Abstract
In mammals, non-visual responses to light have been shown to involve intrinsically photosensitive retinal ganglion cells (ipRGC) that express melanopsin and that are modulated by input from both rods and cones. Recent in vitro evidence suggests that melanopsin possesses dual photosensory and photoisomerase functions, previously thought to be a unique feature of invertebrate rhabdomeric photopigments. In cultured cells that normally do not respond to light, heterologous expression of mammalian melanopsin confers light sensitivity that can be restored by prior stimulation with appropriate wavelengths. Using three different physiological and behavioural assays we show that this in vitro property translates to in vivo melanopsin-dependent non-visual responses. We find that pre-stimulation with long wavelength light not only restores but enhances single unit responses of SCN neurons to 480 nm light, whereas the long-wavelength stimulus alone fails to elicit any response. Recordings in Opn4−/− mice confirm that melanopsin provides the main photosensory input to the SCN and furthermore demonstrate that melanopsin is required for response enhancement since this capacity is abolished in the knockout mouse. The efficiency of the light enhancement effect is wavelength, irradiance and duration dependent. Prior long-wavelength light exposure also enhances short-wavelength induced phase shifts of locomotor activity and pupillary constriction, consistent with the expression of a photoisomerase-like function in non-visual responses to light.
Keywords: Animals; Circadian Rhythm; physiology; Light; Male; Mice; Neurons; physiology; Reflex, Pupillary; physiology; Rod Opsins; physiology; Suprachiasmatic Nucleus; physiology; Time Factors
Keywords: photoreceptor, melanopsin, circadian timing system, pupillary reflex, SCN, electrophysiology, bistable
Introduction
Intrinsically photosensitive melanopsin expressing retinal ganglion cells (ipRGCs) relay photic information to the master circadian clock in the suprachiasmatic nucleus (SCN) as well as to other brain regions that mediate a range of non-visual responses to light (Gooley et al., 2003; Hattar et al., 2003, 2006). The ipRGC’s receive input from rods and cones via amacrine and cone bipolar cells (Belenky et al., 2003, Perez-Leon et al., 2006) that together regulate phase shifts of the circadian oscillator, acute suppression of melatonin, and pupillary constriction, while the absence of these three photopigments completely abolishes these responses (Hattar et al., 2003). Combined morphological and molecular approaches have revealed that the two classes of outer retinal rod and cone photoreceptors and the inner retinal melanopsin ganglion cell have evolved from distinct cell types that employ different light sensitive signalling systems (Arendt, 2003).
Vertebrate rod and cone opsins are ciliary photopigments in which physiological cellular responses are initiated by photoconversion of 11- cis to all-trans-retinal and its subsequent release, rendering the photopigment unresponsive to light. Restoring photosensitivity requires light independent conversion of all-trans- back to 11- cis-retinal (retinoid cycle) a process resident in non-photoreceptor cells of retinal pigment epithelium (RPE) and in Muller cells (McBee et al., 2001; Arshavsky, 2002; Mata et al., 2002).
In contrast, invertebrate photopigments are characterised by a photosensory 11-cis-retinal bound state and a photoisomerase function capable of regenerating all trans to 11-cis-retinal. An essential feature of invertebrate photoreception, this bistable property has been documented by a long history of in vitro investigations (using absorption spectrophotometry, electrophysiology, HPLC) in species such as bee, fly, squid and Limulus (see (Hillman et al., 1983) for review) in which photoisomerase excitation at one wavelength restores photosensory responses at the peak wavelength of the 11-cis-retinal bound photopigment. The invertebrate visual cycle thus requires photon absorption to drive both activation and regeneration pathways, whereas the vertebrate cycle only exploits photons for the activation pathway (Kiselev and Subramaniam, 1994)..
Melanopsin, originally cloned from amphibian melanophores (Provencio et al., 1998) shares extensive amino-acid sequence homologies with invertebrate opsins (Arendt, 2003; Arendt et al., 2004) and has recently been shown to exhibit signal transduction mechanisms and functional properties typical of invertebrate rhabdomeric photopigments (Isoldi et al., 2005; Qiu et al., 2005; Contin et al., 2006) that use light to convert all-trans-retinal back to the active form in an arrestin-dependent manner (Pepe and Cugnoli, 1992; Kiselev and Subramaniam, 1994). Recent in vitro studies using mouse (Panda et al., 2005) and human (Melyan et al., 2005) melanopsin expressed in heterologous cell lines have confirmed that melanopsin confers light sensitivity to the cell that, following initial bleaching by light, can subsequently be restored by light stimulation using appropriate wavelengths. The ability to repeatedly photoconvert expressed amphioxus melanopsin between two spectral absorption states demonstrates an interconvertibility that is consistent with the bistable properties of arthropod and squid rhodopsin (Koyanagi et al., 2005).
While many aspects of the melanopsin phototransduction mechanism remain to be elucidated, a crucial issue in photobiology and chronobiology is to determine whether the capacity of response restoration by light observed for melanopsin in vitro is transposed for melanopsin dependent non-visual responses in vivo (Lucas, 2006; Peirson and Foster, 2006; Berson, 2007; Nayak et al., 2007). To resolve this question, we adapted the classical strategy of successive monochromatic stimulations first employed by Lisman and Sheline (1976) to demonstrate light regeneration of invertebrate photopigments in Limulus to assess photoregeneration of the major non-visual melanopsin-dependent responses in a vertebrate, the mouse. In a first approach, we used single unit recordings to demonstrate that prior exposure to long wavelength light increases the firing rate of SCN neurons to short wavelength blue light. We then established the optimal wavelengths necessary both for conferring enhancement and for eliciting the maximum sensory response. Since this short wavelength region of sensitivity corresponds to that of melanopsin expressing ipRGC’s (Berson et al., 2002; Dacey et al., 2005), we verified the hypothesis that melanopsin is required for response enhancement by demonstrating the absence of this capacity in Opn4−/− mice. We further confirm that the enhanced responsiveness following prior long wavelength exposure translates to other in vivo melanopsin-dependent responses, including the pupillary light reflex and light-induced circadian phase shifts of activity.
Materials and Methods
Animals
Experiments were done with SV129 wild-type male mice (n=33; Charles River Laboratories, l’Arbresle, France) between 6 to 8 weeks of age. Prior to light exposures animals were housed under a 12L/12D light cycle, with food and water ad libitum. Mice (n=3) lacking melanopsin protein expression (Opn4−/−) have been previously described and were from the same strain as those first used by Lucas et al. (Lucas et al., 2003). All treatment of animals was in strict accordance with current international regulations on animal care, housing, breeding and experimentation.
