Abstract
Myelin transcription factor 1 (Myt1) is a zinc-finger DNA binding protein that influences developing oligodendrocyte progenitor (OP) cell proliferation, differentiation, and myelin gene transcription in vitro. The potential of Myt1 to play a role in OP responses leading to remyelination was examined using murine hepatitis virus strain A59 (MHV) to induce spinal cord demyelination and potential relevance to human pathology was evaluated in multiple sclerosis (MS) lesions. In MHV-infected mice, the density of Myt1 expressing cells markedly increased in lesioned areas of spinal cord white matter. Myt1 expressing cells proliferated most extensively during active demyelination and subsequently accumulated to maximal levels during early remyelination. Cells with nuclear Myt1 immunoreactivity were mainly OP cells, identified by co-localization with platelet-derived growth factor alpha receptor, with additional phenotypes being either oligodendrocytes or neural stem cells, identified by CC1 antigen and Musashi1, respectively. The density of OP cells expressing Myt1 was significantly increased in white matter of MHV-infected mice during demyelination and early remyelination then as remyelination advanced the values returned to levels comparable to PBS-injected control mice. In MHV lesions, Myt1 was not expressed in astrocytes, lymphocytes, or macrophage/micro-glial cells. MS lesions demonstrated increased Myt1 expression in both the periplaque white matter adjacent to lesions and within early remyelinating lesions. These results suggest a potential role for Myt1 in the regeneration of oligodendrocyte lineage cells in response to demyelination.
Keywords: remyelination, oligodendroglia, oligodendrocyte progenitor, myelin, multiple sclerosis
INTRODUCTION
Demyelination and oligodendrocyte loss in the CNS can result from multiple sclerosis (MS), spinal cord injury, toxic insults, and neurodegenerative disorders. Demyelination leads to slowing or blockade of action potential conduction. In addition, without the protective effect of myelin, denuded axons are more vulnerable to transection that can lead to permanent neurological impairment (Ferguson et al., 1997; Trapp et al., 1998).
Spontaneous remyelination has been observed in MS lesions following initial episodes of demyelination, yet the capacity for remyelination becomes limited with subsequent prolonged or repeated episodes of demyelination (Bruck et al., 2003). Animal models have shown that remyelination requires oligodendrocyte progenitor (OP) cells in the proximity of demyelinated lesions to proliferate and then differentiate into mature oligodendrocytes that can then myelinate denuded axons (Reynolds et al., 2001; Watanabe et al., 2002). This sequence of cellular responses must be orchestrated through transcriptional control of the complex gene expression patterns that regulate OP proliferation, differentiation, and eventual synthesis of myelin components.
Several previous studies implicate myelin transcription factor 1 (Myt1) as potentially important in regulating OP responses in the developing and adult CNS. Myt1 is a zinc-finger DNA binding protein that was originally identified based upon binding affinity within the promoter region of the proteolipid (PLP) gene, the most abundantly transcribed CNS myelin gene (Kim and Hudson, 1992). During development of the oligodendrocyte lineage, Myt1 is localized within nuclei of immature cells and then downregulated after terminal differentiation and accumulation of myelin proteins in mature oligodendrocytes (Armstrong et al., 1995). Myt1 continues to be expressed in germinal zones of the adult CNS and is upregulated in gliomas (Armstrong et al., 1997) and following spinal cord traumatic injury (Wrathall et al., 1998). In vitro, expression of a dominant negative form of Myt1 showed that Myt1 can regulate a critical transition in oligodendrocyte lineage cell development by modulating OP proliferation relative to terminal differentiation and upregulation of myelin gene transcription (Nielsen et al., 2004).
To examine the potential for Myt1 to play a role in regulating the OP responses required for remyelination, the current study determines the expression of Myt1 relative to the proliferative status and phenotype of cells in white matter during the progression of experimental demyelination, followed by spontaneous remyelination. Murine hepatitis virus strain A59 (MHV) infection was used to induce focal demyelinated lesions of spinal cord white matter from lytic infection of oligodendrocytes with subsequent lymphocytic infiltration and virus clearance (Matthews et al., 2002; Redwine and Armstrong, 1998). Furthermore, MS lesions were examined for Myt1 immunoreactivity relative to stage of disease pathology and control tissues to predict the potential for Myt1 to have a role in human demyelinating disease.
MATERIALS AND METHODS
Animals
C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME) were maintained in the Uniformed Services University of the Health Sciences (USUHS) animal housing facility in accordance with guidelines of the National Institutes of Health and the USUHS Institutional Animal Care and Use Committee.
MHV Model of Spinal Cord Demyelination
As previously described (Armstrong et al., 2005; Redwine and Armstrong, 1998), 4-week-old female C57Bl/6 mice were anesthetized and injected intracranially with MHV diluted in sterile PBS to 1,000 plaque forming units per 10 µL injection volume. Control animals were injected with 10 µL of sterile PBS.
A hang time assay was used to quantify motor impairment and recovery of function that corresponds with histopathological findings of demyelination and remyelination (Armstrong et al., 2005; Frost et al., 2003; Redwine and Armstrong, 1998). Duration of hanging from a cage top was recorded on days 0, 7, 10, 14, 17, 21, 24, 28, and then every 7 days for up to 8 weeks post infection (wpi). On the same days a clinical score was assigned as follows: 0 for no evidence of paresis/paralysis, 1–5 for paresis/ paralysis in 1–5 appendages, and 6 for morbidity (Armstrong et al., 2005; Redwine and Armstrong, 1998). Each mouse used in the study had a clinical score of 2 or greater, indicating impaired limb movement or strength, and hang time values characteristic of severe disability, followed by spontaneous recovery (Frost et al., 2003; Redwine and Armstrong, 1998).
At 2, 4, and 8 wpi, mice were perfused with 4% para-formaldehyde (Sigma, St. Louis, MO). Spinal cords were postfixed in 4% paraformaldehyde at 4°C overnight and cryoprotected prior to cutting as 15 µm transverse sections and mounting onto Superfrost Plus slides (Fisher, Pittsburgh, PA).
In Situ Hybridization of Mouse Tissue Sections
In situ hybridization and preparation of digoxigenin-labeled riboprobes were performed with modifications of methods previously detailed (Redwine and Armstrong, 1998). The antisense riboprobe to detect Myt1 mRNA transcripts was synthesized from a cDNA template (gift from Dr. Lynn Hudson; National Institutes of Health) by in vitro transcription to be complementary to a 1.2 kb mRNA fragment encoding the 3′ untranslated region of murine Myt1 (Kim et al., 1997). Spinal cord cryosections were digested with 20 U/mL proteinase K (Sigma, St. Louis, MO) at 37°C for 40 min prior to hybridization at 53°C overnight. Digoxigenin was detected with an alkaline phosphatase-conjugated sheep anti-digoxigenin antibody (Roche, Indianapolis, IN), followed by reaction with NBT/BCIP overnight (DAKO, Carpinteria, CA).
BrdU Incorporation and Detection in Mice
At 4 and 2 h prior to perfusion, mice were injected intraperitoneally with 200 mg/kg bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO)(Armstrong et al., 2002). Following in situ hybridization detection of Myt1 transcripts, sections were immunostained with anti-BrdU antibody directly conjugated with horseradish peroxidase (Roche, Indianapolis, IN) and detected with DAB (Vector Labs, Burlingame, CA).
