Abstract
Memory tasks can be performed using multiple cognitive strategies, which are mediated by different brain systems. The transverse patterning (TP) task is dependent upon the integrity of the hippocampal system, however, we previously demonstrated successful TP following hippocampal damage using meaningful stimuli and relations (Moses, S.N., Ostreicher, M.L., Rosenbaum, R.S., Ryan, J.D., 2008. Successful transverse patterning in amnesia using semantic knowledge. Hippocampus 18, 121–124). Here, we used magnetoencephalgraphy (MEG) to directly observe the neural underpinnings of TP, and the changes that occur as stimuli and relations become more meaningful. In order to optimize our ability to detect signal from deep, non-dominant, brain sources we implemented the event-related synthetic aperture magnetometry minimum-variance beamformer algorithm (ER-SAM; Cheyne, D., Bakhtazad, L., Gaetz, W., 2006. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Human Brain Mapping 27, 213–229) coupled with the partial least squares (PLS) multivariate statistical approach (McIntosh, A.R., Bookstein, F.L., Haxby, J.V., Grady, C.L., 1996. Spatial pattern analysis of function brain images using partial least squares. NeuroImage 3, 143–157; McIntosh, A.R., Lobaugh, N.J., 2004. Partial least squares analysis of neuroimaging data: Applications and advances. NeuroImage 23, S250–S263). We found that increased meaningfulness elicited reduced bilateral hippocampal activation, along with increased activation of left prefrontal and temporal cortical structures, including inferior frontal (IFG), as well as anterior temporal and perirhinal cortices. These activation patterns may represent a shift towards reliance upon existing semantic knowledge. This shift likely permits successful TP performance with meaningful stimuli and relations following hippocampal damage.
Introduction
Memory tasks can be performed using multiple cognitive strategies, which are mediated by different brain systems. The transverse patterning (TP) task requires memory for relations among stimuli, and is dependent upon the integrity of the hippocampal system (Alvarado and Rudy, 1995; Driscoll et al., 2003; Driscoll et al., 2005; Reed and Squire,1996; Rickard and Grafman,1998; Rickard et al., 2006). We demonstrated intact TP performance following bilateral hippocampal damage using meaningful stimuli and relations, such as playing cards and the childhood game rock–paper–scissors (RPS; Moses et al., 2008). We suggested that a proposition–based cognitive strategy that relies on pre-existing semantic knowledge could be mediated by extra-hippocampal structures.
Here we asked specifically how the relational TP task with meaningful stimuli and relations can be mediated by extra-hippocampal structures. We used magnetoencephalgraphy (MEG) to directly observe the neural underpinnings of TP, and the changes that occur as stimuli and relations become more meaningful. Previous work shows that TP with abstract stimuli elicits hippocampal activation that is detectable with MEG (Hanlon et al., 2003, 2005, 2007), and this activation is found less reliably for TP with RPS stimuli (Hanlon et al., 2005). However, these studies focused predominantly on activation within the hippocampus. We sought to examine the dynamic neural activity across hippocampal and cortical regions supporting TP with abstract stimuli, and the subsequent changes elicited by increased meaningfulness of stimuli and relations. We expected that meaningfulness would recruit extra-hippocampal structures involved in processing semantic information, such as left prefrontal and temporal cortex (Davies et al., 2004; Mummery et al., 2000; Taylor et al., 2006; Wagner et al., 2001), with a concurrent reduction in hippocampal activation.
The sensitivity of MEG to signals from deep neural structures, such as the hippocampus, has been debated because: 1) magnetic field strength decreases with increasing distance between neural sources and MEG sensors and 2) the “spiral” or “spherical” shape of the hippocampus could theoretically lead to cancellation of magnetic signal (for in depth discussions see Stephen et al., 2005; Riggs et al., 2009). However, an increasing body of empirical evidence demonstrates that, although hippocampal activation may be more difficult to detect than superficial sources, it can be reliably detected with a range of experimental paradigms and analysis techniques (Breier et al., 1998, 1999; Hanlon et al., 2003, 2005, 2007; Ioannides et al., 1995; Kirsch et al., 2003; Martin et al., 2007; Mikuni et al., 1997; Nishitani et al., 1999; Papanicolaou et al., 2002; Stephen et al., 2005; Riggs et al., 2008; Tesche, 1997; Tesche and Karhu, 1999, 2000; Tesche et al., 1996).
In order to optimize our ability to detect signal from deep, non-dominant, brain sources we implemented the event-related synthetic aperture magnetometry minimum-variance beamformer (ER-SAM; Cheyne et al., 2006) coupled with the partial least squares (PLS) multivariate statistical approach (McIntosh et al., 1996; McIntosh and Lobaugh, 2004). The ER-SAM algorithm permits detection of weaker sources, as it uses information from all MEG sensors, and does not require a priori assumptions about number or location of sources. The entire brain volume is covered by a grid, and at each grid node, the beamformer enhances sensitivity for the signal from that node and suppresses the signal from other nodes (Huang et al., 2004). The mean-centred PLS analysis is ideal for detecting non-dominant or weaker neural sources. Activation patterns that are unique to individual conditions become enhanced, and patterns that are similar across all conditions (such as strong primary sensory sources) are removed. Further, PLS is more sensitive to distributed signals than traditional univariate statistical approaches. Thus, the application of PLS to ER-SAM source-space solutions is a powerful method for localizing weaker sources that are unique to specific cognitive processes, and reducing contributions from dominant sensory responses that are similar across all conditions.
Materials and methods
Participants
Participants consisted of 22 right-handed individuals (14 female) with no known pathology from the volunteer pool at the Rotman Research Institute. Ages ranged between 20–35 (mean=26).
Post-experimental questionnaires revealed that 18/22 participants were able to correctly report the relationship among the stimuli for all four TP blocks. The remaining participants were unable to correctly report the relationship either on the first block, or the first two blocks. These four participants were considered non-learners, and their data were not included in any of the analyses. Of the 18 remaining participants, 9 participated in the “Progressively meaningful” condition, and 9 participated in the “All abstract” condition.
Stimuli and procedures
Participants performed four independent TP blocks during MEG recording. Three different achromatic stimuli (A, B, and C) were used in each of the four blocks (Fig. 1). Within each block, the stimuli were grouped into three completely overlapping pairs in which A was correct when paired with B, B was correct when paired with C and C was correct when paired with A (A+B−, B+C−, C+A−). Participants were not informed of any relationship among the stimuli, and were required to use button presses to learn the computerized task by trial and error. For each trial, stimuli were presented in pairs, counterbalanced for right and left side presentation. Pairs of stimulus were presented in random order. Stimuli remained on the screen until participants responded, and the intertrial interval following stimulus termination was jittered between 2000–2500 ms (mean 2250 ms). Correct responses were followed by a pleasant chime sound, and incorrect responses were followed by an unpleasant buzz sound. MEG recording continued until participants made 100 or 120 correct responses1. Only correct responses, in which participants responded within 3 s, were retained for analysis. Due to the data parsing procedures and removal of reaction time outliers, an average of 10% of the correct trials were lost. Following completion of all four TP blocks, participants were removed from the shielded room and given four independent post-experimental questionnaires assessing their knowledge of the relationship among the stimuli within each block (Moses et al., 2008).
