Abstract
Excessive production of free radicals by mitochondria is associated with, and likely contributes to, the progression of numerous pathological conditions. Nevertheless, the production of free radicals by the mitochondria may have important biological functions under normal or stressed conditions by activating or modulating redox-sensitive cellular signaling pathways. This raises the intriguing possibility that regulated mitochondrial free radical production occurs via mechanisms that are distinct from pathologies associated with oxidative damage. Indeed, the capacity of mitochondria to produce free radicals in a limited manner may play a role in ischemic preconditioning, the phenomenon whereby short bouts of ischemia protect from subsequent prolonged ischemia and reperfusion. Ischemic preconditioning can thus serve as an important model system for defining regulatory mechanisms that allow for transient, signal-inducing, production of free radicals by mitochondria. Defining how these mechanism(s) occur will provide insight into therapeutic approaches that minimize oxidative damage without altering normal cellular redox biology. The aim of this review is to present and discuss evidence for the regulated production of superoxide by the electron transport chain within the ischemic preconditioning paradigm of redox regulation.
Keywords: Cardiac ischemia, preconditioning, mitochondria, electron transport chain, free radicals, oxidation and reduction
1. Introduction
It is now appreciated that free radicals are not necessarily deleterious molecules, purveyors only of oxidative damage. Free radicals also serve a regulatory function modulating enzymatic activities in a reversible manner. The cellular machinery is adept at reversing certain oxidative modifications, such as protein disulfide bonds, mixed disulfides with glutathione, sulfenic and sulfinic acids, and methionine sulfoxide [1–3], thus allowing for mechanisms of regulation that may be nearly as specific and transient as protein phosphorylation. However, the difference between free radicals produced for regulatory purposes as compared to those that contribute to irreversible oxidation is a fine line, and necessitates a carefully orchestrated, reversible production and removal of pro-oxidants. There are several enzymes potentially capable of producing free radicals or the pro-oxidant hydrogen peroxide under regulated conditions. The major source of free radicals in most cell types is the electron transport chain of the mitochondria (for review see [4]). It is therefore proposed that mitochondria represent an important aspect of redox based signaling that is distinct from its role in oxidative damage.
Mitochondria have long been appreciated as contributors to oxidative damage (for review, see [4]), but their role in producing free radicals in a regulated, limited manner is largely undefined. It is therefore critical to distinguish the sites and mechanisms of mitochondrial free radical production to gain insight into redox-dependent regulated response(s) versus oxidative damage that occurs during the progression of numerous diseases associated with mitochondrial deficits. The strong likelihood that mitochondrial free radical production plays two divergent roles, one beneficial and the other detrimental, underscores the necessity that therapeutic strategies address appropriate selection and delivery of modulators that can prevent oxidative damage without interfering with critical redox-dependent regulatory processes. The importance of these considerations is illustrated by the limited success of antioxidant therapy (for review, see [5–9]).
Perhaps the best documented evidence in support of regulated mitochondrial free radical production is provided by studies investigating cardiac ischemic preconditioning (IPC), the phenomenon whereby short repeated bouts of ischemia and reperfusion protect from cardiac damage associated with prolonged ischemia and reperfusion (for review, see [10–14]). During IPC, free radicals likely produced by the mitochondria appear required for cardioprotection [14–24]. It is not the goal of this review to discuss all of the molecular mechanisms that likely participate in IPC. Rather, we will examine IPC as a paradigm for evaluating mechanisms whereby mitochondria produce free radicals in a regulated fashion. Defining such mechanisms will provide insight into therapeutic options for pharmacological manipulation of IPC as well as establish the importance of mitochondria in participating in redox based cellular signaling pathways under normal and pathophysiological conditions.
2. Reperfusion Injury versus Ischemic Preconditioning
It is well established that alterations in mitochondrial structure and function during reoxygenation of ischemic cardiac tissue accompany and likely contribute to declines in contractile function (for review, see [5, 25, 26]). A consequence of ischemia and reperfusion is the mitochondrial production of oxygen free radicals [27–34]. The rate at which mitochondria produce free radicals, and the resulting oxidative damage, are dependent upon the duration of ischemia [31, 33–39]. Thus, the duration of ischemia and reperfusion likely determines whether there is a shift from reversible to irreversible loss in mitochondrial function. For the purpose of this review, we intend to compare and contrast mitochondrial free radical production mechanisms as this shift occurs.
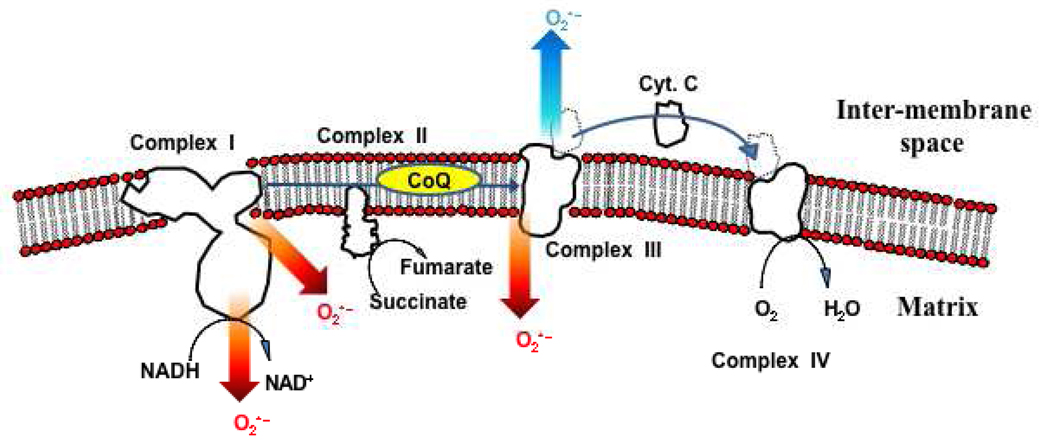
A major source of free radicals that contribute to reperfusion injury is the electron transport chain (Scheme 1). The electron transport chain produces free radicals at a very low rate under basal conditions, but at elevated rates during conditions such as ischemia/reperfusion [27–33]. Reperfusion-induced increases in free radical production are often attributed to declines in the activities of electron transport chain complexes upon ischemia that could, in turn, lead to an impaired overall rate of oxidative phosphorylation [31, 33, 39–42]. Depending on the experimental model and conditions under investigation, various electron transport chain complexes have been identified as sites of ischemia- and/or reperfusion-induced inhibition [33–35, 39–44]. These deficits would be expected to contribute to observed increases in the mitochondrial production of free radicals upon ischemia/reperfusion. However, as discussed later, an elevated rate of free radical production can occur without any observed loss of respiratory activity [35].
Scheme 1.
