Abstract
The inflexibility of existing serological techniques for detection of rabies in surveillance constrains the benefit to be gained from many current control strategies. We analysed 304 serum samples from Tanzanian dogs for the detection of rabies antibodies in a pseudotype assay using lentiviral vectors bearing the CVS-11 envelope glycoprotein. Compared with the widely used gold standard fluorescent antibody virus neutralisation assay, a specificity of 100% and sensitivity of 94.4% with a strong correlation of antibody titres (r = 0.915) were observed with the pseudotype assay. To increase the assay's surveillance specificity in Africa we incorporated the envelope glycoprotein of local viruses, Lagos bat virus, Duvenhage virus or Mokola virus and also cloned the lacZ gene to provide a reporter element. Neutralisation assays using pseudotypes bearing these glycoproteins reveal that they provide a greater sensitivity compared to similar live virus assays and will therefore allow a more accurate determination of the distribution of these highly pathogenic infections and the threat they pose to human health. Importantly, the CVS-11 pseudotypes were highly stable during freeze–thaw cycles and storage at room temperature. These results suggest the proposed pseudotype assay is a suitable option for undertaking lyssavirus serosurveillance in areas most affected by these infections.
Keywords: Rabies virus, Lyssavirus, Africa, Pseudotype
1. Introduction
Rabies is spreading at an alarming rate in some regions of the developing world [1], [2], [3]. Collaborative efforts among human and animal healthcare professionals are required to monitor this situation and allow a timely and proportioned therapeutic response, limiting unnecessary use of valuable vaccines. The combination of rabies awareness campaigns, improved vaccine coverage and disease surveillance has already resulted in the successful elimination of canine rabies in North America [4]. However, vaccines need to be efficacious enough to elicit a response that will confer protection and diagnostic techniques are needed to ensure that adequate levels of virus-neutralising antibodies (VNAbs) have been achieved in response to vaccination. The problem with current tests is that they are not easily used in endemic areas within developing countries because they need to be set up in containment laboratories since highly pathogenic zoonotic viruses are required to perform the assay, or such assays are prohibitively expensive. Those that are e.g., ELISA-based assays, do not have the sensitivity or specificity of the fluorescent antibody virus neutralisation (FAVN) assay that is widely used within Office international des épizooties (OIE) rabies reference laboratories. Therefore, in addition to the need for further implementation and development of improved anti-rabies biologicals, improved techniques to assess seroconversion are required before canine rabies is successfully eliminated in developing countries as it has been in North America.
Within the Lyssavirus genus, classical rabies viruses (genotype 1) are not the only pathogen to cause morbidity and mortality in mammalian populations. Clearly, infection by lyssaviruses of the other genotypes (2–7) can result in a clinical manifestation that is indistinguishable from rabies. The other genotypes are distributed geographically predominantly within African, European and Australian bat populations [5]. Recently, additional variants that are more divergent than genotypes 1–7 have been isolated suggesting further genotypes may yet exist [6], [7], [8], [9], [10]. Isolates representing genotypes 1, 2 and 4–7 have been identified in insectivorous, fruit or vampire bats [5]. Mokola virus (MOKV, genotype 3) along with Lagos bat virus (LBV, genotype 2) and Duvenhage virus (DUVV, genotype 4) comprises the African lyssaviruses. Interestingly, only a few clinical isolates representing these genotypes have been identified to date [11]. The handful of cases that have been reported has given us a limited understanding of the epidemiology and zoonotic threat that these genotypes pose in their respective hosts. Recently, surveillance programs and greater access to serosurveillance techniques have resulted in the discovery of a high seroprevalence against LBV in East and West African megachiroptera [12], [13] and a common presence of LBV in South African bats collected for routine surveillance [11].
These reports and others [2], [14] emphasise that the potential for increased incidence levels of rabies and related lyssavirus infections is a concern in Africa, mainly because of the lack of awareness of these infections in the population. However, there is also poor accessibility to vaccines and post-exposure treatments for those exposed to these viruses and there are difficulties with undertaking (sero)surveillance measures in many countries within Africa [15]. While the most important factor in reducing rabies prevalence is the implementation of vaccination campaigns, it was highlighted at the recent Southern and Eastern African Rabies Group meeting that poor infrastructure becomes a major barrier when attempting to control rabies in Africa [16]. These views are shared by the OIE and World Health Organization (WHO), that list the development of novel diagnostics as an urgent requirement [17], [18].
Serological techniques that can be employed to study naturally occurring or vaccine-induced humoral responses to rabies virus infection include the FAVN assay [19], rapid fluorescent focus inhibition test (RFFIT) [20] and enzyme linked immunosorbant assay (ELISA) [21]. Variations of these assays have been described previously [22], [23]. The routinely used FAVN assay and RFFIT are the current assays of choice with OIE/WHO reference laboratories but must be performed in BSL3/SAPO4 high containment facilities because live virus is handled as part of the assay. While a modified RFFIT that combines green fluorescent protein (GFP) with live recombinant virus can remove the need for expensive conjugates, work with recombinant virus still requires the use of high containment facilities [23]. With the recent addition to the above mentioned set of neutralisation assays of the ELISA-based method that uses plates coated with whole, inactivated virus, the need for live virus has been eliminated. Since both non-neutralising and neutralising antibodies are detected, the level of circulating, protective VNAbs alone cannot be determined. There are also issues with low sensitivity when using the ELISA.