Fig. 1.
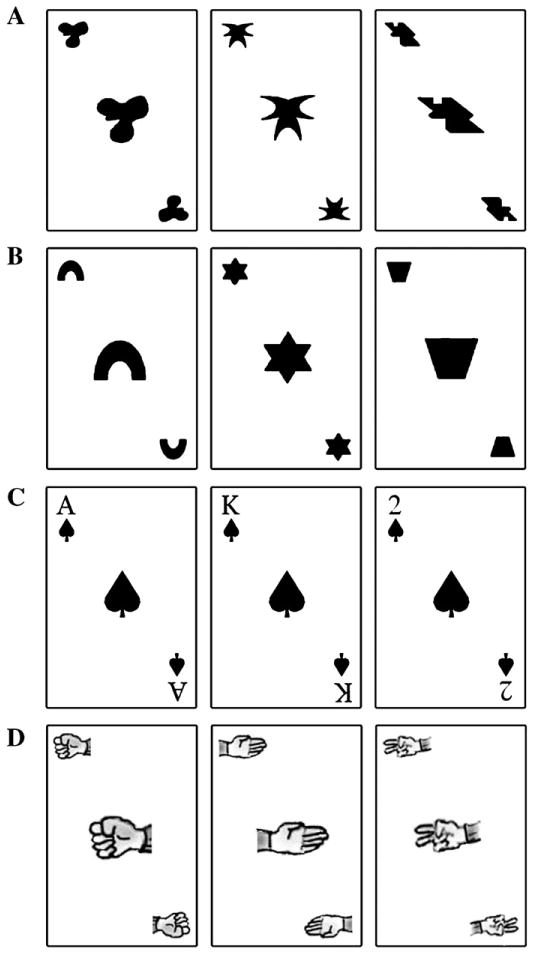
Stimuli used for the four TP blocks in the Meaningful condition. A, Abstract Objects: abstract stimuli with arbitrary relations; B, Geometric Shapes: meaningful stimuli with arbitrary relations; C, Playing Cards: meaningful stimuli with meaningful relations; D, Rock–paper–scissors: meaningful stimuli with meaningful relations. For the All abstract condition, each block contained a unique set of three abstract stimuli.
Half of the participants were exposed to the Progressively meaningful condition, which contained four blocks, each composed of different stimuli that tapped semantic knowledge to varying extents (Fig. 1): 1) abstract objects (novel stimuli with arbitrary relations), 2) geometric shapes (familiar stimuli with arbitrary relations), 3) playing cards (familiar stimuli with meaningful relations), 4) Rock–paper–scissors (RPS; familiar stimuli with meaningful relations). The order of the blocks remained constant for all participants. The other half were exposed to the All abstract condition, which was also composed of four independent TP blocks, however blocks 1–4 each consisted of three different abstract stimuli and no meaningful stimuli were used. Since all aspects of the All abstract and Progressively meaningful conditions were identical with the exception of the stimulus properties, the observed differences are attributable to the meaningfulness of stimuli and relations. Hence, the All abstract condition served as a control condition for changes due to practice with the TP task, not due to the addition of meaningful stimuli and relations.
Data collection
All MEG recordings were performed in a magnetically shielded room at the Rotman Research Institute, Baycrest Hospital for Geriatric Care, using a 151-channel whole-head first order gradiometer CTF system (VSM-Med Tech Inc.) with detection coils uniformly spaced 31 mm apart on a helmet-shaped array. Head position within the MEG was determined by monitoring the position of indicator coils on the nasion and bilateral periauricular points, at the start and end of each recording session. Participants sat in a comfortable chair with a screen 28″ away, with 8° of visual angle for stimuli. A photodiode was implemented to record precise arrival time of the visual stimuli on the screen. MEG data were collected with a bandwidth of 0–208 Hz, at a sampling rate of 625.
A structural MRI was also obtained for each participant in order to specify/constrain the sources of activation as measured by MEG. Structural MRIs were obtained using standard clinical procedures with a 1.5 T MRI system (Signa EXCITE HD 11.0; GE Healthcare Inc., Waukesha, WI) located at Sunnybrook Health Sciences Centre, or a 3 T MRI system (Siemens Magnetom Trio whole-body scanner) located at Baycrest Centre.
Data analysis
Source activity was estimated with an event-related spatial-filtering approach using the synthetic aperture magnetometry minimum-variance beamformer (ER-SAM; Cheyne et al., 2006). The beamformer spatial filter was used to estimate source activity across the whole brain on a grid size of 5 mm, averaged across trials. Source activity was estimated as a pseudo-Z statistic for 0–55 Hz from 200 ms prior to stimulus presentation to 600 ms after. Due to the ambiguity of source polarity during SAM analysis, the absolute pseudo-Z value for each virtual channel was used to create pseudo-Z SAM map of each participant's brain activity (Bardouille and Ross, 2008). These individual functional maps were then transformed to the standard Talairach space, using the same transform applied to the anatomical MR image (using AFNI). The resultant volumetric map of event-related source strength over time was overlaid on the individual participant's structural MRI based on coregistration with the indicator coils placed on the nasion and bilateral periauricular points.
Spatiotemporal changes across TP blocks were characterized using the partial least squares (PLS) multivariate approach (McIntosh et al., 1996; McIntosh and Lobaugh, 2004). The Talairach-transformed individual functional maps for each participant were down-sampled to 125 Hz in order to accommodate computation demands, and used as input for a mean-centred PLS analysis. Mean centring allowed values for the different blocks to be expressed relative to the overall mean. Using this type of analysis, activation patterns that are unique to specific blocks will be emphasized; whereas activations that are consistent across all conditions, such as primary visual activation, will be diminished.
A matrix was constructed with columns containing the pseudo-Z value for every participant at each brain voxel at each time point, embedded horizontally within each independent TP block. For example, the first column contained the value for the first voxel at the first time point, the second column contained of the value of the first voxel at the second time point. With m voxels and t time points, there are m*t columns in the matrix. With n participants and k blocks, there are n*k rows in the matrix. Participants from the Progressively meaningful and All abstract conditions were stacked vertically. An additional matrix contained the grand mean pseudo-Z value, averaged across all four TP blocks, at each brain voxel at each time point, with conditions stacked (note that the means were calculated within each condition, not across conditions). This grand mean matrix was subtracted from the initial matrix.