The electron transport chain. Electrons are introduced into the chain at complex I, in the form of NADH, or at complex II, in the form of succinate. The main sources of superoxide within the electron transport chain are complex I and complex III. Superoxide production from complex I occurs mainly on the matrix side of the membrane. Complex III may release superoxide into either the matrix or inter-membrane space.
Preventing or limiting the extent of reperfusion injury and preserving mitochondrial function is of great therapeutic importance. In this regard, much attention has been given to the ability of short bouts of ischemia and reperfusion prior to a prolonged ischemic event to provide cardioprotection. IPC largely prevents various manifestations of reperfusion injury, including cardiovascular abnormalities, apoptosis, and deficits in mitochondrial functions [11–13, 23, 45–51]. There are two phases of IPC, one that occurs immediately after the brief ischemia/reperfusion episodes, and one that occurs approximately 24 hours later (for review, see [11–13, 20]). The early protection involves acute stress responses, while the later response requires gene transcription/translation [11–13, 20]. It is during the initial phase that IPC-induced protective effects are likely mediated by signal transduction, conformational changes or post-translational modification(s) to protein, and/or subtle differences in protein-protein or protein-membrane interaction(s). The production of transient signaling molecules such as nitric oxide and reactive oxygen species is well documented [11–14, 20]. In addition, several signaling pathways have been implicated, including the activation of numerous protein kinases such as Akt [52], MAP kinases [24, 53, 54], PKA [55], and PKC [56–59]. A central point in ischemic preconditioning mechanisms, though, is a convergence upon mitochondrial functions. For example, PKCε is activated and translocates to mitochondria where it associates with its yet to be identified protein binding partner, RACK [57, 59–62]. PKCε has also been reported to form a signaling module that binds to other kinases, such as MAPK, that are thereby recruited to mitochondria [53]. While there is some discrepancy in the literature concerning the sequence of events, there is an increase in mitochondrial free radical production that may either precede or follow PKC activation [for review, see [14, 20]. An additional target of IPC is cytochrome c, a critical electron carrier in the electron transport chain that, when released from the mitochondria into the cytosol in response to various (patho)physiological stimuli, initiates mitochondrially mediated apoptosis. Indeed, IPC prevents ischemia-induced defects in mitochondrial function and loss of cytochrome c from the mitochondria [46].
3. Reactive Oxygen Species, Mitochondria and Ischemic Preconditioning
Mitochondrial production of reactive oxygen species appears to be a critical component of IPC [14, 19–22, 24, 63]. In in situ and isolated rabbit heart models of IPC, scavenging free radical species upon infusion of the antioxidant, N-2-mercaptopropionylglycine, diminished cardioprotective effects [15]. Infusion of a superoxide generating system, xanthine oxidase and hypoxanthine, prior to a long ischemic event significantly decreased the reperfusion induced infarct size in the isolated rabbit heart model [15]. Similarly, infusion of hydrogen peroxide (2.0 µM) prior to prolonged ischemia/reperfusion significantly improved contractile function in an isolated rat heart model [17]. It is therefore conceivable that free radicals produced upon IPC attenuates excessive mitochondrial free radical production associated with prolonged ischemia and reperfusion, thereby protecting the myocardium from oxidative damage and functional deficits [16].
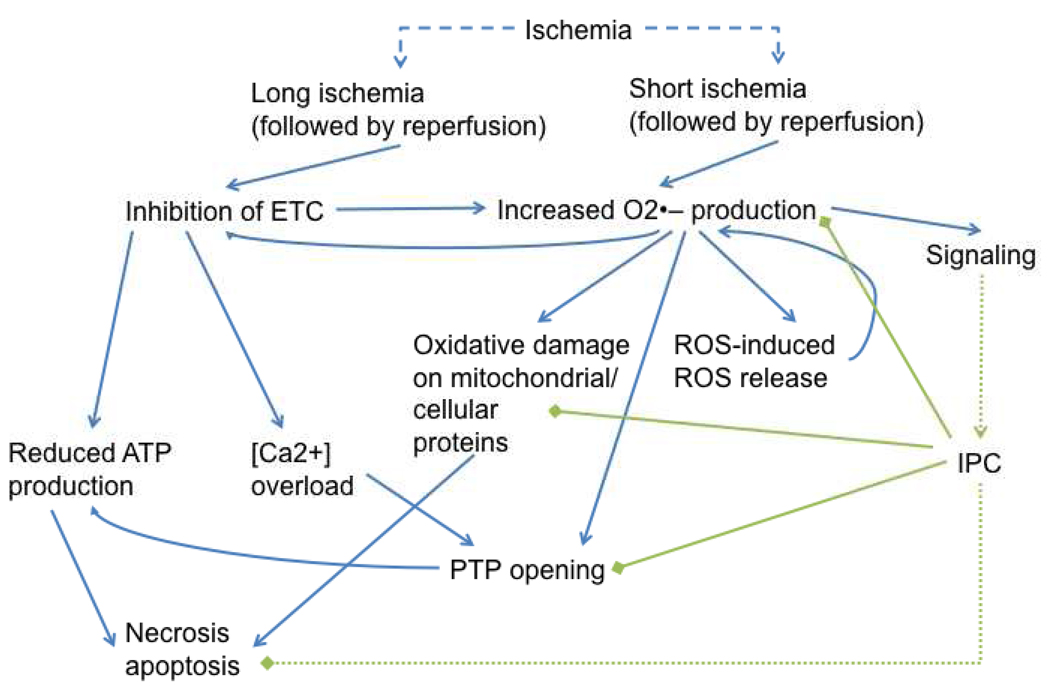
In contrast to extended periods of ischemia and reperfusion, where losses in electron transport chain activities are thought to contribute to deficits in electron flow and contribute to free radical production [27–35, 39–44], IPC may stimulate free radical generation in the absence of such deficits (Scheme 2). In fact, others and we have found that short bouts of ischemia/reperfusion enhance electron transport chain activity [35, 41]. These observations indicate that an increased mitochondrial capacity to produce pro-oxidants is not necessitated by loss of function. Moreover, multiple mechanisms of free radical production likely exist. One mode, induced by long bouts of ischemia and reperfusion, induces an increased rate of free radical production for an extended duration, resulting in oxidative damage [33, 36–39]. In contrast, another mode induced by brief periods of ischemia and reperfusion allows for a limited and controlled increase in free radicals that potentially initiates cardioprotective signaling events. The mechanism(s) by which this latter free radical production occurs is not well understood, but numerous mechanisms have been suggested.
Scheme 2.
Brief and long bouts of ischemia induce increases in mitochondrial free radical production. However, depending upon the ischemic duration, the outcome can either be activation of cardioprotective signals or catastrophic cell death.