We recently described the use of surrogate viruses known as lentiviral pseudotypes as replacements for live or inactivated whole virus to accurately determine anti-rabies VNAb responses in vaccine recipients. The samples tested were taken from vaccinated humans, dogs and cats in the United Kingdom (UK) [24]. Here we report the results of the largest virus neutralisation study published to date using the surrogate lentiviral pseudotypes rather than the live native or recombinant rabies virus with field serum samples from Tanzanian dogs. We further increase the utility of our pseudotype neutralisation assay for laboratories undertaking vaccine trials and serosurveillance in resource-limited, rabies endemic countries by exploring the use of lacZ as a reporter gene and incorporating the glycoproteins of a further three lyssavirus genotypes, in addition to genotype 1, which will allow improved serosurveillance for lyssaviruses other than classical rabies. This report describes a highly sensitive yet flexible platform that can be adapted to allow the evaluation of vaccine and antiviral drugs against highly pathogenic viruses without the need for high level containment facilities or expensive reagents and equipment.
2. Methods
2.1. Study area
Dogs enrolled in this study were selected from domestic animals living in four villages (Ngarawani, Runga’bure, Nyamburi and Bisarara) within 20 km of the Serengeti National Park perimeter in the Serengeti District, northwestern Tanzania (Fig. 1A). In each case the dog owner's consent was sought before enrolling their dog in the study. This project was part of the annual vaccination campaign undertaken by the Viral Transmission Dynamics Project, which works to prevent the spread of diseases through animal populations in the Serengeti region.
Fig. 1.
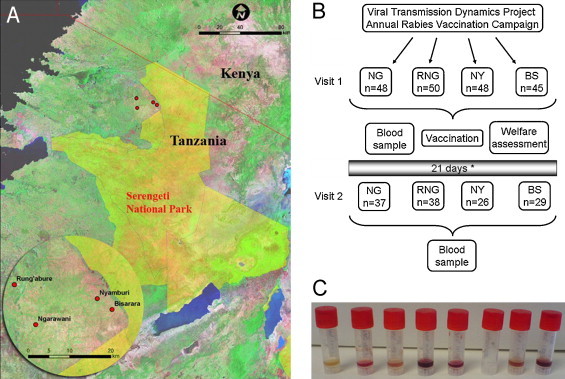
Overview of vaccination trial. (A) As part of an annual rabies vaccination campaign run by the Viral Transmission Dynamics Project, 191 dogs from four villages (Ngarawani [NG], Runga’bure [RNG], Nyamburi [NY] and Bisarara [BS]) near the north western border of the Serengeti National Park were enrolled in this study. The inset shows a larger scale map detailing the location of the four villages. (B) The owners were invited to bring their dogs to two sessions. During the first, samples were collected for baseline measurements, a rabies vaccine was administered and a welfare assessment conducted. Twenty-one days later (* the revisit to BS village was 20 days post-vaccination) the dogs had further samples collected. (C) While all samples were processed using the same protocol, the levels of haemolysis and serum collected were variable but did not affect results.
2.2. Vaccination history
A detailed medical history of each dog enrolled in this study, which included any prior vaccinations, was taken at the first visit. Sixty-six and a half percent (n = 125) of the dogs had not been vaccinated against rabies prior to this study (referred to as “primary”), 27.2% (n = 52) had received at least one rabies vaccine previously (“booster”) and there was no vaccination history available or taken for 7.3% (n = 14; “no record”; Table 1 ). The Nobivac® rabies virus vaccine (donated by Intervet Schering-Plough Animal Health) was administered to all dogs in a single dose inoculation given subcutaneously. It comprises an inactivated vaccine containing >2 IU rabies virus (Pasteur strain).
Table 1.
Details of dogs enrolled from each village for this study.
| Village | Mean age (years) | Gender (% female) | Number of sera collected |
Vaccination history at 1st visit (n/%) |
|||
|---|---|---|---|---|---|---|---|
| First visit | Second visit | Primary | Booster | No record | |||
| Ngarawani | 2.1 | 47.9 | 48a | 37 | 31/64.6 | 6/12.5 | 11/22.9a |
| Runga’bure | 2.65 | 48 | 50 | 38 | 20/40.0 | 27/54.0 | 3/6.0 |
| Nyamburi | 1.4 | 54.2 | 48 | 26 | 41/85.4 | 7/14.6 | 0/0.0 |
| Bisarara | 1.56 | 55.6 | 45b | 29 | 33/73.3 | 12/26.7b | 0/0.0 |
| TOTAL | 1.94 | 51.3 | 191 | 130 | 125/65.5 | 52/27.2 | 14/7.3 |
“Primary” refers to dogs that had never received a rabies vaccination prior to this study, “booster” refers to dogs that had previously received ≥1 rabies vaccination and “no record” means there was no vaccination history available or taken.
One sample was missing on arrival in the UK.
One sample vial was empty on arrival in the UK.
