Abstract
It has been known for decades that cadmium (Cd) must enter the cell to cause damage, but there was no mechanism to explain genetic differences in response to Cd toxicity until 2005. Starting with the mouse Cdm locus associated with differences in Cd-induced testicular necrosis between inbred strains, a 24.6-centiMorgan region on chromosome 3 was reduced ultimately to 880 kb; in this segment is the Slc39a8 gene encoding the ZIP8 symporter. In endothelial cells of the testis vasculature, Cd-sensitive mice exhibit high ZIP8 expression, Cd-resistant mice exhibit very low expression. A 168.7-kb bacterial artificial chromosome (BAC) from a 129S6 (Cd-sensitive) BAC library containing the Slc39a8 gene was inserted into the Cd-resistant C57BL/6J genome: Cd treatment produced testicular necrosis in BAC-transgenic BTZIP8-3 mice but not in non-transgenic littermates, thereby proving that the Slc39a8 gene is indeed the Cdm locus. Cd-induced renal failure also occurred in these BTZIP8-3 mice. Immunohistochemistry showed highly expressed ZIP8 protein in the renal proximal tubular epithelial apical surface, suggesting that ZIP8 participates in Cd-induced renal failure. Slc39a14, most closely evolutionarily related to Slc39a8, encodes differentially-spliced products ZIP14A and ZIP14B that display properties similar to ZIP8. ZIP8 in alveolar cells brings environmental Cd into the organism and ZIP14 in intestinal enterocytes carries Cd into the organism and into the hepatocyte. We believe these two transporters function endogenously as symporters important in combating inflammation and carrying out other physiological functions; Cd is able to displace the endogenous cation, enter the cell, and produce tissue damage and disease.
Keywords: Cadmium, Zinc, Manganese, Copper, Lead, Mercury, Platinum, Uranium, Testicular necrosis, Testicular vasculature endothelial cell, Renal failure, Renal Fanconi syndrome, Renal proximal tubular acidosis, Osteomalacia, Solute carrier (Slc) gene superfamily, ZIP transporter gene family, Slc39 gene family, ZIP8, ZIP14, symporter
Introduction
Relevance to environmental medicine
Cadmium (Cd) is a group IIB metal having an atomic weight of 112.41; it exists in the 0 or 2+ valency states. Cd is found naturally in the earth’s crust and is usually present as inorganic salts. Heavy usage of Cd began with its large-scale applications, dating from the 1940s (Stoeppler, 1991). Other human activities leading to increases in Cd levels in the environment include mining and the burning of fossil fuels (ATSDR, 1999). Today Cd is used principally in combination with nickel: battery manufacturing, pigments, and plastic stabilizers, whereas applications in alloys, solders and electroplating show a decreasing trend (Waisberg et al., 2003).
Major occupational exposures to Cd occur in non-ferrous metal smelters, in Cd production and processing, in Cd alloys and its compounds and, increasingly, in the recycling of electronic waste (Waisberg et al., 2003). In human populations, cigarette smoke is by far the largest source of Cd exposure (Zalups and Ahmad, 2003); curiously, Cd is bioconcentrated in the tobacco plant. Each cigarette generally contains from 1 to 2 μg of Cd, and 40–60% of the Cd in inhaled smoke generally can be taken into the systemic circulation (Lewis et al., 1972; Elinder et al., 1983; ATSDR, 1999). For nonsmokers in the general population, Cd-contaminated food is a major source of Cd exposure: fish, shellfish, organ meat (especially liver and kidney), and grains and cereal products (WHO report, 1992; ATSDR, 1999). The half-life of Cd in humans is estimated to be between 15 and 20 years (Jin et al., 2004).
In humans and other mammals, Cd can damage various organs and tissues—including kidney, liver, lung, pancreas, testis, placenta, and bone—with kidney and liver being the two primary target organs (Zalups and Ahmad, 2003). The importance of Cd as an environmental contaminant was dramatically illustrated by the outbreak at the Jinzu River in Japan. A severe disease (called “itai–itai”, or “ouch–ouch”) occurred that was characterized by severe pain, bone fractures, proteinuria and severe osteomalacia; the disease was caused by eating rice and drinking rice water that had been contaminated with Cd originating from a mine slag. In the most recent listing (http://www.atsdr.cdc.gov/cxcx3.html), Cd is ranked eighth in the “Top 20 Hazardous Substances Priority List” by the Agency for Toxic Substances and Disease Registry and the U.S. Environmental Protection Agency.
Occupational exposure to Cd is associated with cancer of the lung. Associations of Cd-induced cancer of the prostate, pancreas, and kidney have also been suggested, but the conclusion remains controversial. Cd is classified as a category I human carcinogen (WHO report, 1992; IARC, 1993; National Toxicology Program, 2000). In rodents Cd causes tumors in various organs—including the lung after inhalation, prostate and pancreas following subcutaneous injection, testis after oral exposure, and local tumors at various sites of injection (Waisberg, Joseph, Hale, and Beyersmann, 2003). Cd is not a genotoxic carcinogen: it is basically non-mutagenic in bacterial tests and weakly mutagenic in mammalian cell cultures (IARC, 1993); however, Cd is co-mutagenic in mammalian cell tests when combined with genotoxic agents. Possible explanations for this phenomenon include an inhibitory effect of Cd on DNA repair (Hartwig and Schwerdtle, 2002) and on mismatch repair by blocking ATPase activity of the MSH2–MSH6 complex (Banerjee and Flores-Rozas, 2005).
Transport of Cd into the cell
Cd causes damage to a target cell only after influx (Cd uptake) by that cell. Following intestinal (or lung) absorption by the intact animal, Cd binds to various polypeptides such as metallothionein (MT), reduced glutathione (GSH) and other thiol-proteins. These Cd–MT, GS–Cd–SG and Cys–S–Cd–S–Cys complexes in the blood, along with Cd-binding to the surface of red cells, were originally believed to be the principal means by which Cd is transported from the portals of entry; ultimately, Cd is deposited primarily in liver and especially the kidney. Curiously, in mice lacking two metallothionein genes (Mt1 and Mt2), Cd uptake into either liver or kidney is not different from wild-type mice (Liu et al., 2001), supporting the possibility that other forms of Cd transport might be more important than the MT-complexed form.