Immunohistochemistry of Mouse Tissue Sections
Mouse transverse spinal cord tissue sections were immunostained with αMyt1-His rabbit polyclonal antibody (1:100; gift from Dr. Lynn Hudson; National Institutes of Health), which has been characterized in a previous study (Armstrong et al., 1995). OP cells were immunolabeled for platelet-derived growth factor alpha receptor (PDGFαR) antibody (APA5, 1:200; BD Biosciences Pharmingen, San Diego, CA) and mature oligodendrocytes were labeled with CC1 monoclonal antibody (1:20; Oncogene Research Products, Cambridge, MA). Neural stem cells were identified with a monoclonal antibody against Musashi1 (Msi1, 1:2,000; gift from Dr. Hideyuki Okano; Keio University School of Medicine, Tokyo, Japan; Kaneko et al., 2000). Astrocytes were immunostained for glial fibrillary acidic protein (GFAP, 1:20; Roche, Indianapolis, IN). Immune cells were identified with Ox-52 pan T-cell antibody (1:1,000) and CD45R antibody for Bcells (1:25) (both from BD Biosciences Pharmingen, San Diego, CA), and Mac 1 antibody for macrophages/microglia (1:100; Roche, Indianapolis, IN).
All secondary antibodies were purchased from Jackson Immunoresearch (West Grove, PA). PDGFαR, Msi1, O×52, and Mac1 signals were amplified with fluorescein tyramide signal amplification (Perkin Elmer Life Sciences, Boston, MA). Sections were incubated with DAPI (Sigma, St. Louis, MO) to stain nuclei.
Quantitative Analysis in Mouse Tissue Sections
Areas within transverse spinal cord sections were measured using Spot2 software and all labeled cells within each area were counted with a 40× objective to determine cells per mm2. For each condition and time point examined, at least three mice were analyzed with at least three sections included for each mouse. To distinguish significant differences between groups, unpaired Student’s t-tests were used for single time point comparisons and one-way analysis of variance (ANOVA with Tukey’s post hoc test) was used between control mice and multiple MHV disease time points.
MS and Control Tissues
The pathological analysis of MS autopsy and biopsy samples was approved by the Mayo Clinic Institutional Review Board (IRB No. 2067-99). Eight cases were selected from a larger series of MS cases with detailed clinical follow up (n = 130) derived from the MS Lesion Project cohort (Lucchinetti; NMSS grant RG 3185-A2; B3). Control tissue consisted of adult human brain sub-cortical white matter areas chosen from autopsy cases with no known neurological involvement. Two autopsy control cases were obtained from the Brain and Tissue Bank for Developmental Disorders (Baltimore, MD) and one was obtained from the Mayo Clinic.
Histopathology and Staging of Demyelinating Activity in MS Tissues
Specimens were fixed in 4% paraformaldehyde and embedded in paraffin. Sections 4 µm thick were stained with haematoxylin and eosin, Luxol-fast blue, and periodic acid-Schiff as well as Bielschowsky’s silver impregnation. Immunohistochemical staining was performed with a biotin-avidin or an alkaline phosphatase/anti-alkaline phosphatase technique. The primary antibodies used were anti-myelin basic protein (MBP, Boehringer Mannheim, Germany), anti-CD3 (T cells, DAKO, Denmark), anti-CD8 (cytotoxic T cells, DAKO, Denmark), anti-CD20 (B cells, DAKO, Denmark), anti-KiM1P (macrophages/ microglial cells, Dr. Radzun, University of Göttingen, Germany), and anti-MRP 14 (early activated macrophages, BMA Biomedicals, Switzerland). The demyelinating activity was classified according to the presence of minor myelin proteins (MOG, CNPase, MAG) in macrophages (early active lesions), the presence of early remyelination, and the absence of both active demyelination and remyelination (inactive demyelinated) (Brüuck et al., 1995). As white matter lesions might show more than one of these features, a total number of 8 different lesion areas were investigated (3 early active; 3 inactive; and 2 remyelinated).
Myt1 Immunohistochemistry in Human Tissues
Immunostaining with αMyt1-His rabbit polyclonal antibody (1:50; see above) was detected using the Elite ABC kit (Vector Labs; Burlingame, CA) with DAB as the substrate. The specificity of αMyt1-His immunoreactivity in human tissues was characterized by pre-absorption with excess antigen (Armstrong et al., 1997). As a further demonstration of specificity of the immunostaining reaction, selected tissues were similarly immunostained with the αMyt1L-His antibody that recognizes Myt1L, a Myt1 family member that is expressed in neuronal populations but not in oligodendrocyte lineage cells (Kim et al., 1998). Sections were counterstained with eosin.
In Situ Hybridization in Human Tissues
As previously described (Wong et al., 2000), sections processed for in situ hybridization with digoxigenin-labeled riboprobes to detect mRNA transcripts. Antisense riboprobes for human Myt1 (Wrathall et al., 1998) and PLP (Hudson et al., 1987) were generated from plasmids provided by Dr. Lynn Hudson (National Institutes of Health, Bethesda, MD). For combining detection of PLP mRNA and Myt1 protein, sections were processed for PLP mRNA in situ hybridization, followed by immunostaining for Myt1, as described above, without eosin counterstaining.
Human Tissue Analysis
All human tissue sections were analyzed qualitatively and tissues immunostained for Myt1 that exhibited specific and consistent immunoreactivity were also assessed quantitatively. Cells were counted using a 100× oil objective and 10 fields were sampled within each tissue area (i.e. active, remyelinated, or inactive lesion, adjacent periplaque white matter—PPWM, normal white matter—NWM). Characteristics of cell morphology and anatomic localization were used to exclude cells that were expected to be distinct from oligodendrocyte line-age cells, such as lymphocytes, based on comparisons with immunostaining for myelin proteins.
Imaging of Mouse and Human Tissue Sections
All images were acquired with a Spot 2 digital camera mounted to an IX-70 microscope (Olympus, Melville, NY) with narrow band pass and triple band pass filters for fluorescence detection and co-localization studies. Images were prepared as panels using Adobe Photoshop (Mountain View, CA).
RESULTS
Myt1 Expressing Cells Proliferate in Lesions During Demyelination and Early Remyelination
MHV infection in C57Bl/6 mice induces focal areas of demyelination throughout the spinal cord, with subsequent spontaneous remyelination (Frost et al., 2003; Redwine and Armstrong, 1998). To determine whether Myt1 expression was upregulated in correlation with a specific stage of disease progression, cellular expression of Myt1 mRNA transcripts was detected by in situ hybridization in spinal cord sections (Fig. 1). In MHV-infected mice, the density of Myt1 expressing cells in the white matter was increased during the demyelination phase examined at 2 wpi, as compared with PBS-injected controls. This Myt1 expression peaked during the active remyelination phase, which corresponded with 4 wpi in the MHV-infected mice. By 8 wpi, as remyelination progressed in MHV-infected mice the density of cells expressing Myt1 declined to near that of PBS-injected controls.
