Abstract
The use of transgenic mice expressing point mutations demands that the detection of the different alleles is efficient and reliable. In addition, the multiplication of transgenes included in mouse models of human disease underlines the importance of correct controls and the fact that investigators need an accurate and rapid genotyping of the littermates generated. In this study, we demonstrate a powerful alternative for genotyping using presenilin-1 mutant knockin (PS1M146KI) mice as an example.
Mutations in the presenilin-1 (PS1) gene are causally linked to many cases of early-onset inherited Alzheimer’s disease (AD). PS1M146VKI mice that express the PS1 M146V targeted allele at normal physiological levels and triple-transgenic model (3xTg-AD) derived from homozygous PS1M146VKI mice were generated to study the pathogenesis of AD. Genotyping PS1M146VKI line requires many steps and thus a large quantity of DNA. In PS1M146VKI mice, only three nucleotides are modified in the gene. Here we show that this small mutated DNA sequence can affect its secondary structure resulting in altered mobility that can be easily detected on a polyacrylamide gel, by the single-strand conformation polymorphism (SSCP) technique. Our results demonstrate that SSCP is a simple, accurate, repeatable and efficient method for the routine genotyping of this current AD model. This method could be easily applied to other transgenic mice.
Keywords: presenilin 1, genotyping, SSCP, Knock-In, Alzheimer’s Disease
Introduction
Experiments with transgenic mouse models require an accurate and rapid technique for genotyping transgenic mice and littermates. Mutant forms of the presenilin 1 (PS1) gene, which account for the majority of familial early-onset Alzheimer’s disease (FAD) cases, have been used to develop various mouse models of AD. Numerous PS1 models have been developed to replicate amyloid beta-peptide hypersecretion, generally by deriving mice with mutated PS1 overexpression combined with mutated beta-amyloid precursor protein (APP) (Duyckaerts et al., 2007). Among them, mice overexpressing the PS1 M146V mutation have been generated (Duff et al., 1996) and crossed with APP mutant mice to create double transgenic mice (Holcomb et al., 1998) to accelerate AD-like phenotypes. Because the mutant PS1 protein is expressed at normal levels in FAD cases, knock-in mice have been developed. These mice provide a relevant model that more closely reflects FAD than do transgenic mice in which the mutant transgene is overexpressed at supraphysiological levels. Guo et al. (1999) have generated PS1 mutant knockin PS1M146VKI mice that express the PS1M146V targeted allele at normal physiological levels in which a modification of three nucleotides leads to the substitution of Isoleucine and Methionine with two Valines. This “knock-in” model has been used to study the consequences for neuronal function of the M146V mutation of PS1 (Mattson et al., 2000; Xie et al., 2001; LaFontaine et al., 2002; Pigino et al., 2003; Stutzmann et al., 2004; Sun et al., 2005; Wang et al., 2007; Malik et al., 2008).
PS1M146VKI mice have been used to construct a triple-transgenic AD model (3xTg-AD) using single-cell embryos from homozygous PS1M146VKI mice in which two transgenes APP(Swe), and tau(P301L) were microinjected (Oddo et al., 2003). These animals are recognised as a relevant AD model since they show Aβ and tau pathology, which closely resembles that seen in the human AD brain. PS1M146VKI mice are classically genotyped by PCR amplification (Guo et al., 1999; protocol recommended by the Jackson Laboratory). A second step consisting of a PCR product purification and an enzymatic digestion of the amplified DNA with a restriction enzyme is necessary to differentiate the expected wild-type (+/+), heterozygous (+/KI) and homozygous (KI/KI) targeted alleles. This multistep classical method is time-consuming and limited in the detection of the nucleotide variation. We therefore developed a novel method for genotyping the PS1M146VKI line. In the single-strand conformation polymorphism (SSCP) analysis (Orita et al., 1989), double stranded DNA can be denatured to single stranded DNA which adopts a defined secondary structure. DNA sequence differences as small as a single base change can affect the secondary structure and can be detected by electrophoresis in a non-denaturing polyacrylamide gel. Consequently, mutated DNA can be easily detected by SCCP analysis compared to wild-type DNA (Hayashi et al., 1991; Beheshti and al., 1995). Indeed, we show here that the modification of two codons in PS1M146VKI mouse allows the use of SSCP analysis.
We demonstrate that SSCP provides a powerful alternative for genotyping this widely used mouse line.