Singular value decomposition (SVD) was then applied to the resulting mean-centred matrix. SVD yields three matrices, each of which provides unique information. The first, the “design latent variable (LV)”, is composed of contrast coefficients that characterize the difference between blocks, collapsed across the entire brain volume and time epoch. The second matrix, the “brain LV”, contains the covariance of the design with the brain data for each brain voxel at each time point, indicating where and when the differences coded in the design LV are expressed. The third matrix contains the singular values, which are the multivariate covariance of the design and the brain data collapsed across the entire brain volume, time and all blocks.
Statistical assessment for PLS is done using permutation tests and bootstrap estimation of standard errors for the brain LVs (Efron and Tibshirani, 1986; McIntosh et al., 1996; McIntosh and Lobaugh, 2004). The permutation test assesses whether the effect represented in a given LV, captured by the singular value, is sufficiently strong to be different from randomly assigned data. The standard error estimates from the bootstrap tests are used to assess the reliability of the nonzero values in significant LVs.
Results
Behavioural results
We used a mixed-factor repeated measures analysis of variance (ANOVA) to compare reaction time (RT) and accuracy across the within subjects factor “block” and the between subjects factor “meaning” (the Progressively meaningful versus the All abstract stimulus conditions). RT was facilitated due to stimulus meaningfulness. RT decreased as stimuli became more meaningful, but remained constant for subsequent blocks with abstract stimuli (Fig. 2A). This was illustrated by an interaction of “block” by “meaning” F(3,45)=7.8, p=0.005. Follow up comparisons revealed no significant difference among blocks in the All abstract condition (F<1). In contrast, a main effect of “block” occurred in the Progressively meaningful condition (F(3,24)=17.8, p<0.001), with a significant linear contrast of block (F(1,8)=17.8, p=0.003) indicating successive decreases in RT across blocks. A significant quadratic contrast was found as well (F(1,8)=21.7, p=0.002), indicating an increase in RT for the RPS block. Individual contrast showed that RT decreased from the Abstract to the Shapes block (F(1,8)=8.9, p=0.018) and from the Shapes to the Cards block (F(1,8)=9.2, p=0.002), but there was no difference in RT between the Cards and the RPS block (F(1,8)=2.9, p=0.128) or the Shapes and the RPS block (F(1,8)=2.7, p=0.141). The RPS block was significantly faster than the Abstract block (F(1,8)=10.7, p=0.011). One-way ANOVA yielded no significant differences between the Progressively meaningful and All abstract conditions on any individual block (Fs<2.3, ps>0.15).
Fig. 2.
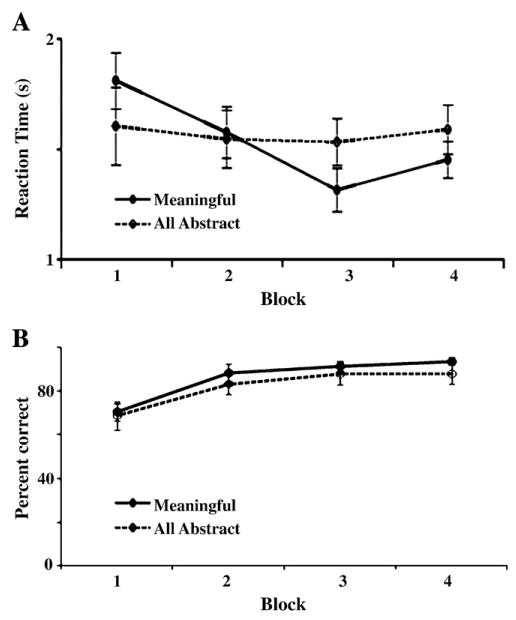
Mean A, reaction times and B, accuracy for TP performance (Meaningful condition: Block 1=Abstract; Block 2=Shapes; Block 3=Cards; Block 4=RPS; All abstract condition: each of Blocks 1–4 contained a unique set of three abstract stimuli). Meaningful stimuli and relations led to decreased reaction times, although a slight increase in RT occurred for RPS). RT remained stable across Blocks 1–4 for the All abstract condition. No differences in reaction times occurred between the Meaningful and All abstract conditions.
In contrast, for both the Progressively meaningful and All abstract conditions, accuracy improved linearly across blocks (Fig. 2B), illustrated by a significant main effect (F(3,45)=4.6, p=0.008) and linear contrast (F(1,16)=5.8, p=0.028) of “block”. There were no differences in accuracy between the Progressively meaningful and All abstract conditions (Fs<1).
MEG results
PLS analysis yielded two significant (p ≤ 0.05) design LVs. The first (LV1) represents a main effect that was expressed similarly across both the Progressively meaningful and All abstract conditions (Fig. 3A). The other (LV2) represents an interaction that differed between the Progressively meaningful and All abstract conditions (Fig. 3B). Note, the contrasts depicted in the LVs were not pre-specified, rather they were derived analytically.
Fig. 3.
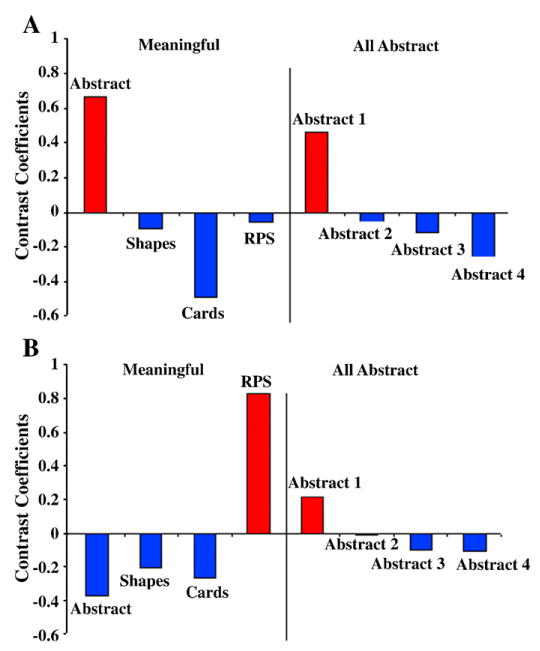
Significant design LVs from PLS analysis. A, LV1 expresses a main effect across conditions, in which the first block showed a unique pattern of brain activation compared to the other blocks regardless of whether the stimuli were abstract or meaningful. B, LV2 expresses an interaction between the conditions, in which a unique pattern of brain activation occurred for the RPS block compared to the other blocks. This effect was absent in the All abstract condition.
LV1
LV1 denotes a contrast between the first TP block versus the last 3 blocks, regardless of condition (Progressively meaningful/All abstract). Greater activation for the first block versus the subsequent blocks was found in right middle frontal gyrus, approximately Brodman's area 10 (BA10), within the first 120 ms following stimulus presentation and between 240–312 ms (Figs. 4A, 5A); as well as in left superior frontal gyrus, also BA 10, between 120–200 ms (Figs. 4B, 5B). Additionally, greater activation for the first block occurred in right fusiform cortex between 488–512 ms (Figs. 4C, 5C).
Fig. 4.