4. The Role of the Mitochondrial KATP Channel in Generating Free Radicals During IPC
It was first demonstrated in 1991 that mitochondria possess ATP-regulated potassium channel (mitoKATP) activity, distinct in its regulation and apparent composition than that contained in the plasma membrane [64]. The identity of protein(s) conveying this activity still remains elusive but is nevertheless an area of great interest. It has been suggested that opening of mitoKATP, such that would occur under ischemic conditions when ATP is depleted, contributes to the mechanism of IPC, in part, by preventing mitochondrial swelling and membrane rupture. This hypothesis is supported by pharmacological manipulation. Diazoxide is a drug that provides cardioprotection from ischemic assaults and mechanistically has been suggested to occur via opening of the mitoKATP [65]. Alternatively, opening of the mitoKATP is accompanied by increased free radical production, which may initiate cardioprotective signaling events [21, 22, 66].
The role of mitoKATP in generating ROS still requires clarification. Reagents implemented to open the channel are pharmacologically nonspecific. For example, diazoxide is a drug that is commonly used to pharmacologically mimic IPC via opening the mitoKATP channel. However, diazoxide is also a potent inhibitor of complex II (succinate dehydrogenase) [67–69]. Furthermore, other inhibitors of complex II can also promote cardioprotection [70–72], and this also corresponds to mitoKATP opening [71, 73]. While complex II itself has been suggested to be a component of the KATP channel [74], it is unclear whether complex II inhibitors provide cardioprotection by promoting influx of potassium into the mitochondria via mitoKATP or by inhibiting the electron transport chain, or by both mechanisms. It is hypothesized that opening of mitoKATP decreases the membrane potential by allowing an influx of potassium. It is unclear, however, how this mild uncoupling of oxidative phosphorylation induces the electron transport chain to produce free radicals at an increased rate. Conclusive molecular characterization of mitoKATP and derivation of pharmacological reagents that specifically modulate its activity are needed.
5. Mitochondrial Permeability Transition Pore
An important physiological consequence of ischemia is a decrease in ATP, resulting in changes in calcium homeostasis which relies on ATP for the uptake of Ca2+ by the sarcoplasmic reticulum during the cycle of contraction and relaxation. A consequence of increased cytosolic Ca2+ concentrations is elevated uptake of Ca2+ by the mitochondria [75–77]. Uncontrolled uptake of calcium in turn results in the opening of the mitochondrial permeability transition pore (MPTP). The protein components of the MPTP have not been fully elucidated. The pore is constituted, at least in part, by the cyclophilin-D protein and perhaps the adenine nucleotide translocase [77, 78]. Opening of the MPTP allows diffusion of molecules less than 1.5 kDa across the inner mitochondrial membrane [77], thereby collapsing the membrane potential and ion gradients. Unchecked, opening of the MPTP is associated with initiation of apoptosis and irreversible cellular damage [78, 79]. The temporal and causal relationship between MPTP opening and free radical production is uncertain. However, it has been demonstrated that an increase in free radicals may promote MPTP opening, which then induces a further increase in free radical production [80]. As with the mitoKATP channel, it is not completely clear how and why the electron transport chain produces free radicals in response to MPTP opening.
Opening of the MPTP has typically been seen as a catastrophic, committed event that results in cell death. More recently, however, it has been proposed that transient opening of the MPTP may occur during IPC [81, 82]. The difference between transient and permanent opening of the MPTP may be dependent on ischemic duration. It is rationalized that brief opening of the MPTP may have the beneficial effect of lowering the membrane potential, thereby limiting the capacity of mitochondria to take up calcium. This would also be expected to reduce free radical production because a drop in membrane potential stimulates electron transport chain activity as mitochondria actively respire to reestablish the proton gradient. Redox centers of actively respiring mitochondria are rapidly cycling between oxidized and reduced states, thereby limiting their ability to produce free radicals. This is in contrast to mitochondria respiring under non-maximal conditions at which the electron transport chain is predominantly in the reduced state and primed for free radical production.
A recent study using a genetically encoded mitochondria-localized superoxide indicator, based on yellow fluorescent protein, provides evidence that individual mitochondria undergo brief periods of increased free radical production due to transient opening of the MPTP [83]. In this experimental system, exposure of cells to hypoxia and reoxygenation resulted in increased ROS production from distinct populations of mitochondria [83]. Whether MPTP increases or decreases ROS production thus remains a point of contention. It is conceivable that free radicals might be produced at different sites and rates, depending on the rate of electron transport. However, a mechanism whereby depolarization of mitochondria would promote free radical production remains elusive.
6. Modulation of Electron Transport Chain Activity as a Means of Regulating Free Radical Production
While evidence suggests that transient opening of mitoKATP and/or MPTP may contribute to cardioprotection afforded by IPC, the ultimate mitochondrial source of oxidants is the electron transport chain. Prolonged ischemia/reperfusion induces deficits in electron transport chain activity, thereby resulting in an increased half-life of reduced components, increased free radical production, and oxidative damage [27–30, 32–44]. In contrast, mitochondrial integrity is maintained during IPC [23, 46–51]. This raises the question, is transient opening of the mitoKATP channel and/or MPTP sufficient to increase free radical production or is IPC accompanied by intrinsic alterations in electron transport chain activity that result in increased rates of superoxide generation? Work from our laboratory has examined this question by measuring the activity of electron transport chain complexes and superoxide production as a function of ischemic duration [35].
Using an isolated rat heart model, hearts were subjected to increasing durations of ischemia. Mitochondria were then isolated and the rates of electron transport chain complexes and superoxide production were measured [35]. The methodology utilized an increase in hydroethidine fluorescence upon oxidation by superoxide anion. Experiments were performed using frozen/thawed mitochondria to allow for direct assessment of electron transport chain derived superoxide production while minimizing interference of mitochondrial antioxidants and to exclude the contributions of MPTP and mitoKATP (for a review of methodology to monitor free radical production, see [84]). As previously reported, an ischemic duration as brief as 4 min was sufficient to induce a significant increase in NADH-driven superoxide production when compared to mitochondria isolated from perfused control hearts [35]. Increases in the duration of ischemia up to 15 min did not result in a further enhancement in the rate of superoxide production. However, with longer ischemic periods (15 to 30 min) the rate increased, indicating a biphasic response to ischemic duration. The rates of overall electron transport activity and individual complexes were examined at each ischemic time point. Brief ischemia induced an increase in electron transport chain activity with a specific increase in activity within the complex III–IV segment of the chain. In contrast, longer ischemic durations were accompanied by loss of complex I activity which was likely responsible, in large part, for the increase in superoxide production observed at ischemic durations greater than 15 min.