Numerous studies on Cd influx into cells have been carried out; however, no direct evidence for the molecular mechanism in the intact animal had been reported until 4 years ago (Dalton et al., 2005). In cultured pituitary cells, the major route of Cd influx was shown to be via voltage-gated calcium (Ca) channels (Hinkle et al., 1987). In hepatocyte cultures, about a third of the internalized Cd enters the cell through this type of Ca channel (Blazka and Shaikh, 1991). The possible role of Ca channels in cellular uptake of Cd has also been demonstrated in rat melanotrophs (Shibuya and Douglas, 1992), hepatic WRL-68 cells (Souza et al., 1997), pheochromocytoma PC12 cells (Hinkle and Osborne, 1994), MDCK cells (Olivi and Bressler, 2000), and mouse Mt1/Mt2(−/−) double-knockout fetal fibroblast cultures (Leslie et al., 2006). SLC11A2 (also called NRAMP2, DMT1), a proton-coupled divalent metal transporter with preference for iron (Fe), has been implicated in Cd uptake and toxicity in mammals; SLC11A2 has a preference for iron, but also transports lead (Pb) and Cd (Bressler et al., 2004). By performing SLC11A2 knockdown studies in human intestinal Caco-2 cells (Bannon et al., 2003), proton-dependent transport of Cd was shown. In vivo studies suggested that SLC11A2 participates in Cd transport in enterocytes (Tallkvist et al., 2001; Elisma and Jumarie, 2001; Park et al., 2002) and renal distal tubular cells (Friedman and Gesek, 1994; Olivi et al., 2001). Consistent with these studies, Cd transport in Xenopus oocytes that express human SLC11A2 produced Michaelis–Menten kinetics with a Km of 1.04 μM (Okubo et al., 2003). However, almost all of these studies were carried out in culture; what happens to Cd in the intact animal cannot be conclusively determined by cell culture and Xenopus oocyte studies.
“Forward” versus “reverse” genetics
The study of reverse genetics is defined as follows: one starts with a gene of interest, determines its (tissue-specific, cell-type-, developmental-specific) expression pattern, and then proves (by mutation or knockout) that the particular gene is responsible for a particular phenotype or disease in the organism. This approach has become increasingly popular since the early 1990s, with the availability of data from the Human Genome Project, as well as from the genomes of many other organisms that have been sequenced.
The study of forward genetics is a much older field: one starts with a phenotype (e.g. enzyme activity), isolates the gene product (protein or mRNA) associated with that trait, and then—by mutating, or by classical genetic quantitative trait loci (QTL) mapping, positional cloning, and complementation studies—finally identifies the gene; subsequently, the gene is proven to be responsible for the trait (or disease) in the organism by generating reporter constructs, mutations, and knockout or knock-in mouse lines. This is a long arduous process with only an occasional success: for example, in a 2005 review of 2050 QTLs mapped in the mouse (Flint et al., 2005), only 15 (0.73%) had been “cloned and characterized to the point of proving plausible gene variation and causation (of the trait or disease).”
The remainder of this review will describe how the mouse phenotype of “Cd-induced testicular necrosis” was traced to specific solute-carrier (Slc39) genes, which encode (ZIP) transporters that play important endogenous roles in Zn and Mn uptake in numerous cell types.
Phenotype: Cd-induced testicular necrosis
Since 1919, it has been known that rodents, given a single injection of Cd at a low dose that has no measurable effect on any other organ system, will exhibit profound testicular damage within 24 to 48 h (Alsberg and Schwartze, 1919/20; Parizek and Zahor, 1956; Parizek, 1957). In fact, Cd-induced toxicity to the seminiferous tubule endothelium is common across all species having testes—including mouse, frog, pigeon, rooster, fish, armadillo and opossum (Chiquoine, 1964). In contrast to exquisite sensitivity to Cd in the testis of every vertebrate examined, in 1969 some inbred strains of mice were found to be resistant (Lucis and Lucis, 1969). Whereas sensitivity to Cd is the wild-type trait inherited as autosomal dominant, resistance to Cd-induced damage in the testis was found to be inherited as an autosomal recessive trait (Taylor et al.,1973). The genetic locus was named Cdm, and a three-point cross—involving the previously mapped amylase-1 (Amy1) and “varitint-waddler” (Va) loci with the Cdm locus—placed Cdm between Amy1 and Va [at distances of Amy1—(17.0± 5.2 centiMorgan, cM)—Cdm—(7.6±3.6 cM)—Va] on mouse chromosome (Chr) 12 (Taylor, 1976). Because the Amy1 and Va loci later were realized to be located on Chr 3 (Bonhomme et al., 1979), the location of the Cdm locus was changed to a ~24.6-cM region of Chr 3 before the end of the 1970s. The Va locus has now become the mucolipin-3 gene (Mcoln3) (Zhu et al., 2003).
Results and discussion
Positional cloning
It was known (Taylor et al., 1973) that the DBA/2J (D2) and 129/SvJ inbred strains are sensitive to Cd, whereas the C57BL/6J (B6) and A/J strains are resistant. Studying the 26 B6×D2-Ty/J recombinant inbred lines, plus several dozen newly identified (at that time) microsatellite markers on mouse chromosome 3, the Nebert laboratory was able to decrease the chromosomal distance from >24 cM to ~0.64 cM (Dalton et al., 2000), which turned out to be 4.97 Mb carrying ~85 genes. Ultimately, by generating 1164 progeny from the (B6D2)F1 ×B6 backcross, and using new additional SNP markers obtained from the Celera mouse genome project, the Cdm-containing segment was refined to 880 kb (Dalton et al., 2005) one of the three candidate genes in this region showed high homology to a “pLIV-related” gene in Arabidopsis thaliana. Bioinformatics analyses showed that this gene is mouse Slc39a8, orthologous to the human SLC39A8, which encodes the 8-transmembrane zinc-related iron-related protein (ZIP8) transporter (Dalton et al., 2005). The D2 and B6 Slc39a8 exons and splice junctions were sequenced and found to contain no nucleotide differences, indicating the inbred strain variation is most likely at the transcriptional level. Whatever mutation causes the difference in response to Cd-induced damage in the testis must therefore be located elsewhere.
Properties of the ZIP8 transporter protein in cell culture and in the intact mouse
The translated region of B6 ZIP8 cDNA was cloned into the pRevTRE vector, which then was used to retrovirally infect mouse fetal fibroblast cultures (rvZIP8 cells); the cDNA-expressed ZIP8 protein was found to enhance Cd uptake by 10-fold and to increase sensitivity to cell killing by 30-fold, as compared with that by a control vector or cDNA expressing a mutant ZIP8 protein (Dalton et al., 2005).