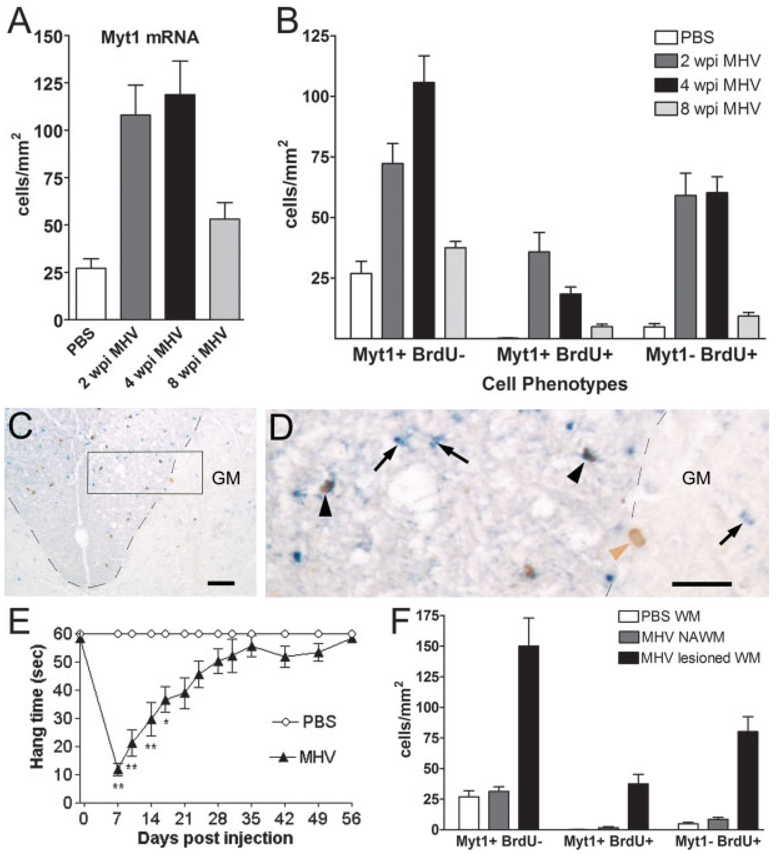
Fig. 1.
Myt1 expression and BrdU incorporation in MHV lesions during demyelination and remyelination. Mice injected with MHV or PBS vehicle were perfused at 2 weeks postinjection (wpi) (active demyelination), at 4 wpi (early remyelination), and at 8 wpi (advanced remyelination). (A) Quantification of Myt1 mRNA expression in white matter of cervical transverse sections throughout MHV disease progression. In MHV-injected mice, as compared to PBS control mice, the density of cells labeled by in situ hybridization for Myt1 mRNA is increased by 2 wpi (P = 0.0323), peaks at 4 wpi (P = 0.0020), and then declines by 8 wpi but is still elevated from controls (P = 0.0297). (B) Quantification ofMyt1 mRNA expression relative to BrdU incorporation throughout MHV disease progression. BrdU was administered during the final 4 h prior to sacrifice to identify actively proliferating cells (BrdU+) among the population of cells expressing Myt1 mRNA (Myt1+). The Myt1+ BrdU+ cell density peaks during active demyelination (2 wpi; P = 0.016) then remains significantly increased at 4 wpi (P = 0.003), relative to the very low density (0.4 cells/mm2) in PBS-injected mice. By 8 weeks, Myt1+ BrdU+ cell density has returned to control levels (P = 0.0791). BrdU+ cells that do not have detectable Myt1 mRNA (Myt1−) are increased over control levels at 2 wpi (P = 0.016) and at 4 wpi (P = 0.0007), but are still slightly elevated at 8 wks (P = 0.017). For panels A and B: PBS, n = 3 mice; MHV 2 wpi, n = 4 mice; MHV 4 wpi, n = 6 mice; MHV 8 wpi, n = 4 mice. (C) Representative image of dorsal column area (border denoted by black dashed line) in transverse section of cervical spinal cord from an MHV-infected mouse at 4 wpi. In situ hybridization showing Myt1 mRNA transcripts (blue) combined with detection of BrdU (brown) to assess proliferation. Increased BrdU incorporation in lesioned areas of the dorsal columns (upper part of image) corresponds with an increased density of cells expressing Myt1, compared to the normal appearing areas in the deep dorsal column white matter. GM = gray matter. Scale bar 5 50 µm. (D) Enlargement of area boxed in panel (C). Cells with detectable Myt1 mRNA are small with an elongated cell body, characteristic of OP cells, and could be found labeled with BrdU (Myt1+ BrdU+, black arrowheads) or may not have incorporated BrdU during this 4-h terminal pulse (Myt1+ BrdU−, black arrows). BrdU labeled clls that do not express Myt1 (Myt1− BrdU+, brown arrowhead) are also common, such as the example with a large oval nucleus that is characteristic of an astrocyte. Dorsal column border with gray matter (GM) is denoted by black dash lines. Scale bar = 25 µm. (E) Disease progression among MHV-infected mice used in the data shown in panels (A) and (B). Motor impairment and recovery was monitored with the hang time test of grip strength and coordination. Control mice injected with PBS can readily hang from bars of a wire cage top for 60 s. Following MHV injection, severely affected mice had significantly reduced hang times when tested on days 7–17. In the following weeks, the hang time scores improve gradually until reaching control levels. ANOVA with repeated measures: **P < 0.01; *P < 0.05; days 7–14: n = 23 mice for MHV, n = 12 mice for PBS; days 17–28: n = 17 mice for MHV, n = 9 mice for PBS; days 35–56: n = 9 mice for MHV, n = 6mice for PBS. (F) Quantification of Myt1 mRNA expression and BrdU incorporation within normal versus lesioned white matter areas. Within transverse spinal cord sections, regions were demarcated as white matter areas of demyelination (MHV lesioned WM), adjacent normal appearing white matter (MHV NAWM), or white matter of PBS-injected controls (PBS WM). Mice were analyzed at 4 wpi after MHV or PBS injection. An increased density of cells with detectable levels of Myt1 mRNA transcripts is mainly restricted to MHV lesion areas, which are significantly elevated compared with NAWM (P = 0.0005) or with PBS WM (P = 0.0081). Myt1+ BrdU+ proliferating cells are also significantly increased specifically within lesion areas relative to NAWM (P = 0.0009) and PBS WM (P = 0.0003). Myt1− BrdU+ cells within MHV lesions are also significantly increased over both NAWM (P = 0.0002) and PBS WM (P = 0.0038). NAWM was not significantly different from PBS WM for any of the values shown. PBS, n = 3 mice;MHV, n = 6 mice.
In models of experimental demyelination, proliferation of OP cells precedes the generation of new oligodendrocytes and remyelination (Reynolds et al., 2001; Watanabe et al., 2002). To evaluate a potential role of Myt1 in proliferation in response to demyelination, in situ hybridization for Myt1 was combined with incorporation of BrdU (Fig. 1). Mice were administered BrdU during the final 4 h before sacrifice, which allowed detection of a sufficient proportion of cells undergoing DNA synthesis while minimizing the potential for differentiation subsequent to BrdU incorporation. The density of cells double labeled for Myt1 mRNA combined with BrdU immunohistochemistry is significantly increased during the active demyelination phase at 2 wpi (Fig. 1B). By 4 wpi, the density of Myt1 and BrdU double labeled cells declined slightly yet remained significantly increased over PBS-injected controls (Fig. 1B). Incorporation of BrdU into cells without detectable Myt1 (Fig. 1B) may correspond with proliferation of astrocytes in MHV lesions (see below; Redwine and Armstrong, 1998). By 8 wpi, BrdU incorporation in sections from MHV-infected mice has declined to the levels observed in sections from PBS-injected control mice (Fig. 1B).