The importance of these results is that they demonstrate two modes of free radical production induced by ischemia. In one mode, caused by brief periods of ischemia, electron transport chain activity is enhanced. With longer durations of ischemia, complex I is susceptible to inactivation. These changes in electron transport chain activity, dependent on the duration of ischemia, are indicative of distinct mechanisms and sites of free radical production. The data suggests that brief periods of ischemia induce superoxide production downstream of complex III, while complex I is the site of production with long bouts of ischemia. As the ischemic duration increases, it is likely that multiple sites participate in superoxide generation [35].
The site of free radical production is likely to induce distinct downstream events. While it remains an active area of investigation, complex I produces superoxide anions at redox active sites which include FeS centers and/or FMN or through generation of the ubisemiquinone radical (for review, see [85]). Complex III produces superoxide at the Qo binding site via the production of ubisemiquinone [86, 87]. Studies examining the topology of superoxide production have shown that complex I produces superoxide predominantly on the matrix side of the inner membrane, while complex III produces superoxide both towards the inner-membrane space and matrix [86, 88] (Scheme 1). Thus, complex III-derived free radicals may be expected to have a greater impact on redox signaling pathways outside of the mitochondrial matrix. In light of this, it is noteworthy that our results demonstrate that the segment between complexes III and IV is a site of superoxide production induced by brief ischemia [35].
Following cardiac ischemia, mitochondria can be isolated and exhibit elevated rates of superoxide production [35]. This is indicative of a stable transformation(s), which may include posttranslational modifications, as discussed below, or structural alterations to the mitochondrial membrane. Because we observed an increase in complex III–IV activity with brief ischemia, a potential point of regulation of activity within this segment is association and disassociation of cytochrome c from the outer surface of the inner-mitochondrial membrane (Scheme 1). It has previously been demonstrated that ischemia induces disassociation of cytochrome c from the membrane where the protein remains free within the intermembrane space [89]. Cytochrome c is found in multiple forms, tightly bound to the inner mitochondrial membrane though hydrophobic interactions, loosely bound through ionic interactions with cardiolipin, and dissociated [90]. Subtle changes in this distribution may modulate the production of superoxide by the complex III–IV segment of the electron transport chain. This may proceed by increasing the half-life of reduced components of complex III responsible for superoxide production, or by direct cytochrome c-mediated production of superoxide [91]. Our data indicates that subtle changes in cytochrome c disassociation, induced in vitro by increasing osmolarity, enhances complex IV activity and superoxide production [35]. This further suggests that increased cycling of cytochrome c between associated and disassociated forms may be an important means of both increasing electron transport chain activity and free radical production.
7. Posttranslational Modifications
Phosphorylation is a common mechanism whereby cells regulate a large variety of processes. Until recently, an understanding of this form of regulation within the mitochondria has been limited almost exclusively to pyruvate dehydrogenase [92, 93]. It is now clear that a large number of mitochondrial proteins can undergo phosphorylation [94, 95]. Thus, a more definitive role of protein phosphorylation in the regulation of mitochondrial function is emerging which has implications for IPC. A number of kinases have been implicated in participating in cardioprotection induced by IPC (reviewed in [96]). Two kinases, PKCε and PKA, are of particular interest to this review because of their reported associations with mitochondria and potential to modulate free radical production.
A kinase that has been well documented as a participant in the cardioprotection afforded by IPC is PKCε. Indeed, PKCε activation and translocation to the mitochondria has been shown to be an important component of IPC mediated cardioprotection [58, 59, 97, 98]. This has been demonstrated by ablating IPC using isoform specific peptides that specifically block interactions of PKCε with binding partners [57–59, 62]. However, the mechanisms by which PKCε modulate mitochondrial function are not well understood but may be mediated through several mechanisms. There is evidence that PKCε interacts with complex IV during IPC and that in vitro, phosphorylation of this complex increases its activity [99]. It was also found that PKCε coimmunoprecipitates with complex IV, suggesting a protein-protein interaction and a possible anchoring site for the kinase [100]. This is particularly interesting in relationship to our observations that brief ischemia induces an increase in complex IV activity [35], further implicating it as a potentially important site of mitochondrial regulation. It is not, however, currently known whether PKCε-mediated phosphorylation of complex IV results in an increase in free radical production by the electron transport chain.
PKCε may regulate other mitochondrial processes that regulate free radical production. For example, PKCε has been reported to associate with mitoKATP and that activation of the kinase also stimulated mitoKATP activity [61]. Other evidence suggests PKCε interacts with and prevents MPTP opening [56]. PKCε was found to coimmunoprecipitate with components of the MPTP, such as VDAC and the adenine nucleotide transporter. At least in vitro, VDAC is a substrate of PKCε [56]. While determination of a functional role of PKCε in mitochondrial physiology is progressing, it is still not clear if PKCε activation and translocation precedes or follows mitochondrial free radical production. Determining the temporal sequence of events will be essential for understanding the mechanism by which mitochondrial reversibly produce radicals and the involvement of PKCε in this process.
Another kinase that has been of particular interest in regulating mitochondrial function is cAMP-dependent protein kinase (PKA). There are reports that PKA is localized to the mitochondrial matrix [101] and/or the outer mitochondrial membrane via interactions with anchoring proteins [102, 103]. A number of electron transport chain components have been identified as PKA substrates in vitro [104–108]. Pertinent to this review, it has been reported that phosphorylation of the 18 kDa subunit of complex I resulted in increased complex I activity, a decrease in ROS production, and an increase in mitochondrial respiratory activity [107]. Interestingly, complex IV has also been demonstrated to be a substrate for PKA phosphorylation. In an isolated rabbit heart model of ischemia, it was observed that there was an increase in the cAMP dependent phosphorylation of several subunits of complex IV, a decrease in mitochondrial respiration, and an increase in ROS production [109]. However, it has been recently reported that PKA-dependent phosphorylation of complex IV has the opposite effect. Phosphorylation occurs with an increase in respiratory activity and a decrease in ROS production [101]. It is also unclear whether PKA activation is important for cardioprotection afforded by IPC, or whether its activity may be a contributor to reperfusion injury. IPC has been demonstrated to induce an increase in myocardial cAMP levels and PKA activity [55], and brief pre-ischemic exposure to pharmacological activators of PKA protect the myocardium in vivo [55, 110]. However, sustained ischemia is also associated with drastically increased cAMP levels and this is detrimental [111]. Interestingly, IPC blocks this increase of cAMP associated with long durations of ischemia, suggesting a potential desensitization mechanism [112]. It is likely that limited activation of the kinase induced by IPC exerts effects distinct from hyperactivation induced by long bouts of ischemia. Clearly, the conditions that influence PKA activation, its downstream substrates, and how phosphorylation affects electron transport chain activity and free radical generation must be resolved in the future.
