Abstract
Impaired P50 gating is thought to reflect a core deficit in schizophrenia, but the relevant neural network is not well understood. The present study used EEG and MEG to assess sensory gating and volumetric MRI to measure hippocampal volume to investigate relationships between them in 22 normal controls and 22 patients with schizophrenia. In the schizophrenia group, anterior but not posterior hippocampal volume was smaller, and both the P50 and M50 gating ratios were larger (worse) than in controls. Independent of group, left-hemisphere M50 gating ratio correlated negatively with left anterior hippocampal volume, and right-hemisphere M50 gating ratio correlated negatively with right anterior hippocampal volume. Schizophrenia diagnosis predicted M50 gating independent of hippocampal volume. These results are consistent with the finding that hippocampus is a critical part of a fronto-temporal circuit involved in auditory gating.
Descriptors: Magnetic resonance imaging (MRI), Hippocampus, Schizophrenia, Sensory gating, Electroencephalography (EEG), Magnetoencephalography (MEG)
Hippocampal structure and function are abnormal in schizophrenia (for recent reviews, see Goldman & Mitchell, 2004; Harrison, 2004). The most conspicuous functional abnormalities associated with hippocampal damage are deficits in learning and memory (especially relational mnemonic ability; Cohen & Eichenbaum, 1993; Moses, Cole, Driscoll, & Ryan, 2005; Rudy & Sutherland, 1995; Rudy & Sutherland, 1989). However, hippocampus is also known to subserve cognition in other ways. For example, there is evidence that hippocampus serves as part of neural networks involved in novelty detection (Knight, 1996; Moses & Ryan, 2006), in orienting attention toward novel auditory stimuli (Knight, 1996), and in novel target detection in tasks such as dichotic listening (Pollmann, Lepsien, Hugdahl, & von Cramon, 2004) and the oddball paradigm (Crottaz-Herbette, Lau, Glover, & Menon, 2005; Paller, McCarthy, Roessler, Allison, & Wood, 1992; Tesche, Karhu, & Tissari, 1996). Hippocampus is also known to be critical for the gating of sensory responses to stimuli. It has been well established that hippocampus is involved in prepulse inhibition (PPI) of startle, sometimes known as sensorimotor gating (Bast & Feldon, 2003; Caine, Geyer, & Swerdlow, 1992; Swerdlow et al., 2001; Zhang, Bast, & Feldon, 2002a, 2002b). However, whether and how hippocampus is involved in gating of the auditory event-related brain potential (ERP) P50 component in a paired-click paradigm is an open question.
Impaired P50 sensory gating has been described as the most robust physiological finding in schizophrenia research (Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; Heinrichs, 2004) and is associated with sensory overload and disruption of higher-order cognitive processing. Gating is typically assessed in a paired-click paradigm, in which two identical click stimuli are played in succession and the P50 response following each is measured. Normally, individuals show a reduced P50 response following the second click, and this reduction of the second response is called sensory gating. Through the simultaneous collection of electroencephalographic (EEG) and magnetoencephalographic (MEG) data, deficits in both P50 (EEG) and left-hemisphere M50 (MEG) gating have been found in schizophrenia patients (Hanlon et al., 2005; Huang et al., 2003; Thoma et al., 2003). Adler et al. (1998) proposed that P50 gating is hippocampal dependent. Studies assessing the rat P50 analog, N40, have shown hippocampal involvement in gating (Adler, Rose, & Freedman, 1986; Bickford-Wimer et al., 1990), and human studies have documented a relationship between hippocampal volume and the presence of a P50 ERP sensory gating deficit in schizophrenia (Waldo et al., 1994) and in traumatic brain injury (Arciniegas et al., 2001). However, because P50 is traditionally measured at a single EEG electrode (Cz), little information is available about hemispheric gating differences and the role the hippocampus may play in them. MEG source localization renders it possible to assess auditory gating in terms of separate right- and left-hemisphere M50 dipoles localizing to superior temporal gyrus (STG; Hanlon et al., 2005; Thoma et al., 2003, 2004, 2005). Research in our laboratory has shown that ERP P50 is primarily a function of neural generators in right- and left-hemisphere STG, best assessed as MEG source dipoles (Edgar et al., 2003; Huang et al., 2003). Presumably, assessing sensory gating in terms of the neural generators instead of a more general scalp ERP measure allows more direct assessment of gating in terms of neural function. M50 sensory gating, assessed during a traditional paired-click paradigm, has resulted in new findings validating sensory gating as a marker of information processing in schizophrenia. For example, left-hemisphere M50 gating is correlated with neuropsychological impairment (Thoma et al., 2003) and positive symptoms (Ricker et al., 2004) in schizophrenia, whereas right-hemisphere M50 gating predicts negative symptoms (Thoma et al., 2005). Further, Hanlon et al. found relationships between M50 and M100 gating within but not across hemispheres, suggesting an intrahemispheric gating circuit.
P50/M50 is dependent on a larger, distributed network, the design of which is integrated with learning and memory systems (Adler et al., 1998; Grunwald et al., 2003). In the animal model, N40 can be robustly recorded from the surface of the skull (Adler et al., 1986, 1998; Bickford-Wimer et al., 1990). Importantly, the rat and mouse N40 component behaves analogously to the human P50 in gating studies and shows “normal” gating in a non-stressed situation (Adler et al., 1986, 1998; Bickford-Wimer et al., 1990; Freedman et al., 1994), with the amplitude of the response to the second stimulus in the pair about 33% of the amplitude of the response to the first stimulus. When depth electrodes are used, there is a polarity reversal of N40 as the electrode passes through hippocampal CA3, indicating that hippocampus is one probable source of the scalp waveform (Bickford-Wimer et al., 1990). These researchers have shown that inhibitory gating in the animal model depends on an intact cholinergic pathway from the septum, interfacing with nicotinic alpha-7 receptors on GABA-b interneurons in hippocampal CA3 (Frazier, Buhler, Weiner, & Dunwiddie, 1998a, Frazier, Rollins, et al., 1998b; Hershman, Freedman, & Bickford, 1995; Miller & Freedman, 1995).
Hippocampal involvement in gating during the paired-click paradigm has also been investigated in research with human subjects. An intracranial study, using hippocampal depth electrodes and subdural strip and grid electrodes in epilepsy patients, documented relatively synchronous gating of 50-ms activity in prefrontal cortex (PFC) and STG sources, followed by sensory gating in hippocampus around 250 ms (Boutros et al., 2005; Grunwald et al., 2003). Hippocampal abnormality in schizophrenia has been associated with abnormal gating in functional imaging studies, and Huang et al. (2003) implicated abnormal gamma-band activity, possibly generated in hippocampus, as a correlate of impaired gating in schizophrenia. Some neural generators of gating have been well defined (i.e., gating of the response in STG; Edgar et al., 2003; Hanlon et al., 2005; Huang et al., 2003; Thoma et al., 2003, 2004, 2005), but the role of hippocampus in sensory gating remains unclear.
