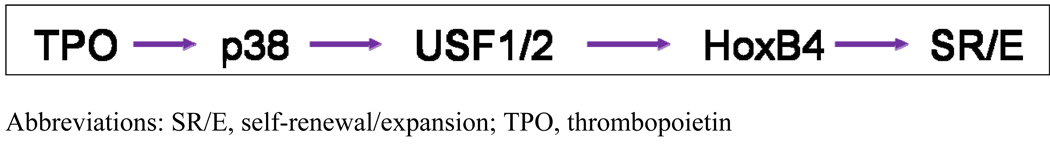
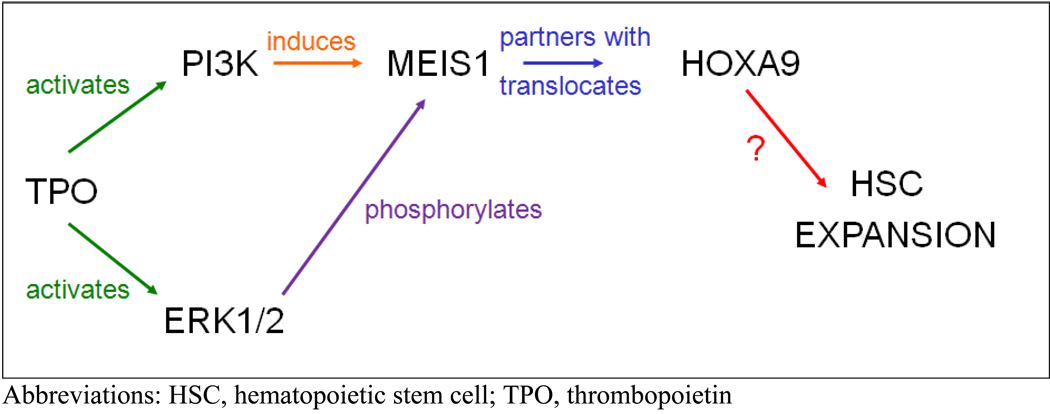
While these pathways were identified using megakaryocytic cell lines and primary cells of that lineage, we began to explore the signals induced by thrombopoietin in HSCs, using a primitive hematopoietic cell line, UT7, and highly purified murine HSCs. The HOX genes are critical for body pattern development during embryogenesis, but their role in adult tissues is less well developed.15 Keith Humphries and Guy Sauvageau identified an important role for HOXB4 and HOXA9 in adult hematopoiesis when they overexpressed or genetically eliminated these genes in murine HSCs and found greatly expanded or reduced numbers of HSCs when transplanted into irradiated recipients.16,17 Of interest, only a 2- to 3-fold overexpression of HOXB4 was required to effect these changes. Based on these observations, we tested whether the favorable HSC effects of thrombopoietin might be mediated by either of these two genes. Using a quantitative real time reverse transcriptase (RT) polymerase chain reaction (PCR), we found that thrombopoietin increased expression of HOXB4 in UT7 cells approximately 3-fold in a p38 MAPK-, USB1/2-dependent fashion (Figure 1). Using this same assay, we found that c- KIT+, SCA+, lineage negative (LIN−) (KSL) murine marrow cells displayed approximately 3-fold less HOXB4 than wild-type KSL cells.18 The levels of HOXA9 mRNA did not change in these experiments, providing a convenient control gene. However, when we next explored whether the subcellular localization of HOXA9 was affected by thrombopoietin, we found that, compared to control cells, KSL cells treated with thrombopoietin displayed greatly enhanced nuclear localization of the transcription factor, an effect mediated by PI3K-based induction of the HOXA9 partner protein MEIS1 and its MAPK-dependent phosphorylation (Figure 2).19
Figure 1.
p38 stimulates HOXB4 in UT7/TPO cells
Figure 2.
Thrombopoietin stimulates HSC expansion through HOXA9
More recently, vascular endothelial cell growth factor (VEGF) was shown by Gerber and colleagues to be essential for HSC renewal.20 As thrombopoietin was previously shown to increase expression of VEGF in platelets, we tested whether this also occurred in HSCs and explored its mechanism. We found that UT7 cells greatly increase platelet-derived growth factor expression in response to thrombopoietin, an effect that is mediated by enhanced stabilization of the well-known VEGF transcriptional activator, hypoxia inducible factor (HIF)1α.21 Subsequent studies have determined that an increase in mitochondrial reactive oxygen species (ROS), mediated by increased glucose flux in the cell, is responsible for the thrombopoietin effect on HIF1α and subsequently, VEGF.22
The downstream effects of PI3K on hematopoiesis have also been extensively studied. One of the known inhibitors of HSC self-renewal is the family of FOXO transcription factors.23 Using UT7 cells, we found that thrombopoietin stimulates the phosphorylation of several isoforms of FOXO3 in a PI3K-dependent fashion.24 Once phosphorylated, FOXO3 is degraded, relieving its enhancing transcriptional effects on several cell cycle inhibitors, including p27. Subsequent experiments confirmed that p27 is an important target of FOXO3 in hematopoietic cells. Thus, at least four transcription factors mediate the favorable effects of thrombopoietin on HSCs.