Materials and methods
PS1 mutant M146V knock-in mice
The derivation and characterization of the PS1M146VKI mice have been described previously (Guo et al., 1999). These mice are maintained on a common homogeneous genetic background (C57BL/6). PS1M146VKI mice were bred and housed in our animal facility in the ‘Université Pierre et Marie Curie’, with 12h/12h light/dark cycle and libitum access to food and water. Heterozygous mice (+/KI) for the PS1 mutation were intercrossed to generate homozygous mutant knock-in mice (KI/KI) and homozygous wild-type mice (+/+). In total, 17 different mice were analysed in the study. Mice genotypes were determined as described previously (Guo et al., 1999). All animal procedures were performed according to the regulations of the Comité National d'Ethique pour les Sciences de la Vie et de la Santé.
PCR primers design
To design the PCR primers, we used the murine PS1 sequence in which the I145V/M146V mutation of three nucleotides in the exon 5 was introduced (Fig. 1). New primers were constructed considering the targeted sequence with the assistance of the AmplifX 1.4 software (Centre de Recherche de Neurobiologie-Neurophysiologie, Marseille, France).
Figure 1. Oligonucleotides designed to amplify the I145V/M146V mutation at 3 nucleotides in the exon 5 murine PS1 gene.
Uppercases indicate exons and lowercases indicate introns. The dash show nucleotides homology in the mutant mouse sequence compared to wild type. The two new designed primers using the AmplifX software are 5’AATCTACACCCCATTCACAG3’ for the forward primer indicated as ⋙ and 5’GCCCCCAACTCTCCCACC 3’ for the reverse primer indicated as ⋘.
DNA extraction
Genomic DNA was extracted from mice tails digested with proteinase K (Qiagen, Courtaboeuf, France) in TSE buffer containing in mM: 25 TrisHCl pH 8.0, 75 NaCl, 25 EDTA pH 8.0 and SDS 1% (Sigma-Aldrich, Saint Quentin, France) overnight at 55°C. DNA fragments were precipitated with isopropanol and washed with 70% ethanol. DNA precipitates were dissolved in 100 µl of distilled water.
PCR
For the amplification reaction, 5 µl of DNA (or water in the negative control) were added to a PCR mix containing 0.032 U/µl Taq polymerase (Applied Biosystems, Courtaboeuf, France) diluted in the manufacturer provided buffer, 0.48 mM of each dNTP (Qiagen, Courtaboeuf, France), and 20 µM of specific designed reverse and forward primers (Eurogentec, Angers, France) in a total reaction volume of 25 µl. Samples were denaturated at 94°C for 3 min and then subjected to 35 cycles of 94°C for 30 s, 60°C for 1 min and 72°C for 1 min, with a final extension step of 5 min at 72°C using a GeneAmp PCR system 2700 (Applied Biosystems, Courtaboeuf, France).
Electrophoresis of PCR products
3 µl of each PCR product mixed with 3 µl of loading buffer (xylene cyanol 0.025%, glycerol 50% in distilled water) was loaded on a 2% agarose gel (Euromedex, Souffelweyersheim, France) containing 0.5 mg/l ethidium bromide (Sigma-Aldrich, Saint Quentin, France). Electrophoresis was performed in 1X TAE (Tris-HCl, Acetate, EDTA) (Euromedex, Souffelweyersheim, France) at 6–7 V/cm for 10–15 min and then imaged under UV transillumination (Fisher Bioblock, Illkirch, France) with a camera (Microvision Instrument, Evry sur Seine, France).
Single strand conformation polymorphism (SSCP) analysis
SSCP analysis of PCR products were performed using the Dcode System (Biorad, Marnes, France), according to the manufacturer instructions, as follows: 10 µl of PCR products were mixed with 10 µl of denaturing loading dye (0.05% xylene cyanol, 0.05% bromophenol blue, 20 mM EDTA; pH 8.0 in formamide) (Sigma-Aldrich, Saint Quentin, France), denatured at 95°C for 6 min and rapidly cooled to 4°C using an ice bath for 6 min.