Amplification of Myt1 expressing cells is specifically associated with MHV lesion areas (Fig. 1F). Lesions in spinal cord white matter were identified using dark field and phase contrast microscopy to image areas of myelin loss and vacuolation (not shown; Armstrong, 2000; Redwine and Armstrong, 1998). The overall density of cells expressing Myt1 mRNA is greatly increased within lesions. In contrast, normal appearing white matter (NAWM) of MHV-infected mice had a relatively low density of cells expressing Myt1, which was similar to values from the white matter of PBS-injected controls.
Myt1 is Localized to the Nuclei of Immature Cell Types in MHV Lesions
Immunohistochemistry for Myt1 demonstrated that Myt1 protein was increased in cells associated with white matter lesions of MHV-infected mice during demyelination (2 wpi) and early remyelination (4 wpi) (Fig. 2). Double immunolabeling for cell type-specific antigens was used to characterize cell types with Myt1 immunoreactivity (Fig. 2, Fig 3, and Fig 4). The nuclear pattern of Myt1 immunoreactivity was confirmed by co-localization with DAPI staining of nuclei (data not shown). As expected during demyelination and early remyelination in MHV-infected mice, the density of oligodendrocytes was significantly lower in lesioned areas (Fig. 2B) and corresponded with a reduction in the density of CC1+ oligodendrocytes in the total white matter area, as compared with PBS controls (Fig. 3C). Among the mature oligodendrocytes immunolabeled with CC1 only a small proportion exhibited Myt1 immunoreactivity (Fig. 3C and Fig 4). The small proportion of Myt1+ CC1+ double labeled cells is consistent with our previous study showing that during CNS development Myt1 immunoreactivity is downregulated after OP cells undergo terminal differentiation into mature oligodendrocytes (Armstrong et al., 1995).
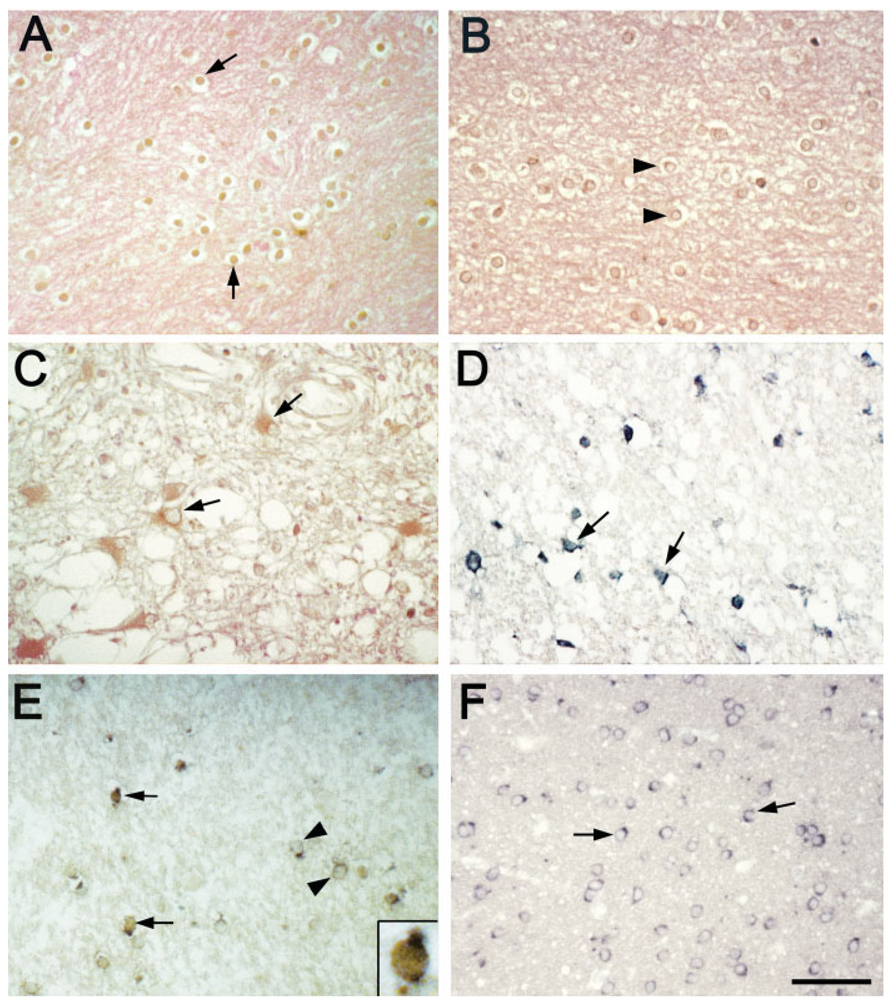
Fig. 2.
Characterization of cell types expressing Myt1. Myt1 immunodetection alone (A) and in combination with immunostaining for CC1 to identify mature oligodendrocytes (B), Msi1 to identify immature neural stem cells (C), and PDGFαR to identify OP cells (D). (A) Low power of a transverse hemi-section of cervical spinal cord during the early remyelination stage at 4 wpi of MHV. The approximate boundary of the dorsal columns is denoted by white dash marks. The density of Myt1 immunoreactive cells is highest in lesioned areas of the superficial dorsal columns and the ventrolateral white matter. Scale bar = 100 µm. (B) In a higher magnification of dorsal column white matter from a 2 wpi mouse, the dorsal column border with the gray matter (GM) is noted by white dash marks. Normal CC1 immunolabeling (green) of oligodendrocytes is seen in the deep dorsal column white matter, which is not lesioned. In the more superficial dorsal column region, the lesion area is evident by the lack of CC1 immunolabeled oligodendrocytes (top right of panel) where a high density of Myt1+ CC1− cells (red) are present (examples at arrow-heads). Along the lesion border, CC1 immunoreactivity is detected in cells with nuclear Myt1 (CC1+ Myt1+ examples at arrows). Scale bar = 50 µm. The inset shows an enlarged view of several CC1+ cells and a pair of CC1+ Myt1+ cells. Scale bar = 10 µm. (C) Msi1 immunoreactivity (green) is increased in reactive astrocytes and neural stem cells in the superficial part of the dorsal column white matter of a 2 wpi mouse. Cells expressing Myt1 (red; arrowheads) are more prevalent in the lesion area than in the nonlesioned deep dorsal column white matter. Reactive astrocytes express Msi1 (detected with immunostaining for glial fibrillary acidic protein; data not shown). However, Msi1+ Myt1+ cells (examples at arrows) are likely to be neural stem cells since in these tissues Myt1 was not co-expressed in astrocytes (data not shown). Scale bar = 50 µm. The inset shows an enlarged view of two cells with cytoplasmic Msi1 and nuclear Myt1 as well as three cells with only nuclear Myt1 immunoreactivity. Scale bar = 10 µm. (D) The density of OP cells expressing PDGFαR (green) is increased in the lesioned area of the superficial dorsal column in a 4 wpi mouse. The density of cells with nuclear Myt1 immunoreactivity (red) is also increased in the lesion area. Examples of cells double labeled for PDGFαR and Myt1 are indicated by arrows while PDGFα R-Myt1+ cells are shown by arrowheads. Scale bar = 50 µm. The inset shows a pair of PDGFαR1 cells with nuclear Myt1 immunoreactivity. Scale bar = 10 µm.