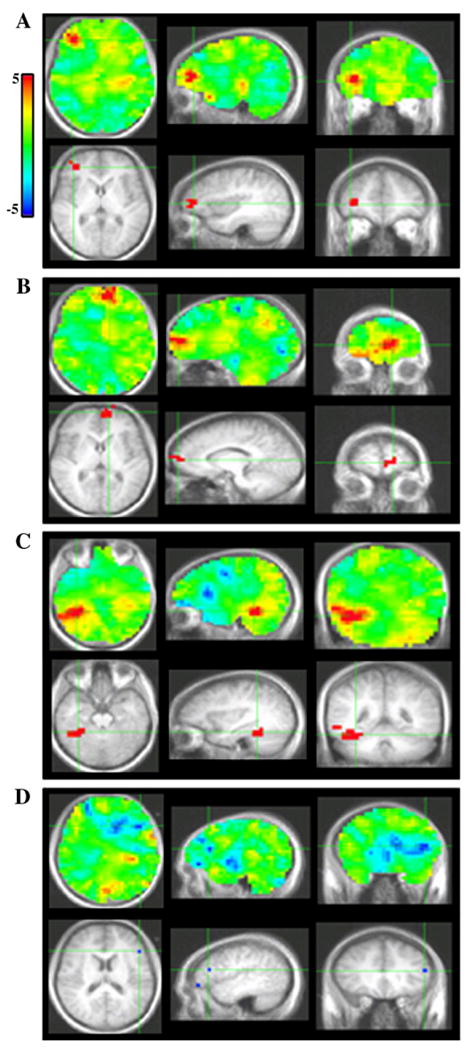
PLS bootstrap ratio plots from LV1. Unthresholded images (top); images thresholded at a bootstrap ratio of 4.5. Sources in A, right area 10, B, left area 10, C, right fusiform gyrus, D, left inferior frontal gyrus.
Fig. 5.
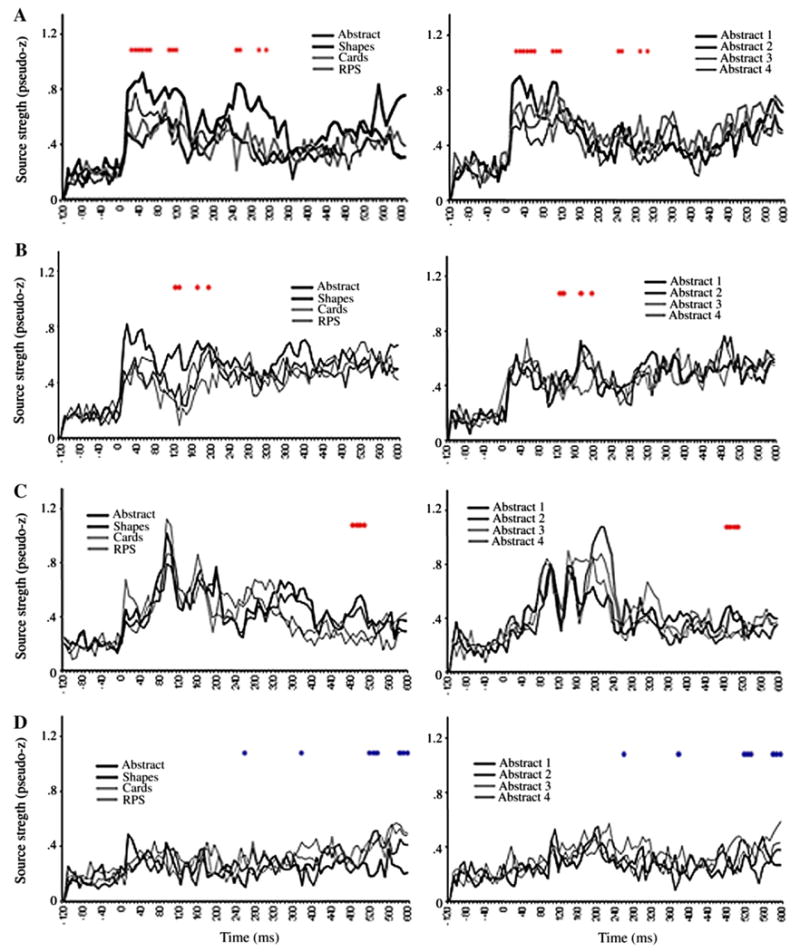
ER-SAM waveforms from LV1 sources. Red dots denote bootstrap ratios >3, and blue dots <−3. Waveforms from A, right area 10, B, left area 10, C, right fusiform gyrus, D, left inferior frontal gyrus.
In contrast, greater activation for the last 3 blocks versus the first was found in left inferior frontal gyrus (IFG), in the operculum region (Brodman's area 45) at 256 and 376 ms, as well as between 520–600 ms (Figs. 4D, 5D).
LV2
LV2 illustrates a contrast between the final TP block versus the first 3 blocks, that is only expressed within the Progressively meaningful condition. Hence, this LV represents a difference between performance of RPS versus the other conditions.
Greater activation for RPS versus the other conditions was found in left IFG (operculum) at 112, 144 and 408 ms, as well as between 512–568 ms (Figs. 6A, 7A). The finding of a significant interaction between conditions in the IFG suggests that the main effect observed in this region in LV1 was primarily driven by the Progressively meaningful condition and was not expressed strongly in the All abstract condition. This notion is further supported upon inspection of the ER-SAM waveforms.
Fig. 6.
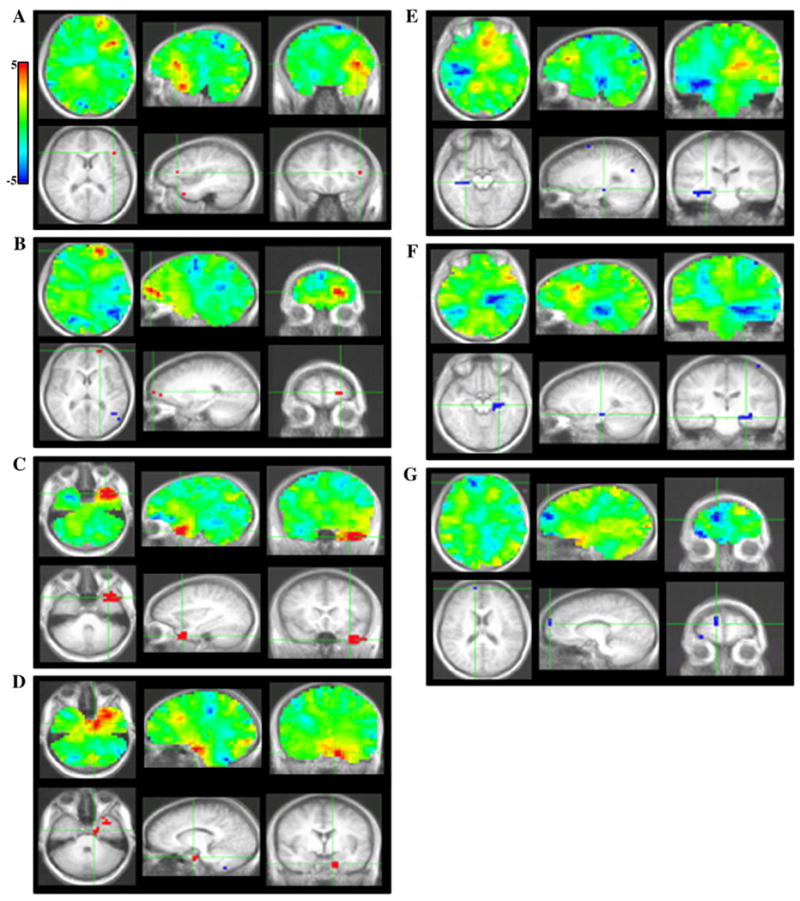
PLS bootstrap ratio plots from LV2. Unthresholded images (top); images thresholded at a bootstrap ratio of 4.5. Sources in A, left inferior frontal gyrus, B, left area 10, C, left anterior temporal cortex, D, left perirhinal cortex, E, right hippocampus, F, left hippocampus, G, right area 10.