The denatured samples were loaded in a 12% polyacrylamide gel (ratio 37.5:1). The buffer was chilled by connecting the Dcode electrophoresis cooling tank to an external chiller (Multitemp III, Amersham Biosciences, Aulnay, France). The coolant used in the chiller was 50% ethylene glycol in distilled water. The fragments were electrophoresed at 65 mA overnight at 4°C. This method can be applied to other systems of electrophoresis as long as the migration is performed at 4°C. After electrophoresis, gels were stained using a silver staining kit (Amersham Biosciences, Aulnay, France) according to the instructions of the supplier. Processing of the gel involved fixation in “fixing solution” (30 min), impregnation in “staining solution” (30 min), washing in distilled water (1 min), development in sodium carbonate solution (formaldehyde 37%, sodium thiosulphate 2%) for 7 min, and stopping reaction in “stopping/preserving solution”. Photomicrographs of the gels were obtained with an Epson Perfection 2580 Scanner.
Results
1. Design and feasibility of SCCP technique for genotyping
To test the feasibility of the new method to genotype PS1KIM146V mice, we first systematically analysed three mice (+/+, +/KI and KI/KI), whose genotypes were determined using the classical method (Guo et al., 1999). Each mouse was tested in triplicate.
1.1 Primer design and PCR amplification
It has been shown that the amplicon size detected by the SSCP technique must be small; approximatively 95% of mutations are detected by SSCP analysis for amplicons of 100–250 bp (Orita et al., 1990; Gasser and Zhu, 1999). The amplicon size obtained using the classical primers in the PS1M146V gene is 530 bp, which can not be applied to SSCP analysis. Using the AmplifX software (Centre de Recherche de Neurobiologie-Neurophysiologie, Marseille, France) we found that the classical primers may match to different regions of the murine PS1 gene, some of which are outside of the targeted nucleotides. Consequently, we have designed new reverse and forward primers which exclusively match the targeted region of the murine PS1 sequence. DNA was amplified by PCR with the following two primers: 5’AATCTACACCCCATTCACAG 3’ and 5’GCCCCCAACTCTCCCACC 3’ (Fig 1). The size of the new amplicon (231 bp) was determined using the same software. We systematically performed agarose electophoresis to check the successful DNA amplification using these new primers. Amplification was efficient at 100%.
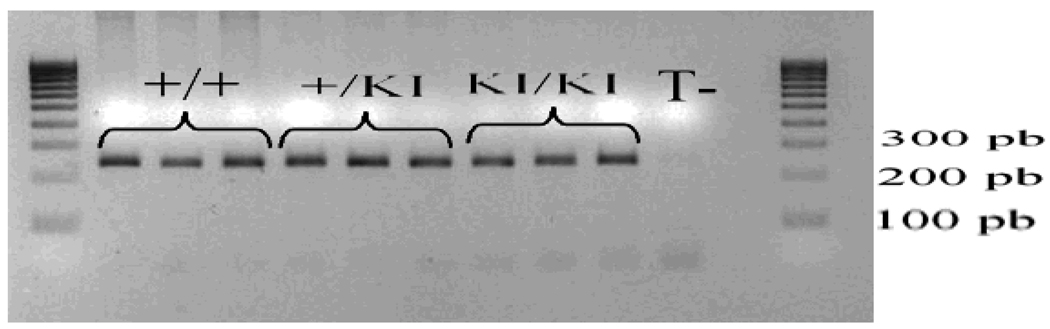
The three mice (+/+, +/KI and KI/KI) were analysed to test the efficiency of the new primers. As shown in figure 2, the new set of primers designed for PS1 gene generated a band at the expected size for each sample after PCR amplification. The specific band was absent in the negative control lane (T−).
Figure 2. Agarose electrophoresis of PS1 DNA amplified by PCR using the designed primers.
The specific set of primers designed for PS1 gene generated a 231 bp PCR product in three different DNA samples tested whose genotypes were determined using the classical method (+/+, +/KI, KI/KI). PCR was performed in triplicate for each sample. No PCR product was detected in the negative control lane (T−).
1.2 Detection of the PS1 mutation by SSCP analysis
DNA sequence differences as small as a single base change can affect its secondary structure and can be detected by a SSCP analysis. In the case of the PS1 mutation, 3 nucleotides are substituted. Thus, differences in mobility of the single strands between wild-type DNA (+/+) and homozygous mutant DNA (KI/KI) should be detectable. For the heterozygous DNA (+/KI), two bands corresponding to wild-type and mutant DNA must be obtained.