2.3. Serum samples
At the first visit, a blood sample was taken from each dog before the rabies virus vaccine was administered. The second visit was 20–21 days post-vaccination, the optimum time to detect an immune response stimulated by the vaccine. The overall study protocol is shown in Fig. 1B.
Blood was drawn from the cephalic or jugular vein, stored on ice, and processed at the end of each day (centrifugation at 3000 rpm for 10 min). Serum was prepared, inactivated at 57 °C for 30 min and then frozen for transport. In total, 321 samples were taken from the enrolled dogs, blinded and sent to University College London (UCL) and Veterinary Laboratories Agency (VLA) for testing. The OIE standard reference dog serum diluted to 0.5 international units/ml (IU/ml) with PBS was used as a positive control.
Rabbit anti-sera raised against LBV isolate RV1 (n = 2) and DUVV isolate RV131 (n = 4) were used in neutralisation assays with African lyssavirus pseudotypes. Anti-sera to MOKV were not available but anti-LBV serum is known to cross-neutralise MOKV. One serum raised against uninfected tissue culture supernatant and another against Australian bat lyssavirus (RV634) were used in the same panel as the LBV and DUVV sera.
2.4. Infection and neutralisation assays
Human embryonic kidney 293T cells [25] were used for production of the lentiviral pseudotypes (lyssavirus surrogates). Neutralisation assays were undertaken on baby hamster kidney 21 cells clone 13 (BHK; [26]). The NP2 human glioma cell line expressing CD4 and CXCR4 was used as target cells for HIV-1 pseudotypes [27].
2.4.1. FAVN assays
Live virus experiments were undertaken using a restricted version of the FAVN that is identical to the existing FAVN assay [19] but with serum samples diluted 3-fold to a final titre of 1:81, roughly equivalent to 5.92 IU/ml.
2.4.2. Pseudotype assays
Standard plasmids and the transfection protocol for lentiviral pseudotype (lyssavirus surrogate) production have been described elsewhere [24]. Additionally, glycoprotein (G) gene sequences from LBV (LBV.SA2004; accession number EF547428), MOKV (MOKV.98/071 RA361; accession number GQ500108) and DUVV (DUVV.RSA2006; accession number EU623444) were amplified by PCR using specific primers (detailed in Supplementary Table S1). Pseudotypes containing the HIV-1 envelope gp160 gene were generated using pSVIIIenv [28]. G gene sequence analysis was undertaken using ClustalW [29] and Treeview [30].
Infection and neutralisation assays using lyssavirus pseudotypes were performed as previously described [24] with the following three modifications: (1) plates were centrifuged at 500 rpm for 10 s after the virus was added and once more following addition of BHK cells; (2) to ensure the OIE standard reference dog serum recorded an IC100 at a 1:40 dilution, approximately 30× TCID50 of pseudotype was used; (3) serum was diluted 5-fold to a final titre of 1:640.
2.5. Pseudotype reporter genes
2.5.1. β-Galactosidase
To enable pseudotype particle detection using the appropriate β-galactosidase (β-gal) substrates, we constructed pCSLZW, which contains the lacZ gene from pMFG-nls-lacZ (a kind gift from Dr. Yasuhiro Takeuchi; [31]) cloned into pCSGW (primers detailed in Supplementary Table S1). Where β-gal was the reporter protein, β-gal-pseudotype-infected cells were detected using 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-gal; Sigma), chlorophenol red-beta-d-galactopyranoside (CPRG; Sigma) or o-nitrophenyl-b-d-galactopyranoside (ONPG; Sigma). Detection using the X-gal substrate was accomplished by fixing the infected cell monolayer and then staining the cells with substrate buffer (10 mM deoxycholic acid, 5 mM potassium ferrocynate, 5 mM potassium ferricynate, 4.3 mM magnesium chloride and 0.02% (v/v) NP40) containing 1 mg/ml X-gal. For CPRG and ONPG substrates, nuclei were lysed and 50 μl of reaction buffer (120 mM Na2HPO4·2H2O, 80 mM NaH2PO4·H2O, 2 mM MgCl2, 100 mM β-mercaptoethanol) was added containing CPRG or ONPG at a final concentration of 29.2 and 1.3 mg/ml, respectively. This reaction was inhibited, after 1–2 h at 37 °C, by adding stop solution (1 M sodium carbonate).
2.5.2. GFP and luciferase
GFP and luciferase reporter genes were employed to detect GFP-positive cells, which were visualised using a fluorescent microscope and FACSCalibur (BD Biosciences) and luciferase expression, which was detected using the Bright-Glo reagent and GloMax 96 microplate luminometer (Promega).
3. Results
3.1. Serum samples analysed
Of the 191 dogs enrolled in this study, the mean age was 1.94 years and 51.3% were female (Table 1). In total, 321 samples were taken during both visits (Table 1). However, in transport one sample disappeared (#27) and one leaked so no serum remained (#192). Of the remaining 319 samples, the volume in 12 was too small to test in the FAVN assay and a further three samples could not initially be tested in the pseudotype assays because of contamination and toxicity. The remaining 304 samples were tested in both assay types.