Electrophysiological Recording
Anesthesia was induced using an intraperitoneal injection of a mixture of ketamine (1.5–3.0 mg) /xylazine (0.1–0.2 mg). Heart rate and temperature were recorded to monitor the level of anesthesia and supplemental injections of ketamine were administered as necessary to maintain stable heart and respiratory rates. Electrophysiological recordings began between ZT4 and ZT12. A dorsal craniotomy was made to descend the microelectrode into the SCN. Stereotaxic coordinates of the SCN were: 0.4 mm anterior to bregma, 0.1 mm lateral to the midline and 5.5 to 6.5 mm ventral to the cortical surface. The eyelids were retracted and pupils were dilated by application of 1% atropine sulfate on the cornea. The condition of the eye was periodically verified and the cornea and brain surface were maintained hydrated using topical application of 0.9% saline. Extracellular single neuron activity was recorded using glass insulated, gold-platinum plated tungsten electrodes (0.7–1 Mω, Ainsworth Inc. UK). Response patterns and experimental sequences were controlled by a computerized system (TDT systems). A multi-spike form discriminator was used (Alpha-omega-MSD) to sort out and follow the activity of individual neurons. Once a single neuron was isolated, light responsiveness was tested using to a stimulation protocol consisting of 3 light steps each lasting 30 sec at an irradiance of 3.1014 photons/cm2/s. Neurons unable to respond under these conditions were not investigated.
Firing rate (spikes/sec) was calculated as the mean number of spikes per 5 sec bins. Results are expressed as percentage variation (V) between test stimulation and reference stimulation calculated as follows: V=(((T-BL)−(R-BL))/(R-BL))×100 where R and T are the mean spike discharge rate respectively during the reference (first 480nm light exposure) and the test stimulation (subsequent 480 nm light exposure) and BL is the baseline corrective factor (mean spike discharge rate during the 1 minutes darkness preceding the reference and test stimulations). Subtraction of the baseline firing rate of the neuron in darkness allowed comparison between neurons with different baseline firing rates. Results are expressed as mean ± S.E.M.)
Monochromatic Light Stimulation
Stimulation duration and irradiance were computer controlled and monochromatic light was produced with a tungsten halogen light source, collimating lenses, neutral density and Schott interference filters (10 nm half band width). Light was projected through an opal diffuser providing a uniform, pattern-less stimulus that encompassed the entire binocular visual field. Mice were submitted to successive 480 nm light stimulations separated by periods of darkness or stimulation with other wavelengths (400, 420, 440, 460, 480, 500, 530, 560, 590, 620 nm) of different durations (30s to 5 min) or irradiances (1011 to 3.0 × 1014 photons/cm2/s). In order to allow the neuron to return to a stable baseline state, pre-stimulation exposures included 2 min periods of darkness before and after monochromatic stimulation. Irradiance levels and spectral distribution were monitored with a photometer (International Light) and spectrophotometer (Ocean Optics).
Histology
At the end of the experiment the last electrode position was marked with electrolytic lesions. The animal was given a lethal dose of sodium pentobarbital, the brain was removed and fixed by immersion in Zamboni fixative (4% phosphate buffered paraformaldehyde, picric acid) during 24 hours. Brains were then cryoprotected by immersion in 30% sucrose in PB (0.01 M; pH 7.4) for 24 hours. Serial coronal sections were made at 50 μm on a freezing microtome. The distance between the electrode tract lesions and the SCN were measured to verify localization of recorded single units in the nucleus.
Behavioral phase shifting assay
Adult (6 to 8 week-old) male mice were exposed to an initial period of 12L/12D light-dark cycle with broad-spectrum fluorescent lighting to entrain the circadian locomotor activity. For monitoring locomotor activity, mice were housed individually in cages equipped with infrared motion captors placed over the cages and continuous data collected in 1 min bins using a computerized data acquisition system (Circadian Activity Monitoring System, INSERM, Lyon). Activity records were analysed with the Clocklab software package (Actimetrics, Evanston, IL). After entrainment to the 12L/12D light cycle for 25–30 days mice received 15-min monochromatic light pulses (10 nm half bandwidth) at ZT16 (Aschoff type II paradigm). The stimulator (light source and chamber) has been described previously (Dkhissi-Benyahya et al., 2000). Animals were given a 15-min pulse of either 480 nm or of 620 nm light or 15-min of one of the wavelengths followed by the other, separated by 2 minutes of darkness. All groups and the dark controls remained in the stimulator for the same length of time before being returned to the home cage. Activity was the monitored in constant darkness (DD) for an additional 15–20 days to calculate the amplitude of the light induced phase shift.
Consensual pupillary light constriction measures
Pupillometry assays were made in unanaesthetized mice between 2–6 hrs prior to lights off (12/12 light dark cycle; see (Lucas et al., 2001)). One eye of the animal was monitored under infrared illumination using a video camera in a plane parallel to the cornea and pupil area was measured on line using Arrington Eye tracking system. Sampling rate was 30 images/sec. The pupil of the other eye was dilated by topical application of 1% atropine sulfate on the cornea and stimulated by 1 min light pulses using the same stimulus apparatus as in electrophysiology (see above). To correct for individual variations, data were normalized to maximal pupil area during the sequence of exposures. Results are expressed as the mean variation in constriction between the first 480 nm stimulation (reference stimulus) and the consecutive 480 nm stimulation (test stimulus). Irradiance values are 1012 photons/cm2/s at 480 nm and 1013photons/cm2/s at 620 nm.
Results
Prior long-wavelength stimulation increases the responsiveness of SCN neurons to light
We recorded responses to light from single SCN neurons in mice stimulated with successive 480 nm light pulses separated by a period of darkness or by stimulations using a different wavelength. In this and in the following experimental paradigms, the initial 480 nm stimulus used is defined as the reference stimulus while an identical subsequent 480 nm light pulse constitutes the test stimulation. We chose 480 nm monochromatic light since this wavelength corresponds to the peak of ipRGC sensitivity (Berson et al., 2002; Dacey et al., 2005). Results are expressed as the variation in response (mean ± SEM) between the reference and the test stimulations and the statistics are provided in the figure legends.