After PCR amplification, the PCR products of the three mice (+/+, +/KI and KI/KI) were denaturated and loaded in a polyacrylamide gel. Different conditions for the polyacrylamide gel were tested in order to optimize the SSCP analysis. The optimal composition of the gel to reveal differences in mobility of the two single strands is 12% polyacrylamide and 37.5:1 ratio (mono – bis acrylamide)
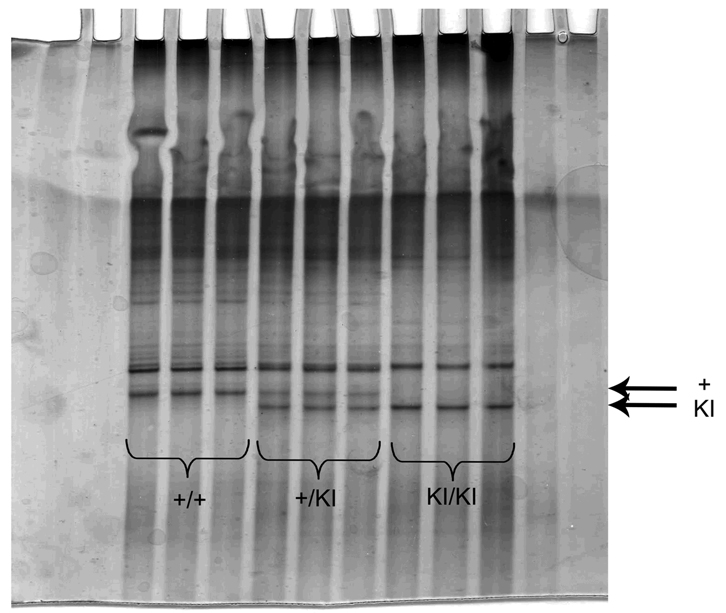
As shown on figure 3, two bands corresponding to the wild-type (upper band) and mutated (lower band) single strand are clearly detected, in the middle part of the gel, using the SSCP technique. Thus, +/+ mice are identified by one band in upper position, KI/KI mice by one band in lower position and +/KI mice by the two bands (Fig. 3). In addition, we also detected a non specific band. As shown by previous authors, gel lanes often showed additional artifactual bands comprised of reannealed double-stranded DNA or metastable 'ghost' bands of incompletely denatured single strands (Hongyo et al., 2003). This band does not prevent a good lecture of the genotypes (Fig. 3).
Figure 3. Feasibility of SSCP analysis: differences in the migration of wild-type and mutated PS1.
Photomicrograph of a polyacrylamide gel of SSCP electrophoresis. Migration was stopped when the loading dye had totally migrated outside of the gel (i.e. overnight). The lower part of the gel was cut off. Arrows indicate targeted specific bands. The upper band represents wild-type allele and the lower band the mutated allele KI of PS1
2. Study of the repeatability and efficiency of the SSCP analysis
To test the efficiency of our new genotyping method, we first established that we were able to obtain the same genotype as that identified by classical method using the same sample of tail. Genotypes of 17 animals including 7 wild-type mice (+/+), 4 heterozygous mutant mice (+/KI) and 6 homozygous mutant mice (KI /KI) were analysed blind using the SSCP analysis. Fragments of tail obtained from the 17 animals were cut into two pieces and a number was attributed for each tail from 1 to 33 in order to test all the samples blind to the investigator (table 1).
Table 1. Efficiency of SSCP analysis for genotyping.
Description of the tails (samples) tested corresponding to the number of lane and the genotypes obtained by classical PCR in order to confirm the efficiency of SSCP method. In total, 33 fragments of tails were analysed from the three genotypes blind to the tester: 7 fragments from +/+ mice among which 6 were duplicated; 4 fragments from +/KI mice among which 4 were duplicated and 6 fragments from KI/KI mice among which 6 were duplicated.
The efficiency of SSCP method for genotyping was clearly confirmed (100% match).