Fig. 7.
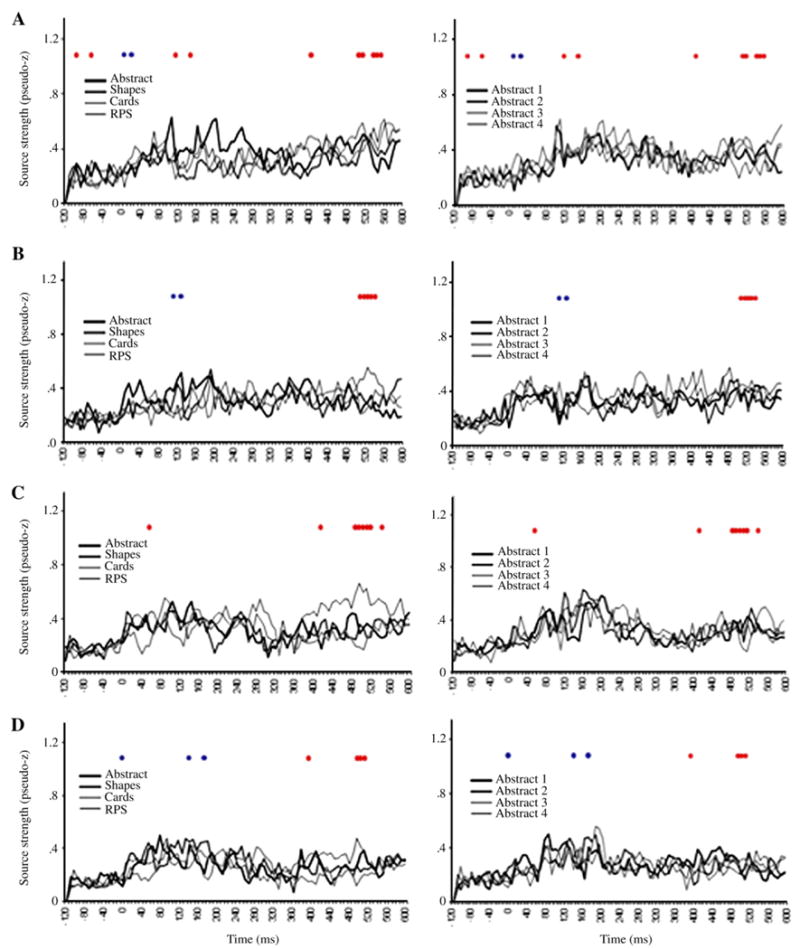
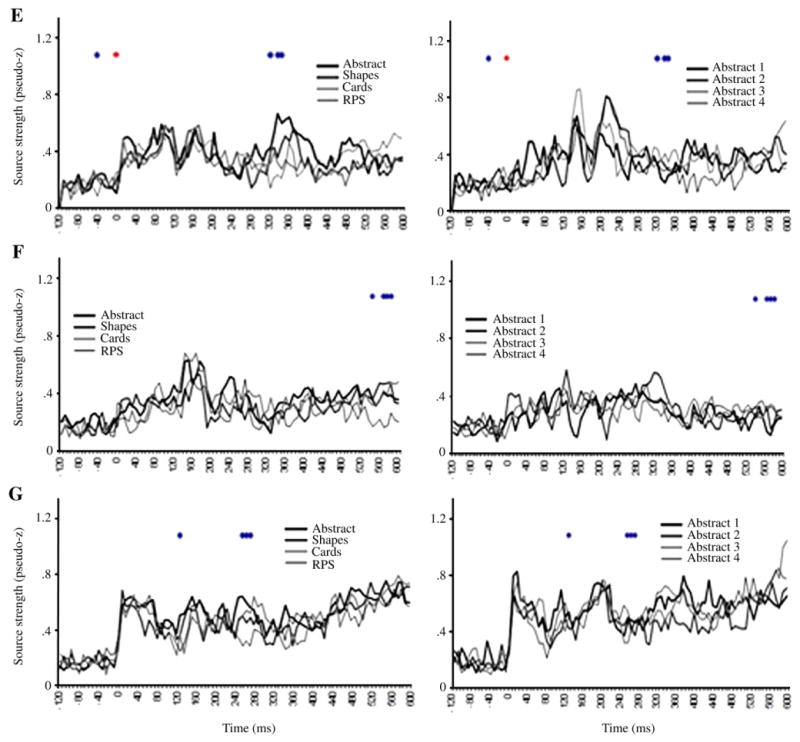
ER-SAM waveforms from LV2 sources. Red dots denote bootstrap ratios >3, and blue dots <−3. Waveforms from A, left inferior frontal gyrus, B, left area 10, C, left anterior temporal cortex, D, left perirhinal cortex, E, right hippocampus, F, left hippocampus, G, right area 10.
Greater activation for RPS versus the other conditions was also found in left superior frontal gyrus/BA 10 at 512–544 ms (Figs. 6B, 7B). Additionally, the same source showed a reduction in activation for RPS versus the other condition at an earlier time epoch of 112–136 ms. This time epoch overlaps that in which the main effect for the left BA 10 source described above was found. As with the left IFG source, this interaction, along with inspection of the ER-SAM waveforms, suggests that the main effect in LV1 was driven more strongly by the Progressively meaningful than the All abstract condition.
Greater activation for RPS versus the other conditions similarly occurred in left temporal cortex. This effect was found in left anterior temporal cortex at 56 and 416 ms, and between 488–552 ms (Figs. 6C, 7D), and in left perirhinal cortex at 392 ms, and between 496–520 ms (Figs. 6D, 7D). For the perirhinal cortex source, an additional effect of reduced activation for RPS versus the other conditions occurred at earlier latencies of 136 and 168 ms.