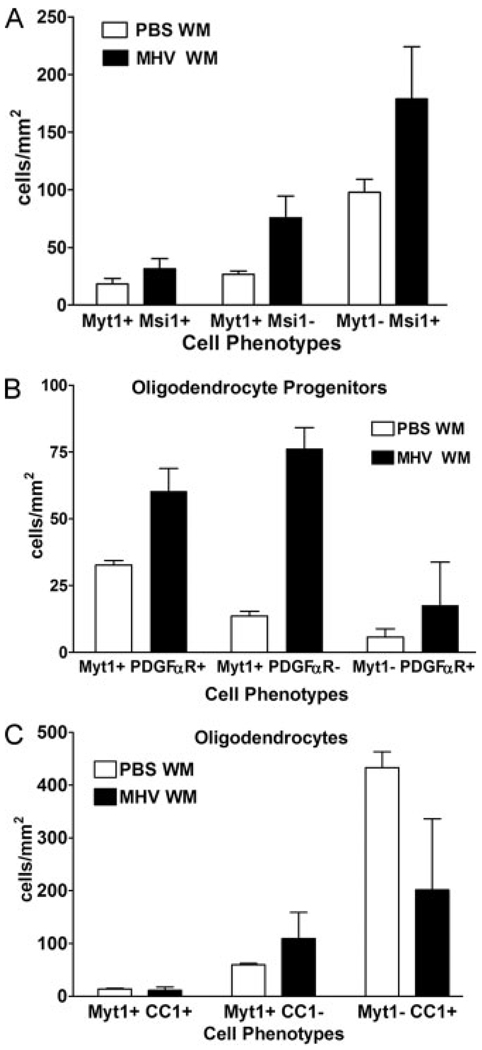
Fig. 3.
Quantitative analysis of Myt1 expression relative to cell type specific markers. At 4 wpi with MHV or PBS vehicle, mice were sacrificed and the white matter (WM) of transverse cervical sections was analyzed to determine the density of Myt1 immunolabeled cells in combination with cell type-specific markers. (A) Myt1 expressing immature neural stem cells were detected with Musashi1 (Myt1+ Msi1+). Astrocytes express Msi1 but not Myt1 (i.e. Myt1− Msi+) and so can be distinguished from neural stem cells (see text). (B) The majority of OP cells expressing PDGFαR are mostly also immunolabeled for Myt1 (i.e. Myt1+ PDGFαR+ relative to Myt1− PDGFαR+). (C) The density of mature oligodendrocytes, identified by CC1 immunolabeling, is very high in PBS WM but greatly reduced in MHV WM. Relatively few mature oligodendrocytes have detectable Myt1 expression (i.e. Myt1+ CC1+ relative to Myt1− CC1+). PBS, n = 3 mice; MHV, n = 3 mice.
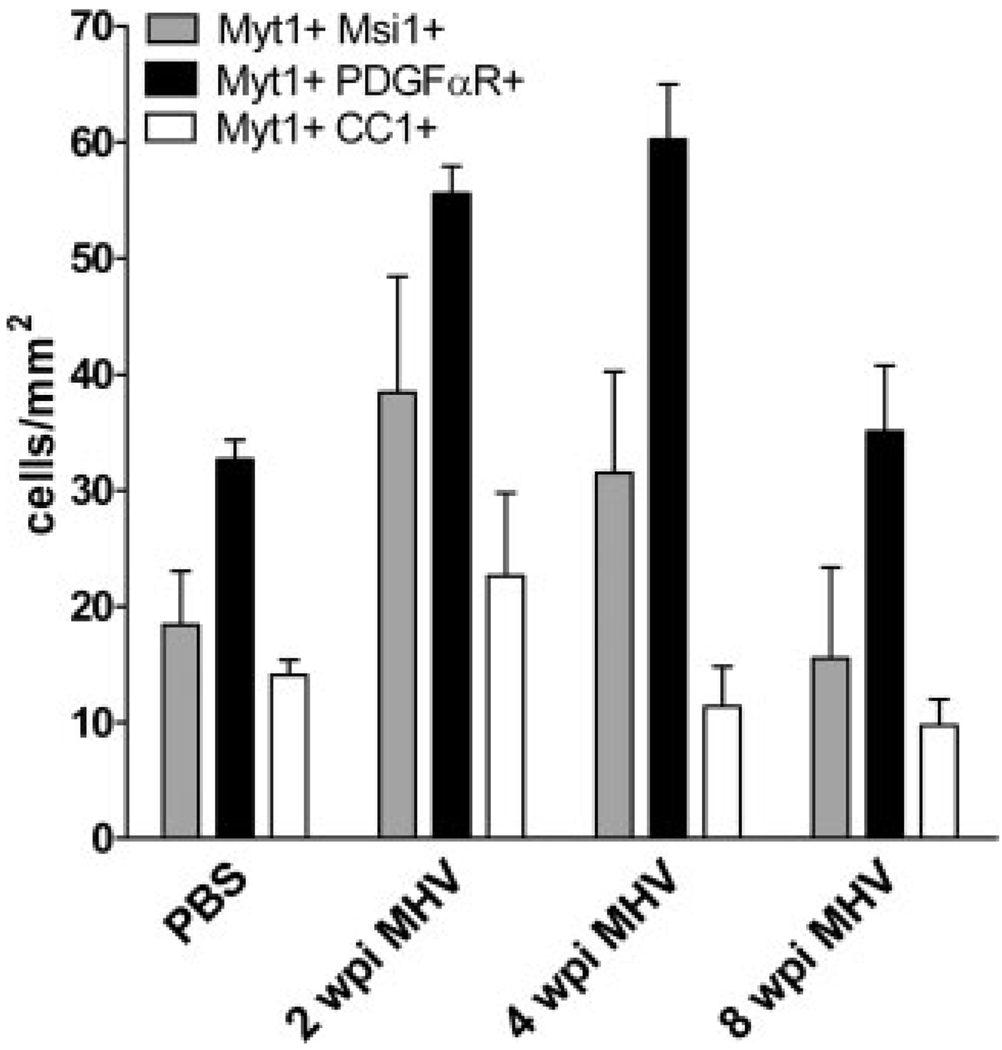
Fig. 4.
Comparison among cell types exhibiting nuclear Myt1 immunoreactivity in PBS control mice and MHV mice throughout disease progression. OP cells immunolabeled with PDGFαR were the principal cell type that expressed Myt1. In addition, the population of Myt1+ PDGFαR+ OP cells increased significantly at 2 wpi (P < 0.001) and 4 wpi (P < 0.001) before returning to PBS control levels at 8 wpi (p > 0.05). Musashi1 (Msi1) neural stem cell marker detected a smaller population of Myt1 expressing cells that was greatest at 2 wpi. Relatively few cells continued to exhibit nuclear Myt1 immunoreactivity after differentiating into mature oligodendrocytes, as indicated by CC1 immunolabeling. PBS, n = 3 mice; MHV, n = 3 mice.
Our results correlating Myt1 transcript expression with a proliferative population of cells in MHV lesions (Fig. 1) indicated that immature cell types may be correlated with Myt1 expression in MHV lesions. Indeed, in spinal cord white matter, the majority of OP cells identified by immunolabeling for PDGFαR; (Fig. 2D) exhibited nuclear Myt1 immunoreactivity (Fig. 3B; 85.2% in PBS WM; 77.4% in MHV WM). The density of cells double immunolabeled for Myt1 and PDGFαR was significantly increased in sections from MHV mice at 2 and 4 wpi, as compared to PBS-injected control mice (Fig. 4).