The basic response pattern of an SCN neuron to light (Meijer et al., 1998, Drouyer et al., 2007) closely resembles that of in vitro recordings of ipRGCs, with phasic On-Off components derived from rods and cones, and a sustained response to continued light exposure based on melanopsin photoresponse properties (Berson et al., 2002; Warren et al., 2003; Dacey et al., 2005; Tu et al., 2005). An initial (reference) light stimulation using 480 nm monochromatic light elicits this typical increase in the firing rate of a single SCN neuron compared to the baseline spike rate in darkness (Fig. 1A). Following the initial phasic burst, the firing rate remains at a sustained level throughout the entire 5 min duration of light exposure, consistent with the melanopsin derived tonic photoresponse (Berson et al., 2002; Dacey et al., 2005; Tu et al., 2005, Drouyer et al., 2007). During the subsequent 7 minute period of total darkness, the mean firing rate reverts back to a baseline level similar to that observed during the dark period preceding the light stimulation. Administration of the second test light exposure at 480 nm again increases the sustained firing rate to a level equivalent to that observed during the previous reference stimulation at this wavelength. When longer periods of darkness are allowed between the first reference and second test 480 nm stimulations, no significant increases in firing rate (2 minutes: 1.1±7.8 %, n=6; 9 minutes: 7,3± 6.6%, n = 14) are observed between the two light exposures illustrating a lack of recovery of the response in the absence of light. In the second part of this experimental sequence (Fig 1A), long-wavelength stimulation (620 nm, 3 min) leads to a neuronal firing rate that is similar to the mean baseline level observed in darkness. However, following this long-wavelength exposure, the responsiveness of the neuron to a renewed 480 nm light stimulation now increases by 60% compared to the previous identical stimulation at this same wavelength and irradiance. These results demonstrate that previous stimulation with long-wavelength light only, but neither with 480 nm light nor a period of prolonged darkness, is capable of increasing the responsiveness of SCN neurons to a subsequent short-wavelength light exposure.
Figure 1.
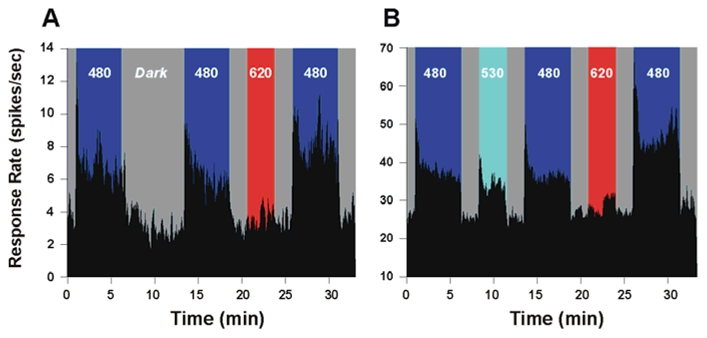
Enhancement of the short-wavelength light response of representative single SCN neurons (A, B) following long-wavelength light exposure. In this sequence of stimulations the first 480 nm stimulation serves as a reference for the second 480 nm exposure, and subsequently the second as a reference for the third stimulation. (A) shows that the first 480 nm light exposure (5 min) dramatically increases the firing rate of a single neuron (6.84+/−0.1 spikes/sec). During subsequent darkness (dark) the firing rate of the neuron returns to the mean baseline level (3.3 ±0.2 spikes/sec) but again increases during the second 480 nm exposure (5 min) to a rate similar to that observed for the first short-wavelength light exposure (6.21+/− 0.1spikes/sec after dark. Stimulation with 620 nm light (3 min) has no effect on the firing rate of the neuron (3.6+/−0.1) spikes/sec), which is identical to that the baseline rate observed during the periods of darkness. This pre-stimulation with long-wavelength red light results in a 60% increase in the firing rate of the neuron (7.98+/−0.1 spikes/sec), during the following (third) short-wavelength stimulation as compared to the previous 480 nm stimulation of equal irradiance. (B) When an SCN neuron is tested using the same sequence as above with 530 nm light the response rate increases from 18.4 ±0.7 to 28.3 ±0.3 spikes/sec) to a value which is 52.9% greater than the baseline rate but slightly less than the response rate at 480 nm (32.6±0.3 spikes/sec). However, in contrast to a pre-stimulation with red light, the subsequent responsiveness of the neuron to 480 nm light (31.2±0.3 spikes/sec) is slightly decreased by 9.9%. A pre-stimulation exposure with 620 nm light enhances the response of the neuron (43.4 ±0.4 spikes/sec) to the following short-wavelength stimulation by 96.0% similar to the increase seen in the previous neuron shown in A.. Both neurons shown in A and B also exhibit a clear phasic ON response to light onset with 480 and 530 nm, which is absent at 620 nm stimulation. Irradiance values are 1014 photons/cm2/s for 480 nm and 530 nm and 3.0 × 1014 photons/cm2/s for 620 nm.
In our experimental design the increase of neuronal firing occurs following the long-wavelength pulse flanked by two dark pulses, which are necessary so as to allow the neuronal firing rate to return to a stable baseline level (see methods). In order to verify that the observed enhancement of the response during the short-wavelength exposure is due to the pre-stimulation with long-wavelength light and not simply the result of the sequence of dark-light-dark stimulation, we substituted the 620 nm stimulus with a mid-wavelength 530 nm stimulus (Fig. 1B). In contrast to the effect of long-wavelength stimulation, previous exposure to this mid-wavelength light results in a 9.9% decrease in firing rate during the subsequent 480 nm test stimulation period. Repeating the long-wavelength stimulation is again capable of significantly increasing the firing rate (96.0%) in the following test exposure to short-wavelength 480 nm light. In this sequence it should be noted that stimulation with 530 nm light increases the neuronal firing rate by 52.9% compared to the dark baseline level, whereas no change in the mean baseline firing rate is observed during the 620 nm light exposures. In addition, the phasic ON response of SCN neurons observed immediately following 480 and 530 nm light onset that is derived from cone input (Drouyer et al., 2007) is absent during the 620 nm stimulation. This lack of response to long wavelength 620 nm light was further confirmed by comparing SCN response levels during 5 minute duration exposures for increasing irradiances to the neuron’s baseline firing rate in darkness. For irradiances from 1011 – 3.0 × 1014 photons/cm2/sec no significant difference in the firing rate from the baseline rate is observed (data not shown). This result agrees with the reduction in sensitivity reported for several non-visual responses at wavelengths greater than 590–600 nm (phase shift (Provencio and Foster, 1995; Yoshimura and Ebihara, 1996), melatonin suppression (Brainard et al., 1984) and pupil constriction (Lucas et al., 2001)). To fully characterize the long wavelength enhancing effect, we explored the wavelength, irradiance, and duration dependence properties of the response.