THROMBOPOIETIN IN MYELOPROLIFERATIVE DISORDERS
Multiple lines of evidence establish that the primary myeloproliferative diseases (MPDs), polycythemia vera (PV), essential thrombocythemia (ET), and idiopathic myelofibrosis (IMF), are disorders of the HSC. Three years ago, the hematopoietic cells of patients with each of these diseases were found to express an acquired somatic mutation in the JAK2 kinase, in which a valine is mutated to phenylalanine at position 617 (V617F).25–28 This mutation resides in the pseudokinase domain of JAK2, a region that down-modulates the activity of the adjacent kinase domain of the molecule. Discovery of this mutation brought much-needed understanding to the field, explaining the hypersensitivity of marrow and blood progenitor cells to hematopoietic growth factors in patients with MPDs and the frequent finding that many of the pro-survival and pro-proliferative signaling molecules stimulated by thrombopoietin and other growth factors discussed above are found in a stimulated state in the absence of growth factors in the marrow cells of these patients.29–31
The assumption that the mutant JAK2 is both necessary and sufficient for the pathogenesis of MPDs was questioned, however, and the intracellular receptors employed by the mutant kinase, if any, were unknown. To address this latter question, Gilliland and Lodish constructed a series of cell lines that express wild-type JAK2 or JAK2V617F and the homodimeric hematopoietic receptors for erythropoietin (EPOR), granulocyte colony-stimulating factor (G-CSF) or c-MPL, or the heterodimeric receptor for interleukin (IL)-3.32 They found that only the homodimeric receptors could support growth factor hypersensitivity in the presence of JAK2V617F. We found a similar result for c-MPL compared to the IL-3R. Taken together, these results suggest that a homodimeric hematopoietic growth factor receptor is required for JAK2 V617F- induced growth factor hypersensitivity. Of the three homodimeric hematopoietic receptors, EPOR, G-CSFR, and c-MPL, only the latter is found and functions in HSCs. This finding strongly suggests that the thrombopoietin receptor plays an important role in the MPDs. This conclusion helps explain a 13-year-old observation: inhibition of c-MPL expression in the marrow cells of patients with ET greatly reduces growth factor hypersensitivity in these cells.33 Moreover, there is little doubt that c-MPL is involved in the pathogenesis of ET and IMF in the minority of patients that bear acquired somatic mutations in the thrombopoietin receptor, c-MPL W515L.34 Finally, very recent experiments suggest that dimerization of the c-MPL receptor, not simply passive tethering of the mutant JAK2 to its cytoplasmic domain, is necessary to manifest the effect of JAK2 V617F on hematopoiesis.
CONCLUSION
Much is now known of the intracellular circuitry responsible for the favorable effects of thrombopoietin on hematopoiesis. A number of signaling pathways common to most or all of the hematopoietic growth factors, such as PI3K, MAPKs, and PKC isoforms, stimulate cell survival and proliferation. For thrombopoietin, additional pathways that support HSC survival and expansion have been uncovered, such as HOXB4, HOXA9 and VEGF. Moreover, these pathways were identified using a candidate gene strategy; it is almost certain that the use of a systematic approach to signaling will net even more information. Nevertheless, it is also clear that even our current understanding of thrombopoietin signaling has yielded additional insights into neoplastic hematopoiesis, as seen in the chronic MPDs in humans. These results also lay the groundwork for manipulating thrombopoietin and its receptor in health and disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357:2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 3.Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 4.Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- 5.Ku H, Yonemura Y, Kaushansky K, et al. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87:4544–4551. [PubMed] [Google Scholar]
- 6.Solar GP, Kerr WG, Zeigler FC, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92:4–10. [PubMed] [Google Scholar]
- 7.Fox N, Priestley G, Papayannopoulou T, et al. Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest. 2002;110:389–394. doi: 10.1172/JCI15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushansky K, Lin N, Grossmann A, et al. Thrombopoietin expands erythroid, granulocyte-macrophage, and megakaryocytic progenitor cells in normal and myelosuppressed mice. Exp Hematol. 1996;24:265–269. [PubMed] [Google Scholar]