The present study addressed the question of hippocampal involvement in the schizophrenia sensory gating deficit, specifically a relationship between hemisphere-specific M50 auditory sensory gating ratios and hippocampal volume. It was predicted that in schizophrenia patients (1) P50 and left-hemisphere M50 gating would be impaired, (2) hippocampal volume would be smaller, and (3) hippocampal volume would correlate with the extent of P50 and M50 gating impairment. Further, (4) it was predicted that this effect would be most robust within hemispheres. That is, left-hemisphere gating would correlate more with left-hemisphere hippocampal volume, and right-hemisphere gating would correlate more with right-hemisphere hippocampal volume.
Methods
Participants
Twenty-two schizophrenia and 22 healthy control participants matched on age, education, and gender participated in this study (see Table 1). Group membership was determined with the Structured Clinical Interview for DSM-IV, Clinician Version (SCID-CV; First, Spitzer, Gibbon, & Williams, 1997). No participant had a history of head injury, neurological disorder, or severe medical illness.
Table 1.
Demographics
| Control | Schizophrenia | t (p) | |
|---|---|---|---|
| Age (years) | 41.55 | 39.38 | 0.52 (p = .61) |
| Sex | Male = 16 | Male = 17 | 1.03 (p = .31) |
| Female = 6 | Female = 5 | ||
| Education (years) | 13.56 | 12.47 | 1.47 (p = .15) |
The schizophrenia group consisted of volunteers, some referrals, who were relatively stable outpatients well known to their providers. They met criteria for clinical stability, treatment with the same antipsychotic medications for at least 3 months and no inpatient stays during the past year. Absence of other current psychiatric disorders was determined via SCID-CV. One schizophrenia subject was removed from the sample due to implausibly high S2 amplitude resulting in an extreme value for gating ratio (S2/S1 = 5.47, more than 3 standard deviations above the group mean value). All patients with schizophrenia were taking antipsychotic medications, either haloperidol (n = 4), olanzapine (n = 5), risperidone (n = 5), clozapine (n = 6), or quetiapine (n = 2).
The control group consisted of healthy volunteers recruited via advertisements in local newspapers. Control participants were screened with the SCID-CV for the presence of Axis I and Axis II psychopathology by a licensed clinical psychologist (Thoma) or a psychology graduate student under his direct supervision. During the clinical interview, potential control participants were asked if they had a first-degree relative with schizophrenia or other psychotic disorder and if so were not included in the study.
Institutional Review Board approval was obtained before the experiment commenced, and volunteers were informed that they could leave the study at any time. Appropriate informed consent was obtained from all participants.
Magnetic Resonance Images
All structural magnetic resonance images (MRI) were collected with a 1.5 Tesla Picker Edge Imager at the New Mexico Veterans Affairs Health Care System Magnetic Source Imaging Center. A three-dimensional gradient echo pulse sequence was used to acquire sagittal section with the following parameters: TR = 15 ms; TE = 4.4 ms; FOV = 256 mm; 192 × 256 matrix, flip angle = 25°; slice thickness = 1.5 mm, no gap.
Structural MRI Analysis
MRIs were first resliced into coronal images 1.0 mm thick. The skull was then stripped from each MRI using Brain Extraction Tool software (BET: fMRIB Image Analysis Group, Oxford, UK). Intracranial volume was calculated from the mask produced from this program. Images were then segmented using an automated k-means clustering segmentation algorithm, and the volumes of gray matter (GM; not including cerebellum), white matter (WM; not including cerebellum), cerebrospinal fluid (CSF), and total intracranial brain volume (ICV; including cerebellum) were determined by the number of pixels in each of their respective clusters (Petropoulos, Sibbitt, & Brooks, 1999). Pixels that could not be assigned exclusively to GM or CSF were considered partial volume (PV). The number of PV pixels was divided in half and then added to the GM for a final count.
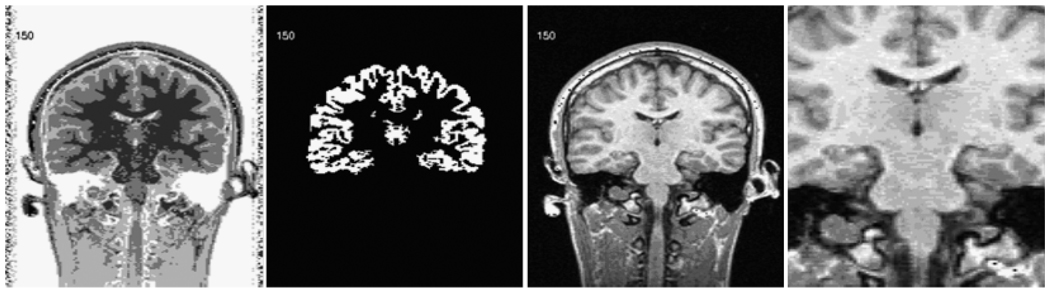
The k-means algorithm segmented GM from WM to assist raters in selecting the hippocampus. As a result, hippocampal white matter was excluded from the overall hippocampal volume measurement. Two independent raters used Segmentation And Visualization for Research Advancement (SAVRA; Petropoulos et al., 1999) interactive software to conduct the volumetric assessment of hippocampus using the already k-means segmented coronal T1-weighted images. SAVRA allows the user to select a segmented brain section (Figure 1), magnify the area of interest, and remove sections of segmented data from the volumetric analysis, thus allowing specification of brain structures and easy quantification of the selection via a pixel counting algorithm.
Figure 1.
SAVRA (Segmentation And Visualization for Research Advancement) software made several views of brain tissue available simultaneously for each slice to enhance users’ judgment regarding tissue selection.
Raters followed the anatomical guidelines of Watson et al. (1992) to measure hippocampal volume, except for the posterior hippocampal definition. Watson et al. defined the most posterior slice as where the crux of the fornix separates from the hippocampus. This method, however, eliminates the most posterior slices of the hippocampus. Consequently, the most posterior slice was defined in this study as the slice where the hippocampus connects laterally to the lateral ventricle and medially to the midline. Hippocampal volume was determined for total, right, left, anterior (anterior nine slices), and posterior (posterior nine slices) regions (Maguire et al., 2000). Total hippocampal volume was the sum of the measurements collected for the images available, around 40 (1.0-mm) slices. The mean of the measurements from the two raters was used. Interrater reliability between two raters was established in a subset of 20 hippocampi (alpha = .82).