Enhanced responsiveness of SCN neurons is wavelength dependent
First, we analysed the capacity of different monochromatic wavelengths of light to alter the response to short-wavelength blue light. Fig. 2A shows a typical recording sequence for a single neuron using light stimulation ranging from 480 to 620 nm at equal photon densities. The response shows two distinct trends in the different components of the wavelength-dependent responses. First, during the stimulation phases at different wavelengths (in between the 480 nm pulses) a clear reduction in the response amplitude is observed with increasing wavelength. This is coherent with the known spectral response properties of the ipRGCs (Berson et al., 2002; Dacey et al., 2005; Tu et al., 2005) and non-visual responses for behavioural phase shifts (Provencio and Foster, 1995; Yoshimura and Ebihara, 1996), FOS induction in the SCN (Rieux C, 2000), pupillary reflex (Lucas et al., 2001) and a previous electrophysiological study of SCN neurons by Aggelopoulous and Meissl (2000). Secondly, during the successive exposures, longer wavelengths generate the inverse trend of an increase in neuronal responsiveness to 480 nm light. The rate of increase of the wavelength-dependent enhancing effect is a mirror image of the wavelength-dependent decrease in responsiveness of the neuron (dashed arrows, Fig. 2A).
Figure 2.
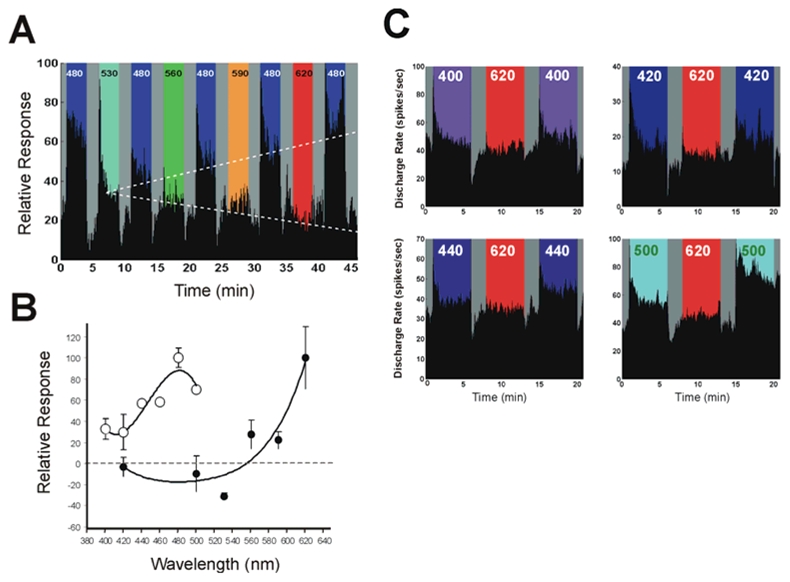
The wavelength dependence of the enhancing effect of light is shown in the sequences of responses of a single SCN neuron to blue light exposure (A) and summarized for SCN neurons stimulated with different wavelengths in random order (B). The relative response rate of the neuron to a 480 nm stimulus (3 min duration, 1014 photons/cm2/s) is compared before and after a pre-stimulation phase (3 min, 1014 photons/cm2/s) using a different wavelength flanked by 2 min dark periods necessary to establish a stable baseline level. A sequence of stimulations with wavelengths from 530 nm to 620 nm shows two wavelength dependent trends. During the pre-stimulation exposure, the firing rate response of the neuron decreases with increased wavelength associated with a progressive decrease of the phasic excitatory ON and inhibitory OFF responses that are absent at wavelengths > 560 nm. In contrast, the short-wavelength response of the neuron progressively increases with longer wavelengths. The dashed lines show regression lines fitted through the mean response level illustrating the two trends of decreased response to the progressively longer wavelength reference exposures (y = −4.08× + 42.18, r2 = 0.93) and the increased responsiveness during the consecutive 480 nm light test exposures (y = 5.83x + 35.66, r2 = 0.98). The relative spectral efficiency (B) is shown for the long wavelength enhancing effect by using different wavelengths to assess percent variation in the response for 480nm (^) as reference and test pulses and in the opposite direction by using wavelengths from 400−500 nm (○) as the reference and test pulses before and after 620 nm exposure. Several examples of this latter effect for individual neurons are illustrated in C. In A and C, the dark gray shading corresponds to complete darkness.
Construction of the spectral response function (Fig. 2B) of the percent increase in responsiveness at 480 nm following pre-stimulation at different wavelengths ranging from 420 nm to 620 nm (randomly administrated) illustrates that the short-wavelength enhancing effect is clearly wavelength-dependent. Wavelengths above 560 nm enhance the response, whereas wavelengths of 530 nm or shorter lead to a slight reduction in the response. The characteristic shape of this curve is similar to the difference spectra described for insect photopigments (Stavenga, 1975). We then used 620 nm, the most efficient wavelength for eliciting the effect, to assess the enhancement response in the short wavelength region by using the inverse strategy of stimulation. The change in responsiveness to a given wavelength was measured prior and following 620 nm stimulation (see examples for several wavelengths in figure 2C). The maximum response was obtained at 480 nm (Fig. 2B), which is consistent with the region of peak sensitivity of melanopsin ipRGCs (Berson et al., 2002; Dacey et al., 2005).
Melanopsin is required for the enhancing effect
In order to confirm whether melanopsin, rather than cones or rods are involved in the photosensory response, we assessed whether response enhancement is conserved in a mouse in which melanopsin is invalidated but outer retinal photoreceptors remain intact (Hattar et al., 2003). First, we confirmed that SCN neurons conserve light-responsiveness by stimulating with broadband white light (Fig 3A). It can be seen that the typical phasic-On and phasic-Off responses originating from cone and/or rod inputs (Berson et al., 2002; Dacey et al., 2005; Drouyer et al., 2007) are robustly expressed. In contrast, the sustained response corresponding to the melanopsin based photoresponse of ipRGCs is no longer detectable in the SCN of Opn4−/− mice. Comparison of the response to white light in the wild-type and in the Opn4−/− mouse shows that a majority (roughly 90%) of photic responsiveness in the SCN can be attributed to melanopsin (Fig 3B). In particular, both the sustained and post stimulus persistent response components are absent in the Opn4−/− mouse. Compared to the enhancement of the response in wild-type mice, previous exposure to 620 light in the Opn4−/− mouse results in a slight decrease (27.3 ± 20.1%) in the neuronal responsiveness to 480 nm light (Fig 3C,D), consistent with a partial bleaching of rod and/or cone photopigments (Drouer et al., 2007). These results support the conclusion the light restoration of photic responses requires the presence of melanopsin and that rods and cones alone are insufficient for expression of the response.
Figure 3.