- 9.Akahori H, Shibuya K, Obuchi M, et al. Effect of recombinant human thrombopoietin in nonhuman primates with chemotherapy-induced thrombocytopenia. Br J Haematol. 1996;94:722–728. doi: 10.1046/j.1365-2141.1996.d01-1842.x. [DOI] [PubMed] [Google Scholar]
- 10.Drachman JG, Griffin JD, Kaushansky K. The c-Mpl ligand (thrombopoietin) stimulates tyrosine phosphorylation of Jak2, Shc, and c-Mpl. J Biol Chem. 1995;270:4979–4982. doi: 10.1074/jbc.270.10.4979. [DOI] [PubMed] [Google Scholar]
- 11.Drachman JG, Millett KM, Kaushansky K. Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem. 1999;274:13480–13484. doi: 10.1074/jbc.274.19.13480. [DOI] [PubMed] [Google Scholar]
- 12.Geddis AE, Fox NE, Kaushansky K. Phosphatidylinositol 3-kinase is necessary but not sufficient for thrombopoietin-induced proliferation in engineered Mpl-bearing cell lines as well as in primary megakaryocytic progenitors. J Biol Chem. 2001;276:34473–34479. doi: 10.1074/jbc.M105178200. [DOI] [PubMed] [Google Scholar]
- 13.Rojnuckarin P, Miyakawa Y, Fox NE, et al. The roles of phosphatidylinositol 3-kinase and protein kinase Czeta for thrombopoietin-induced mitogen-activated protein kinase activation in primary murine megakaryocytes. J Biol Chem. 2001;276:41014–41022. doi: 10.1074/jbc.M106508200. [DOI] [PubMed] [Google Scholar]
- 14.Pallard C, Gouilleux F, Benit L, et al. Thrombopoietin activates a STAT5-like factor in hematopoietic cells. EMBO J. 1995;14:2847–2856. doi: 10.1002/j.1460-2075.1995.tb07284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botas J. Control of morphogenesis and differentiation by HOM/Hox genes. Curr Opin Cell Biol. 1993;5:1015–1022. doi: 10.1016/0955-0674(93)90086-6. [DOI] [PubMed] [Google Scholar]
- 16.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence HJ, Helgason CD, Sauvageau G, et al. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–1930. [PubMed] [Google Scholar]
- 18.Kirito K, Fox N, Kaushansky K. Thrombopoietin stimulates Hoxb4 expression: an explanation for the favorable effects of TPO on hematopoietic stem cells. Blood. 2003;102:3172–3178. doi: 10.1182/blood-2003-03-0944. [DOI] [PubMed] [Google Scholar]
- 19.Kirito K, Fox N, Kaushansky K. Thrombopoietin induces HOXA9 nuclear transport in immature hematopoietic cells: potential mechanism by which the hormone favorably affects hematopoietic stem cells. Mol Cell Biol. 2004;24:6751–6762. doi: 10.1128/MCB.24.15.6751-6762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerber HP, Malik AK, Solar GP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 21.Kirito K, Fox N, Komatsu N, et al. Thrombopoietin enhances expression of vascular endothelial growth factor (VEGF) in primitive hematopoietic cells through induction of HIF-1alpha. Blood. 2005;105:4258–4263. doi: 10.1182/blood-2004-07-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida K, Kirito K, Yongzhen H, et al. Thrombopoietin (TPO) regulates HIF-1alpha levels through generation of mitochondrial reactive oxygen species. Int J Hematol. 2008;88:43–51. doi: 10.1007/s12185-008-0091-6. [DOI] [PubMed] [Google Scholar]
- 23.Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakao T, Geddis AE, Fox NE, et al. PI3K/Akt/FOXO3a pathway contributes to thrombopoietin-induced proliferation of primary megakaryocytes in vitro and in vivo via modulation of p27(Kip1) Cell Cycle. 2008;7:257–266. doi: 10.4161/cc.7.2.5148. [DOI] [PubMed] [Google Scholar]
- 25.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 26.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 28.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 29.Roder S, Steimle C, Meinhardt G, et al. STAT3 is constitutively active in some patients with Polycythemia rubra vera. Exp Hematol. 2001;29:694–702. doi: 10.1016/s0301-472x(01)00637-3. [DOI] [PubMed] [Google Scholar]
- 30.Silva M, Richard C, Benito A, et al. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338:564–571. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]
- 31.Dai C, Chung IJ, Krantz SB. Increased erythropoiesis in polycythemia vera is associated with increased erythroid progenitor proliferation and increased phosphorylation of Akt/PKB. Exp Hematol. 2005;33:152–158. doi: 10.1016/j.exphem.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Lu X, Levine R, Tong W, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Hetet G, Kiladjian JJ, et al. Proto-oncogene c-mpl is involved in spontaneous megakaryocytopoiesis in myeloproliferative disorders. Br J Haematol. 1996;92:60–66. doi: 10.1046/j.1365-2141.1996.00297.x. [DOI] [PubMed] [Google Scholar]
- 34.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]