While the serum process times were kept as constant as possible there were varying degrees of haemolysis and volumes of sera produced varied with each collection (Fig. 1C). Haemolysis did not affect the results obtained with either the FAVN or the pseudotype assays.
3.2. Sensitivity and specificity of CVS-11 pseudotypes using Tanzanian canine sera
Sera that failed to achieve an antibody titre of 1:10 by the pseudotype assay, the lowest recordable result from the dilutions that were used, were given an arbitrary titre of 1:5 for this analysis. The OIE positive control serum routinely neutralised 100% of CVS-11 pseudotype particles at a dilution of 1:40 (in this study the OIE mean dilution = 37.5 and standard deviation = ±14.4) and 100% of live CVS-11 at a dilution of 15.59, normally equivalent to 0.5 IU/ml, for the FAVN assay (in this study the OIE mean dilution = 17.2 and standard deviation = ± 4.0). The lowest titre a serum sample was given by the FAVN assay in this study is 0.07 IU/ml. Any dogs with VNAb levels that attained an IC100 at a dilution of greater than or equal to 1:40 (by pseudotype assay) or a titre of 0.5 IU/ml (by FAVN assay) were considered positive (also referred to as “adequate” in this study).
When results from the FAVN and pseudotype assays were compared, we observed a concurrent increase in VNAb titres reported by the pseudotype assay as the FAVN titre increased (Fig. 2A). There were 17 discordant samples, classified as negative by the pseudotype assay but positive with the FAVN assay (Table 2 ). As a result, the average titre of samples classified as borderline positive by the FAVN assay (0.5 IU/ml) is 1:25, a borderline negative result with the pseudotype assay. While these discordant samples reduced the sensitivity of the pseudotype neutralisation assay to 94.4%, the specificity of the assay was 100% (n = 304). The majority of the discrepancies clustered around 0.5 IU/ml, the titre achieved using the OIE positive control serum and currently used to classify VNAb responses as inadequate (levels below that achieved by the OIE positive control serum) or adequate (levels greater than or equal to that achieved by the OIE positive control serum). Twelve of the discordant results (71%) were in the lowest dilution classified as positive by the FAVN assay (0.5 IU/ml), one (5.9%) fell within the 0.66 IU/ml category, three (17.6%) in the 0.87 IU/ml category and one (5.9%) scored 1.14 IU/ml by the FAVN assay (Table 3 ). There were no discrepant results achieved using the FAVN or pseudotype assays and samples from the second visit.
Fig. 2.
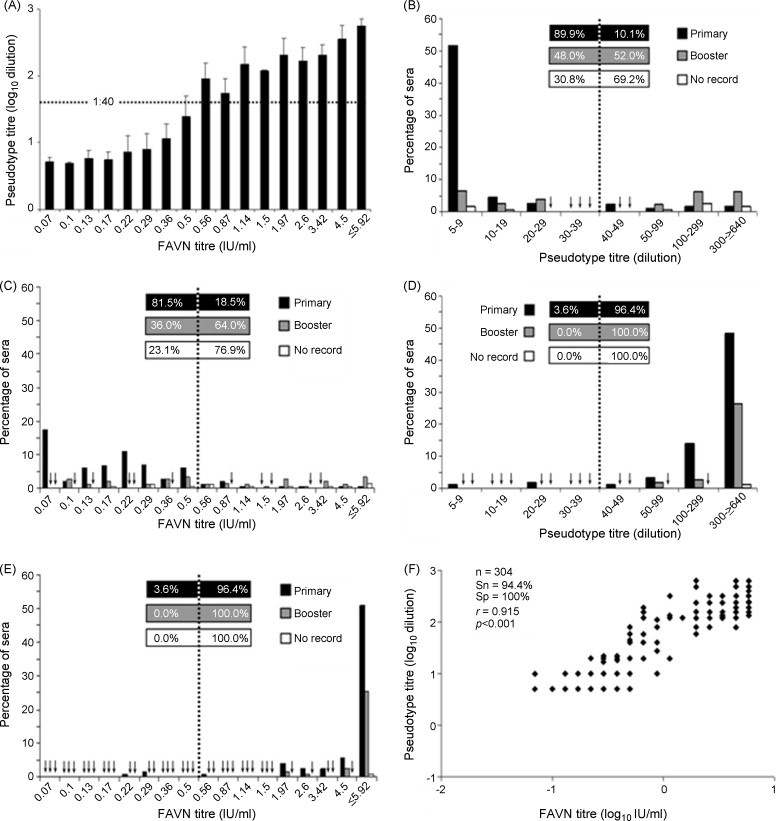
Strong correlation between FAVN and pseudotype neutralising antibody titres using Tanzanian dog sera. (A) Neutralising titres achieved with the pseudotype assay increase concordantly with those detected in the FAVN assay. The distribution, according to VNAb titres determined by the (B) pseudotype and (C) FAVN assays, of sera collected at the first visit from primary (dogs that had not received a previous rabies vaccination—black columns), booster (dogs that had received ≥1 rabies vaccination previously—grey columns) or dogs with no vaccination record (white columns) is shown. Similar analyses for samples collected at the second visit are shown in (D) and (E) for the pseudotype and FAVN assays, respectively. The dotted line marks the level of VNAb that was achieved by the OIE positive control serum. Percents of samples with an inadequate or adequate VNAb response are given left and right of the dotted line, respectively. Vaccination history was available for 169 dogs (first visit: primary n = 119, booster n = 50, no record n = 13; second visit: primary n = 84, booster n = 37, no record n = 1). (F) Results from the pseudotype neutralisation and FAVN assays reveal a high degree of concordance (Sn: sensitivity and Sp: specificity) and a strong correlation (r) between titres. Sn and Sp are relative to the 0.5 IU/ml threshold and r/p values were calculated using Pearson's product–moment correlation. Arrows indicate VNAb titres for which there were no serum samples containing that level of antibodies.