Reduced activation for RPS versus the other conditions was found in the right hippocampus between 320–352 ms (Figs. 6E, 7E), and left hippocampus at 544 ms, and between 568–592 ms (Figs. 6F, 7F). A similar reduction of activation for RPS versus the other conditions was also found in right superior frontal gyrus/BA 10 at 128 ms, and at a later epoch between 256–272 ms (Figs. 6G, 7G).
Discussion
We investigated how TP is supported by extra-hippocampal structures, using meaningful stimuli and relations. Increased meaningfulness generally facilitated RT. Meaningfulness also induced bilateral decreases in hippocampal activation, with increased left inferior frontal and temporal cortical activation. Changes in prefrontal and fusiform activation accompanied initial learning versus subsequent performance, regardless of whether stimuli were abstract or meaningful. These effects will be expanded upon below.
Effects of semantically meaningful stimuli and relations
Semantic meaningfulness led to changes in behavioural responding and brain activation. No changes in RT occurred with repeated exposure to abstract TP blocks. With meaningful stimuli, however, RT decreased linearly from abstract to the shapes, and from shapes to cards. Therefore, the addition of meaningful stimuli, and subsequently meaningful relations, each facilitated performance.
Subsequently, RT increased for RPS. This pattern of RT replicates previous findings using identical stimuli, but with the blocks presented in reverse order (Ostreicher et al., in review). Despite RPS being presented first and abstract last, RT was longest for abstract, then shapes, and RPS fell between shapes and cards. Thus, the current pattern of RT cannot be attributed to floor effects. Rather, it indicates the use of a unique cognitive strategy for RPS. Our neuroimaging data support this hypothesis.
PLS analysis yielded an LV that dissociated brain activation patterns unique to RPS versus the other three blocks. Reductions in bilateral hippocampal and right BA 10 activation occurred for RPS, accompanied by increased activation in left BA 10, IFG, anterior temporal, and perirhinal cortices. This pattern was not found when all four TP blocks were abstract.
Our detection of hippocampal activation reinforces prior MEG findings of hippocampal activation at similar latencies during TP, and of earlier right versus left activation onset (Hanlon et al., 2003, 2005, 2007). Maximum differentiation between RPS and the other conditions occurred at approximately 300 ms in the right, and 550 ms in the left hemisphere, although hippocampal peaks were apparent as early as 120 ms. Thus, meaningfulness affected later processing, rather than the earliest hippocampal peaks. The reduction of hippocampal activation for RPS coincides with previous findings of less reliable hippocampal activation for RPS versus abstract stimuli (Hanlon et al., 2005); although Hanlon et al. analyzed data at the individual participant level, and did not directly compare activation strength across conditions. Additionally, they did not investigate which extra-hippocampal structures support TP with meaningful stimuli.
We found that decreased hippocampal activation was accompanied by increased activation in left hemisphere structures implicated in processing semantic information, including left IFG, anterior temporal and perirhinal regions. Interestingly, the maximum differentiation between RPS and the other conditions in these left cortical regions occurred at 500–550 ms, which coincides with the observed reduction in left hippocampal activation. This pattern of results is consistent with the conception of a tradeoff between two systems, one that is involved in the formation of novel relations in memory and one involved in the processing of relations already stored in semantic memory (Ryan and Cohen, 2003, 2004). This pattern is also congruent with reports of reduced hippocampal and increased IFG activation as relations become familiar, versus when relations remain novel (Doeller et al., 2005). Doeller et al. (2005) found effects in right hippocampal and prefrontal cortex, likely because stimuli were spatial, whereas ours incorporated semantic information.
Activation of left IFG, as reported here, occurs during semantic memory retrieval, and bilateral BA 10 activation occurs during semantic, working and episodic memory tasks, as well as problem solving (Cabeza and Nyberg, 2000; Green et al., 2006; Nyberg et al., 2003). Additionally, anterior temporal and perirhinal cortices participate in processing semantic information, and damage to these structures leads to impairments such as semantic dementia (Davies et al., 2004; Mummery et al., 2000). Further, left perirhinal cortex, specifically, is activated by semantically meaningful multimodal information (Taylor et al., 2006). Thus, as hippocampal activation decreased, frontal and temporal cortical structures supporting semantic memory increased. This pattern suggests that RPS taps semantic memory to a greater extent than the other conditions.
Previous studies have found that perirhinal cortex plays a crucial role in TP (Alvarado and Bachvalier, 2005a; Saksida et al., 2007). Saksida et al. (2007) found impaired TP in monkeys with perirhinal cortex lesions, and facilitation with hippocampal lesions; which contradicts multiple reports of impaired TP with hippocampal lesions (Alvarado and Bachvalier, 2005b; Alvarado and Rudy, 1995; Driscoll et al., 2005; Reed and Squire, 1996; Rickard and Grafman, 1998; Rickard et al., 2006). This discrepancy may be due to the role of the perirhinal cortex in perception and memory for complex item identity (Buckley and Gaffan, 1998a,b; Bussey and Saksida, 2002, 2005; Gaffan and Parker, 1996; Pihlajamäki et al., 2004), including the creation of configural representations composed of multiple items (Alvarado and Bachvalier, 2005b). Without crucial input from the perirhinal cortex for interpreting ambiguous stimuli, the hippocampus could not support performance (Buckley and Gaffan, 1998b; Moses et al., 2005). Note that the RPS condition in our study differs from the TP paradigm in Saksida et al. (2007) because it uses familiar stimuli which invoke previously acquired semantic relations, and does not require the creation of novel object or relational representations. Therefore, perirhinal cortex participates in the processing of pre-existing relations stored in semantic memory, in addition to creating novel object representations. In support of this, Taylor et al. (2006) demonstrate a greater role for perirhinal cortex for processing semantically meaningful association compared to arbitrary relationships.
Along with decreased hippocampal activation, a decrease also occurred in right BA 10 for RPS. Maximum differentiation between RPS and the other conditions occurred at approximately 250 ms, which slightly preceded activation differences within the right hippocampus and far preceded activation differences observed in structures related to semantic processing. Thus, the altered right BA 10 recruitment was associated with an earlier stage of activation than the other sources, and perhaps represents a shift in perceptual processes. The observed reduction in both right BA 10 and hippocampal activation may reflect a shift away from a predominantly right-lateralized hippocampal-frontal network to a left-lateralized anteriotemporal-frontal network. A comparable shift away from right lateralized hippocampal activation for abstract TP towards bilateral activation for RPS, and left lateralization for verbal TP was observed by Hanlon et al. (2005, 2007). Additionally, right-lateralized frontal and temporal activation is consistently found for non-nameable stimuli, with a shift towards bilateral activation in these regions for namable objects, and left-lateralized activation for verbal stimuli (Golby et al., 2001; Kelley et al., 1998; Wagner et al., 1998). Thus, the current right–left shift may be related to increased verbalization with RPS stimuli.