MEG Data Collection
Subjects were asked to refrain from smoking for at least 1 h before the examination. To ensure compliance, subjects were asked to report to the facility an hour before recordings commenced. During that time, subjects were familiarized with the equipment and procedures and were prepared for the data recording session. MEG data were recorded in a magnetically and electrically shielded room using a whole-cortex, 122-channel biomagnetometer system (Elekta-NeuroMag, Helsinki, Finland). At the start of each test session, subjects were fitted with an electrode cap to which three small coils were attached. These coils provided specification of the position and orientation of the MEG sensors relative to the head. Nineteen EEG channels (referenced to right mastoid and re-referenced off-line to linked mastoids; Miller, Lutzenberger, & Elbert, 1991) and a bipolar oblique electrooculogram (EOG) channel were recorded simultaneously with MEG. Electrode impedances were maintained below 10 kΩ. Because it was important that the subject’s head remain fixed in the same place in the dewar across the recording session, a number of precautions were taken to ensure head stability. Patients were comfortably seated in a reclining, padded, nonmagnetic chair. Foam wedges were inserted between the subjects’ face and the inside of the dewar. In addition, a Velcro strap running under the subject’s chin and a knee bolster under the subject’s legs were used to ensure immobility and comfort. The MRI data described above were also used for MEG localization purposes.
Paired-Click Design
Stimuli were presented in pairs (S1, S2) with a 500-ms interstimulus interval and an intertrial interval between S1 onsets that varied randomly in 1-s steps between 8 and 12 s. Stimuli were binaural 3-ms clicks, created and delivered using NeuroStim software and Etymotic earphones placed in the subject’s ear canal. Before data collection, each subject’s hearing threshold was determined, and peak click intensity was set 30 dB above threshold. To minimize motion-related artifact produced by the plastic tubes connected to the Etymotic earphones, the tubes were taped to the subject’s face and ear. Uncontaminated click-pair epochs (N = 150) were collected during approximately 30 min, with some variability in number of trials presented depending on the number of epochs rejected online because of artifact. Epochs were rejected if peak-to-peak signal strength exceeded 150 µV in any EOG or EEG channel or 3000 fT in any MEG channel. EEG and MEG data were digitized at 500 Hz per channel for 1000 ms beginning 100 ms before S1.
EEG Data Analysis
Filters for EEG data analysis were designed to approximate those described by Adler et al. (1982) in order to enable a comparison of present results with those of that group’s many sensory gating studies. For P50 peak scoring, cross-trial average ERPs were digitally filtered using Chebyshev Type 2 IIR filters with a 4-point moving-average and a recursive high-pass filter (A = 0.85; Coppola, 1979). Filter parameters were as follows: Fstop = 1 Hz, Fpass = 4 Hz for the high-pass and Fstop = 60 Hz, Fpass = 55 Hz for the low-pass. The ripple for the pass band (4–55 Hz) was less than 1 dB, and signal loss for the stop bands (0.1 and 0.60 Hz) was more than 60 dB. Each filter was applied twice, in the forward and reverse direction, to increase roll-off and to preserve waveform latency by avoiding the introduction of a phase shift. The prestimulus baseline (− 100 to − 10 ms) was removed.
P50 was defined as the most positive peak at Cz between 40 and 80 ms after S1 onset. If two equal-amplitude peaks were present, the later peak was scored, but this was unnecessary in the present sample. Amplitude was measured relative to the immediately preceding negativity. Thus, S1 amplitude was calculated as the difference between the positive peak and immediately preceding negative trough. Following Judd, McAdams, Budnick, and Braff (1992), this trough did not have a latency of less than 30 ms following onset of S1 (i.e., the trough search is stopped if a horizontal tangent line is not encountered by 30 ms poststimulus, and the 30-ms point is then used as the start of the P50 component). For the S2 score, the most positive point at Cz following S2 onset within 10 ms of the latency of the S1 P50 peak was selected. This range ensured that the same response was measured for S1 and S2. If there was no peak in that range, the amplitude was scored as zero. As with S1, the S2 score was calculated relative to the immediately preceding negative trough. P50 gating ratio was calculated as S2 amplitude divided by S1 amplitude.
MEG Source Localization
To coregister MEG and MRI data, three anatomical landmarks (nasion and right and left preauriculars) were measured for each subject by using the Probe Position Identification System (Polhemus; Colchester, VT) before data collection. The same three points were identified in the subject’s MRI, and a subject-specific transformation matrix that involved rotation and translation between the MEG and MRI coordinate systems was used. To increase the reliability of the MEG–MRI coregistration, approximately 50 points from across the scalp were digitized with the Polhemus system, colocalized to the three anatomical landmarks, and stored as part of the MEG data file. In a two-step procedure, first the three standard landmarks were initially fitted to each individual’s 3D MRI head and, second, the 50 additional points were used to fine tune coregistation, allowing additional points of reference from scalp, face, and ears.
A 5–55-Hz bandpass filter and a − 100 to − 10 ms baseline adjustment were applied to each subject’s cross-trial-averaged MEG data. A spherical MEG head model (Cardenas, Gerson, & Fein, 1993) was used for dipole source modeling in which a sphere is fitted to the inner surface of the skull (obtained from each subject’s structural MRI). The inverse problem of MEG source localization involves reconstructing the sources for a given magnetic field distribution measured by an array of MEG sensors. The equivalent current dipole model was adopted, with the assumption that the neuronal sources were focal. Dipolar sources were identified in left and right hemisphere for S1 M100 responses to each click (a dipole oriented downward peaking 80–120 ms poststimulus). M50 was defined as the first upward-oriented dipole occurring before M100, 40–80 ms poststimulus. Determination of the strength, location, and peak latency of the M50 source in the left and right hemispheres was accomplished by fitting each dipole separately over the left and right hemispheres using subsets of 34 planar gradiometers over each temporal lobe. For modeling S1 M50, 4 ms of data before the M50 peak and 4 ms of data following the M50 peak were selected. Equivalent current dipoles were then determined separately for each hemisphere by using the aforementioned source localization routines. Only equivalent current dipoles with goodness-of-fit values (a measure of the correlation between calculated and measured signal) exceeding 75% for S1 were accepted. Peak strength of the source over the 8-ms period was then determined. S2 M50 was identified by using a procedure outlined by Cardenas et al. (1993), in which the location of the S2 dipole was assumed to be the same as that of the S1 dipole, and the strength of S2 M50 was determined over the same 8-ms period. M50 suppression for each hemisphere was expressed as a ratio similar to that of P50: S2 dipole peak strength divided by S1 dipole peak strength.
Results
P50 Sensory Gating Ratio
A t test was used to test for group differences in ERP P50 sensory gating ratios. The schizophrenia group (M = .56, SD = .36) was found to have larger P50 sensory gating ratio than the control group (M = .34, SD = .18), t(42) = 2.351, p = .024. S1 and S2 P50 latency and amplitude did not differ by group.