In addition to phosphorylation, a posttranslational modification of potential relevance is the formation of mixed disulfides between glutathione and cysteines on protein, termed glutathionylation. Complex I of the mitochondrial electron transport chain has previously been shown to undergo glutathionylation when isolated mitochondria were exposed to diamide in combination with glutathione, conditions of mild oxidative stress (e.g. superoxide generated by the electron transport chain), or high concentrations of oxidized glutathione [113, 114]. Complex I glutathionylation correlated with loss of enzyme activity [113, 114]. However, depending on the experimental conditions evaluated, the rate of free radical production associated with complex I glutathionylation increased [114] or decreased [113]. It remains to be determined whether IPC and/or ischemia/reperfusion induce similar modifications to electron transport chain components (see [115] for a review of thiol modifications during IPC), certain of which may alter the mitochondrial capacity to generate (pro)oxidants provoking either regulated, beneficial free radical production (IPC) or oxidative damage (ischemia/reperfusion).
Posttranslational regulation by the pro-oxidant nitric oxide (NO) is of particular interest in IPC because of the innate ability of mitochondria to produce the molecule [116, 117], and its suggested role in regulating electron transport chain function [118–120]. In in vitro studies, NO inhibits electron transport activity and increases superoxide production [121]. It has been found in a variety of tissues and model systems that nitric oxide inhibits at a variety of sites within the electron transport chain (for review, see [122]), but is best understood in terms of reversible inhibition of cytochrome c oxidase (complex IV) [120, 123–125]. Upon preconditioning, a number of components of the electron transport chain have been identified as targets of S-nitrosylation [119], the reaction of nitric oxide with sulfur groups. However, it should also be noted that the main effect of NO on electron transport activity is inhibition, and as discussed above, IPC occurs without mitochondrial deficits. It is thus still unclear how S-nitrosation or other reactions of nitric oxide with redox active sites modulates free radical production during IPC.
8. Additional Mechanisms to Regulate the Level of Pro-Oxidants
Given the emerging interest in free radical species as signaling molecules, the role of antioxidant systems must be reevaluated. In the past, when free radicals were viewed almost exclusively from the perspective of the damage they could induce, antioxidant enzymes were considered as constitutively active exhibiting little regulation. Nevertheless, the level of pro-oxidants present in a biological system is dependent on the relative rate of generation and removal. If free radicals are to act as signaling molecules, then one might expect that antioxidant systems may be modulated as an additional means of controlling the concentration of specific free radical species. Numerous studies have explored the effects of exogenously supplied antioxidants on the outcomes of cardiac ischemia and reperfusion. These studies have had limited success [5–9], likely due to numerous complicating factors. These include differences in experimental conditions and models of ischemia-reperfusion as well as in the ability of various antioxidants and/or antioxidant enzymes to reach regions of ROS production [126] and the strict requirement for appropriate ratios of antioxidants for efficient function. Critical to the current discussion, (pro)oxidants may exert certain beneficial effects that are blunted by antioxidant modulators.
An underappreciated aspect of electron transport derived free radical production that must be considered is the supply of reducing equivalents, the ultimate mitochondrial source of (pro)oxidants. Changes in the activities of Krebs cycle components may therefore represent important means for controlling the rate of mitochondrial free radical production. Evidence in support of this possibility is provided by previous findings that isolated cardiac mitochondria exposed to conditions of mild oxidative stress exhibit a loss in the rates of NADH-supported mitochondrial oxidative phosphorylation [127–129]. This was due to glutathionylation and inhibition of the rate-limiting Krebs cycle enzyme, α-ketoglutarate dehydrogenase (KGDH) [127–129]. Upon normalization of the mitochondrial redox status, KGDH underwent deglutathionylation and reactivation, enabling full return of mitochondrial respiratory rates to control values [127–129]. In addition, aconitase is well known to be susceptible to oxidative inactivation. However, it has more recently been shown that aconitase can be reversibly regulated in cardiac mitochondria in response to mild oxidative stress and ischemia/reperfusion [36, 130, 131]. Thus, supply of reducing equivalents for electron transport and, consequently, free radical production can be regulated by altered redox status. This may represent an elegant feedback mechanism to prevent unmitigated free radical production and eventual oxidative damage.
9. Conclusions
The current state of research supports a role of mitochondrially-derived free radicals as important participants in redox based signaling. This may be particularly true for ischemic preconditioning, where the limited production of free radicals is believed to play a critical role in initiating cardioprotective signaling cascades. A number of mechanisms have been purported to regulate free radical production during IPC, including transient opening of mitoKATP and/or the MPTP. However, it is yet to be determined how opening of either the channel or the pore modulates electron transport chain activity, specifically within distinct sites, to increase superoxide production. Evidence suggests that modulation of electron transport chain activity, either by post-translational modifications or disassociation of cytochrome c from the inner-mitochondrial membrane, may regulate the rate at which superoxide is formed. This hypothesis is supported by evidence demonstrating that enhanced superoxide production induced by short durations of ischemia is retained following isolation of mitochondria. It is likely that regulated free radical production will be tightly coupled to antioxidant capacity. Thus, inhibition of redox signaling may have the benefit of reducing oxidative damage, but likely carries the unintended consequence of blocking the initiation of important pro-survival cascades. As mechanisms of regulated mitochondrial free radical production are elucidated, insight will emerge regarding therapeutic options that limit oxidative damage while promoting pro-survival redox signaling pathways.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free radical biology & medicine. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxidants & redox signaling. 2005;7(3–4):348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 3.Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free radical biology & medicine. 1995;18(1):93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- 4.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 5.Reimer KA, Jennings RB. Myocardial ischemia, hypoxia, and infarction. In: Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, editors. The Heart and Cardiovascular System. New York: Raven; 1992. pp. 1875–1973. [Google Scholar]
- 6.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. The Journal of clinical investigation. 2005;115(3):500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milei J, Grana DR, Forcada P, Ambrosio G. Mitochondrial oxidative and structural damage in ischemia-reperfusion in human myocardium. Current knowledge and future directions. Front Biosci. 2007;12:1124–1130. doi: 10.2741/2131. [DOI] [PubMed] [Google Scholar]