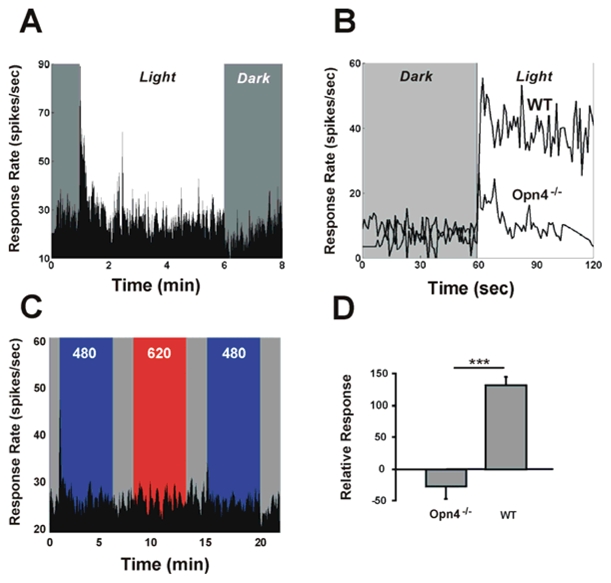
Mice lacking melanopsin also lack the capacity for response enhancement by light. Neurons of the SCN in Opn4−/− mouse conserve the capacity to respond to white light (tungsten light source), although response amplitude is significantly reduced (A). This is shown in (B) which compares typical normalized responses to a pulse of white light in the wild-type and in the Opn4−/− mouse. The reduction in responsiveness is mainly observed for the sustained response, since the initial phasic-on response is present in both strains (A, B). When exposed to the sequence of prior light stimulation using 620 nm light, neurons in the SCN of the Opn4−/− mouse fail to show any potentiation of the response to the 480 nm test stimulus (C). Note that the phasic-ON response is reduced by about 50% during the second 480 nm stimulation (same neurone shown in A and C). D shows that the mean percent change in response to short wavelength light for this stimulation sequence (shown in C) leads to a 132.7±12.1% (n=5) increase in wild-type mice, but 27.3±20.1% (n=11) decrease in the Opn4−/− mouse. (*** =p<0.001, t-test)
Enhanced responsiveness is irradiance and duration dependent
To further determine the irradiance and duration dependence of the response enhancement effect in the wild-type mouse, we used 480 nm as the reference and test stimuli and a 620 nm pulse as the conditioning stimulus. First, light exposures of increasing irradiances were applied while maintaining a constant 5 min duration. Fig. 4A clearly illustrates the increased responsiveness of four individual SCN neurons at 480 nm subsequent to a pre-stimulation with long-wavelength light of different irradiance levels (summarized for all the neurons studied in the irradiance dose response curve shown in Fig. 4B). The firing rate increases significantly by 45.8±8.1% at the lowest irradiance used (1011 photons/cm2/sec) compared to the dark control (7.3±6.6%). This enhancing effect rises sharply after 1013 photons/cm2/sec (66.7±11.1%) and saturates at roughly 1 log unit higher resulting in a 150% increase in the firing rate compared to the previous short-wavelength reference stimulation. The next step assessed whether the enhancing effect of long-wavelength light on short-wavelength light responsiveness is stimulus-duration dependent. Fig. 4C illustrates for a single neuron the increase in responsiveness at short-wavelength light following successive pre-stimulation at 620 nm of equal photon flux (3 ×1014 photons/cm2/s) with increasing durations from 30 sec to 5 min. Again the baseline firing rate is similar during the dark exposures and during the successive series of red light stimulations. The mean responses from all the neurons stimulated with different durations (randomly administered) show a dose dependent increase that is expressed after only 3 minutes of prior light exposure, indicating a rapid mechanism of restoration of the response (histogram, Fig. 4D).
Figure 4.
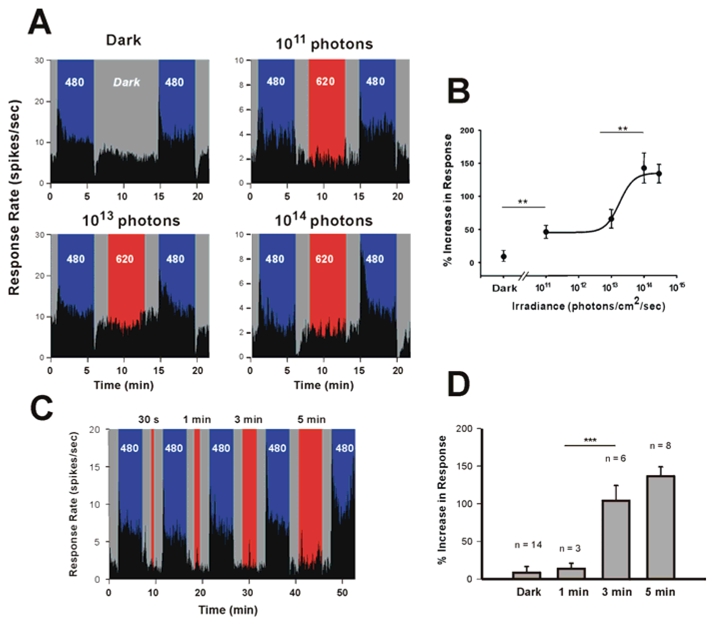
The enhancing effect of prior long-wavelength light on short-wavelength light responsiveness is irradiance and duration dependent. Increasing irradiance levels of the 620 nm long-wavelength pre-stimulation (5 min) results in an increase in the short-wavelength responsiveness of single SCN neurons (A). After 9 minutes dark exposure the firing rate at 480 nm (1014 photons/cm2/sec, 5 min) is slightly increased from baseline (10.1%). Irradiances of 1011, 1013 and 1014 photons/cm2/sec lead to an increase of the firing rate during the second 480 nm stimulation of 37.6 %, 79.5%, and 188.9% respectively for the neurons illustrated in A. During each long wavelength stimulation the firing rate of the neuron is similar to the baseline firing rate during darkness. Note also the distinct phasic-ON response to 480 nm light in all four neurons and the phasic-OFF response to light in 3 of the 4 neurons which are absent during red light stimulation. The summary dose-response curve (B) shows the pooled data from several neurons (randomly stimulated with different irradiances). The firing rate increases significantly by 45.8±8.1% (n=9, t test p<0.003**) at the lowest irradiance used (1011 photons/cm2/sec) compared to the dark control (7.2±6.6%, n=14). This enhancing effect rises sharply after 1013 photons/cm2/sec (66.7±11.1%, n=11) and saturates at roughly a 1 log unit higher irradiance (142.8±22.5% at 1014 photons/cm2/sec, n=5, ANOVA, post hoc Newman-Keuls test p<0.003**) level resulting in a 150% increase in the firing rate compared to a previous short-wavelength stimulation (curve fit using a 4-parameter Naka-Rushton function). The enhancing effect of long wavelength light to short wavelength light (480 nm, 1014 photons/cm2/s) responsiveness is also stimulus-duration dependent as illustrated by the responses of a single neuron (C) to successively increased durations of pre-stimulation using a 620 nm light (3×1014 photons/cm2/s, light gray shading). The histogram (D) summarizes for pooled data from several neurons stimulated with different durations. Although an effect of long wavelength light is seen after 1 minute (11.7 ±6.0%, n=3), a significant enhancing effect requires at least 3–5 minutes duration (102.5 ±24.6%, n=6 and 129.0 ±13.8%, n=8, ANOVA followed by Newman-Keuls post hoc-test, p<0.001***). For variance homogeneity in statistical analyses data were first log-transformed (log (X+1)).