Thus, the Nebert lab was convinced that the Slc39a8 gene was the same as the Cdm locus and is responsible for the phenotype (Cd-induced testicular damage), but how could this be proven? ZIP8 mRNA levels were found to be highest in lung, testis and kidney; by Northern blot hybridization or qRT-PCR analysis, however, no differences in mRNA levels were seen among two sensitive inbred strains (D2, 129/SvJ) and two resistant strains (B6, A/J). We next reasoned that—if the defect is cell-type-specific and resides in only a small group of cells—this difference would not be detectable by Northern blot hybridization or qRT-PCR analysis. Using in situ hybridization, therefore, we found that the two Cd-sensitive mouse inbred strains (D2, 129/SvJ) exhibited high ZIP8 expression in vascular endothelial cells of the testis, whereas the two Cd-resistant strains (B6, A/J) showed very low ZIP8 mRNA levels in these cells (Dalton et al., 2005). Endothelial cell injury would result in vascular leakage, which would include red blood cell extravasation and platelet plugging, ultimately causing testicular ischemia, followed by necrosis (Dalton et al., 2000). The in situ data therefore led to the hypothesis that differential endothelial cell expression of the ZIP8 transporter in blood vessels of the testis is the consequence of a DNA variant site(s) within an intron or in a 5′- or 3′-flanking region cis to the Slc39a8 gene (Dalton et al., 2005).
There have been several reports confirming that Cd-sensitive inbred mouse strains accumulate more 109Cd in their testes than Cd-resistant strains; five Cd-sensitive (CBA/J, DBA/1J, DBA/2J, 129/J and CD-1) and five Cd-resistant (C57BL/6J, BALB/cJ, C3H, A/J and C3H/HeJ) were included in these experiments (Lucis and Lucis, 1969; Hata et al., 1980; Chellman et al., 1984; Shaikh et al., 1993). These studies used sub-toxic levels of subcutaneous Cd, i.e., no testicular damage was induced.
Unique properties attributed to the testis vasculature endothelial cells
The blood-brain barrier (BBB) and the blood-testis barrier (BTB) comprise two vascular cell types that are (evolutionarily and developmentally) unique to these two locations in vertebrate animals. Hence, testicular endothelial cells differ from “regular” endothelial cells located throughout the rest of the animal. Certain tissues, such as brain and testis, require a highly “protected” environment for their physiological functions. Thus, a biological barrier is required to prevent the free exchange of solutes between blood and tissue. For the BBB, the barrier function is primarily achieved by the endothelium. For the BTB, the first barrier is the endothelium, impeding substances in the blood from moving easily into the interstitial space; most importantly, the second barrier—Sertoli cells and their inter-cellular tight junctions—further prevent the substances in the interstitial fluid from easily entering the seminiferous tubules (Ploen and Setchell, 1992). In the BTB, the endothelium is continuous, not fenestrated, and has long junctional profiles; yet, compared with the BBB, the testicular endothelial cells still show greater permeability (Holash et al., 1993). Barrier function, however, is just one part of the story. The endothelial cells in the BBB (which contain, e.g. high numbers of glucose transporters) versus the BTB undoubtedly contain quite different profiles of transporters, compared with those from “regular” endothelial cells; it is not yet well known, but the BBB and BTB undoubtedly carry transporters that differ from one other. Another possible need for high Zn levels in the testis might be associated with the production of fertile spermatocytes. These, therefore, are possible reasons why ZIP8 displays such a highly tissue-and cell-type-specific expression pattern.
Only the presence of an efficient Cd transporter on the apical surface of BTB (but not BBB) endothelial cells would result in relevant amounts of Cd influx into the testis but not the brain. Once inside, intracellular Cd2+ causes striking oxidative stress—via mechanisms not entirely understood—thus forming disulfide bonds on many proteins that contain sulfhydryl groups. Consequently (unlike what happens in the brain vasculature), large concentrations of Cd in these vasculature endothelial cells lead quickly to vascular collapse of the testis, as well as extravasation of Cd into other portions of the organ. The bottom line is that there is no means of proving, in a test tube or in cell culture, that the Cdm locus is the Slc39a8 gene. Proof must be obtained from experiments in the intact animal.
Proof that the Cdm locus is indeed the Slc39a8 gene
The Cd-sensitivity trait is dominant over the Cd-resistance trait (Taylor et al., 1973; Dalton et al., 2000). Therefore, the Slc39a8 gene was isolated on a bacterial artificial chromosome (BAC) clone derived from a BAC library constructed from the genome of the Cd-sensitive 129S6/SvEvTac (abbreviated 129S6) strain. The BAC (measuring ~168.7 kb) was inserted into the resistant B6 mouse genome, and lines having three, five and six copies of the 129S6 Slc39a8 gene (in addition to the two copies of the B6 Slc39a8 gene) were generated (Wang et al., 2007). Mice having five or six additional copies were able to become pregnant but unable to give birth to viable offspring. It was thus concluded that having seven or more Slc39a8 gene copies causes infertility and/or very early embryolethality. We have found that ZIP8 is highly expressed in placenta (B.W. and D.W.N., manuscript in preparation), affects the development of hematopoiesis, and also contributes to zinc (Zn) uptake. It is therefore likely, in untreated BTZIP8-5 and BTZIP8-6 mice, that Zn perturbation by ZIP8 massive overexpression in the placenta or yolk sac can lead to infertility and/or very early embryolethality. During erythropoietin-induced differentiation of splenic erythroid progenitor cells into reticulocytes, ZIP8 mRNA expression was shown to be elevated within 2 to 6 h in these cell membranes (Ryu et al., 2008); ZIP8 massive overexpression thus could perturb normal hematopoiesis in the developing embryo. Intriguingly, ZIP8 overexpression has also been associated with detrimental effects on the normal differentiation of stem cells (Zhu et al., 2007).
Our BTZIP8-3 line (having three copies of the 129S6 Slc39a8 gene plus two copies of the B6 Slc39a8 gene) was healthy and produced viable offspring. Low doses of Cd produced testicular necrosis in the BTZIP8-3 mouse but the non-transgenic littermates remained resistant; moreover, twice as much Cd accumulated in the kidneys of BTZIP8-3 mice, compared with that of the non-transgenic littermates (Wang et al., 2007). The overexpression of ZIP8 in the testis vascular endothelial cells of the BTZIP8-3 mouse, compared with the nontransgenic littermate, was proven by in situ hybridization and immunohistochemistry. The phenotype of “Cd-resistance” was reverted to “Cd-sensitivity”, thereby demonstrating unequivocally that the Slc39a8 gene represents indeed the Cdm locus (Wang et al., 2007.
Where is the mutation located?
Our original data (Wang et al., 2007 indicate that one DNA variant, most likely one single-nucleotide polymorphism (SNP) in the 168,722 bp of this BAC must be responsible for this difference in phenotype. Fig. 1 (top) illustrates the recent locations of the various haplotype blocks across the genome wherein this Slc39a8-containing BAC is derived, identified in 15 inbred mouse strains (Frazer et al., 2007). The colored blocks (bottom) denote the specific haplotype structure found in the Cd-resistant B6 and A/J and Cd-sensitive D2 and 129S6 across the region spanning the BAC. Because “Cd-sensitivity” is wild-type and “Cd-resistance” is the mutant, it is very likely that the “resistance” mutation happened only one time during evolution of the inbred mouse strains. Consistent with this idea, all resistant mouse strains should have the same haplotype in the region carrying the “Cd-resistance” SNP. Fig. 1 shows that this is absolutely true for B6 and A/J —as indicated by their having the same color across all three blocks (A–B, B–C, and C–D) present in the BAC.