Table 2.
Distribution of sera according to FAVN neutralising antibody titre.
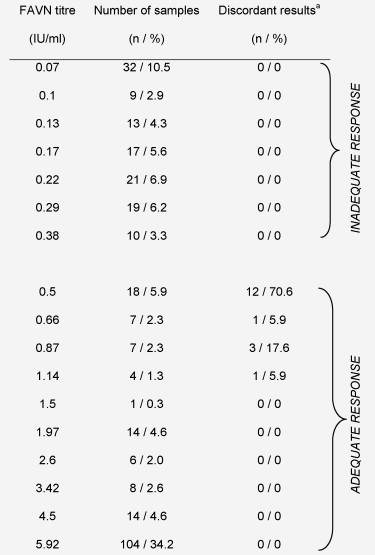
Percentages given are the proportion in that category out of the total number for the column.
aSamples that were positive by FAVN assay but negative by the pseudotype assay.
Table 3.
Titres recorded for discordant sera using the FAVN and pseudotype assays.
| Sample ID | FAVN titre (IU/ml) | Pseudotype titre (dilution) |
|---|---|---|
| 3a | 0.5 | 5 |
| 41b | 0.5 | 20 |
| 60b | 0.5 | 5 |
| 73 | 0.5 | 25 |
| 75 | 0.5 | 5 |
| 81 | 0.5 | 20 |
| 94 | 0.5 | 5 |
| 157b | 0.5 | 5 |
| 171b | 0.5 | 5 |
| 189b | 0.5 | 5 |
| 198b | 0.5 | 5 |
| 200b | 0.5 | 5 |
| 69b | 0.66 | 20 |
| 42b | 0.87 | 30 |
| 102b | 0.87 | 10 |
| 165 | 0.87 | 30 |
| 168 | 1.14 | 20 |
Vaccination history was not available for this dog (“no record”).
Serum samples taken from dogs with no rabies vaccination history prior to this study (“primary”).
Prior to vaccination at the first visit, results using the pseudotype assay classified 89.9% of the dogs that had never been vaccinated (“primary”) as having inadequate levels of VNAbs (Fig. 2B). This compares to 81.5% as determined by the FAVN assay (Fig. 2C). There was a roughly equal split of inadequate vs adequate levels of VNAbs (48% vs 52%) for the dogs that had received one or more rabies vaccination(s) previously (“booster”) when tested using the pseudotype assay (Fig. 2B). The discrepant results described above caused this balance to shift in favour of adequate VNAb levels (36% vs 64%) when the same sera were analysed by the FAVN assay (Fig. 2C). In sera from the second visit both assays detected three dogs (2.5%) that failed to achieve a level of VNAbs that was greater than the OIE control serum despite receiving the vaccination (Fig. 2D and E). None of the three dogs had been given a rabies vaccination prior to this study and their level of circulating VNAbs was inadequate at both visits. Analysis of all results showed that the titres recorded for each serum by FAVN and pseudotype assays correlated strongly (r = 0.915, p < 0.001 [Pearson's product–moment correlation]; Fig. 2F).
3.3. African lyssavirus pseudotypes for surveillance
We cloned the G gene sequences of LBV, MOKV and DUVV into our expression plasmid and tested their ability to be incorporated into lentiviral pseudotype particles. Using GFP as a marker for infection of BHK cells we observed comparable titres for LBV and MOKV pseudotypes as we achieved using CVS-11 (3.3 × 105, 3.6 × 105 and 3.2 × 105 IFU/ml, respectively; Fig. 3 ). The titre recorded for pseudotypes expressing the DUVV G was 1.5-fold higher at 5.2 × 105 IFU/ml. To test the specificity of these African lyssavirus pseudotypes, neutralisation assays were run with sera raised against LBV (RV1; serum nos. 1 and 2) and DUVV (RV131; serum nos. 3, 4, 111 and 112) isolates. Neutralising titres with the pseudotypes correlated strongly with the titres observed using the FAVN assay (Table 4 ). Anti-LBV sera cross-neutralised MOKV in both the FAVN and pseudotype assays. However, the FAVN assay failed to detect anti-DUVV VNAb in serum 4 and 111 and only low levels in serum 3 (IC100 = 8) compared to the pseudotype assay, which recorded IC100 titres of 160, 8 and 28 for sera 3, 4 and 111, respectively (Table 4). The negative control and Australian bat lyssavirus (ABLV) sera did not achieve an IC100 against any genotype, in either assay, and no cross-neutralisation of DUVV by the LBV sera was observed (Table 4).