Prior long-wavelength light exposure enhances short-wavelength induced phase shifts and pupillary constriction
A critical question remaining was to determine whether the response enhancement previously reported from in vitro expression studies (Melyan et al., 2005) and here observed in single SCN neurons are transduced to behavior. We hypothesized that this property would be amenable to experimental demonstration for non-visual melanopsin-dependent responses in a mammal since (1) melanopsin, which is required for the enhancement response, is the only known photopigment in the mammalian retina of rhabdomeric origin (Arendt, 2003; Arendt et al., 2004) that displays two photoreversible pigment states (Koyanagi et al., 2005) and (2) the region of optimal response for the short wavelength response function is sufficiently distinct from the long wavelength mediated enhancement function (Fig. 2). We thus applied the same light stimulation strategy as used previously to assay light induced phase shifts, but with stimulus durations and irradiance levels known to produce a 50% saturating response for a 480 nm light-induced phase shift (Provencio and Foster, 1995; Yoshimura and Ebihara, 1996). First, we confirmed that a 15 min stimulation with red light (620 nm) alone fails to induce a phase shift in the onset of the behavioral activity (Fig. 5). Second, exposure to blue (480 nm) light leads to an expected mean phase shift of 0.95±0.11 hrs in agreement with previous behavioural studies. Finally, when this same blue light stimulation is preceded by a red light (620 nm) exposure, the mean phase shift is increased nearly two-fold (1.57 ±0.15 hrs; Fig. 5B). Of particular importance, reversing the order of a blue followed by a red light exposure leads to phase shift (0.93 ±0.06 hrs) identical to that induced by the blue light exposure alone, indicating that the enhancing effect of the response requires the prior long wavelength stimulation and not merely exposure to the two wavelengths.
Figure 5.
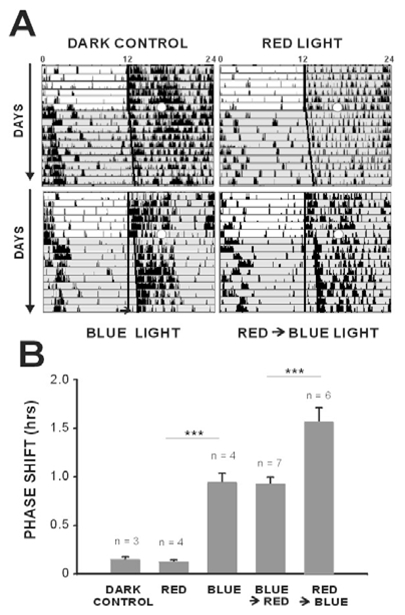
Pre-stimulation with red light enhances the amplitude of a behavioural phase shift to a subsequent light exposure at short-wavelength light. Typical actograms are shown on (A) and mean data are illustrated in the histogram (B). Light pulses (open white circle) were administered at CT16. Similar to the dark control animals, a 15 minute light pulse with 620 nm light (5× 1011 photons/cm2/s) fails to induce a phase shift in the onset of locomotor activity (dark = 0.15 ±0.02 min, red light = 0.13 ±0.02 min, n=3 and 4 respectively, ns). Exposure to a pulse of blue light (480 nm, 15 min, 5 ×1011 photons/cm2/s) induces a significant phase shift of 0.95± 0.11 min (n=4, ANOVA, post hoc Newman-Keuls test p<0.001***). When this same blue light exposure is preceded by the red light pulse which alone fails to induce a behavioural response, a significant increase is observed in the amplitude of the phase shift of locomotor activity (1.57± 0.15 min, n=6, ANOVA, post hoc Newman-Keuls test p<0.001***) to blue light (A, B). In contrast, stimulation with blue light followed by red light produces a phase shift which is equivalent to that observed with the blue light exposure alone (0.93± 0.06 min, n=7).
To further confirm the in vivo enhancement of non-visual responses we pursued our strategy to test the pupillary light reflex for which melanopsin ipRGC’s have also been shown to be involved in the response (Hattar et al., 2003; Lucas et al., 2003; Panda et al., 2003). We observe the same degree of pupillary constriction in the present study for stimulations with the blue and red wavelengths-irradiance levels (480 nm=1012 photons/cm2/s; 620 nm=1013photons/cm2/s) as in the study by Lucas et al (Lucas et al., 2001). When the eye receives a prior exposure to 620 nm light the increase in pupillary constriction at 480 nm corroborates the results of the cellular SCN electrophysiological and behavioural phase shift studies (Fig. 5). The comparatively reduced magnitude of the effect observed for pupil constriction (23%), may be related to the higher proportion of melanopsin ipRGC’s versus non-melanopsin expressing RGC’s projecting to the SCN compared to the projection to the olivary pretectal nucleus (Hattar et al., 2006).
Discussion
Our results demonstrate a novel, robust in vivo effect of an enhancement of the major non-visual responses to short-wavelength light by previous exposure to long-wavelength light. The absence of this effect in melanopsin knockout mice argues against the involvement of rods and cones for which prior light exposure exerts the opposite effect by reducing rod and cone sensitivities due to photopigment bleaching (Fain et al., 2001; Drouyer et al., 2007). Possible post-stimulus chromatic interactions also appear unlikely. A rare example of this phenomenon is transient tritanopia (originally described by Stiles) in which prior long wavelength (yellow light) exposure causes the opposite effect of a decrease in sensitivity to blue light (Pugh and Mollon, 1979). Furthermore, the light independent regeneration of the photopigments involving the retinoid cycle is a relatively slow process requiring mobility of the chromophore out of the photoreceptor towards the RPE or in Muller cells (McBee et al., 2001; Arshavsky, 2002).