A substantial population of cells expressing Myt1 did not appear to be OP cells (i.e. Myt1+ PDGFαR−) yet could not be accounted for by the relatively few Myt1+ cells that had acquired CC1 immunolabeling as oligodendrocytes (i.e. Myt1+ CC1+) (Fig. 3). Therefore, expression of Myt1 at a more immature lineage stage was examined. Immunolabeling for Musashi1 (Msi1) was used as a marker of adult neural stem cells prior to the OP cell stage (Kaneko et al., 2000; Pincus et al., 1998; Sakakibara and Okano, 1997). A population of cells with nuclear immunoreactivity for Myt1 was immunolabeled for Msi1 (Fig. 2, panel C). The cell density of these Myt1+ Msi1+ cells was increased in lesioned white matter, particularly early in the disease progression (Fig. 4). Msi1 is known to be expressed in reactive astrocytes (Sakakibara and Okano, 1997), which was confirmed in the MHV lesioned tissues using double immunolabeling for glial fibrillary acidic protein (GFAP) to identify reactive astrocytes (data not shown). However, reactive astrocytes did not exhibit Myt1 immunoreactivity (data not shown) and so did not complicate the interpretation of the Myt1+ Msi1+ cells as neural stem cells.
The overall population of Myt1 expressing cells can be appreciated from the cell densities quantified for each cell type from white matter of MHV-infected mice throughout the disease course as compared to PBS control white matter (Fig. 4). Myt1 expression appears to begin during the neural stem cell stage (Myt1+ Msi1+) and continue throughout the OP cell stage (Myt1+ PDGFαR+), which comprises the majority of cells with nuclear Myt1 immunoreactivity. In contrast, Myt1 is expressed in only a small proportion of the mature oligodendrocyte population (Myt1+ CC1+), possibly reflecting expression during a transitional stage of OP differentiation into mature oligodendrocyte with subsequent down regulation. The combined densities of Myt1+ cells is lower than the density of oligodendrocytes in non-lesioned tissue (Fig. 3C) but could be sufficient for regenerating the observed oligodendrocyte population with proliferation in subsequent weeks.
MHV lesions also contain substantial populations of infiltrating and endogenous immune cells (Matthews et al., 2002; Redwine and Armstrong, 1998). Myt1 immunoreactivity was not detected in Mac1 immunolabeled macrophage/microglial cells, or within lymphocytes immunostained with either Ox-52 for T-cells or CD-45 receptor for B-cells (data not shown).
Myt1 Expression in MS Lesions and Control Cases
MS lesions of differing stages of demyelinating activity (active, inactive, and remyelinated) and control cases were analyzed by immunostaining and in situ hybridization to detect Myt1 expression (Fig. 5 and Table 1). In MS cases, Myt1 nuclear immunoreactivity was frequently observed in cells located in the PPWM adjacent to active or inactive lesions, or within early remyelinating lesions (Fig. 5A and Table 1). In control adult white matter, nuclear Myt1 immunostaining was much less frequently observed (Fig. 5B and Table 1), consistent with our previous report (Armstrong et al., 1997). In a minority of lesions, nuclear Myt1 immunoreactivity was detected among infiltrating lymphocytes (data not shown) or reactive astrocytes had cytoplasmic Myt1 immunoreactivity (Fig. 5C). No immunostaining was detected above background levels when the Myt1 primary antibody was omitted or was replaced with an antibody against Myt1L, a closely related Myt1 family member that is not expressed in oligodendrocytes (Kim et al., 1998 and data not shown). The increased frequency of Myt1 immunostaining in MS tissues was confirmed using in situ hybridization to detect Myt1 mRNA transcripts (Fig. 5D).
Fig. 5.
Myt1 expression in multiple sclerosis (MS) and control cases. Representative results are shown from adult human brain sections that were immunostained for Myt1 (A–C), processed for in situ hybridization for Myt1 mRNA (D), or analyzed by immunostaining for Myt1 combined with in situ hybridization for proteolipid protein (PLP) mRNA as a marker of oligodendrocytes (E,F). Nuclear immunostaining for Myt1 (brown DAB with pink eosin tissue counter stain) was seen in small round cells in the periplaque white matter (PPWM) adjacent to MS lesions (A, PPWM; case 9, Table 1), at an increased density relative to white matter control areas (B; case 2, Table 1). In some MS lesions (C, lesion area; case 6, Table 1), reactive astrocytes exhibited cytoplasmic immunoreactivity for Myt1 (brown DAB with pink eosin tissue counter stain), which was never seen in control cases. In situ hybridization for Myt1 mRNA (D, lesion edge; dark blue; case 4, Table 1) in MS lesions indicated expression of Myt1 in a pattern similar to Myt1 immunoreactivity (A). Within MS lesions, some cells with nuclear Myt1 expressed PLP mRNA transcripts (E, lesion; Myt1 brown, PLP dark blue; case 10, Table 1). Co-labeling for Myt1 among oligodendrocytes expressing PLP mRNA was generally not observed in adult human control white matter (F; case 3, Table 1). Scale bars = 50 µm for (A–F), as shown in (F). (A–F) Arrows indicate examples of cells expressing Myt1 protein (A–C,E) or mRNA transcripts (D). Arrowheads (B,E,F) indicate examples of cells that are not expressing Myt1.
TABLE 1.
Density of Cells with Myt1 Nuclear Immunoreactivity in White Matter of Human Controls and MS Lesions
| Case | Lesion stage | Lesion cells (mm2) | PPWM cells (mm2) | NWM cells (mm2) |
|---|---|---|---|---|
| 1 | Control | 15 | ||
| 2 | Control | 101 | ||
| 3 | Control | 86 | ||
| 4 | Demyelinated inactive | 101 | 321 | |
| 5 | Demyelinated inactive | 113 | 284 | |
| 6 | Demyelinated inactive | 57 | 309 | |
| 7 | Early active | 193 | 371 | |
| 8 | Early active | 113 | 365 | |
| 9 | Early active | 38 | 309 | |
| 10 | Early remyelination | 536 | n/a |
PPWM, periplaque white matter adjacent to lesions in MS tissues. NWM, normal white matter in non-neurological controls. n/a, samples are each from a biopsy of the lesion area so PPWM tissue is not available.
Myt1 expression may activate transcription of myelin-specific genes as OP cells differentiate into mature oligodendrocytes (Kim and Hudson, 1992; Nielsen et al., 2004). To identify cells with nuclear Myt1 localization relative to myelin-specific gene transcription, immunostaining for Myt1 was combined with in situ hybridization for PLP mRNA (Figs. 5E,F). In the combined protocol, specific signal was maintained although the number of cells identified was reduced, relative to the separate detection methods. Individual cells could be clearly identified as double labeled for Myt1 immunoreactivity and PLP mRNA transcripts in MS tissues (Fig. 5E and inset), which was not observed in the white matter of control cases (Fig. 5F). A similar protocol to double label OP cells for PDGFαR mRNA and Myt1 was not feasible because of the low abundance of PDGFαR transcripts per cell. Importantly, as in our mouse data of Myt1+ CC1+ cells (Fig. 2–Fig 4), Myt1 expression in a subset of oligodendrocytes identified by PLP mRNA may indicate ongoing OP differentiation into mature oligodendrocytes and support a potential role for Myt1 in oligodendrocyte regeneration in MS lesions.
DISCUSSION
In experimental and human demyelinating diseases, the CNS is capable of effective oligodendrocyte regeneration, remyelination, and recovery of function in viable axons. However, this capacity for repair becomes limited with repeated or chronic episodes of demyelination. To better understand how successful remyelination can be accomplished, the current study examines the cellular responses during spontaneous remyelination following transient experimental demyelination. Recent studies have identified an emerging array of transcription factors that are associated with OP proliferation, induction of differentiation toward an oligodendrocyte phenotype, and elevated transcription of myelin-specific genes during myelination (Gohkan et al., 2005; Ligon et al., 2006). Analysis of developmental myelination has predicted specific transcription factors that have been shown to be involved in oligodendrocyte lineage responses to demyelination (Arnett et al., 2004; Fancy et al., 2004; Watanabe et al., 2004).