It may be surprising that, despite the presence of meaningful stimuli and relations within both RPS and cards, behavioural and neuroimaging results single out RPS as unique and group cards with the other conditions. Importantly, our results do not imply that TP with cards and the abstract/shapes stimuli rely on identical networks; they only demonstrate that the largest difference occurred between RPS and the other conditions. Nevertheless, this pattern is congruent with previous findings using identical TP blocks, that following hippocampal damage, only RPS stimuli led to performance that was similar to controls on 2/3 test sessions (Moses et al., 2008). Although accuracy for cards was well above chance, it was significantly lower than controls on all test sessions. Moreover, the relations among the stimuli were correctly reported for RPS, but incorrectly for cards. Thus, hippocampal damage affected test performance, and explicit knowledge about relations, for cards to a greater extent than RPS. This suggests that RPS may be performed using a cognitive strategy that relies on the hippocampus to a lesser extent, and supports the present behavioural and neuroimaging findings.
We speculate that, the crucial difference between RPS and cards is that the relations among the RPS stimuli can be inferred unambiguously based on their identity and inherent function (i.e. rocks crush scissors, scissors cut paper, paper covers rock). Each stimulus is associated with an action that dictates its relationship to all other stimuli (Hanlon et al., 2005). In contrast, although the relations among the cards are meaningful and over-learned, their identities are not associated with specific functions that unambiguously dictate an action they will inflict, or a relationship they will have (i.e. there is nothing inherent to the identity/function of a “two” which indicates whether it will beat an “ace”). This increase in relational ambiguity leads to an increased reliance on the hippocampus, although the task can be solved independently of the hippocampus; albeit less efficiently. A similar effect of varying degrees of relational ambiguity affecting compensation following hippocampal lesions has been demonstrated by McDonald and White (1995). Conversely, the unambiguous object–action relations invoked by RPS tap extra-hippocampal structures and invoke alternate cognitive strategies that rely on existing semantic memory.
Learning versus performance
PLS analysis also yielded an LV that dissociated activation patterns unique to the initial block in which participants learned the task, from those unique to the subsequent blocks in which participant were already familiar with the task. These patterns occurred regardless of whether the stimuli and relations were abstract or meaningful.
Initial learning of TP elicited bilateral activation of BA 10, although only the right hemisphere activation was present across both conditions, while the left hemisphere activation was primarily driven by the Progressively meaningful condition. Differentiation between the first block and the last three occurred extremely early, between approximately 35–150 ms, perhaps indicative of a role in perceptual aspects of problem solving such as encoding and retrieval of visual representations. Activation of BA 10 is observed in many memory tasks, including working memory, semantic memory and episodic memory using either verbal or nonverbal stimuli (Cabeza and Nyberg, 2000; Nyberg et al., 2003), and is engaged during problem solving (Cabeza and Nyberg, 2000; Green et al., 2006). Thus, the finding of BA10 activation elicited by acquisition of TP concurs with previous work. Activation during learning also occurred in the right fusiform cortex. Differentiation between the first block and the last three occurred at latency later than those associated with initial perceptual processing, at approximately 490 ms. Activation of this region is found for tasks with rely on object identity information (Danckert et al., 2007; Pihlajamäki et al., 2004; Tyler et al., 2004). Hence, activation in this region is likely related to the reliance on object identity information and the requisite for in depth feature processing to permit item discrimination.
Conclusions
TP with meaningful stimuli and relations elicited increased activation of left prefrontal and temporal cortices, and reduced hippocampal activation. These activation patterns may represent a shift in strategy supported by existing semantic knowledge. This shift likely permits successful TP with meaningful stimuli and relations following hippocampal damage (Moses et al., 2008).
Acknowledgments
The authors wish to thank Christina Villate, Melanie Ostreicher, Bernhard Ross and Wilkin Chau for their advice and technical assistance. This work was funded by the J.S. McDonnell Foundation Collaborative Grant 22002082 awarded to ARM; by Natural Science and Engineering Research Council and Canada Research Chair grants awarded to JDR; and grants from The Mind Research Network/University of New Mexico and the NCRR Center on Neural Mechanisms of Schizophrenia, COBRE Grant No. P20 RR021938 awarded to FMH.
Footnotes
Accuracy was similar regardless of whether 100 or 120 trials were administered (F> 1); therefore, all participants were combined.
References
- Alvarado MC, Bachvalier J. Selective neurotoxic damage to the hippocampal formation impairs performance of the transverse patterning and location memory tasks in rhesus macaques. Hippocampus. 2005a;15:118–131. doi: 10.1002/hipo.20037. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Bachvalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J Neurosci. 2005b;24:1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Rudy JW. Rats with damage to the hippocampal-formation are impaired on the transverse-patterning problem but not on elemental discriminations. Behav Neurosci. 1995;109:204–211. doi: 10.1037//0735-7044.109.2.204. [DOI] [PubMed] [Google Scholar]
- Bardouille T, Ross B. MEG imaging of sensorimotor areas using inter-trial coherence in vibrotactile steady-state responses. NeuroImage. 2008;42:323–331. doi: 10.1016/j.neuroimage.2008.04.176. [DOI] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Relative timing of neuronal activity in distinct temporal lobe areas during a recognition memory task for words. J Clin Exp Neuropsychol. 1998;20:782–790. doi: 10.1076/jcen.20.6.782.1116. [DOI] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Lateralization of cerebral activation in auditory verbal and non-verbal memory tasks using magnetoencephlography. Brain Topogr. 1999;12:89–97. doi: 10.1023/a:1023458110869. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs visual object identification. J Neurosci. 1998a;115:776–785. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998b;6:535–546. doi: 10.1016/s0028-3932(97)00120-6. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesion in perirhinal cortex. Eur J Neursci. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: an alternative approach. Curr Opin Neurobiol. 2005;15:730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp. 2006;27:213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckert SL, Gati JS, Menon RS, Köhler S. Perirhinal and hippocampal contributions to visual recognition memory can be distinguished from those of occipito-temporal structures based on conscious awareness of prior occurrence. Hippocampus. 2007;17:1081–1092. doi: 10.1002/hipo.20347. [DOI] [PubMed] [Google Scholar]