Our findings overwhelmingly support a role for melanopsin as the photosensory pigment in response enhancement following prior long wavelength light exposure (Berson, 2007). The response kinetics, consistent with an arrestin dependant response (Kiselev and Subramaniam, 1994, Panda et al., 2005), favor a mechanism involving a rapid process of intracellular chromophore photoregeneration. The most plausible hypothesis, suggested in several recent reviews, is that melanopsin is capable of both photosensory and photoisomerase functions (Lucas, 2006; Peirson and Foster, 2006; Berson, 2007; Nayak et al., 2007). This is based on, and consistent with, the photic restoration of responsiveness to light in heterologously expressed melanopsin (Melyan et al., 2005; Panda et al., 2005) and the ability to photoconvert melanopsin between two distinct spectral absorption states (Koyanagi et al., 2005). This hypothesis is further consolidated by studies demonstrating that melanopsin ipRGCs can autonomously regenerate the chromophore independent of RPE visual retinoid cycle. Rpe65−/− or lrat−/− mice that lack that enzymatic capacity to convert retinal from the all-trans to the 11-cis state conserve inner retinal ipRGC light sensitivity (Doyle et al., 2006; Tu et al., 2006). In addition, in transgenic mice incapable of chromophore regeneration coupled with non-functional rod and cone opsin transduction pathways (rpe65−/− gnat1−/− cnga3−/− mice), administration of all-trans retinal is sufficient and necessary to restore photosensitivity (Fu et al., 2005).
The possibility that rods and cones participate in response recovery and enhancement though as yet unknown interactions with melanopsin could be validated in mice lacking functional rods and cones. However, this possibility seems unlikely since the enhancing effect observed after prior 620 nm light exposure, fail to elicit any detectable neuronal response at the irradiances used in this study. Use of a mouse with melanopsin as the exclusive photopigment would consolidate the hypothesis that melanopsin alone is sufficient for enhancement, although this would not exclude the possible involvement of other putative retinal photoisomerases either intrinsic to the ipRGCs and/or within other cells and operating in concert with melanopsin (Kumbalasiri and Provencio, 2005; Terakita, 2005). Peropsin is present in mammalian RPE and Koyanagi et al. (2002) have shown that an Amphioxus peropsin homologue can act as a photoisomerase. In the mouse, however, light restores photic responses of melanopsin expressed in Neuro-2a cells that lack known mammalian photoisomerases including peropsin and RGR-opsin (Melyan et al., 2005). Mouse RGR-opsin is localised in both RPE and in Muller cells and, although it has been shown to act as a photoisomerase in vitro (Chen et al., 2001), this has not been confirmed in vivo (Maeda et al., 2003). An alternative possibility could be an extrinsic enzymatic regeneration of the photoexcitable pigment involving, for example, the retinoid cycle resident in Muller cells (Arshavsky, 2002), although this process would presumably imply longer response kinetics for release, isomerisation and recapture of retinal by the photopigment.
Ultimate proof that melanopsin is capable of acting as both the photosensory and photosomerase photopigment awaits biochemical investigation by in vitro expression and reconstitution of the pigment extract with 11-cis-/all trans-retinal and spectrophotometric measurements. When carried out for invertebrate photopigments, these measures can provide the absorption spectra of the two underlying photoreversible R and M pigment states (Hillman et al., 1983). The current results from heterologous expression studies (Melyan et al., 2005; Panda et al, 2005) and photoreversability of melanopsin photopigment states (Koyanagi et al., 2005) provide compelling evidence that melanopsin is a bistable photopigment. The key issue addressed in the present study is whether this property of bistability is functionally translated to in vivo physiology and behavior. The concurring evidence of response restoration and enhancement for several circadian and non-visual melanopsin-dependent responses supports this hypothesis.
In invertebrates, the physiologically measured responses of bistable pigments typically represent a photoequilibrium state that depends on the ratios and spectral properties of the underlying R and M states. All previously assayed melanopsin-dependent non-visual responses, including our enhancement spectrum for SCN neurons, consistently show a peak near 480 nm. The obtained long wavelength dominated function for eliciting enhancement (Figure 2B) presumably reflects the differences in the extinction spectra of the pigment states (difference spectrum). This electropysiologically derived function contains the typical features of invertebrate difference spectra, including positive values at longer wavelengths and a region of negative values at shorter wavelengths. Although it is theoretically possible to extrapolate from the difference and photoequilibrium spectra the putative R and M spectra (Stavenga, 1975), further knowledge of the underlying biochemical properties would be required to obtain an accurate unbiased estimation (Hillman et al., 1983). In this respect, caution should be used to interpret the profile of the curve for the long wavelength enhancement effect (difference spectrum) that may vary according to the particular stimulus protocol condition.
Additional evidence of melanopsin bistability could be obtained from study of the post-stimulus persistence of the response that is an intrinsic feature of melanopsin dependent responses (ipRGC: Berson et al, 2003, PLR: Gamlin et al, 2007, SCN single unit: Drouyer et al, 2007, present study, see fig 3). As recently stressed by Berson (2007), this post-stimulus persistence closely resembles the persistent depolarizing after potential (PDA) observed in invertebrate photopigments (for review see Hillman et al, 1983). This effect, related to metarhodopsin stability, can be abolished by exposure to long wavelengths causing a shift back to the 11-cis bound opsin. Investigation of this after effect would be better suited to an in vitro study of isolated ipRGCs.
The electrophysiological recordings in the Opn4−/− mouse offer the additional novel finding that melanopsin provides the main photosensory input to the SCN compared to rods and cones. Our findings provide an important advance towards understanding how different photoreceptors contribute to regulation of the circadian system by light (Dkhissi-Benyahya et al., 2007) and add insight to the potential response of the circadian system to changing light spectra throughout the day. In particular, the response of melanopsin appears appropriately tuned to benefit from the sequence of a relative predominance of long wavelength light at dawn and dusk along the horizon (due to atmospheric absorption) followed by a subsequent enrichment of shorter wavelengths during the day (Roenneberg and Merrow, 2002). In humans, the implications for optimising the effects of light (Gronfier et al., 2007) on the circadian system and for the development of strategies for the treatment of seasonal affective or other chronobiological disorders are far reaching.
Figure 6.