| Mice | Genotypes by classical PCR (Guo et al., 1999) |
Sample numbers (lanes) |
Genotypes determined by SSCP analysis |
|---|---|---|---|
| 339 | +/+ | 1 | +/+ |
| 339 | +/+ | 19 | +/+ |
| 440 | +/+ | 6 | +/+ |
| 440 | +/+ | 21 | +/+ |
| 409 | +/+ | 7 | +/+ |
| 409 | +/+ | 30 | +/+ |
| 338 | +/+ | 9 | +/+ |
| 338 | +/+ | 14 | +/+ |
| 285 | +/+ | 16 | +/+ |
| 285 | +/+ | 27 | +/+ |
| 286 | +/+ | 25 | +/+ |
| 286 | +/+ | 29 | +/+ |
| 410 | +/+ | 32 | +/+ |
| 405 | +/KI | 2 | +/KI |
| 405 | +/KI | 18 | +/KI |
| 433 | +/KI | 4 | +/KI |
| 433 | +/KI | 24 | +/KI |
| 432 | +/KI | 8 | +/KI |
| 432 | +/KI | 12 | +/KI |
| 456 | +/KI | 28 | +/KI |
| 456 | +/KI | 33 | +/KI |
| 378 | KI/KI | 26 | KI/KI |
| 378 | KI/KI | 31 | KI/KI |
| 319 | KI/KI | 3 | KI/KI |
| 319 | KI/KI | 11 | KI/KI |
| 447 | KI/KI | 5 | KI/KI |
| 447 | KI/KI | 13 | KI/KI |
| 371 | KI/KI | 10 | KI/KI |
| 371 | KI/KI | 20 | KI/KI |
| 379 | KI/KI | 23 | KI/KI |
| 379 | KI/KI | 15 | KI/KI |
| 530 | KI/KI | 17 | KI/KI |
| 530 | KI/KI | 22 | KI/KI |
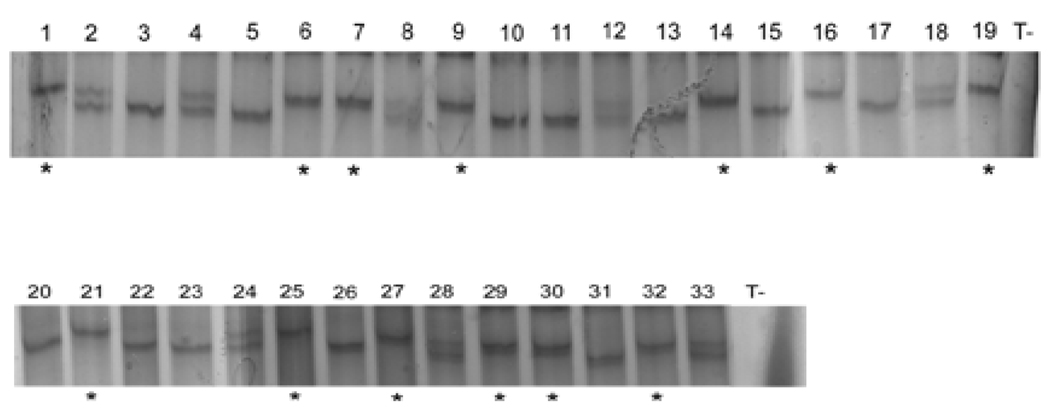
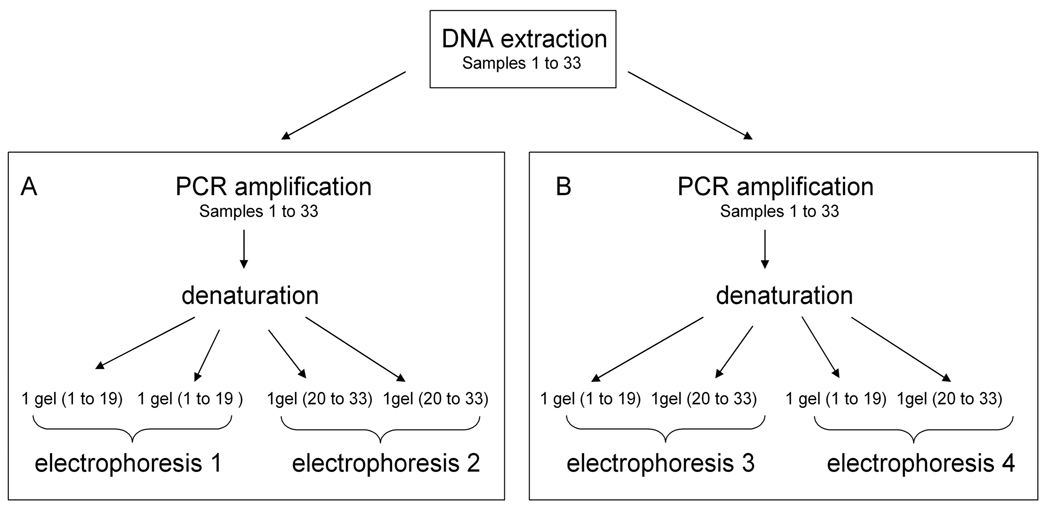
Firstly, for each animal, bands corresponding to the different alleles were successfully detected for the samples 1 to 33, as shown on figure 4. All the genotypes determined by the SSCP analysis match with genotypes determined using the classical method (Guo et al, 1999) (100% match) (Table 1). Secondly, to evaluate the reproducibility of PCR and SSCP electrophoresis, we undertook the following approach (Fig. 5): two different PCR amplifications were made from one DNA extraction for each sample 1 to 33. Each PCR product was denaturated and loaded to gels four separate times (Fig. 5A, B). Samples 1 to 19 were loaded on two gels migrating in a same electrophoresis, as were samples 20 to 33 (Fig. 5A). These were then repeated with sample 1 to 19 and 20 to 33 in the same electrophoresis (Fig. 5B). Consequently, eight gels were analysed.