- Davies RR, Graham KS, Xuereb JH, Williams GB, Hodges JR. The human perirhinal cortex and semantic memory. Eur J Neurosci. 2004;20:2441–2446. doi: 10.1111/j.1460-9568.2004.03710.x. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Opitz B, Krick CM, Mecklinger A, Reith W. Prefrontal-hippocampal dynamics involved in learning regularities across episodes. Cereb Cortex. 2005;15:1123–1133. doi: 10.1093/cercor/bhh211. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Prusky GT, Rudy JW, Sutherland RJ. Seahorse wins all races: hippocampus participates in both linear and non-linear visual discrimination learning. Behav Brain Res. 2005;164:29–35. doi: 10.1016/j.bbr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- Gaffan D, Parker A. Interaction of perirhinal cortex with the fornix-fimbria: memory for objects and “objects-in-place” memory. J Neurosci. 1996;16:5864–5869. doi: 10.1523/JNEUROSCI.16-18-05864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN. Frontopolar cortex mediates abstract integration in analogy. Brain Res. 2006;1096:125–137. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Huang M, Lee RR, Moses SN, Paulson KM, Thoma RJ, Miller GA, Cañive JM. A non-invasive method for observing hippocampal function. NeuroReport. 2003;14:1957–1960. doi: 10.1097/00001756-200310270-00015. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Yeo RA, Huang M, Lee RR, Thoma RJ, Moses SN, Paulson KM, Petropoulos H, Miller GA, Cañive JM. A specific test of hippocampal deficit in schizophrenia. Behav Neurosci. 2005;119:863–875. doi: 10.1037/0735-7044.119.4.863. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Euler MJ, Bantz RL, Lundy SL, Thoma RJ, Weisend MP, Mayer AR, Bustillo JR, Miller GA, Tesche CD. Assessment of lateralized hippocampal function in schizophrenia. International Congress on Schizophrenia Research Abstracts 2007 [Google Scholar]
- Huang MX, Shih JJ, Lee RR, Harrington DL, Thoma RJ, Weisend MP, Hanlon FM, Paulson KM, Li T, Martin K, Miller GA, Cañive JM. Commonalities and differences among vectorized beamformers in electromagnetic source imaging. Brain Topogr. 2004;16:139–158. doi: 10.1023/b:brat.0000019183.92439.51. [DOI] [PubMed] [Google Scholar]
- Ioannides AA, Liu MJ, Liu LC, Bamidis PD, Hellstrand E, Stephan KM. Magnetic field tomography of cortical and deep processes: examples of “real time mapping” of averaged and single trial MEG signals. Int J Psychophysiol. 1995;20:161–175. doi: 10.1016/0167-8760(95)00031-3. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Peterson SE. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Achenbach C, Kirsch M, Heinzmann M, Schienle A, Vaitl D. Cerebellar and hippocampal actiation during eyeblink conditioning depends on the experimental paradigm: a MEG study. Neural Plast. 2003;10:291–301. doi: 10.1155/NP.2003.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, McDaniel MA, Guynn MJ, Houck JM, Woodruff CC, Bish JP, Moses SN, Kicić D, Tesche CD. Brain regions and their dynamics in prospective memory retrieval: a MEG study. Int J Psychophysiol. 2007;64:247–258. doi: 10.1016/j.ijpsycho.2006.09.010. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behav Neurosci. 1995;109:579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of function brain images using partial least squares. NeuroImage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Mikuni N, Nagamine T, Ikeda A, Terada K, Taki W, Kimura J, Kikuchi H, Shibasaki H. Simultaneous recording of epileptiform discharges by MEG and subdural electrodes in temporal lobe epilepsy. NeuroImage. 1997;5:298–306. doi: 10.1006/nimg.1997.0272. [DOI] [PubMed] [Google Scholar]
- Moses SN, Cole C, Ryan JD. Relational memory for object identity and spatial location in rats with lesions of perirhinal cortex, amygdala and hippocampus. Brain Res Bull. 2005;65:501–512. doi: 10.1016/j.brainresbull.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ostreicher ML, Rosenbaum RS, Ryan JD. Successful transverse patterning in amnesia using semantic knowledge. Hippocampus. 2008;18:121–124. doi: 10.1002/hipo.20378. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Nishitani N, Ikeda A, Nagamine T, Honda M, Mikuni N, Taki W, Kimura J, Shibasaki H. The role of the hippocampus in auditory processing studied by event-related electric potentials and magnetic fields in epilepsy patients before and after temporal lobectomy. Brain. 1999;122:687–707. doi: 10.1093/brain/122.4.687. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Ostreicher ML, Moses SN, Rosenbaum RS, Ryan JD. Unpublished manuscript in review. Remediation of age-related deficits in relational memory [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Katz JS, Wright AA. The hippocampus and memory of verbal and pictorial material. Learn Memory. 2002;9:99–104. doi: 10.1101/lm.44302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki M, Tanila H, Könönen M, Hänninen T, Hämäläinen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal subareas in humans. Eur J Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired transverse patterning in human amnesia is a special case of impaired memory for two-choice discrimination tasks. Behav Neurosci. 1996;113:3–9. doi: 10.1037//0735-7044.113.1.3. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Grafman J. Losing their configural mind: Amnesic patients fail on transverse patterning. J Cogn Neurosci. 1998;10:509–516. doi: 10.1162/089892998562915. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Verfaellie M, Grafman J. Transverse patterning and human amnesia. J Cogn Neurosci. 2006;18:1723–1733. doi: 10.1162/jocn.2006.18.10.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs L, Moses SN, Bardouille T, Herdman AT, Ross B, Ryan JD. A complementary analytic approach to examining medial temporal lobe sources using magnetoencephalography. NeuroImage. 2009;45:627–642. doi: 10.1016/j.neuroimage.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. The contribution of long-term memory and the role of frontal-lobe systems in on-line processing. Behav Brain Sci. 2003;26:756. [Google Scholar]
- Ryan JD, Cohen NJ. The nature of change detection and on-line representations of scenes. J Exp Psychol : Hum Percept Perform. 2004;30:988–1015. doi: 10.1037/0096-1523.30.5.988. [DOI] [PubMed] [Google Scholar]
- Saksida LM, Busey TJ, Buckmaster CA, Murray EA. Impairment and facilitation of transverse patterning after lesions of the perirhinal cortex and hippocampus, respectively. Cereb Cortex. 2007;17:108–115. doi: 10.1093/cercor/bhj128. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Ranken DM, Aine CJ, Weisend MP, Shih JJ. Differentiability of simulated MEG hippocampal, medial temporal and neocortical temporal epileptic spike activity. J Clin Neurophysiol. 2005;22:388–401. [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proc Natl Acad Sci U S A. 2006;21:8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche CD. Non-invasive detection of ongoing neuronal population in normal human hippocampus. Brain Res. 1997;749:53–60. doi: 10.1016/s0006-8993(96)01286-3. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Karhu J. Interactive processing of sensory input and motor input in the human hippocampus. J Cogn Neurosci. 1999;11:424–436. doi: 10.1162/089892999563517. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci U S A. 2000;97:919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche CD, Karhu J, Tissari SO. Non-invasive detection of neuronal population activity in human hippocampus. Cogn Brain Res. 1996;4:39–47. doi: 10.1016/0926-6410(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE. Processing objects at different levels of specificity. J Cogn Neurosci. 2004;16:351–362. doi: 10.1162/089892904322926692. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldrige LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. NeuroReport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]