M50 Sensory Gating Ratio
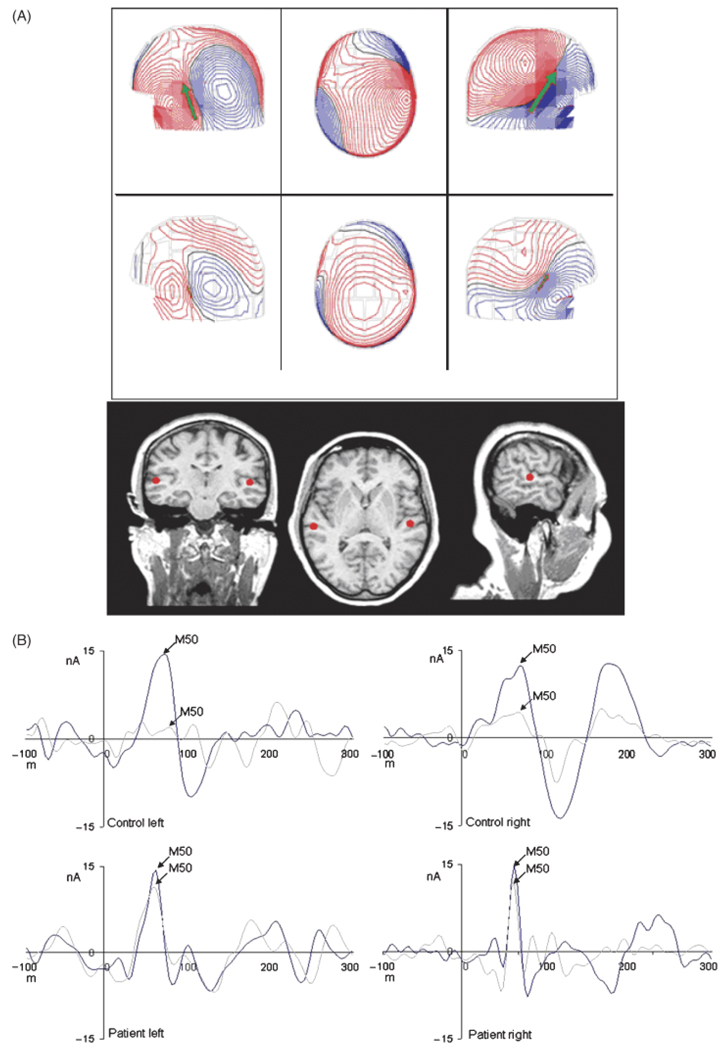
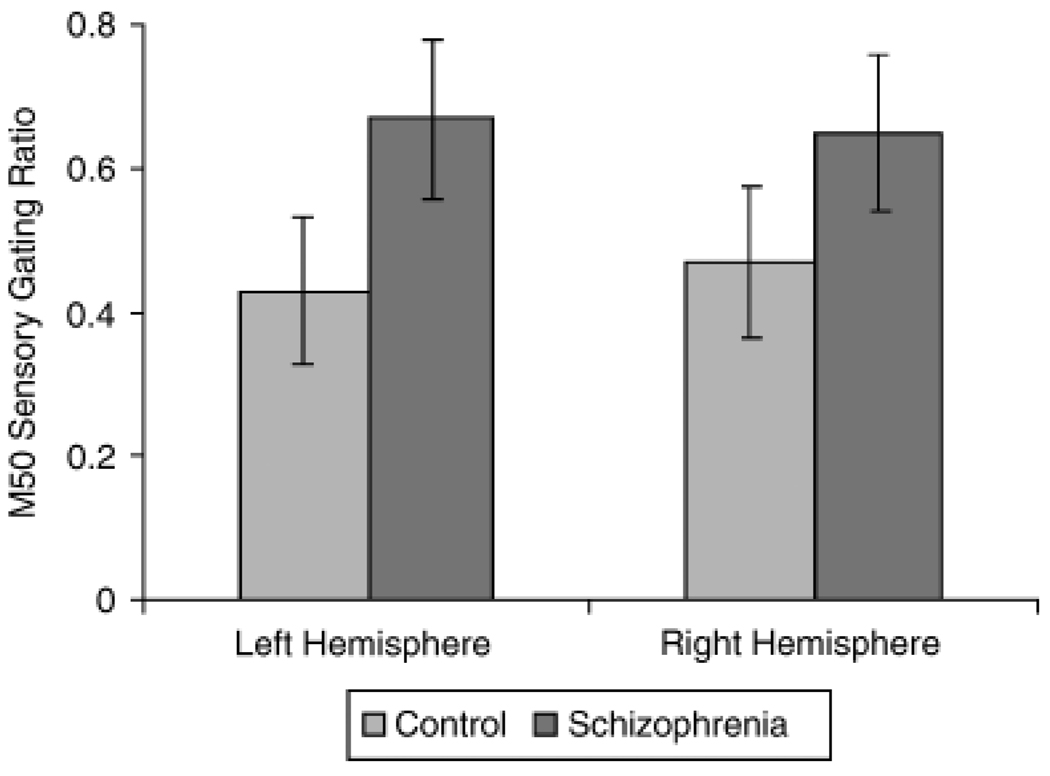
For every subject, two source dipoles associated with M50 reliably localized to bilateral auditory cortex in left and right STG (see Figure 2). A Group (controls and patients) × Hemisphere (right and left STG) ANOVA indicated that gating ratios for the schizophrenia group (M = .67, SD = .19) were larger than those for the control group (M = .44, SD = .17), F(1,41) = 15.116, p < .001 (see Figure 3). There was no hemisphere or Group X Hemisphere effect.
Figure 2.
A: An example of the magnetic field pattern and cortical localization for left and right M50 equivalent current dipoles. For all subjects, the M50 response localized bilaterally to auditory cortex. B: An example of left and right hemisphere equivalent current dipole waveforms for a representative subject from the control group and one subject from the schizophrenia group.
Figure 3.
M50 sensory gating is expressed as a ratio of S2/S1. M50 sensory gating was impaired bilaterally in the schizophrenia group (error bars = 1 SD).
M50 Amplitude and Latency
A Group × Hemisphere × Stimulus (S1 and S2) ANOVA testing M50 amplitude (nAm) confirmed overall gating, with S2 smaller than S1, F(1,41) = 147.027, p < .001. A Group × Stimulus interaction, F(1,41) = 9.815, p = .003, indicated that the group difference in gating was carried entirely by S2:S1 amplitude and was the same for the control (M = 14.34 nAm, SD = 6.64) and schizophrenia (M = 14.21 nAm, SD = 6.69) groups, whereas controls’ S2 (M = 6.54 nAm, SD = 4.40) was smaller than patients’ S2 (M = 9.52, SD = 8.22). No other effects approached significance. For M50 latency (ms), no main effects or interactions approached significance.
Hippocampal Volume
A Group × Hemisphere × Region ANOVA for hippocampal volume found a main effect for Region, with anterior hippocampus larger than posterior hippocampus, F(1,41) = 229.702, p < .001. Table 2 provides simple-effects tests exploring a Group × Region interaction, F(1,41) = 4.29, p = .045, showing that groups differed only in anterior hippocampus (patients smaller). Left anterior and left posterior hippocampal volumes were slightly smaller than the corresponding regions in the right hemisphere, with marginal significance, F(1,42) = 3.936, p = .054. Hemisphere × Region, Group × Hemisphere, and Group × Hemisphere × Region interactions were not significant.
Table 2.