Fig. 1.
Haplotype blocks (denoted by the “h” numbers) identified across the region of the Chr 3 Slc39a8 gene among 15 inbred mouse strains (Frazer et al., 2007). The large bold arrow signifies the genomic location (and 5′ to 3′ orientation) of our Slc39a8-containing BAC; the BAC begins at 135,396 k (just 5 kb 3′-ward of the start of haplotype block h12757). The orientations of the Nfkb1 gene (left) and small portion of the Bank1 gene (right) indicate that these two genes are located on the negative strand. ENSMUSG00000045520, LOC100039350, and LOC383923 represent putative protein-coding genes not yet identified. The bottom colored rectangles represent the specific haplotype blocks of the Cd-resistant B6 and A/J strains and Cd-sensitive D2 and 129S6 strains—across the BAC-spanning region. Segments B–E show that the same haplotype exists in all four strains. Segment A–B shows that B6 and A/J carry the same haplotype block (yellow), while a second haplotype block occurs in the D2 (green) and a third haplotype block occurs in the 129S6 strain (blue). Subtle haplotype–block differences might exist (Threadgill et al., 1997) between the 129S6/SvEvTac sub-line from which the BAC was isolated (Wang et al., 2007) and the 129S1/SvImJ sub-line resequenced by Perlegen (Frazer et al., 2007).
For the “Cd-sensitivity” alleles, however, it cannot be said that they would all carry the same haplotype structure, because they are wild-type. Consequently, there could be many polymorphic wild-type alleles in the “resistance” SNP-containing region. Indeed, D2 and 129S6 are not identical to each other nor to the B6 and A/J haplotype in segment A–B (Fig. 1): for the 3′ one-eighth of this BAC (segments B–C, C–D), given that D2 and 129S6 do share the same haplotype structure as B6 and A/J, it is reasonable to conclude that the “resistance” SNP cannot be located in this region. Throughout the entire 168.7-kb BAC (Supplementary Fig. 1) there are 112 SNPs that show an identical base for the two mutant “Cd-resistance” strains, versus a different base for the one or both of the wild-type “Cd-sensitivity” inbred strains. Given the haplotype analysis from the latest inbred mouse data (Frazer et al., 2007 and Fig. 1), the 3′ end of haplotype block h12757 is located in intron 6 of the Slc39a8 gene. Thus, we are confident that the number of possible SNPs can only be reduced from 112 to 109 SNPs. Although we would like to precisely identify the SNP that causes the variation in phenotype, we have been denied funding to pursue this endeavor.
Characterization of the ZIP8 transporter protein
Cd influx by ZIP8 in rvZIP8 cells showed temperature-dependence, was maximal at pH 7.2, and could be completely inhibited by cyanide, implicating a requirement for ATP (He et al., 2006). In Hanks balanced salt solution, the ZIP8 transporter in rvZIP8 cells displayed a Km of ~0.62 μM for Cd uptake and ~2.2 μM for Mn uptake. In the Madin–Darby canine kidney (MDCK) polarized epithelial cell line, ZIP8 was demonstrated to be localized on the apical surface. Interestingly, Cd or Mn uptake is absolutely dependent on in the medium. Therefore, it was suggested that the ZIP8 endogenous function might be a symporter, which Cd is able to highjack and thus gain entrance into cells, using a gradient (He et al., 2006).
In ZIP8 cRNA-injected Xenopus oocyte cultures (Liu et al., 2008), it was shown that ZIP8-mediated Cd and Zn uptake exhibit very low Km values of ~0.48 and ~0.26 μM, respectively. Moreover, ZIP8-mediated Cd2+ uptake is most highly inhibited by Zn2+, second-best inhibited by Cu2+, Pb2+ and Hg2+, and not inhibited by Mn2+ or Fe2+. These data went beyond the findings that were possible in rvZIP8 cells, i.e. Zn uptake was only modestly demonstrated in rvZIP8 cells—probably because of such a high background due to numerous cell-surface Zn transporters in mammalian cells in culture; also, inhibition of Cd uptake by Cu2+ and Pb2+ was not unequivocally determined in mouse rvZIP8 cells, whereas inhibition of Cd uptake by Mn2+ had been shown in rvZIP8 cells but for unknown reasons not in the frog eggs (Liu et al., 2008).
As mentioned earlier, Cd transport by human SLC11A2 (DMT1) expressed in Xenopus oocytes gave a Km of ~1.04 μM (Okubo et al., 2003). Cd transport by mouse ZIP8 in frog eggs is ~0.48 μM (Liu et al., 2008). In other words, ZIP8 has at least a 2-fold higher affinity than SLC11A2. The Cd transport affinity of ZIP8 is at least an order of magnitude higher than that of Ca channels (Kim et al., 2004; Wang et al., 2004). Given that environmental exposures of Cd are usually extremely low, ZIP8 transport of Cd would thus be favored over SLC11A2 or calcium channel transport. One additional stipulation, of course, would be the concentration of any of these transporters that is expressed—in a particular tissue or cell type.
In ZIP8-expressing Xenopus oocyte cultures (Liu et al., 2008), electrogenicity studies have shown an influx of two anions per one Cd2+ (or one Zn2+) cation, i.e. electroneutral complexes. In MDCK polarized epithelial cells retrovirally-infected with ZIP8 cDNA and tagged with hemagglutinin at the C-terminus, it was shown that—similar to ZIP4 (Kim et al., 2004; Wang et al. 2004—the ZIP8 eight-transmembrane protein is largely internalized during Zn2+ homeostasis, but moves predominantly to the cell surface membrane (trafficking) under conditions of Zn2+ depletion in the culture medium (Liu et al., 2008).
Cd-induced renal disease
Testing the BTZIP8-3 line for Cd-induced testicular necrosis led to an additional finding: acute renal failure occurred and actually preceded damage to the testis by several hours; this happened in the BTZIP8-3 mice but not in the nontransgenic littermates (Wang et al., 2007). Furthermore, a substantially greater abundance of ZIP8 mRNA (via in situ hybridization) and protein (via immunohistochemistry) was demonstrated on the apical surface of the renal proximal tubular epithelial cells of BTZIP8-3 mice compared with nontransgenic littermates (Wang et al., 2007). It is therefore quite likely that ZIP8 might be an important metal transporter in causing Cd-induced renal metabolic acidosis and kidney damage—conditions often seen in human populations chronically exposed to environmental Cd.