- 8.Levonen AL, Vahakangas E, Koponen JK, Yla-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation. 2008;117(16):2142–2150. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 9.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000;47(3):446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 10.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 11.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104(24):2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 12.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104(25):3158–3167. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 13.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83(4):1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 14.Penna C, Mancardi D, Rastaldo R, Pagliaro P. Cardioprotection: A radical view Free radicals in pre and postconditioning. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbabio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. Journal of molecular and cellular cardiology. 1997;29(1):207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 16.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284(2):H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 17.Yaguchi Y, Satoh H, Wakahara N, Katoh H, Uehara A, Terada H, Fujise Y, Hayashi H. Protective effects of hydrogen peroxide against ischemia/reperfusion injury in perfused rat hearts. Circ J. 2003;67(3):253–258. doi: 10.1253/circj.67.253. [DOI] [PubMed] [Google Scholar]
- 18.da Silva MM, Sartori A, Belisle E, Kowaltowski AJ. Ischemic preconditioning inhibits mitochondrial respiration, increases H2O2 release, and enhances K+ transport. Am J Physiol Heart Circ Physiol. 2003;285(1):H154–H162. doi: 10.1152/ajpheart.00955.2002. [DOI] [PubMed] [Google Scholar]
- 19.Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86(5):541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- 20.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767(8):1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004;286(1):H468–H476. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 22.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87(6):460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 23.Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60(24):2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 24.Yue Y, Qin Q, Cohen MV, Downey JM, Critz SD. The relative order of mK(ATP) channels, free radicals and p38 MAPK in preconditioning's protective pathway in rat heart. Cardiovasc Res. 2002;55(3):681–689. doi: 10.1016/s0008-6363(02)00452-2. [DOI] [PubMed] [Google Scholar]
- 25.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 26.Zhao ZQ, Nakamura M, Wang NP, Velez DA, Hewan-Lowe KO, Guyton RA, Vinten-Johansen J. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J Surg Res. 2000;94(2):133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. The Journal of biological chemistry. 1993;268(25):18532–18541. [PubMed] [Google Scholar]
- 28.Das DK, George A, Liu XK, Rao PS. Detection of hydroxyl radical in the mitochondria of ischemic-reperfused myocardium by trapping with salicylate. Biochem Biophys Res Commun. 1989;165(3):1004–1009. doi: 10.1016/0006-291x(89)92702-2. [DOI] [PubMed] [Google Scholar]
- 29.Ueta H, Ogura R, Sugiyama M, Kagiyama A, Shin G. O2-. spin trapping on cardiac submitochondrial particles isolated from ischemic and non-ischemic myocardium. Journal of molecular and cellular cardiology. 1990;22(8):893–899. doi: 10.1016/0022-2828(90)90120-q. [DOI] [PubMed] [Google Scholar]
- 30.Pietri S, Culcasi M, Cozzone PJ. Real-time continuous-flow spin trapping of hydroxyl free radical in the ischemic and post-ischemic myocardium. Eur J Biochem. 1989;186(1–2):163–173. doi: 10.1111/j.1432-1033.1989.tb15191.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free radical biology & medicine. 2006;40(6):976–982. doi: 10.1016/j.freeradbiomed.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Otani H, Tanaka H, Inoue T, Umemoto M, Omoto K, Tanaka K, Sato T, Osako T, Masuda A, Nonoyama A, et al. In vitro study on contribution of oxidative metabolism of isolated rabbit heart mitochondria to myocardial reperfusion injury. Circ Res. 1984;55(2):168–175. doi: 10.1161/01.res.55.2.168. [DOI] [PubMed] [Google Scholar]
- 33.Venditti P, Masullo P, Di Meo S. Effects of myocardial ischemia and reperfusion on mitochondrial function and susceptibility to oxidative stress. Cell Mol Life Sci. 2001;58(10):1528–1537. doi: 10.1007/PL00000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294(2):C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 35.Matsuzaki S, Szweda LI, Humphries KM. Mitochondrial superoxide production and respiratory activity:Biphasic response to ischemic duration. Arch Biochem Biophys. 2009;484:87–93. doi: 10.1016/j.abb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulteau AL, Lundberg KC, Ikeda-Saito M, Isaya G, Szweda LI. Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc Natl Acad Sci U S A. 2005;102(17):5987–5991. doi: 10.1073/pnas.0501519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. Journal of molecular and cellular cardiology. 2001;33(11):1919–1927. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
- 38.Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1998;95(2):510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadek HA, Humphries KM, Szweda PA, Szweda LI. Selective inactivation of redox-sensitive mitochondrial enzymes during cardiac reperfusion. Arch Biochem Biophys. 2002;406(2):222–228. doi: 10.1016/s0003-9861(02)00446-0. [DOI] [PubMed] [Google Scholar]
- 40.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94(1):53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 41.Veitch K, Hombroeckx A, Caucheteux D, Pouleur H, Hue L. Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Anoxic preperfusion protects against ischaemic damage. Biochem J. 1992;281(Pt 3):709–715. doi: 10.1042/bj2810709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardy L, Clark JB, Darley-Usmar VM, Smith DR, Stone D. Reoxygenation-dependent decrease in mitochondrial NADH:CoQ reductase (Complex I) activity in the hypoxic/reoxygenated rat heart. Biochem J. 1991;274(Pt 1):133–137. doi: 10.1042/bj2740133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas DT, Szweda LI. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc Natl Acad Sci U S A. 1999;96(12):6689–6693. doi: 10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piper HM, Sezer O, Schleyer M, Schwartz P, Hutter JF, Spieckermann PG. Development of ischemia-induced damage in defined mitochondrial subpopulations. Journal of molecular and cellular cardiology. 1985;17(9):885–896. doi: 10.1016/s0022-2828(85)80102-4. [DOI] [PubMed] [Google Scholar]
- 45.Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta AJ, Dobson GP. Preconditioning and postconditioning: innate cardioprotection from ischemia-reperfusion injury. J Appl Physiol. 2007;103(4):1441–1448. doi: 10.1152/japplphysiol.00642.2007. [DOI] [PubMed] [Google Scholar]
- 46.Lundberg KC, Szweda LI. Preconditioning prevents loss in mitochondrial function and release of cytochrome c during prolonged cardiac ischemia/reperfusion. Arch Biochem Biophys. 2006 doi: 10.1016/j.abb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Boengler K, Gres P, Dodoni G, Konietzka I, Di Lisa F, Heusch G, Schulz R. Mitochondrial respiration and membrane potential after low-flow ischemia are not affected by ischemic preconditioning. Journal of molecular and cellular cardiology. 2007 doi: 10.1016/j.yjmcc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Crestanello JA, Doliba NM, Babsky AM, Niibori K, Osbakken MD, Whitman GJ. Mitochondrial function during ischemic preconditioning. Surgery. 2002;131(2):172–178. doi: 10.1067/msy.2002.119490. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka-Esposito C, Chen Q, Moghaddas S, Lesnefsky EJ. Ischemic preconditioning does not protect via blockade of electron transport. J Appl Physiol. 2007;103(2):623–628. doi: 10.1152/japplphysiol.00943.2006. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Cherednichenko G, Hernandez L, Halow J, Camacho SA, Figueredo V, Schaefer S. Preconditioning limits mitochondrial Ca(2+) during ischemia in rat hearts: role of K(ATP) channels. Am J Physiol Heart Circ Physiol. 2001;280(5):H2321–H2328. doi: 10.1152/ajpheart.2001.280.5.H2321. [DOI] [PubMed] [Google Scholar]