Myt1 warranted analysis as a transcription factor regulating oligodendrocyte regeneration and remyelination based upon findings indicating a role in proliferation, differentiation, and activation of myelin-specific gene transcription during the generation of oligodendrocytes in the developing CNS (Armstrong et al., 1995; Nielsen et al., 2004). A role for Myt1 in human development has been predicted from the pattern of Myt1 expression in the developing human brain and from correlation of Myt1 immunolabeling with the attempted regenerative response of immature oligodendrocyte lineage cells in cases of periventricular leukomalacia (Hirayama et al., 2003). In the adult human CNS, Myt1 is expressed in germinal zones and is upregulated in gliomas, indicating potential function in immature proliferative cell types (Armstrong et al., 1997).
We report increased expression of Myt1 as a local white matter response to demyelination. We used the MHV model of experimental demyelination to assess the oligodendrocyte regenerative response in the context of a complex lesion environment that includes gliosis, inflammation, and breakdown of the blood-brain barrier to reflect the complex pathology of MS lesions (Lucchinetti et al., 2005; Morales et al., 2006). Myt1 expression corresponded with incorporation of BrdU, indicative of a proliferative phenotype. Consistent with this finding, the majority of cells with nuclei immunolabeled for Myt1 were double immunolabeled for PDGFαR, a marker of OP cells, with a substantial response also noted in cells immunolabeled for Msi1, a marker of neural stem cells. Furthermore, a role for Myt1 in the oligodendrocyte lineage response in human injury and disease is suggested by our observation of increased expression of Myt1 associated with MS lesions.
In the current study, a role for Myt1 in remyelination is supported by correlation with increased expression of Myt1 in proliferating cells and localization of Myt1 in nuclei of neural stem cells and OP cells. Interestingly, only nuclear immunoreactivity for Myt1 was observed in the adult mouse tissues, even though a transition to cytoplasmic localization was observed in rat developing oligodendrocytes (Armstrong et al., 1995). The present quantitative cellular analysis demonstrating Myt1 expression in OP cells extends an earlier report of increased abundance of mRNA transcripts for both Myt1 and PDGFαR during remyelination following ethidium bromide induced demyelination in rats (Sim et al., 2002). In addition, similar to our current quantification of Myt1 and BrdU incorporation, our previous MHV study demonstrated that PDGFαR+ cell proliferation in response to demyelination was localized within and near lesions (Redwine and Armstrong, 1998). However, Myt1 is expressed in additional populations of responding cells that were not identified by PDGFαR immunolabeling. We identified a subset of Myt1 expressing cells by co-immunolabeling for Msi1. Msi1 is an RNA-binding protein that has been used as a marker of neural stem cells in the developing and adult CNS of rodents and humans (Kaneko et al., 2000; Pincus et al., 1998; Sakakibara and Okano, 1997). Therefore, the initial expression of Myt1 in MHV lesions is expected to be in neural stem cells that may represent a relatively small but potentially important population of cells that is responsive to demyelination.
While the current analysis has not directly demonstrated a lineage progression for the expression of Myt1 during MHV disease progression, a possible scenario from current data and the available literature is that Myt1 is expressed in Msi1+ neural stem cells that may differentiate into PDGFαR+ OP cells then amplify in response to demyelination to generate CC1+ oligodendrocytes to accomplish remyelination. Myt1 expression prior to the OP stage has been shown with in vitro analysis of cultured neonatal cells (Armstrong et al., 1995). In vitro, Myt1 continued to be expressed at the OP stage, followed by downregulation in mature oligodendrocytes after accumulation of myelin proteins (Armstrong et al., 1995). In vivo, the proliferative response during demyelination has been clearly associated with the OP stage in MHV lesions, other models of experimental demyelination, and MS lesions (Redwine and Armstrong, 1998; Reynolds et al., 2001; Solanky et al., 2001; Watanabe et al., 2002). In this MHV model, cells that incorporated thymidine analogues, as indicators of proliferation, during demyelination have been shown to express PDGFαR and then maintain labeling during the transition into oligodendrocytes in the remyelination phase (Godfraind et al., 1989; Redwine and Armstrong, 1998). In the current MHV study, expression of Myt1 was observed mainly in PDGFαR+ OP cells and in only a small proportion of CC1+ oligodendrocytes, which may be cells that recently differentiated and had not yet downregulated Myt1.
The current finding that the increased density of Myt1 cells is specifically localized to areas of white matter lesions is consistent with the local generation of oligodendrocytes reported in several studies of remyelination. In focally demyelinated spinal cord, newly generated remyelinating cells appeared to be generated from a local ring of normal tissue surrounding the lesion (Franklin et al., 1997). Similarly, retroviral labeling of remyelinating cells indicated recruitment from less than 500 µm from the lesion site in adult rat corpus callosum (Gensert and Goldman, 1997). A preliminary study of mice administered cuprizone indicated that Myt1 expression is upregulated during acute demyelination in the corpus callosum in areas of increased OP proliferation (data not shown). This comparison indicates that increased Myt1 expression may occur during OP amplification in response to diverse demyelinating pathologies.
Analysis of MS lesions demonstrated the highest density of Myt1 expressing cells in early remyelinating lesions, consistent with our findings in MHV and cuprizone. Furthermore, Myt1 expression was increased in PPWM relative to active or inactive lesion areas. The variability of Myt1 expression in MS lesions may reflect the reported differences of immature OP populations (Chang et al., 2002; Maeda et al., 2001). In contrast to MHV lesions, some MS lesions exhibited Myt1 immunoreactivity in lymphocytes and astrocytes. In the unusual cases in which astrocytes expressed Myt1, the immunoreactivity was localized in the cytoplasm. Similarly, Myt1 immunoreactivity in astrocyte cytoplasm was observed in highly reactive astrocytes of epileptic foci in human temporal lobe tissue (Armstrong et al., 1997). It is not clear whether this astrocytic expression of Myt1 is the result of a species difference or possibly due to differences in the pathology of some MS lesions.
Further work is required to understand the regulation of Myt1 in development and pathology, and the function of Myt1 in each context. Myt1 acted as a transcriptional activator to increase luciferase levels from the promoter of a myelin-specific gene, 2′,3′-cyclic nucleotide 3′-phosphodiesterase, in transient transfection assays (Nielsen et al., 2004). However, Myt1 may act in concert with other transcription factors or co-factors that contribute to effects on transcriptional controls (Koyano-Nakagawa et al., 1999; Schneider et al., 2001). In addition, Myt1 may form complexes with Sin3B, a protein that mediates repression by recruiting histone deacetylases (Romm et al., 2005). Therefore, the function of Myt1 may depend upon the levels of other nuclear proteins that may be differentially regulated in cells relative to stage of differentiation or environmental context. The current study documenting Myt1 expression corresponding to regenerative responses of the oligodendrocyte line-age in animal models and MS lesions suggests that Myt1 may have functional importance in remyelination.
ACKNOWLEDGMENTS
Dr. Lynn D. Hudson, National Institutes of Health, generously provided the Myt1 antibody and plasmids for Myt1 and PLP.