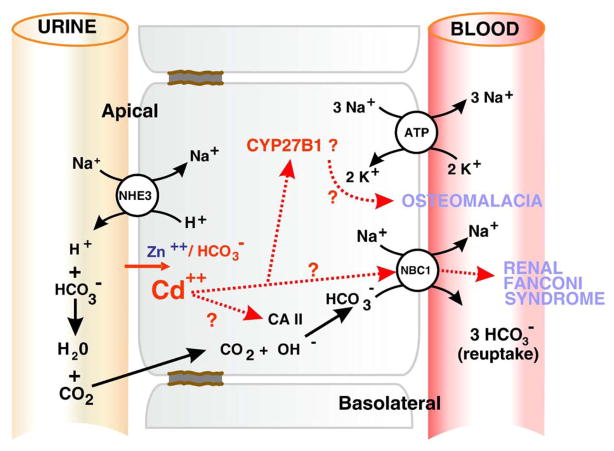
Fig. 2 summarizes our current thoughts. At the apical surface of the renal proximal tubular epithelial cell, ZIP8 normally moves Zn (and perhaps Mn) with anions from the glomerular filtrate into the cell. Dissolved pCO2 in the glomerular filtrate can also enter the cell and be converted by carbonic anhydrase to HCO3− (Fig. 2). HCO3− anions are moved from the epithelial cell into the blood by the symporter NBC1 located on the cell’s basolateral surface.
Fig. 2.
Diagram of bicarbonate reabsorption in the renal proximal tubular epithelial cell. The glomerular filtrate moves vertically down the left side of the diagram, next to the apical surface of the epithelial cell. Solute moving back into the blood across the basolateral surface is shown at right. NHE3, sodium/hydrogen exchanger-3 (encoded by SLC9A3). NBC1, sodium/bicarbonate cotransporter-4 (encoded by SLC4A4). CA II, carbonic anhydrase-2 (encoded by CA2 gene). Na,K-ATPase is shown at upper right on basolateral side. CYP27B1, mitochondrial 25-hydroxy-D3 1α-hydroxylase.
If Cd is environmentally present, it can displace either endogenous Zn or Mn and enter the epithelial cell. Cd deposition occurs largely in the renal proximal tubule. Consistent with this phenomenon, concentrations are ~24 mM as the glomerular filtrate enters segment 1 (S1) of the proximal tubule, are decreased to ~4 mM before the glomerular filtrate passes through S2 and S3, and are negligible by the time the filtrate reaches the ascending loop of Henle. Predominantly by means of oxidative stress, Cd can destroy numerous enzymes and other proteins by forming disulfide bonds. If NBC1 becomes nonfunctional, cannot cross the basolateral membrane and proximal tubular metabolic acidosis is likely to occur. If 25-hydroxy-D3 1α-hydroxylase (CYP27B1) becomes nonfunctional, the most potent ligand for the vitamin D3 receptor, 1α,25-dihydroxy-D3, would not be produced, leading to bone disease (Fig. 2). The combination of renal proximal tubular acidosis and osteomalacia is called renal Fanconi syndrome, which has numerous genetic and environmental etiologies.
Intriguingly, along with Cd, Hg and Pb are known to cause renal proximal tubular acidosis and osteomalacia, and Hg and Pb are competitive inhibitors of ZIP8-mediated Cd and Zn uptake; the only other metals known to cause this human condition are platinum and uranium (Bergeron et al., 2008). Our current plans include to study Pt and U uptake by ZIP8 in Xenopus oocytes. We also plan to test whether Cd in the proximal renal tubule does indeed render nonfunctional such proteins as carbonic anhydrase, CYP27B1, and NBC1.
Slc39a14 gene is most closely related to the Slc39a8 gene
The SLC39 gene family has 14 highly conserved members in the human and mouse genomes (Girijashanker et al., 2008). As compared to the other 12 family members (Fig. 3), ZIP14 is evolutionarily most closely related to ZIP8. Interestingly, the mouse and human SLC39A14 genes both contain two exons 4, giving rise to ZIP14A and ZIP14B alternatively-spliced variants. C57BL/6J mouse ZIP14A expression is highest in liver, duodenum, kidney and testis; ZIP14B expression is highest in liver, duodenum, brain and testis (Girijashanker et al., 2008).
Fig. 3.
Nearest-neighbor joining (NNJ)-generated phylogenetic tree of the 14 mouse ZIP domains of the Slc39 gene-encoded proteins. In terms of evolution, the mouse Slc39a14 and Slc39a8 genes can be seen to be most closely related. ZIP8 and ZIP14 diverged from one another some time after the land animal–sea animal split ~425 million years ago, because the puffer fish (Takefugu rubripes) genome contains one gene having almost equal identity to both mouse genes (Girijashanker et al., 2008). The human SLC39A14 and SLC39A8 genes (not shown) are homologous to the mouse orthologs (Girijashanker et al., 2008).
Studying both rvZIP14A and rvZIP14B cell lines, the Nebert lab proceeded to demonstrate that these ZIP14 transporters take up Cd by a temperature- and energy-dependent mechanism and function most optimally at pH 7.2. Both ZIP14 proteins (especially the B isoform) exhibit high affinity for Cd. ZIP14-mediated Cd uptake is most inhibited by Zn2+, and next by Mn2+ and Cu2+. As was found with ZIP8, ZIP14-mediated Cd uptake is dependent on extracellular , implicating ZIP14A and ZIP14B as symporters (in which M2+ represents Zn, Mn or Cd). Moreover, ZIP14A and ZIP14B are located on the apical surface of MDCK cells (Girijashanker et al., 2008). A comparison of various parameters of the mouse versus human SLC39A8 and SLC39A14 genes, and the mouse ZIP8 and ZIP14 protein transporters, is summarized in Table 1.
Table 1.