Group Comparison of Volume of Hippocampus, ICV, GM, WM, and CSF in Cubic Centimeters
| Measurement | Control mean (SD) | Schizophrenia mean (SD) | F | P value |
|---|---|---|---|---|
| Anterior hippocampus | 1.93 (0.53) | 1.61 (0.37) | 5.28 | .027 |
| Posterior hippocampus | 0.70 (0.24) | 0.72 (0.28) | 0.034 | .854 |
| Total hippocampus | 7.17 (1.04) | 6.62 (1.07) | 2.83 | .100 |
| Intracranial volume | 1514.40 (144.55) | 1465.68 (150.71) | 0.395 | .552 |
| Gray matter | 539.05 (59.82) | 512.76 (76.56) | 0.713 | .403 |
| White matter | 556.28 (73.88) | 545.09 (71.31) | 0.209 | .650 |
| Cerebrospinal fluid | 181.39 (38.00) | 190.552 (35.08) | 1.914 | .173 |
A second ANOVA was used to rule out the possibility that group differences in overall ICV account for the difference in hippocampus volume. There was no group difference in ICV (see Table 2). When ICV was added to the Group × Hemisphere × Region ANOVA as a covariate (appropriate because the groups did not differ on the covariate; Miller & Chapman, 2001), anterior hippocampal volume remained smaller in the schizophrenia group, F(1,40) = 4.416, p = .024.
P50 Gating and Hippocampal Volume Relationships
Hierarchical regressions were done separately for the two hemispheres, each investigating whether there were relationships between P50 gating ratio and hippocampal volume. P50 gating was first regressed on left anterior hippocampal volume, R2 = .145, p = .013. When added second to the model, group did not account for additional variance. When added third to the model, the Group × Left Anterior Hippocampal Volume interaction term did not account for additional variance.
P50 gating was regressed on right anterior hippocampal volume, R2 = .234, p = .001. When added second and third to the model, neither group nor the Group × Right Anterior Hippocampal Volume interaction accounted for additional variance.
M50 Gating and Hippocampal Volume Relationships
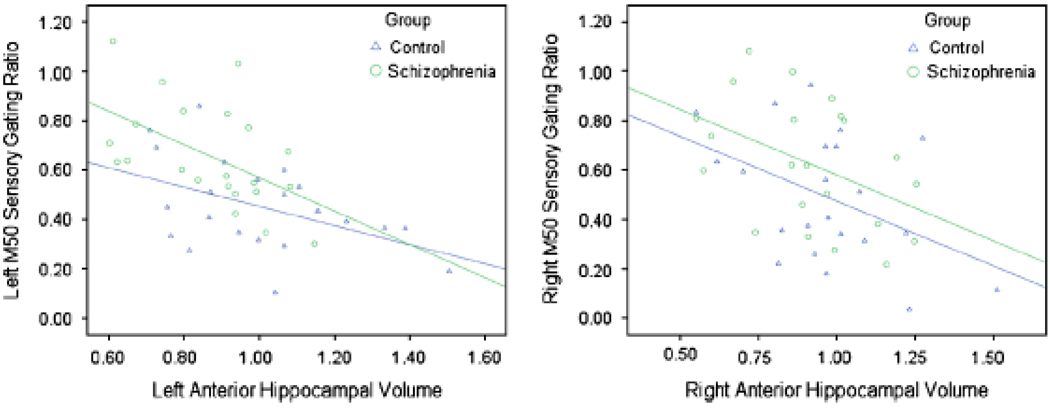
A similar regression approach was used with M50. In combination, left anterior hippocampal volume and diagnostic group controlled substantial variance in left M50 gating ratio, R2 = .400, p < .001. Each predictor added unique variance (volume ΔR2 = .312, p < .001, see Figure 4; group, R2 = .09, p = .018). Importantly, then, patients had higher gating ratios than controls even with hippocampal volume variance removed. When added third to the model, the Group × Left Anterior Hippocampal Volume interaction term did not account for additional variance.
Figure 4.
Scatterplots depicting the relationship between anterior hippocampal volumes and M50 sensory gating ratios in the control and schizophrenia groups.
To investigate a possible independent effect for contralateral right-hemisphere M50 gating ratio, the regression was reversed, and left-hemisphere hippocampal volume was regressed on gating ratios. Left-hemisphere M50 gating ratio had a significant effect (see above). When right-hemisphere M50 gating ratio was added as a second predictor (with left-hemisphere M50 gating ratio added first), right-hemisphere M50 gating ratio did not account for additional variance.
In the right-hemisphere analysis, right-hemisphere M50 gating ratio was the dependent variable. In combination, right anterior hippocampal volume and group controlled significant variance in right M50 gating ratio, R2 = .221, p = .009. Each predictor added unique variance (volume R2 = .142, p = .015, see Figure 4; group, R2 = .08, p = .057). Importantly, then, patients had higher gating ratios than controls even with hippocampal volume variance removed. When added third to the model, the Group × Right anterior hippocampal volume interaction term did not account for additional variance.
To investigate a possible independent effect for contralateral left-hemisphere M50 gating ratio, the regression was reversed, and left-hemisphere hippocampal volume was regressed on gating ratios. Right-hemisphere M50 gating ratio had a significant effect (see above). When left-hemisphere M50 gating ratio was added as a second predictor (with right-hemisphere M50 gating ratio added first), right-hemisphere M50 gating ratio did not account for additional variance.
To investigate a possible independent effect of left-hemisphere M50 gating ratio, it was added second to the model (with right-hemisphere anterior hippocampal volume added first). Left-hemisphere M50 gating ratio did not account for additional variance in right-hemisphere M50 ratio.
Although not a primary hypothesis, t tests were used to investigate possible differences in sensory gating ratios and volumes in patients taking typical versus atypical antipsychotic medications. Patients prescribed atypical antipsychotic medications had smaller (better) gating ratios in both hemispheres, but the group difference reached significance only in the left, t(19) = 2.23, p = .038. Patients prescribed atypical antipsychotic medications had larger anterior hippocampal volumes in both hemispheres, but the group difference only approached significance in the left hemisphere, t(19) = − 1.86, p = .082.
Intra-run responses and gating ratios are known to be stable across relatively long MEG data collection sessions (Lysne et al., 2006). However, if there were group differences in session length, it is conceivable that attentional effects or differential habituation would skew results. Session length was recorded for unselected subgroups of 12 controls and 9 patients. There was not a significant difference in mean run time (control M = 30.42 min, SD = 2.67; patient M = 33.11 min, SD = 4.76), t(19) = − 1.65, p = .115, indicating comparable session lengths and number of trials in schizophrenia and control subjects.