- 51.Yabe K, Nasa Y, Sato M, Iijima R, Takeo S. Preconditioning preserves mitochondrial function and glycolytic flux during an early period of reperfusion in perfused rat hearts. Cardiovasc Res. 1997;33(3):677–685. doi: 10.1016/s0008-6363(96)00269-6. [DOI] [PubMed] [Google Scholar]
- 52.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288(2):H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 53.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90(4):390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 54.Nagy N, Shiroto K, Malik G, Huang CK, Gaestel M, Abdellatif M, Tosaki A, Maulik N, Das DK. Ischemic preconditioning involves dual cardio-protective axes with p38MAPK as upstream target. J Mol Cell Cardiol. 2007;42(5):981–990. doi: 10.1016/j.yjmcc.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Lochner A, Genade S, Tromp E, Podzuweit T, Moolman JA. Ischemic preconditioning and the beta-adrenergic signal transduction pathway. Circulation. 1999;100(9):958–966. doi: 10.1161/01.cir.100.9.958. [DOI] [PubMed] [Google Scholar]
- 56.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92(8):873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCepsilon-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol. 2007;293(4):H2056–H2063. doi: 10.1152/ajpheart.00403.2007. [DOI] [PubMed] [Google Scholar]
- 58.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81(3):404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 59.Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Cepsilon (PKCepsilon): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007;35(Pt 5):1052–1054. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- 60.Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36(12):2781–2790. doi: 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]
- 61.Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99(8):878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 62.Dorn GW, 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci U S A. 1999;96(22):12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambrosio G, Tritto I, Chiariello M. The role of oxygen free radicals in preconditioning. Journal of molecular and cellular cardiology. 1995;27(4):1035–1039. doi: 10.1016/0022-2828(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 64.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352(6332):244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 65.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81(6):1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 66.Krieg T, Qin Q, Philipp S, Alexeyev MF, Cohen MV, Downey JM. Acetylcholine and bradykinin trigger preconditioning in the heart through a pathway that includes Akt and NOS. Am J Physiol Heart Circ Physiol. 2004;287(6):H2606–H2611. doi: 10.1152/ajpheart.00600.2004. [DOI] [PubMed] [Google Scholar]
- 67.Schafer G, Wegener C, Portenhauser R, Bojanovski D. Diazoxide, an inhibitor of succinate oxidation. Biochem Pharmacol. 1969;18(10):2678–2681. [PubMed] [Google Scholar]
- 68.Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial K(ATP) channel change the function of mitochondria in situ? The Journal of biological chemistry. 2000;275(47):37291–37295. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- 69.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542(Pt 3):735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochim Biophys Acta. 2008;1777(7–8):882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol. 2009;104(2):121–129. doi: 10.1007/s00395-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turan NN, Basgut B, Aypar E, Ark M, Iskit AB, Cakici I. Chemical preconditioning effect of 3-nitropropionic acid in anesthetized rat heart. Life Sci. 2008;82(17–18):928–933. doi: 10.1016/j.lfs.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Wojtovich AP, Burwell LS, Sherman TA, Nehrke KW, Brookes PS. The C. elegans mitochondrial K+(ATP) channel: a potential target for preconditioning. Biochem Biophys Res Commun. 2008;376(3):625–628. doi: 10.1016/j.bbrc.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101(32):11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delcamp TJ, Dales C, Ralenkotter L, Cole PS, Hadley RW. Intramitochondrial [Ca2+] and membrane potential in ventricular myocytes exposed to anoxia-reoxygenation. Am J Physiol. 1998;275(2 Pt 2):H484–H494. doi: 10.1152/ajpheart.1998.275.2.H484. [DOI] [PubMed] [Google Scholar]
- 76.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61(3):372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 77.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34(Pt 2):232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 78.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777(7–8):946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. Journal of molecular and cellular cardiology. 2003;35(4):339–341. doi: 10.1016/s0022-2828(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 80.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192(7):1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saotome M, Katoh H, Yaguchi Y, Tanaka T, Urushida T, Satoh H, Hayashi H. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2009;296(4):H1125–H1132. doi: 10.1152/ajpheart.00436.2008. [DOI] [PubMed] [Google Scholar]
- 82.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109(14):1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 83.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134(2):279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757(5–6):553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 86.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. The Journal of biological chemistry. 2004;279(47):49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 87.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237(2):408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 88.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. The Journal of biological chemistry. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 89.Czerski LW, Szweda PA, Szweda LI. Dissociation of cytochrome c from the inner mitochondrial membrane during cardiac ischemia. The Journal of biological chemistry. 2003;278(36):34499–34504. doi: 10.1074/jbc.M302021200. [DOI] [PubMed] [Google Scholar]
- 90.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99(3):1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao Y, Wang ZB, Xu JX. Effect of cytochrome c on the generation and elimination of O2*- and H2O2 in mitochondria. The Journal of biological chemistry. 2003;278(4):2356–2360. doi: 10.1074/jbc.M209681200. [DOI] [PubMed] [Google Scholar]
- 92.Reed LJ, Hackert ML. Structure-function relationships in dihydrolipoamide acyltransferases. The Journal of biological chemistry. 1990;265(16):8971–8974. [PubMed] [Google Scholar]
- 93.Roche TE, Baker JC, Yan X, Hiromasa Y, Gong X, Peng T, Dong J, Turkan A, Kasten SA. Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Progress in nucleic acid research and molecular biology. 2001;70:33–75. doi: 10.1016/s0079-6603(01)70013-x. [DOI] [PubMed] [Google Scholar]