Parameters of the SLC39A8 and SLC39A14 genes and their ZIP8 and ZIP14 proteins.
| Properties | ZIP8 | ZIP14A | ZIP14B | |
|---|---|---|---|---|
| Tissues in which gene is expressed most highly (assessed by Northern and qRT-PCR) | Lung=testis>kidney≫liver>brain>duodenum | Liver>duodenum>kidney>testis>brain=lung | Liver=duodenum>brain=testis>kidney=lung | |
| Cations transported by mammalian cells in culture (always with ) | Zn, Mn, Cd | Zn, Mn, Cd | Zn, Mn, Cd | |
| Km values for M2+ uptake in mammalian cell culture | Mn 2.2 μM; Cd 0.62 μM | Mn 18 μM; Cd 1.1 μM | Mn 4.4 μM; Cd 0.14 μM | |
| Cation inhibitors of Cd uptake in mammalian cell culture | Mn>Hg≫Pb=Cu=Zn=Cs | Zn≫Mn>Cu | Zn≫Mn>Cu | |
| Cations transported by Xenopus oocytes | Zn, Cd | Zn, Cd | Zn, Cd | |
| Km values for Cd uptake in Xenopus oocytes | Zn 0.26 μM; Cd 0.48 μM | Zn 0.38 μM; Cd 0.46 μM | Cd 0.30 μM | |
| Cation inhibitors of Cd uptake in Xenopus oocytes | Zn>Cu=Pb=Hg | Hg=Pb>Zn>Cu>Fe>Mn | Hg=Pb>Zn>Fe>Cu>Mn | |
| Mouse Slc39a8 gene | Mouse Slc39a14 gene | |||
| Chromosomal location | Chr 3; nt 135488243(+) | Chr 14; nt 70751231(−) | ||
| Transcript distance spanneda | 64,860 bp | 92,502 bp | ||
| Size of mRNA | 3.1–3.3 kb | 2.0 kb | ||
| Molecular weight of protein | 50,082 Da | 53,754 versus 53,962 Dab | ||
| Number of amino acids in protein | 462 | 489 | ||
| Human SLC39A8 gene | Human SLC39A14 gene | |||
| Chromosomal location | 4q22–q24 | 8p21.2 | ||
| Transcript distance spanned | 88,148 bp | 57,942 bp | ||
| Size of mRNA | 3.3–3.5 kb | 4.6 kb | ||
| Molecular weight of protein | 49,631 Da | 54,056 versus 54,212 Dab | ||
| Number of amino acids in protein | 461 | 492 | ||
Transcript spans from the 5′ end of the 5′-most exon 1 to the end of the last exon (exon 9). Exons 1b and 1c of human SLC39A14 have not yet been found (Girijashanker et al., 2008).
Molecular weight for ZIP14A versus ZIP14B, respectively.
We predict that ZIP14 will also transport electroneutral complexes. We expect that ZIP14A and ZIP14B will participate just like ZIP8 does in the trafficking of Cd and Zn, as a function of extracellular metal concentration. Why these two splice variants have evolved—with two different functional transporter proteins, having slightly different levels of expression in the various tissues that we have examined—remains to be understood. As with virtually everything else in evolution, however, there must be some reason for these splice variants to persist in the mouse and human.
Conclusions and future directions
If a transporter is demonstrated in MDCK cell cultures to be located on the apical surface or basolateral surface, then that transporter protein is likely localized to the same surface of all polarized epithelial cells in the intact animal that express this gene product. The above notion was demonstrated to be true for the ZIP8 protein, in MDCK cell cultures using a hemagglutinin C-terminus tag, and then in the intact animal via immunohistochemistry (in the kidney). The endogenous functions of ZIP8 and ZIP14 are those of a Zn2+/HCO3− symporter, perhaps also as a Mn2+/HCO3− symporter. Cd (due to its very high affinity) can displace either Zn or Mn and be pumped into the cell along with HCO3−. ZIP8 and ZIP14 might also play an important role in the influx of Hg2+ and Cu2+ into these specific cell types. Whether Pt2+ and/or U3+ might be transported by ZIP8 or ZIP14 remains to be determined.
Interestingly, the ZIP8 and ZIP14 transporters are conveniently located between the environment and cells at portals of entry (Fig. 4). ZIP8 expression is highest in lung—where the transporter protein presumably exists on the apical surfaces of alveolar cells. Hence, incoming cigarette smoke or other Cd-contaminated dust would be transported into alveolar cells by ZIP8, which therefore most likely plays a pivotal role in Cd-induced human cancer. ZIP14 expression is very high in gut, again where the transporter protein presumably exists on the apical surfaces of enterocytes, facing the intestinal lumen. At least as much, if not more, cigarette smoke particles that are inhaled—actually end up in the intestine rather than lung (Chen et al., 1989). Cd present in cigarette smoke, contaminated food, or ingested soil would be moved into the enterocytes and then onward toward the liver via the mesenteric vessels and portal vein. ZIP14 expression is also very high in hepatocytes, thereby removing Cd from the blood and influxing the metal into liver cells. As described earlier, Cd is transported in the blood as Cd–MT, GS–Cd–SG and Cys–S–Cd–S–Cys complexes, being moved from the portals of entry (lung and intestine), toward becoming deposited in liver and especially kidney cells. ZIP8 is critical for Cd influx into the renal proximal tubular epithelial cell and the testis vascular endothelial cell (Fig. 4). Both ZIP8 and ZIP14 participate in Cd transport into the central nervous system, including the nasal epithelium and inner ear (Ma et al., 2008).
Fig. 4.
Diagram of the anatomy of various organs important in Cd uptake. ZIP14 participates principally in the small intestine and hepatocyte; ZIP8 participates predominantly in the lung, kidney and testis; both ZIP14 and ZIP8 participate in the central nervous system. Cd is carried as GS–Cd–SG and MT–Cd–MT complexes in the blood. Cd passes from the small intestine to the liver, then to the right heart and through the lung to the arterial side of the body; in addition, inhaled Cd passes from the lung to the heart and then to the arterial side of the body. Cd ultimately becomes stored to some degree in the liver and to a much greater degree in the kidney.
The endogenous function of ZIP8 and ZIP14 at the apical surfaces of these specific cell types (Fig. 4) is most likely Zn influx; Zn homeostasis is particularly important during anti-inflammatory activities. It is therefore evolutionarily feasible as to why these Zn2+/HCO3− symporters are likely to exist at these portals of entry and other specialized cell types shown in Fig. 4. Of course, Zn transporters also occur on other cell surfaces, because Zn is important in nutrition as well as in numerous other mechanisms of metabolic homeostasis—including many enzymes as well as Zn-finger-containing transcription factors.
Although Mn is an essential metal (needed as a cofactor in the function of certain enzymes), excess amounts of Mn as an occupational hazard are known to cause neurotoxicity—including ataxia and deafness (Santamaria et al., 2007). We therefore postulate that the ZIP8 and ZIP14 transporters might be important as the mediators in helping to cause certain Mn-induced occupational diseases. In addition to Cd- and Mn-induced diseases, it is possible that other heavy metal cations are also efficiently transported by ZIP8 and ZIP14 and lead to occupational or other environmental diseases. It is our hope that these studies on the Slc39a8 and Slc39a14 genes encoding the ZIP8 and ZIP14 transporters, respectively, will be useful to other investigators in this field and help to provide a conceptual framework for future studies.