Figure 4. Representative gels of SSCP electrophoresis of samples 1 to 33.
Same genotypes were successfully detected for each duplicate for the samples 1 to 33, as referred in table 1. The reproducibility was also verified on different PCR and SSCP electrophoresis. Different secondary structures of amplified DNA in the lanes 1–33 were detected showing the three genotypes (+/+; +/KI and KI/KI; 100% match). Wild type allele is indicated by *. No bands were detected in the negative control lanes (T−).
Figure 5. Repeatability approaches.
Two different PCR amplifications were realized from one DNA extraction for the samples 1 to 33. Each PCR product was denaturated and loaded on gels four separate times.
(A) Samples 1 to 19 were loaded on two gels migrating in a same electrophoresis (1) as for samples 20 to 33 (electrophoresis 2).
(B) Samples 1 to 33 were loaded on two gels (1 to 19 and 20 to 33). Two electrophoresis were realized (3 and 4).
The same genotype was obtained for each duplicate introduced in the study blind to the tester. In conclusion, using the SSCP analysis, from a small quantity of DNA, we were able to identify reproducibly wild-type and mutated alleles of the PS1 gene.
Discussion
In this study, we developed a novel strategy for genotyping the PS1M146VKI mouse line, a current AD model. The classical method for genotyping PS1 mice, described by Guo et al. (1999) and recommended by the Jackson Laboratories is a multistep method. It necessitates a PCR amplification followed by a PCR product purification, an enzymatic digestion and an agarose electrophoresis. The purification step necessitates a large quantity of amplified DNA. Futhermore, three DNA fragments of 180, 350 and 530 bp are obtained for the PS1 targeted allele, which include a large range of sizes. This method is thus limited and may lead to genotype misreading. Taken together, these limitations emphasize the importance of developing a new method for genotyping PS1M146VKI mice. Our new method makes two improvements: greater specificity of the primers and greater sensitivity of the detection system.
The AmplifX software used to design our primers revealed that the classical primers may match different regions of the murine PS1 gene outside of the targeted mutated nucleotides and consequently may fail to amplify mutant DNA by classical PCR.
The PS1M146VKI mouse has a mutation of two codons which can be identified by the SSCP analysis. We successfully designed specific primers to the targeted PS1 sequence, avoiding possible impairment in primers matching during PCR, and limiting amplicon size.
We have obtained differences in mobility of the wild-type and mutant single strands. We have clearly demonstrated the efficiency of the SSCP analysis testing the reproducibility of the technique. The three genotypes targeted (+/+, +/KI and KI/KI) were correctly identified without any errors.
Futhermore, the present study confirmed that the SSCP technique has many advantages to detect mutations as small as a single base change. Its optimization is neither time-consuming nor does it require complicated instruments or specialized techniques. We used Multitemp III electrophoresis tank from Amersham Biosciences, but it can also be performed with a simple electrophoresis tank at 4°C, overnight.
In addition, visualisation of the mobility shift of wild-type and mutated strands performed using a silver staining in place of ethidium bromide transillumination strongly enhances the sensitivity of the technique (Han et al., 2008; Byun et al., 2009).
Our study highlights a new method for genotyping PS1M146VKI mice using the SSCP technique. SSCP is a simple and rapid method reducing the number of steps that permits the differential display, on non-denaturing gels, of the different PS1 targeted genotypes. We strongly recommend this technique to genotype PS1M146VKI mice
Finally, this new approach can be applied to other transgenic or mutant mice that differ in sequence by as few as one nucleotide. For example, the SSCP analysis is currently used in our laboratory to genotype another transgenic model (Lurcher mutant) in which only one nucleotide is substituted (Zuo et al., 1997; Selimi et al., 2000).