Discussion
There was a sensory gating deficit in the schizophrenia group, whether assessed with P50, left-hemisphere M50, or right-hemisphere M50. Huang et al. (2003) demonstrated that the ERP P50 in a paired-click paradigm is largely comprised of signal generated by two sources localizing to left and right STG auditory regions. Considering gating in terms of lateralized sources, it has been shown that left-hemisphere M50 gating was related to attention and working memory deficits in schizophrenia (Thoma et al., 2003), and that right-hemisphere M50 gating was associated with schizophrenia negative symptoms (Thoma et al., 2005). Measurement of gating in terms of individual neural generators affords greater specificity of measurement and has resulted in the discovery of relationships between gating and clinical variables that were not evident in single-electrode ERP studies. In the present study, this approach allowed investigation of structure–function relationships between hippocampal volume and lateralized gating ratios within hemispheres.
It is thought that gating is dependent on a fronto-temporal network comprised of prefrontal, medial frontal, STG, and medial temporal cortex (Grunwald et al., 2003). Based on an assumption that the extent of hippocampal impairment in schizophrenia could be assessed in terms of reduced hippocampal volume, the present hypothesis was that reduced size of hippocampus in that group would reduce the functional capacity of the entire network—and that reduced function would be measurable as poorer gating. Indeed, gating accounted for significant variance in ipsilateral anterior hippocampal volume, not just for patients but for the control group. That is, smaller anterior hippocampal volume occurred with higher M50 gating ratios for both groups. This effect was hemisphere specific: left-hemisphere M50 gating accounted for variance only in left-hemisphere anterior hippocampal volume, and right-hemisphere M50 gating accounted for variance only in right-hemisphere hippocampal volume.
Waldo et al. (1994) demonstrated an indirect association between hippocampal volume and P50 gating in schizophrenia. Although no correlations were reported, a group of schizophrenia-spectrum patients with reduced hippocampal volume also had impaired gating. The improved specificity of measurement provided by dense-array MEG revealed the presence of positive correlations between hippocampal volumes and functional gating measures in the present data. This effect was found specifically for anterior hippocampus, whereas Waldo et al. did not distinguish subhippocampal regions, working only with whole hippocampal volumes. There is considerable evidence based on animal studies demonstrating the importance of hippocampus for sensory gating. For example, it has been well established that hippocampus is involved in PPI of startle, a more comprehensively studied gating paradigm than that associated with P50 (Bast & Feldon, 2003; Caine et al., 1992; Perlstein, Fiorito, Simons, & Graham, 1993; Perlstein, Simons, & Graham, 2001; Swerdlow et al., 2001; Zhang, Bast, & Feldon, 2002a, 2002b). There is strong evidence that ventral hippocampus plays a role in the gating of the startle response in rats (Caine et al., 1992; Swerdlow et al., 2001, 2004). Rat ventral hippocampus is analogous to anterior hippocampus in humans. There is also evidence of entorhinal cortex involvement in PPI (Goto, Ueki, Iso, & Morita, 2002; Swerdlow et al., 2001), which led these researchers to suggest that polymodal sensory information, exchanged by entorhinal cortex and ventral hippocampal CA3, is vital for any gating effect to occur. Neurochemical challenge studies also support the activities of these regions in the activity of a gating circuit. For example, Swerdlow et al. (1995) found increased sensitivity to the disruptive effects of apomorphine on PPI in rats with either medial PFC (mPFC) or ventral hippocampal lesions. Alternately, chemical stimulation of the ventral hippocampal-mPFC circuit disrupts PPI (Shoemaker et al., 2005). Entorhinal cortex has extensive, direct connections to mPFC. Entorhinal cortex may serve to integrate information from frontal cortex into ventral hippocampus. In the clinical literature, increasing evidence has demonstrated a robust link between sensory gating and the presence and extent of hippocampal nicotinic receptor functioning in schizophrenia (particularly in hippocampal CA3; Martin, Kem, & Freedman, 2004), all of which suggests that proper nicotinic receptor functioning is critical for normal sensory gating. The present results suggest that overall anterior hippocampal volume may reflect the quality and extent of hippocampal nicotinic receptor functioning.
That there is distinct hippocampal variance associated with gating was not surprising, but that patients with schizophrenia had higher gating ratios than controls, even with the hippocampal variance removed, indicates that significant schizophrenia variance remains to be accounted for in terms of brain structure or function. Based on this finding, further research is warranted to investigate other candidate brain regions that are known to be functionally impaired in schizophrenia. For example, intrahemispheric gating ratio has been linked to reduced schizophrenia STG cortical thickness (Thoma et al., 2004), and recent depth-electrode research in patients with epilepsy has suggested that medial frontal and sensory-motor cortex are also critical for gating (Kurthen et al., 2007).
There has been some evidence that anterior hippocampus is involved in novelty detection and encoding, whereas posterior hippocampus is more involved with familiarity (Strange, Fletcher, Henson, Friston, & Dolan, 1999; Zeineh, Engel, Thompson, & Bookheimer, 2003). However, it is not clear what the functional implications of having a small anterior hippocampus are in normal healthy control subjects. There has been considerable research in patients with schizophrenia suggesting that reduced hippocampus is an integral characteristic of the disorder (Csernansky et al., 1998; DeLisi, Dauphinais, & Gershon, 1988; Hanlon et al., 2005; Rossi et al., 1994; Shenton et al., 1992; Suddath et al., 1989, 1990). Reduced hippocampal volume is associated with abnormality on neuropsychological tests typically associated with impaired fronto-temporal function (Bilder & Degreef, 1991; Szeszko et al., 2002; Weinberger, Berman, Suddath, & Torrey, 1992). It has been suggested that the effect is primarily due to reduction in anterior hippocampal volume (Pegues, Rogers, Amend, Vinogradov, & Deicken, 2003). In present data, there was no difference in posterior hippocampal volume, accounting for ambiguity in earlier findings of reduced size of overall hippocampus (Szeszko et al., 2002). Szeszko et al. (2002) suggested that these neuropsychological findings make sense if one considers the extensive feedfoward and feedback pathways between hippocampus and PFC, further documenting a fronto-temporal disconnection in schizophrenia. Auditory gating has also been associated with neuropsychological measures typically associated with frontal lobe function (Thoma et al., 2003; Yee & White, 2001). Thus, a fronto-temporal disconnection may also account for the present results.
The present patient group was too small for strong conclusions about how medication type interacts with hippocampal volume and sensory gating. Typical antipsychotic medications appear to enlarge thalamic and striatal brain structures but have not yet been shown to have any direct effect on hippocampus volume or the volume of other cortical features (Flashman & Green, 2004). Atypical antipsychotic medications are thought to improve sensory gating ratios (Adler et al., 2004). In present data, patients on typical antipsychotic medications (n = 4) had smaller hippocampi and poorer left hemisphere sensory gating ratios than patients on atypical medications (n = 18). This was not a treatment study, and patients were accepted regardless of the type of antipsychotic prescribed by their doctor. Thus, it may be that smaller hippocampus is a marker for a subcategory of patients with schizophrenia that have a better response to typical medications.