Fig. 3.
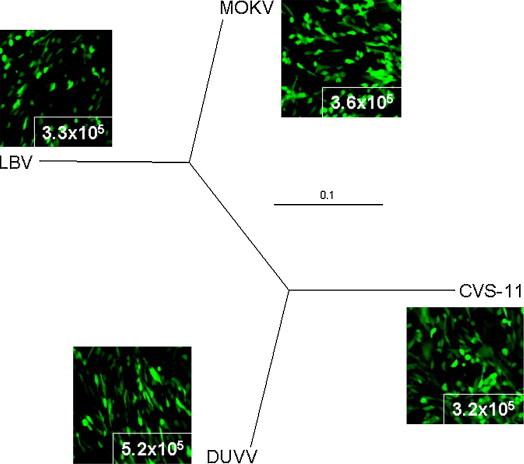
Radial tree and panels showing degree of glycoprotein identity and viral titre of lyssavirus pseudotypes. The envelope G genes from LBV, MOKV and DUVV were cloned into pI.18 and titres were compared with that achieved by pseudotypes bearing the CVS-11 G. Identity of the full-length G amino acid sequence was used to construct the tree. Viral titres were assessed using GFP carrying pseudotypes and are given in IFU/ml. The branch lengths and scale correspond to the number of amino acid substitutions per site.
Table 4.
Levels of African lyssavirus-neutralising antibodies in sera from immunised rabbits as determined by FAVN and pseudotype assays.
| Sample ID | Immunogen | Lagos bat virus |
Mokola virus |
Duvenhage virus |
|||
|---|---|---|---|---|---|---|---|
| FAVN titre | Pseudotype titre | FAVN titre | Pseudotype titre | FAVN titre | Pseudotype titre | ||
| 1 | RV 1 (LBV) | 128 | 508 | 128 | 640 | • | • |
| 2 | RV 1 (LBV) | 362 | 508 | 64 | 80 | • | • |
| 3 | RV 131 (DUVV) | • | • | • | • | 8 | 160 |
| 4 | RV 131 (DUVV) | • | • | • | • | • | 8 |
| 5 | RV634 (ABLV) | • | • | • | • | • | • |
| 6 | Negative control | • | • | • | • | • | • |
| 111 | RV 131 (DUVV) | • | • | • | • | • | 28 |
| 112 | RV 131 (DUVV) | • | • | • | • | – | 12 |
(•): Failed to record a positive titre. (–): Experiment not undertaken. Titres are given as the geometric mean dilution that achieved an IC100.
3.4. Utility of rabies (CVS-11) pseudotypes carrying the lacZ reporter gene
To broaden the lyssavirus pseudotype platform, we cloned the lacZ gene as a reporter. Using a panel of 33 serum samples selected to contain low, medium and high VNAb titres, we ran parallel assays and determined that using lacZ as the reporter gene, instead of luciferase, did not alter the high correlation (r = 0.918, p < 0.001 [Pearson's product–moment correlation]; Fig. 4A) or sensitivity and specificity with respect to the titres achieved using the FAVN assay.
Fig. 4.
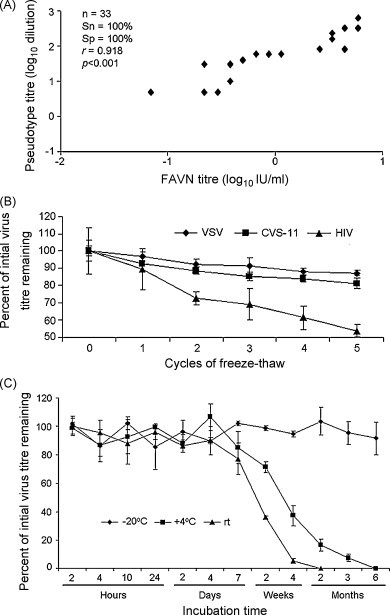
Validation of lacZ-based pseudotypes as a tool for in-field diagnostics to detect neutralising antibodies. (A) Selected samples were run using the lacZ-based CVS-11 pseudotypes. The results show an identical correlation (r) between the FAVN results and those achieved using luciferase-based pseudotypes. Sensitivity (Sn) and specificity (Sp) relative to the 0.5 IU/ml cut-off are given and r/p values were calculated using Pearson's product–moment correlation. The stability of CVS-11 lacZ-pseudotypes was tested by (B) subjecting aliquots to round of freeze–thaw or (C) storing aliquots at different temperatures over time. Results for pseudotypes bearing the VSV and HIV-1 envelope proteins are shown in (B) and CVS-11 pseudotype titres for the time course (C) are given relative to the titre of stocks kept at −80 °C. rt: room temperature.