- 94.Pagliarini DJ, Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci. 2006;31(1):26–34. doi: 10.1016/j.tibs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 95.Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45(8):2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12(3–4):217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 97.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98(20):11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ping P, Zhang J, Zheng YT, Li RC, Dawn B, Tang XL, Takano H, Balafanova Z, Bolli R. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ Res. 1999;85(6):542–550. doi: 10.1161/01.res.85.6.542. [DOI] [PubMed] [Google Scholar]
- 99.Ogbi M, Johnson JA. Protein kinase Cepsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393(Pt 1):191–199. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, Caldwell RW, Johnson JA. Protein kinase C-epsilon coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am J Physiol Heart Circ Physiol. 2007;293(4):H2219–H2230. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- 101.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9(3):265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perkins GA, Wang L, Huang LJ, Humphries K, Yao VJ, Martone M, Deerinck TJ, Barraclough DM, Violin JD, Smith D, Newton A, Scott JD, Taylor SS, Ellisman MH. PKA, PKC, and AKAP localization in and around the neuromuscular junction. BMC Neurosci. 2001;2:17. doi: 10.1186/1471-2202-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang LJ, Wang L, Ma Y, Durick K, Perkins G, Deerinck TJ, Ellisman MH, Taylor SS. NH2-Terminal targeting motifs direct dual specificity A-kinase-anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. J Cell Biol. 1999;145(5):951–959. doi: 10.1083/jcb.145.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papa S, De Rasmo D, Scacco S, Signorile A, Technikova-Dobrova Z, Palmisano G, Sardanelli AM, Papa F, Panelli D, Scaringi R, Santeramo A. Mammalian complex I: a regulable and vulnerable pacemaker in mitochondrial respiratory function. Biochim Biophys Acta. 2008;1777(7–8):719–728. doi: 10.1016/j.bbabio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Sardanelli AM, Technikova-Dobrova Z, Scacco SC, Speranza F, Papa S. Characterization of proteins phosphorylated by the cAMP-dependent protein kinase of bovine heart mitochondria. FEBS Lett. 1995;377(3):470–474. doi: 10.1016/0014-5793(95)01407-1. [DOI] [PubMed] [Google Scholar]
- 106.Maj MC, Raha S, Myint T, Robinson BH. Regulation of NADH/CoQ oxidoreductase: do phosphorylation events affect activity? Protein J. 2004;23(1):25–32. doi: 10.1023/b:jopc.0000016255.17077.2c. [DOI] [PubMed] [Google Scholar]
- 107.Raha S, Myint AT, Johnstone L, Robinson BH. Control of oxygen free radical formation from mitochondrial complex I: roles for protein kinase A and pyruvate dehydrogenase kinase. Free radical biology & medicine. 2002;32(5):421–430. doi: 10.1016/s0891-5849(01)00816-4. [DOI] [PubMed] [Google Scholar]
- 108.Chen R, Fearnley IM, Peak-Chew SY, Walker JE. The phosphorylation of subunits of complex I from bovine heart mitochondria. J Biol Chem. 2004;279(25):26036–26045. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- 109.Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome C oxidase function and augments hypoxia and myocardial ischemia related injury. The Journal of biological chemistry. 2005 doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanada S, Kitakaze M, Papst PJ, Asanuma H, Node K, Takashima S, Asakura M, Ogita H, Liao Y, Sakata Y, Ogai A, Fukushima T, Yamada J, Shinozaki Y, Kuzuya T, Mori H, Terada N, Hori M. Cardioprotective effect afforded by transient exposure to phosphodiesterase III inhibitors: the role of protein kinase A and p38 mitogen-activated protein kinase. Circulation. 2001;104(6):705–710. doi: 10.1161/hc3201.092216. [DOI] [PubMed] [Google Scholar]
- 111.Makaula S, Lochner A, Genade S, Sack MN, Awan MM, Opie LH. H-89, a non-specific inhibitor of protein kinase A, promotes post-ischemic cardiac contractile recovery and reduces infarct size. J Cardiovasc Pharmacol. 2005;45(4):341–347. doi: 10.1097/01.fjc.0000156825.80951.14. [DOI] [PubMed] [Google Scholar]
- 112.Sandhu R, Thomas U, Diaz RJ, Wilson GJ. Effect of ischemic preconditioning of the myocardium on cAMP. Circ Res. 1996;78(1):137–147. doi: 10.1161/01.res.78.1.137. [DOI] [PubMed] [Google Scholar]
- 113.Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. The Journal of biological chemistry. 2008;283(36):24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. The Journal of biological chemistry. 2003;278(22):19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 115.Eaton P, Bell RM, Cave AC, Shattock MJ. Ischemic preconditioning: a potential role for protein S-thiolation? Antioxid Redox Signal. 2005;7(7–8):882–888. doi: 10.1089/ars.2005.7.882. [DOI] [PubMed] [Google Scholar]
- 116.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. The Journal of biological chemistry. 1998;273(18):11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 117.Navarro A, Boveris A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv Drug Deliv Rev. 2008;60(13–14):1534–1544. doi: 10.1016/j.addr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 118.Novalija E, Hogg N, Kevin LG, Camara AK, Stowe DF. Ischemic preconditioning: triggering role of nitric oxide-derived oxidants in isolated hearts. J Cardiovasc Pharmacol. 2003;42(5):593–600. doi: 10.1097/00005344-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 119.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101(11):1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 120.Boveris A, Costa LE, Poderoso JJ, Carreras MC, Cadenas E. Regulation of mitochondrial respiration by oxygen and nitric oxide. Ann N Y Acad Sci. 2000;899:121–135. doi: 10.1111/j.1749-6632.2000.tb06181.x. [DOI] [PubMed] [Google Scholar]
- 121.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328(1):85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 122.Burwell LS, Brookes PS. Mitochondria as a Target for the Cardioprotective Effects of Nitric Oxide in Ischemia-Reperfusion Injury. Antioxidants & redox signaling. 2007 doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 123.Wilson MT, Torres J, Cooper CE, Sharpe MA. Interactions of cytochrome c oxidase with nitric oxide: reactions of the 'turnover' intermediates. Biochem Soc Trans. 1997;25(3):905–909. doi: 10.1042/bst0250905. [DOI] [PubMed] [Google Scholar]
- 124.Torres J, Davies N, Darley-Usmar VM, Wilson MT. The inhibition of cytochrome c oxidase by nitric oxide using S-nitrosoglutathione. J Inorg Biochem. 1997;66(3):207–212. doi: 10.1016/s0162-0134(96)00206-1. [DOI] [PubMed] [Google Scholar]
- 125.Poderoso JJ, Peralta JG, Lisdero CL, Carreras MC, Radisic M, Schopfer F, Cadenas E, Boveris A. Nitric oxide regulates oxygen uptake and hydrogen peroxide release by the isolated beating rat heart. Am J Physiol. 1998;274(1 Pt 1):C112–C119. doi: 10.1152/ajpcell.1998.274.1.C112. [DOI] [PubMed] [Google Scholar]
- 126.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19(9):1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 127.Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. The Journal of biological chemistry. 2001;276(26):23357–23361. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]
- 128.Nulton-Persson AC, Starke DW, Mieyal JJ, Szweda LI. Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry. 2003;42(14):4235–4242. doi: 10.1021/bi027370f. [DOI] [PubMed] [Google Scholar]
- 129.Applegate MA, Humphries KM, Szweda LI. Reversible Inhibition of alpha-Ketoglutarate Dehydrogenase by Hydrogen Peroxide: Glutathionylation and Protection of Lipoic Acid. Biochemistry. 2008;47(1):473–478. doi: 10.1021/bi7017464. [DOI] [PubMed] [Google Scholar]
- 130.Bulteau AL, Ikeda-Saito M, Szweda LI. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42(50):14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- 131.Bulteau AL, O'Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305(5681):242–245. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]