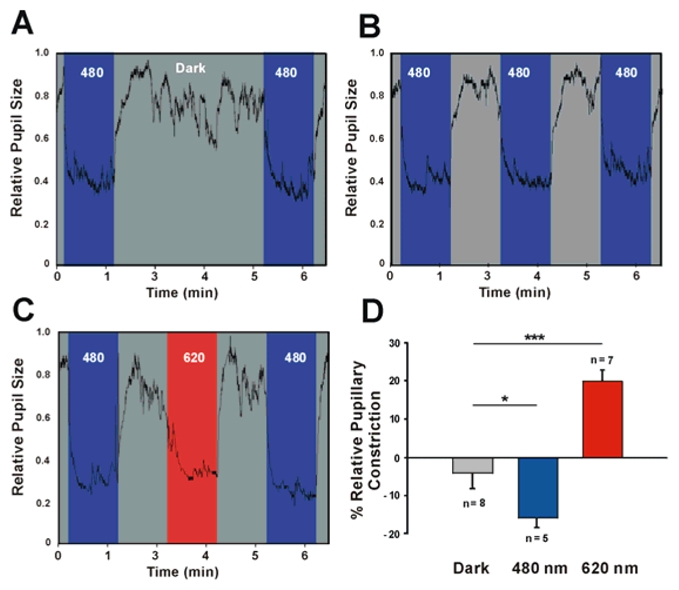
Consensual pupillary constriction in the mouse in response to 480 nm and 620 nm. Note that pupil surface area is measured continuously at a 30 Hz sampling rate. A first baseline 480 nm exposure with blue light (1 min) is followed by 3 minutes of darkness (A), or 1 min of either 480 nm (B) or 620 nm (C) flanked by 1 min periods of darkness, subsequently followed by the 480 nm test exposure. (D) Dark exposure fails to alter pupillary constriction (−3.9± 4.1%, n=9, ns), whereas exposure to blue light reduces the response by 15.6 ± 2.8%, n=5, ANOVA, post hoc Newman-Keuls test p<0.05*). 620 nm exposure results in an increased amplitude of the response (20.2±2.9%, n=7, ANOVA, post hoc Newman-Keuls test P<0.001***). Irradiance values are 480 nm (1012 photons/cm2/s) and 620 nm (1013photons/cm2/s).
Acknowledgments
This work was supported by grants from FP6-EUCLOCK, ACI MNRT, INSERM ACT, GIS-Longévité, Emergence-Rhône-Alpes.
References
- Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523:211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003;47:563–571. [PubMed] [Google Scholar]
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- Arshavsky V. Like night and day: rods and cones have different pigment regeneration pathways. Neuron. 2002;36:1–3. doi: 10.1016/s0896-6273(02)00937-6. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson DM. Phototransduction in ganglioncell photoreceptors. Pflugers Arch. 2007 Mar 10; doi: 10.1007/s00424-007-0242-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Richardson BA, King TS, Reiter RJ. The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res. 1984;294:333–339. doi: 10.1016/0006-8993(84)91045-x. [DOI] [PubMed] [Google Scholar]
- Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, Fong HK. A photic visual cycle of rhodopsin regeneration is dependent on RGR. Nat Genet. 2001;28(3):256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- Contin MA, Verra DM, Guido ME. An invertebrate-like phototransduction cascade mediates light detection in the chicken retinal ganglion cells. Faseb J. 2006;20:2648–2650. doi: 10.1096/fj.06-6133fje. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Sicard B, Cooper HM. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: temporal summation and reciprocity. J Neurosci. 2000;20:7790–7797. doi: 10.1523/JNEUROSCI.20-20-07790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Castrucci AM, McCall M, Provencio I, Menaker M. Nonvisual light responses in the Rpe65 knockout mouse: rod loss restores sensitivity to the melanopsin system. Proc Natl Acad Sci U S A. 2006;103:10432–10437. doi: 10.1073/pnas.0600934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of SCN neurons to light and dark adaptation: relative contributions of melanopsin and rod-cone inputs. J Neuroscience. 2007 doi: 10.1523/JNEUROSCI.1391-07.2007. manuscript accepted with revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci U S A. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23(18):7093–106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci U S A. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman P, Hochstein S, Minke B. Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol Rev. 1983;63:668– 772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- Isoldi MC, Rollag MD, Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci U S A. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev A, Subramaniam S. Activation and regeneration of rhodopsin in the insect visual cycle. Science. 1994;266:1369–1373. doi: 10.1126/science.7973725. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Terakita A, Kubokawa K, Shichida Y. Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores. FEBS Lett. 2002;531(3):525–528. doi: 10.1016/s0014-5793(02)03616-5. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Kumbalasiri T, Provencio I. Melanopsin and other novel mammalian opsins. Exp Eye Res. 2005;81:368–375. doi: 10.1016/j.exer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Sheline Y. Analysis of the rhodopsin cycle in limulus ventral photoreceptors using the early receptor potential. J Gen Physiol. 1976;68:487–501. doi: 10.1085/jgp.68.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ. Chromophore regeneration: melanopsin does its own thing. Proc Natl Acad Sci U S A. 2006;103:10153–10154. doi: 10.1073/pnas.0603955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Maeda T, Van Hooser JP, Driessen CA, Filipek S, Janssen JJ, Palczewski K. Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J Neurochem. 2003;85(4):944–956. doi: 10.1046/j.1471-4159.2003.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20(4):469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci. 1998;18(21):9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Nayak SK, Jegla T, Panda S. Role of a novel photopigment, melanopsin, in behavioral adaptation to light. Cell Mol Life Sci. 2007;64(2):144– 154. doi: 10.1007/s00018-006-5581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Peirson S, Foster RG. Melanopsin: another way of signaling light. Neuron. 2006;49:331–339. doi: 10.1016/j.neuron.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Pepe IM, Cugnoli C. Retinal photoisomerase: role in invertebrate visual cells. J Photochem Photobiol B. 1992;13:5–17. doi: 10.1016/1011-1344(92)80035-t. [DOI] [PubMed] [Google Scholar]
- Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Lane Brown R. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24(4):1117–23. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 1995;694:183– 190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN, Jr, Mollon JD. A theory of the pi1 and pi3 color mechanisms of Stiles. Vision Res. 1979;19:293–312. doi: 10.1016/0042-6989(79)90175-5. [DOI] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745– 749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Rieux CCN, Cooper HM. Spectral sensitivity of the mouse circadian system using early gene expression: evidence for the involvement of two photopigments. In: Neurosci EJ, editor. Federation of European Neurosciences Meeting “FENS2000”. Brighton: 2000. [Google Scholar]
- Roenneberg T, Merrow M. Light reception: discovering the clock-eye in mammals. Curr Biol. 2002;12:R163–165. doi: 10.1016/s0960-9822(02)00731-5. [DOI] [PubMed] [Google Scholar]
- Stavenga DG. Derivation of photochrome absorption spectra from absorbance difference measurements. Photochem Photobiol. 1975;21(12):105–110. doi: 10.1111/j.1751-1097.1975.tb06636.x. [DOI] [PubMed] [Google Scholar]
- Terakita A. The opsins. Genome Biol. 2005;6(3):213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Tu DC, Owens LA, Anderson L, Golczak M, Doyle SE, McCall M, Menaker M, Palczewski K, Van Gelder RN. Inner retinal photoreception independent of the visual retinoid cycle. Proc Natl Acad Sci U S A. 2006;103:10426–10431. doi: 10.1073/pnas.0600917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17(9):1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+) mice. J Comp Physiol [A] 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]