Acknowledgements
This work was supported by funds from “Centre National de la Recherche Scientifique” (CNRS), Université Pierre et Marie Curie (UPMC), ”Fondation de le la Recherche Médicale’’ (FRM) and “Fondation de la Recherche sur le Cerveau” (FRC), and the Intramural Research Program of the National Institute on Aging of the NIH. We thank the animal facility of Université Pierre et Marie Curie. We thank Mrs. S. Jezequel and Mohamed Mughal for technical assistance, and Dr. Rachel Sherrard for her critical reading of the paper.
References
- Beheshti I, Hanson NQ, Copeland KR, Garg U, Tsai MY. Single-strand conformational polymorphisms (SSCP): studies of the genetic polymorphisms of exon 4 of apolipoprotein C III. Clin Biochem. 1995;28(3):303–307. doi: 10.1016/0009-9120(94)00083-8. [DOI] [PubMed] [Google Scholar]
- Byun SO, Fang Q, Zhou H, Hickford JGH. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Analytical Biochemistry. 2009;385:174–175. doi: 10.1016/j.ab.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Potier M, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser RB, Zhu XQ. Sequence-based Analysis of Enzymatically Amplified DNA Fragments by Mutation Detection Techniques. Parasitology Today. 1999;15:462–465. doi: 10.1016/s0169-4758(99)01536-7. [DOI] [PubMed] [Google Scholar]
- Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5(1):101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- Han YC, Teng CZ, Hu ZL, Song YC. An optimal method of DNA silver staining in polyacrylamide gels. Electrophoresis. 2008;29(6):1355–1358. doi: 10.1002/elps.200700558. [DOI] [PubMed] [Google Scholar]
- Hayashi K. PCR-SSCP: a simple and sensitive method for detection of mutations in the genomic DNA. PCR Methods Appl. 1991;1:34–38. doi: 10.1101/gr.1.1.34. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Hongyo T, Buzard GS, Calvert RJ, Weghorst CM. 'Cold SSCP': a simple, rapid and non-radioactive method for optimized single-strand conformation polymorphism analyses. Nucleic Acids Res. 1993;21(16):3637–3642. doi: 10.1093/nar/21.16.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFontaine MA, Mattson MP, Butterfield DA. Oxidative stress in synaptosomal proteins from mutant presenilin-1 knock-in mice: implications for familial Alzheimer's disease. Neurochem Res. 2002;27(5):417–421. doi: 10.1023/a:1015560116208. [DOI] [PubMed] [Google Scholar]
- Malik B, Currais A, Soriano S. Cell cycle-driven neuronal apoptosis specifically linked to amyloid peptide Abeta1–42 exposure is not exacerbated in a mouse model of presenilin-1 familial Alzheimer's disease. J Neurochem. 2008;106(2):912–916. doi: 10.1111/j.1471-4159.2008.05446.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci. 2000;20(4):1358–1364. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Orita M, Suzuki Y, Sekiya T, Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989;86:2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M, Sekiya T, Hayashi K. DNA sequence polymorphisms in Alu repeats. Genomics. 1990;8(2):271–278. doi: 10.1016/0888-7543(90)90282-y. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23(11):4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimi F, Vogel MW, Mariani J. Bax Inactivation in Lurcher Mutants Rescues Cerebellar Granule Cells But Not Purkinje Cells or Inferior Olivary Neurons. The Journal of Neuroscience. 2000;20(14):5339–5345. doi: 10.1523/JNEUROSCI.20-14-05339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24(2):508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Beglopoulos V, Mattson MP, Shen J. Hippocampal spatial memory impairments caused by the familial Alzheimer's disease-linked presenilin 1 M146V mutation. Neurodegener Dis. 2005;2(1):6–15. doi: 10.1159/000086426. [DOI] [PubMed] [Google Scholar]
- Wang Y, Greig NH, Yu QS, Mattson MP. Presenilin-1 mutation impairs cholinergic modulation of synaptic plasticity and suppresses NMDA currents in hippocampus slices. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Chang X, Zhang X, Guo Q. Aberrant induction of Par-4 is involved in apoptosis of hippocampal neurons in presenilin-1 M146V mutant knock-in mice. Brain Res. 2001;915(1):1–10. doi: 10.1016/s0006-8993(01)02803-7. [DOI] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388(6644):769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]