Present findings demonstrate, first, that smaller anterior hippocampus volume relates to P50 and intrahemispheric M50 sensory gating. Contrary to prediction, this relationship was true for both groups. Thus, the second main finding of the present study is that the P50/M50 gating deficit in schizophrenia is not merely an artifact of diagnostic group differences in hippocampal volume: Diagnosis and hippocampal volume make distinct contributions to gating. Third, at least a portion of the relevant circuitry is intrahemispheric.
Normal reduction in the response to a second stimulus in a gating experiment is related to stimulus redundancy, and the gating effect is diminished or disappears entirely when the paired stimuli are dissimilar (Boutros, Belger, Campbell, D’Souza, & Krystal, 1999). Wepropose that, for successful gating to occur, at minimum, a healthy mPFC–hippocampal network is necessary, with mPFC involved in attentional control associated with stimulus perception and hippocampus involved in the modulation and preservation of stimulus traces. Future research may involve hippocampal lesion studies to investigate the necessity of hippocampus for intrahemispheric gating, investigation of relationships between responses in stimulus novelty paradigms associated with anterior hippocampal function and those involving gating, and further investigation of the role for frontal cortical regions in gating.
Acknowledgments
We thank Jessica Irwin, Fernando Torres, Robin Douglas, Kim Paulson, Ira Driscoll, and Laura Rowland for advice and assistance with all stages of this research. This project was funded by a NARSAD Young Investigator Award and NIAAA grant 1 K23 AA016544-01 to Dr. Thoma, an NIMH grant R01 MH-65304 to Dr. Cañive, and grants from the Mental Illness and Neuroscience Discovery (MIND) Institute to Drs. Thoma and Cañive. Helen Petropoulos was supported in part by National Institutes of Health grants NS039123, AG16418, NS35708, NS41390, and HD41237.
REFERENCES
- Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, et al. Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. American Journal of Psychiatry. 2004;161:1822–1828. doi: 10.1176/ajp.161.10.1822. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtmen E, Franks R, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Adler LE, Rose G, Freedman R. Neurophysiological studies of sensory gating in rats: Effects of amphetamine, phencyclidine, and haloperidol. Biological Psychiatry. 1986;21:787–798. doi: 10.1016/0006-3223(86)90244-1. [DOI] [PubMed] [Google Scholar]
- Arciniegas DB, Topkoff JL, Rojas DC, Sheeder J, Teale P, Young DA, et al. Reduced hippocampal volume in association with p50 nonsuppression following traumatic brain injury. Journal of Neuropsychiatry and Clinical Neuroscience. 2001;13:213–221. doi: 10.1176/jnp.13.2.213. [DOI] [PubMed] [Google Scholar]
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Progress in Neurobiology. 2003;70:319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, et al. Auditory sensory gating in hippocampal neurons: A model system in the rat. Biological Psychiatry. 1990;27:183–192. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Degreef G. Morphologic markers of neurodevelopmental paths to schizophrenia. In: Mednick SA, Canon TD, Barr CE, LaFosse JM, editors. Developmental neuropathology of schizophrenia. New York: Plenum; 1991. pp. 167–190. [Google Scholar]
- Boutros NN, Belger A, Campbell D, D’Souza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: A preliminary report. Psychiatry Research. 1999;88:119–130. doi: 10.1016/s0165-1781(99)00074-8. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Trautner P, Rosburg T, Korzyukov O, Grunwald T, Schaller C, et al. Sensory gating in the human hippocampal and rhinal regions. Clinical Neurophysiology. 2005;116:1967–1974. doi: 10.1016/j.clinph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Hippocampal modulation of acoustic startle and prepulse inhibition in the rat. Pharmacology Biochemistry and Behavior. 1992;43:1201–1208. doi: 10.1016/0091-3057(92)90503-8. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Gerson J, Fein G. The reliability of P50 suppression as measured by the conditioning testing ratio is vastly improved by dipole modeling. Biological Psychiatry. 1993;33:335–344. doi: 10.1016/0006-3223(93)90322-5. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Coppola R. Isolating low frequency activity EEG spectrum analysis. Electroencephalography and Clinical Neurophysiology. 1979;46:224–226. doi: 10.1016/0013-4694(79)90073-7. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Lau KM, Glover GH, Menon V. Hippocampal involvement in detection of deviant auditory and visual stimuli. Hippocampus. 2005;15:132–139. doi: 10.1002/hipo.20039. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proceedings of the National Academy of Sciences, USA. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Dauphinais ID, Gershon ES. Perinatal complications and reduced size of brain limbic structures in familial schizophrenia. Schizophrenia Bulletin. 1988;14:185–191. doi: 10.1093/schbul/14.2.185. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Huang MX, Weisend M, Sherwood A, Miller GA, Adler LE, et al. Interpreting abnormality: An EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biological Psychology. 2003;65:1–20. doi: 10.1016/s0301-0511(03)00094-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), clinician version. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Flashman LA, Green MF. Review of cognition and brain structure in schizophrenia: Profiles, longitudinal course, and effects of treatment. Psychiatric Clinics of North America. 2004;27:1–18. doi: 10.1016/S0193-953X(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. Journal of Neuroscience. 1998a;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. Journal of Neuroscience. 1998b;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum CM, et al. Schizophrenia and nicotinic receptors. Harvard Review of Psychiatry. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Mitchell CP. What is the functional significance of hippocampal pathology in schizophrenia? Schizophrenia Bulletin. 2004;30:367–392. doi: 10.1093/oxfordjournals.schbul.a007086. [DOI] [PubMed] [Google Scholar]
- Goto K, Ueki A, Iso H, Morita Y. Reduced prepulse inhibition in rats with entorhinal cortex lesions. Behavioral Brain Research. 2002;134:201–207. doi: 10.1016/s0166-4328(02)00039-6. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, et al. Neuronal substrates of sensory gating within the human brain. Biological Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Miller GA, Thoma RJ, Irwin J, Jones A, Moses SN, et al. Distinct M50 and M100 auditory gating deficits in schizophrenia. Psychophysiology. 2005;42:417–427. doi: 10.1111/j.1469-8986.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. Meta-analysis and the science of schizophrenia: Variant evidence or evidence of variants? Neuroscience and Biobehavioral Reviews. 2004;28:379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hershman KM, Freedman R, Bickford PC. Gaba(B) antagonists diminish the inhibitory gating of auditory response in the rat hippocampus. Neuroscience Letters. 1995;190:133–136. doi: 10.1016/0304-3940(95)11523-y. [DOI] [PubMed] [Google Scholar]
- Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, et al. Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clinical Neurophysiology. 2003;114:835–850. doi: 10.1016/s1388-2457(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Judd LL, McAdams L, Budnick B, Braff DL. Sensory gating deficits in schizophrenia—New results. American Journal of Psychiatry. 1992;149:488–493. doi: 10.1176/ajp.149.4.488. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kurthen M, Trautner P, Rosburg T, Grunwald T, Deitl T, Kuhn K-U, et al. Towards a functional topography of sensory gating areas: Invasive P50 recording and electrical stimulation mapping in epilepsy surgery candidates. Psychiatry Research/Neuroimaging. 2007;155(2):121–133. doi: 10.1016/j.pscychresns.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysne PA, Montano R, Hanlon FM, Bantz R, Lundy L, Euler M, et al. Intra-run stability of the M50 auditory gating response in a paired-click paradigm. Poster session presented at the annual BioMag Meeting; Vancouver, British Columbia, Canada. 2006. Aug, [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences, USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: Potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174(1):54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory-stimuli. Neuroscience. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller GA, Lutzenberger W, Elbert T. The linked-reference issue in EEG and ERP recording. Journal of Psychophysiology. 1991;5:273–276. [Google Scholar]
- Moses SN, Cole C, Driscoll I, Ryan JD. Differential contributions of hippocampus, amygdala and perirhinal cortex to recognition of novel objects, contextual stimuli and stimulus relationships. Brain Research Bulletin. 2005;67:62–76. doi: 10.1016/j.brainresbull.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus. 2006;16:43–65. doi: 10.1002/hipo.20131. [DOI] [PubMed] [Google Scholar]
- Paller KA, McCarthy G, Roessler E, Allison T, Wood CC. Potentials-evoked in human and monkey medial temporallobe during auditory and visual oddball paradigms. Electroencephalography and Clinical Neurophysiology. 1992;84:269–279. doi: 10.1016/0168-5597(92)90008-y. [DOI] [PubMed] [Google Scholar]
- Pegues MP, Rogers LJ, Amend D, Vinogradov S, Deicken RF. Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophrenia Research. 2003;60:105–115. doi: 10.1016/s0920-9964(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Perlstein W, Fiorito E, Simons RF, Graham FK. Lead stimulation effects on reflex blink, exogenous brain potentials, and loudness judgments. Psychophysioloqy. 1993;30:347–358. doi: 10.1111/j.1469-8986.1993.tb02056.x. [DOI] [PubMed] [Google Scholar]
- Perlstein WP, Simons RF, Graham FK. Prepulse effects as a function of cortical projection system. Biological Psychology. 2001;56:83–111. doi: 10.1016/s0301-0511(01)00075-8. [DOI] [PubMed] [Google Scholar]
- Petropoulos H, Sibbitt WL, Brooks WM. Automated T2 quantitation in neuropsychiatric lupus erythematosus: A marker of active disease. Journal of Magnetic Resonance Imaging. 1999;9:39–43. doi: 10.1002/(sici)1522-2586(199901)9:1<39::aid-jmri5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Lepsien J, Hugdahl K, von Cramon DY. Auditory target detection in dichotic listening involves the orbitofrontal and hippocampal paralimbic belts. Cerebral Cortex. 2004;14:903–913. doi: 10.1093/cercor/bhh049. [DOI] [PubMed] [Google Scholar]
- Ricker DA, Thoma RJ, Hanlon FM, Moses SN, Edgar CJ, Irwin JM, et al. Auditory sensory gating deficit and negative symptoms in schizophrenia. Poster session presented at the biannual meeting of the International Conference on Schizophrenia Research; Colorado Springs, CO. 2004. Apr, [Google Scholar]
- Rossi A, Stratta P, Mancini F, Gallucci M, Mattei P, Core L, et al. Magnetic resonance imaging findings of amygdala-anterior hippocampus shrinkage in male patients with schizophrenia. Psychiatry Research. 1994;52:43–53. doi: 10.1016/0165-1781(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. The hippocampal-formation is necessary for rats to learn and remember configural discriminations. Behavioural Brain Research. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: An appraisal and reconfiguration. Hippocampus. 1995;5:375–389. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: A quantitative magnetic resonance imaging study. New England Journal of Medicine. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shoemaker JM, Saint Marie RL, Bongiovanni MJ, Neary AC, Tochen LS, Swerdlow NR. Prefrontal D1 and ventral hippocampal N-methyl-D-aspartate regulation of startle gating in rats. Neuroscience. 2005;135:385–394. doi: 10.1016/j.neuroscience.2005.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proceedings of the National Academy of Sciences, USA. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR, Jr, Weinberger DR. Temporal lobe pathology in schizophrenia: A quantitative magnetic resonance imaging study. American Journal of Psychiatry. 1989;146:464–472. doi: 10.1176/ajp.146.4.464. [DOI] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. New England Journal of Medicine. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hanlon FM, Henning L, Kim YK, Gaudet I, Halim ND. Regulation of sensorimotor gating in rats by hippocampal NMDA: Anatomical localization. Brain Research. 2001;898:195–203. doi: 10.1016/s0006-8993(01)02143-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Lipska BK, Weinberger DR, Braff DL, Jaskiw GE, Geyer MA. Increased sensitivity to the sensori-motor gating-disruptive effects of apomorphine after lesions of medial prefrontal cortex or ventral hippocampus in adult rats. Psychopharmacology. 1995;122:27–34. doi: 10.1007/BF02246438. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Noh HR, Ma L, Gaudet I, Munson M, et al. The ventral hippocampal regulation of prepulse inhibition and its disruption by apomorphine in rats are not mediated via the fornix. Neuroscience. 2004;123:675–685. doi: 10.1016/j.neuroscience.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, et al. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. American Journal of Psychiatry. 2002;159:217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Karhu J, Tissari SO. Non-invasive detection of neuronal population activity in human hippocampus. Cognitive Brain Research. 1996;4:39–47. doi: 10.1016/0926-6410(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang M, Irwin J, et al. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon F, Moses S, Ricker D, Huang M, Edgar C, et al. M50 sensory gating predicts negative symptoms in schizophrenia. Schizophrenia Research. 2005;73:311–318. doi: 10.1016/j.schres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Sanchez N, Weisend MP, Huang M, Jones A, et al. Auditory sensory gating deficit and cortical thickness in schizophrenia. Neurology and Clinical Neurophysiology. 2004;62:1–7. [PubMed] [Google Scholar]
- Waldo MC, Cawthra E, Adler LE, Dubester S, Staunton M, Nagamoto H, et al. Auditory sensory gating, hippocampal volume, and catecholamine metabolism in schizophrenics and their siblings. Schizophrenia Research. 1994;12:93–106. doi: 10.1016/0920-9964(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: A magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. American Journal of Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Yee CM, White PM. Experimental modification of P50 suppression. Psychophysiology. 2001;38:531–539. doi: 10.1017/s0048577201981454. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. Effects of hippocampal N-methyl-D-aspartate infusion on locomotor activity and prepulse inhibition: Differences between the dorsal and ventral hippocampus. Behavioral Neuroscience. 2002a;116:72–84. doi: 10.1037//0735-7044.116.1.72. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. Prepulse inhibition in rats with temporary inhibition/inactivation of ventral or dorsal hippocampus. Pharmacology, Biochemistry, Behavior. 2002b;73:929–940. doi: 10.1016/s0091-3057(02)00936-x. [DOI] [PubMed] [Google Scholar]