Supplementary Material
Acknowledgments
We thank our colleagues for valuable discussions during the course of this work and careful readings of this manuscript. The Cd project was initiated in 1994 and continues today. We especially thank Tim Dalton for his help in moving this project forward during the time he was with our lab (1997–2006) and Professor Manoocher Soleimani who continues to work with us. Portions of this work were presented at the Society of Toxicology Annual Meeting in March 2007, Charlotte, NC. This work has been supported by NIH Grants R01 ES010416 (D.W.N.) and P30 ES06096 (D.W.N.).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.taap.2009.02.017.
References
- Alsberg CL, Schwartze EW. Pharmacological action of cadmium. J Pharmacol Exp. 191920;13:504–505. [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry. Atlanta, GA: 1999. Toxicological profile for cadmium. [PubMed] [Google Scholar]
- Banerjee S, Flores-Rozas H. Cadmium inhibits mismatch repair by blocking the ATPase activity of the MSH2–MSH6 complex. Nucleic Acids Res. 2005;33:1410–1419. doi: 10.1093/nar/gki291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon DI, Abounader R, Lees PS, Bressler JP. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am J Physiol Cell Physiol. 2003;284:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- Bergeron MJ, Simonin A, Bhrzle M, Hediger MA. Inherited epithelial transporter disorders—an overview. J Inherit Metab Dis. 2008;31:178–187. doi: 10.1007/s10545-008-0861-6. [DOI] [PubMed] [Google Scholar]
- Blazka ME, Shaikh ZA. Differences in cadmium and mercury uptakes by hepatocytes: role of calcium channels. Toxicol Appl Pharmacol. 1991;110:355–363. doi: 10.1016/s0041-008x(05)80018-3. [DOI] [PubMed] [Google Scholar]
- Bonhomme F, Benmehdi F, Britton-Davidian J, Martin S. Genetic analysis of interspecific crosses Mus musculus L. ×Mus spretus Lataste: linkage of Adh-1 with Amy-1 on chromosome 3 and Es-14 with Mod-1 on chromosome 9. CR Seances Acad Sci D. 1979;289:545–548. [PubMed] [Google Scholar]
- Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D. Divalent metal transporter-1 in lead and cadmium transport. Ann N Y Acad Sci. 2004;1012:142–152. doi: 10.1196/annals.1306.011. [DOI] [PubMed] [Google Scholar]
- Chellman GJ, Shaikh ZA, Baggs RB. Decreased uptake and altered subcellular disposition of testicular cadmium as possible mechanisms of resistance to cadmium-induced testicular necrosis in inbred mice. Toxicology. 1984;30:157–169. doi: 10.1016/0300-483x(84)90126-4. [DOI] [PubMed] [Google Scholar]
- Chen BT, Weber RE, Yeh HC, Lundgren DL, Snipes MB, Mauderly JL. Deposition of cigarette smoke particles in the rat. Fundam Appl Toxicol. 1989;13:429–438. doi: 10.1016/0272-0590(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Chiquoine AD. Observations on the early events of cadmium necrosis of the testis. Anat Rec. 1964;149:23–35. doi: 10.1002/ar.1091490104. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Miller ML, Wu X, Menon A, Cianciolo E, McKinnon RA, Smith PW, Robinson LJ, Nebert DW. Refining the mouse chromosomal location of Cdm, the major gene associated with susceptibility to cadmium-induced testicular necrosis. Pharmacogenetics. 2000;10:141–151. doi: 10.1097/00008571-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA. 2005;102:3401–3406. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder CG, Kjellstrom T, Lind B, Linnman L, Piscator M, Sundstedt K. Cadmium exposure from smoking cigarettes: variations with time and country where purchased. Environ Res. 1983;32:220–227. doi: 10.1016/0013-9351(83)90209-8. [DOI] [PubMed] [Google Scholar]
- Elisma F, Jumarie C. Evidence for cadmium uptake through NRAMP2: metal speciation studies with Caco-2 cells. Biochem Biophys Res Commun. 2001;285:662–668. doi: 10.1006/bbrc.2001.5245. [DOI] [PubMed] [Google Scholar]
- Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat Rev, Genet. 2005;6:271–286. doi: 10.1038/nrg1576. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- Friedman PA, Gesek FA. Cadmium uptake by kidney distal convoluted tubule cells. Toxicol Appl Pharmacol. 1994;128:257–263. doi: 10.1006/taap.1994.1205. [DOI] [PubMed] [Google Scholar]
- Girijashanker K, He L, Soleimani M, Reed JM, Li H, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig A, Schwerdtle T. Interactions by carcinogenic metal compounds with DNA repair processes: toxicological implications. Toxicol Lett. 2002;127:47–54. doi: 10.1016/s0378-4274(01)00482-9. [DOI] [PubMed] [Google Scholar]
- Hata A, Tsunoo H, Nakajima H, Shintaku K, Kimura M. Acute cadmium intoxication in inbred mice: a study on strain differences. Chem-Biol Interact. 1980;32:29–39. doi: 10.1016/0009-2797(80)90066-6. [DOI] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- Hinkle PM, Osborne ME. Cadmium toxicity in rat pheochromocytoma cells: studies on the mechanism of uptake. Toxicol Appl Pharmacol. 1994;124:91–98. doi: 10.1006/taap.1994.1012. [DOI] [PubMed] [Google Scholar]
- Hinkle PM, Kinsella PA, Osterhoudt KC. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J Biol Chem. 1987;262:16333–16337. [PubMed] [Google Scholar]
- Holash JA, Harik SI, Perry G, Stewart PA. Barrier properties of testis microvessels. Proc Natl Acad Sci USA. 1993;90:11069–11073. doi: 10.1073/pnas.90.23.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. Meeting of the IARC working group on beryllium, cadmium, mercury and exposures in the glass manufacturing industry. Scand J Work Environ Health. 1993;19:360–363. doi: 10.5271/sjweh.1461. [DOI] [PubMed] [Google Scholar]
- Jin T, Wu X, Tang Y, Nordberg M, Bernard A, Ye T, Kong Q, Lundstrom NG, Nordberg GF. Environmental epidemiological study and estimation of benchmark dose for renal dysfunction in a cadmium-polluted area in China. BioMetals. 2004;17:525–530. doi: 10.1023/b:biom.0000045732.91261.e2. [DOI] [PubMed] [Google Scholar]
- Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Liu J, Klaassen CD, Waalkes MP. Acquired cadmium resistance in metallothionein-I/II(−/−) knockout cells: role of the T-type calcium channel CACNα1G in cadmium uptake. Mol Pharmacol. 2006;69:629–639. doi: 10.1124/mol.105.014241. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Coughlin LL, Jusko WJ, Hartz S. Contribution of cigarette smoking to cadmium accumulation in man. Lancet. 1972;1:291–292. doi: 10.1016/s0140-6736(72)90294-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu J, Klaassen CD. Metallothionein-null and wild-type mice show similar cadmium absorption and tissue distribution following oral cadmium administration. Toxicol Appl Pharmacol. 2001;175:253–259. doi: 10.1006/taap.2001.9244. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li H, Soleimani M, Girijashanker K, Reed JM, He L, Dalton TP, Nebert DW. Cd2+ versus Zn2+ uptake by the ZIP8 dependent symporter: kinetics, electrogenicity and trafficking. Biochem Biophys Res Commun. 2008;365:814–820. doi: 10.1016/j.bbrc.2007.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucis OJ, Lucis R. Distribution of cadmium-109 and zinc-65 in mice of inbred strains. Arch Environ Health. 1969;19:334–336. doi: 10.1080/00039896.1969.10666853. [DOI] [PubMed] [Google Scholar]
- Ma C, Schneider SN, Miller M, Nebert DW, Lind C, Roda SM, Afton SE, Caruso JA, Genter MB. Manganese accumulation in the mouse ear following systemic exposure. J Biochem Mol Toxicol. 2008;22:305–310. doi: 10.1002/jbt.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program. National Toxicology Program. Research Triangle Park, NC: NIEHS, NIH; 2000. Ninth report on carcinogens; pp. 1–85. [Google Scholar]
- Okubo M, Yamada K, Hosoyamada M, Shibasaki T, Endou H. Cadmium transport by human NRAMP2 expressed in Xenopus laevis oocytes. Toxicol Appl Pharmacol. 2003;187:162–167. doi: 10.1016/s0041-008x(02)00078-9. [DOI] [PubMed] [Google Scholar]
- Olivi L, Bressler J. Maitotoxin stimulates Cd influx in Madin–Darby kidney cells by activating Ca-permeable cation channels. Cell Calcium. 2000;27:187–193. doi: 10.1054/ceca.1999.0115. [DOI] [PubMed] [Google Scholar]
- Olivi L, Sisk J, Bressler J. Involvement of DMT1 in uptake of Cd in MDCK cells: role of protein kinase C. Am J Physiol Cell Physiol. 2001;281:C793–C800. doi: 10.1152/ajpcell.2001.281.3.C793. [DOI] [PubMed] [Google Scholar]
- Parizek J. The destructive effect of cadmium ion on testicular tissue and its prevention by zinc. J Endocrinol. 1957;15:56–63. doi: 10.1677/joe.0.0150056. [DOI] [PubMed] [Google Scholar]
- Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature. 1956;177:1036. doi: 10.1038/1771036b0. [DOI] [PubMed] [Google Scholar]
- Park JD, Cherrington NJ, Klaassen CD. Intestinal absorption of cadmium is associated with divalent metal transporter-1 in rats. Toxicol Sci. 2002;68:288–294. doi: 10.1093/toxsci/68.2.288. [DOI] [PubMed] [Google Scholar]
- Ploen L, Setchell BP. Blood-testis barriers revisited. An homage to Lennart Nicander. Int J Androl. 1992;15:1–4. doi: 10.1111/j.1365-2605.1992.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Ryu MS, Lichten LA, Liuzzi JP, Cousins RJ. Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J Nutr. 2008;138:2076–2083. doi: 10.3945/jn.108.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS. State-of-the-science review: does manganese exposure during welding pose a neurological risk? J Toxicol Environ Health B Crit Rev. 2007;10:417–465. doi: 10.1080/15287390600975004. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, Jordan SA, Tewari PC. Cadmium disposition and metallothionein induction in mice: strain-, sex-, age- and dose-dependent differences. Toxicology. 1993;80:51–70. doi: 10.1016/0300-483x(93)90076-5. [DOI] [PubMed] [Google Scholar]
- Shibuya I, Douglas WW. Calcium channels in rat melanotrophs are permeable to manganese, cobalt, cadmium, and lanthanum, but not to nickel: evidence provided by fluorescence changes in FURA-2-loaded cells. Endocrinology. 1992;131:1936–1941. doi: 10.1210/endo.131.4.1327724. [DOI] [PubMed] [Google Scholar]
- Souza V, Bucio L, Gutierrez-Ruiz MC. Cadmium uptake by a human hepatic cell line (WRL-68 cells) Toxicology. 1997;120:215–220. doi: 10.1016/s0300-483x(97)00057-7. [DOI] [PubMed] [Google Scholar]
- Stoeppler M. Metals and their compounds in the environment. In: Merian E, editor. Cadmium. VCH, Weinheim; New York, Basel, Cambridge: 1991. pp. 803–851. [Google Scholar]
- Tallkvist J, Bowlus CL, Lonnerdal B. DMT1 gene expression and cadmium absorption in human absorptive enterocytes. Toxicol Lett. 2001;122:171–177. doi: 10.1016/s0378-4274(01)00363-0. [DOI] [PubMed] [Google Scholar]
- Taylor BA. Linkage of the cadmium resistance locus to loci on mouse chromosome 12. J Hered. 1976;67:389–390. doi: 10.1093/oxfordjournals.jhered.a108759. [DOI] [PubMed] [Google Scholar]
- Taylor BA, Heiniger HJ, Meier H. Genetic analysis of resistance to cadmium-induced testicular damage in mice. Proc Soc Exp Biol Med. 1973;143:629–633. doi: 10.3181/00379727-143-37380. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Yee D, Matin A, Nadeau JH, Magnuson T. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm Genome. 1997;8:390–393. doi: 10.1007/s003359900453. [DOI] [PubMed] [Google Scholar]
- Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Wang F, Kim BE, Dufner-Beattie J, Petris MJ, Andrews G, Eide DJ. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum Mol Genet. 2004;13:563–571. doi: 10.1093/hmg/ddh049. [DOI] [PubMed] [Google Scholar]
- Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP, He L, Miller ML, Stringer KF, Soleimani M, Richardson DD, Nebert DW. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am J Physiol Cell Physiol. 2007;292:C1523–C1535. doi: 10.1152/ajpcell.00409.2006. [DOI] [PubMed] [Google Scholar]
- WHO report. Cadmium: environmental health criteria. WHO Report. 1992;134:117–120. [Google Scholar]
- Zalups RK, Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol. 2003;186:163–188. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King BL, Parvizi B, Brunk BP, Stoeckert CJ, Jr, Quackenbush J, Richardson J, Bult CJ. Integrating computationally assembled mouse transcript sequences with the Mouse Genome Informatics (MGI) database. Genome Biol. 2003;4:R16. doi: 10.1186/gb-2003-4-2-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Yang H, Owen MR. Combined microarray analysis uncovers self-renewal related signaling in mouse embryonic stem cells. Syst Synth Biol. 2007;1:171–181. doi: 10.1007/s11693-008-9015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.