While the flexibility to use different read-outs is a key requirement of any assay to be used in Africa, it must also be robust, able to withstand fluctuations in temperatures. Therefore, we tested the stability of the pseudotype stocks stored under different conditions. Freeze-thaw cycles of CVS-11 pseudotypes revealed an average decrease of 3.7% in viral titre per cycle, while pseudotypes bearing the VSV or HIV-1 envelope proteins lost 2.6% and 9.2%, respectively (Fig. 4B). Compared to the CVS-11 pseudotype stock stored at −80 °C, aliquots stored at room temperature (average of 23 °C) had a half-life of 1–2 weeks, which increased to 2–4 weeks for aliquots at +4 °C. Pseudotypes stored at −20 °C were relatively stable for over 6 months (Fig. 4C). This stability was similar to that observed with pseudotypes bearing the VSV envelope glycoprotein and was 7-fold greater than the half-life of pseudotypes bearing the HIV envelope glycoprotein stored at +4 °C or room temperature (data not shown).
The inclusion of lacZ as a reporter gene increased the applicability of our pseudotype system in resource-limited laboratories as it allows the determination of VNAb titres without the need for expensive regents or equipment. Cells were infected with lacZ-based pseudotypes and subsequently stained with one of three β-gal substrates. Punctate blue staining of infected cell nuclei was achieved in the presence of X-gal (Fig. 5 , far left panels). We also adapted the assay to allow the β-gal colorimetric substrates CPRG and ONPG to be used (Fig. 5, second left and middle panels). These changes, and resulting VNAb titres, were recorded using a microplate reader, reading at 550 nm (CPRG) or 405 nm (ONPG), or simply by eye. The incorporation of lacZ is an additional option to the much used GFP and luciferase reporter genes, which allow a more high-throughput approach but at a far greater expense (Fig. 5, second right and far right panels, respectively).
Fig. 5.
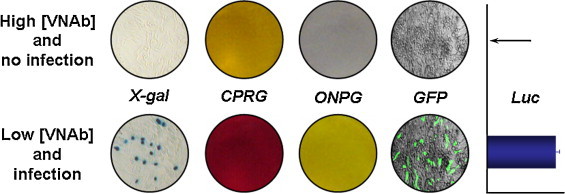
Repertoire of reporter genes that can be carried within pseudotypes. We have expanded the range of reporter genes to enable the neutralisation assay to be performed in a much greater number of laboratories than previously possible. LacZ-based pseudotypes can be detected with X-gal, CPRG or ONPG β-gal substrates that turn blue, red or yellow in the presence of the enzyme, respectively. GFP appears as green cytoplasmic staining while the oxidation of the luciferase substrate results in light emission that can be detected in a luminometer. [VNAb]: virus-neutralising antibody concentration. The arrow indicates zero luciferase activity.
4. Discussion
While mass culling of stray dogs has previously been shown to be ineffective in controlling the spread of rabies [33], [34], this practice is once again being adopted by a number of countries. On the contrary, dog vaccination programs have been enormously successful in controlling canine rabies [35], [36], [37], and improved diagnostics to enable rapid serosurveillance in countries undertaking these programs are therefore important. The assay reported here offers a practical, effective and robust solution for rapid lyssavirus serosurveillance. Using lentiviral pseudotypes we can accurately measure the concentration of VNAbs and, coupled with the use of lacZ as the reporter gene for pseudotype particle production, removes the need for high containment laboratories and expensive equipment or reagents. This allows the assay to be undertaken in laboratories previously unable to use the existing FAVN. Furthermore, the assay is not just restricted to lyssavirus serosurveillance as other highly pathogenic viruses that have been pseudotyped could be incorporated into this platform [24], [38], [39], [40], [41].
To our knowledge this is the largest study published to date using pseudotypes in a diagnostic format for serosurveillance. Of the 319 samples received, titres for three could not be determined using the pseudotype assay: one had low level contamination and two caused cellular cytotoxicity in the assay. These issues were overcome using higher concentrations of antibiotics and increasing the number of BHK cells used 2-fold, to 2 × 104 per assay. In comparison, there were 12 samples that could not be titrated with the FAVN assay because of insufficient serum volume. While serum volume varied between samples, there was sufficient volume for each sample to run duplicate pseudotype neutralisation assays. This highlights another major advantage of using pseudotypes as surrogate viruses in neutralisation assays, only small serum volumes (5–10-fold less than live virus assays) are required for each assay.
An additional important benefit of pseudotypes is the flexibility to choose the reporter gene depending on the operator's requirements. For this study, luciferase-based CVS-11 pseudotypes were used, which allowed high-throughput screening of the samples. This permitted many plates to be analysed in a short period, however, both the luciferase reagent and the plate reader are expensive. In contrast, the reagent for the lacZ reporter system is over 30 times less expensive than that for luciferase and 3 times less expensive than the fluorescein isothiocyanate conjugate used in the FAVN assay. Additionally, as neutralisation assays using pseudotypes are safer, not requiring BSL3/SAPO 4 facilities, the overall cost of using them is many fold lower compared to performing the FAVN assay, thereby allowing expansion of rabies serosurveillance in resource-limited laboratories. In addition, since pseudotypes carrying the lacZ reporter can be detected using X-gal, CPRG or ONPG, the flexibility of the assay is further increased. With colorimetric substrates it is possible to record VNAb titres by simply looking at the assay plate and recording the colour change. Neutralisation assays using pseudotypes with lacZ as the reporter can also be used for semi-high-throughput screening when using an ELI-SPOT (X-gal staining) or microplate reader (CPRG and ONPG staining). However, the flexibility offered by packaging lacZ, GFP or luciferase into the pseudotype particle alone makes this assay an attractive option for use in any laboratory undertaking lyssavirus serosurveillance.