Grant sponsor: USUHS; Grant numbers: RO70PC; Grant sponsor: National Institutes of Health; Grant numbers: NS39293National Multiple Sclerosis Society; Grant numbers: RG3515 and RG 3185-A2, B3.
REFERENCES
- Armstrong RC. Potential roles of trophic factors in CNS development and recovery from demyelinating disease. In: Mocchetti I, editor. Neurotrophic factors. Mountain Home, TN: Graham Publishing; 2000. pp. 417–437. [Google Scholar]
- Armstrong RC, Kim JG, Hudson LD. Expression of myelin transcription factor I (MyTI), a “zinc-finger” DNA-binding protein, in developing oligodendrocytes. Glia. 1995;14:303–321. doi: 10.1002/glia.440140407. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J Neurosci. 2002;22:8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Migneault A, Shegog ML, Kim JG, Hudson LD, Hessler RB. High-grade human brain tumors exhibit increased expression of myelin transcription factor 1 (MYT1), a zinc finger DNA-binding protein. J Neuropathol Exp Neurol. 1997;56:772–781. [PubMed] [Google Scholar]
- Armstrong RC, Redwine JM, Messersmith DJ. Coronavirus-induced demyelination and spontaneous remyelination: Growth factor expression and function. In: Lavi E, Constantinescu CS, editors. Experimental models of multiple sclerosis. Norwell, MA: Springer Science; 2005. p. 2005. [Google Scholar]
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Bruck W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. doi: 10.1016/s0022-510x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27:247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Part 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gilson JM, Blakemore WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res. 1997;50:337–344. doi: 10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Frost EE, Nielsen JA, Le TQ, Armstrong RC. PDGF and FGF2 regulate oligodendrocyte progenitor responses to demyelination. J Neurobiol. 2003;54:457–472. doi: 10.1002/neu.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Godfraind C, Friedrich VL, Holmes KV, Dubois-Dalcq In vivo analysis of glial cell phenotypes during a viral demyelinating disease in mice. J Cell Biol. 1989;109:2405–2416. doi: 10.1083/jcb.109.5.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhan S, Marin-Husstege M, Yung SY, Fontanez D, Casaccia-Bonnefil P, Mehler MF. Combinatorial profiles of oligodendrocyte-selective classes of transcriptional regulators differentially modulate myelin basic protein gene expression. J Neurosci. 2005;25:8311–8321. doi: 10.1523/JNEUROSCI.1850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama A, Oka A, Ito M, Tanaka F, Okoshi Y, Takashima S. Myelin transcription factor 1 (MyT1) immunoreactivity in infants with periventricular leukomalacia. Brain Res Dev Brain Res. 2003;140:85–92. doi: 10.1016/s0165-3806(02)00585-0. [DOI] [PubMed] [Google Scholar]
- Hudson LD, Berndt JA, Puckett C, Kozak CA, Lazzarini RA. Aberrant splicing of proteolipid protein mRNA in the dysmyelinating jimpy mutant mouse. Proc Natl Acad Sci USA. 1987;84:1454–1458. doi: 10.1073/pnas.84.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Sakakibara S, Imai T, Suzuki A, Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T, Okano H. Musashi1: An evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- Kim JG, Armstrong RC, Berndt JA, Kim NW, Hudson LD. A secreted DNA-binding protein that is translated through an internal ribosome entry site (IRES) and distributed in a discrete pattern in the central nervous system. Mol Cell Neurosci. 1998;12:119–140. doi: 10.1006/mcne.1998.0701. [DOI] [PubMed] [Google Scholar]
- Kim JG, Armstrong RC, v Agoston D, Robinsky A, Wiese C, Nagle J, Hudson LD. Myelin transcription factor 1 (Myt1) of the oligodendrocyte lineage, along with a closely related CCHC zinc finger, is expressed in developing neurons in the mammalian central nervous system. J Neurosci Res. 1997;50:272–290. doi: 10.1002/(SICI)1097-4547(19971015)50:2<272::AID-JNR16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kim JG, Hudson LD. Novel member of the zinc finger superfamily: A C2-HC finger that recognizes a glia-specific gene. Mol Cell Biol. 1992;12:5632–5639. doi: 10.1128/mcb.12.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano-Nakagawa N, Wettstein D, Kintner C. Activation of Xenopus genes required for lateral inhibition and neuronal differentiation during primary neurogenesis. Mol Cell Neurosci. 1999;14:327–339. doi: 10.1006/mcne.1999.0783. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci USA. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti CF, Parisi J, Bruck W. The pathology of multiple sclerosis. Neurol Clin. 2005;23:77–105. doi: 10.1016/j.ncl.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Solanky M, Menonna J, Chapin J, Li W, Dowling P. Platelet-derived growth factor-a receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Ann Neurol. 2001;49:776–785. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- Matthews AE, Weiss SR, Paterson Y. Murine hepatitis virus-a model for virus-induced CNS demyelination. J Neurovirol. 2002;8:76–85. doi: 10.1080/13550280290049534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales Y, Parisi JE, Lucchinetti CF. The pathology of multiple sclerosis: Evidence for heterogeneity. Adv Neurol. 2006;98:27–45. [PubMed] [Google Scholar]
- Nielsen JA, Berndt JA, Hudson LD, Armstrong RC. Myelin transcription factor 1 (Myt1) modulates the proliferation and differentiation of oligodendrocyte lineage cells. Mol Cell Neurosci. 2004;25:111–123. doi: 10.1016/j.mcn.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, Sakakibara S, Okano H, Nedergaard M, Goldman SA. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFαR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Cenci di Bello I, Dawson M, Levine J. The response of adult oligodendrocyte progenitors to demyelination in EAE. Prog Brain Res. 2001;132:165–174. doi: 10.1016/s0079-6123(01)32073-3. [DOI] [PubMed] [Google Scholar]
- Romm E, Nielsen JA, Kim JG, Hudson LD. Myt1 family recruits histone deacetylase to regulate neural transcription. J Neurochem. 2005;93:1444–1453. doi: 10.1111/j.1471-4159.2005.03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: Implications of their roles in neuronal and glial cell development. J Neurosci. 1997;17:8300–8312. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Turner DL, Vetter ML. Notch signaling can inhibit Xath 5 function in the neural plate and developing retina. Mol Cell Neurosci. 2001;18:458–472. doi: 10.1006/mcne.2001.1040. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanky M, Maeda Y, Ming X, Husar W, Li W, Cook S, Dowling P. Proliferating oligodendrocytes are present in both active and chronic inactive multiple sclerosis plaques. J Neurosci Res. 2001;65:308–317. doi: 10.1002/jnr.1155. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hadzic T, Nishiyama A. Transient upregulation of Nkx2.2 expression in oligodendrocyte lineage cells during remyelination. Glia. 2004;46:311–322. doi: 10.1002/glia.20006. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Wong K, Armstrong RC, Gyure KA, Morrison AL, Rodriguez D, Matalon R, Johnson AB, Wollmann R, Gilbert E, Le TQ, Bradley CA, Crutchfield K, Schiffmann R. Foamy cells with oligodendroglial phenotype in childhood ataxia with diffuse central nervous system hypomyelination syndrome. Acta Neuropathol (Berl) 2000;100:635–646. doi: 10.1007/s004010000234. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Li W, Hudson LD. Myelin gene expression after experimental contusive spinal cord injury. J Neurosci. 1998;18:8780–8793. doi: 10.1523/JNEUROSCI.18-21-08780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]