The stability of pseudotypes reported here also makes this assay suitable for use in countries where the infrastructure and cold-chain may be unreliable. If the stocks of pseudotype particles were to be thawed and re-frozen or to be stored at temperatures below −80 °C, they would still be viable for use in subsequent neutralisation assays. This is a major advantage over live virus, where the virus titre decreases rapidly on freeze-thaw or storage above −80 °C. However, where possible stocks of pseudotypes should be maintained at −80 °C and stored in aliquots to reduce the number of times each vial is thawed. A future consideration is to freeze dry the pseudotype particles to avoid cold-chain storage and to increase ease of transport.
The number of inadequate versus adequate vaccine responders, detected by both types of assays, was comparable at either visit for collection of serum. However, 17 discordant results were observed between the pseudotype and FAVN assays, which can partially be explained by the fact that they were all close to the 0.5 IU/ml threshold. Assay variability with the FAVN assay means that samples scoring 0.5 IU/ml had a variable range of 0.38–0.66 IU/ml if the test was repeated. Twelve out of these 17 samples that had a 0.5 IU/ml titre in the FAVN assay might have been classified as negative if re-tested, which would result in a new sensitivity score of 98.3%. Conversely, it is also possible that the scores of 0.38–0.66 IU/ml obtained with the FAVN assay and the corresponding titres achieved using the pseudotype assay could change if re-tested. It is worth noting the 17 dogs that were classified as having inadequate VNAb responses using the pseudotype assay, in disagreement with the FAVN results, were sampled before vaccination in this trial. In addition, 10 out of the 17 samples were from dogs with no history of previous rabies vaccination and 6 had been vaccinated ≥1 year before the study began, therefore their levels of VNAbs could have decreased over time. One did not have a vaccination history collected during the trial (#3). These discordant results coupled with neutralisation data showing DUVV pseudotypes can detect VNAb in anti-DUVV serum not detected by the FAVN assay suggests that the pseudotype assay may be more specific and sensitive for detection of seroconversion than the FAVN assay when a 0.5 IU/ml cut-off is used. However, to confirm this observation further studies are required using analogous viral isolates and serum, and larger sample sizes.
The introduction of the LBV, MOKV and DUVV G as binding antigens into our pseudotype platform, coupled with existing CVS-11, EBLV-1 and EBLV-2 pseudotypes, means that serum can now be screened to detect VNAbs against six different lyssavirus genotypes. This is of particular importance because large proportions of these assays are performed on sera from bats, from which only small volumes of serum are available.
At a time when emerging infectious disease outbreaks are becoming more frequent and have the potential to spread faster through international travel, technological advances in infectious disease research are making assays more rapid facilitating more automation. However, countries where the outbreaks are most likely to occur are often unable to utilise these new techniques. We have addressed these limitations by developing an assay that can be used for infectious disease serosurveillance and for monitoring vaccine responses within low-containment laboratories. Neutralisation assays based on pseudotypes as a source of target antigen are a useful platform to use at the start of a new outbreak, not only for rabies, but also other enveloped RNA viruses such as SARS coronaviruses, influenza and Ebola viruses. Incorporating the lyssaviral envelope protein (G) into the pseudotype platform creates an assay that allows rapid screening and vaccine evaluation to be performed within weeks of the start of an epidemic.
Acknowledgements
We thank Denise Marston for help with DNA sequence analysis, Nigel Temperton for constructive discussion and Olivia Avdis and the Viral Transmission Dynamics Project for assistance with sample collection. We are indebted to Tanzania Government ministries, Tanzania National Parks, Tanzania Wildlife Research Institute and Tanzania Commission for Science and Technology for permission to undertake this research. This research was supported by the UK Medical Research Council (grant number G0801176), the UK Department for Environment, Food and Rural Affairs (grant number SEV3500), partial funding from EU FP7 Epizone project, the Royal College of Veterinary Surgeons Trust (grant number GR000 683), the Wellcome Trust, the University of Edinburgh Development Trust and Lincoln Park Zoo.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2009.09.024.
Appendix A. Supplementary data
References
- 1.Dodet B. Preventing the incurable: Asian rabies experts advocate rabies control. Vaccine. 2006;24(16):3045–3049. doi: 10.1016/j.vaccine.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Knobel D.L., Cleaveland S., Coleman P.G., Fevre E.M., Meltzer M.I., Miranda M.E. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83(5):360–368. [PMC free article] [PubMed] [Google Scholar]
- 3.Si H., Guo Z.M., Hao Y.T., Liu Y.G., Zhang D.M., Rao S.Q. Rabies trend in China (1990–2007) and post-exposure prophylaxis in the Guangdong province. BMC Infect Dis. 2008;8:113. doi: 10.1186/1471-2334-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanton J.D., Hanlon C.A., Rupprecht C.E. Rabies surveillance in the United States during 2006. J Am Vet Med Assoc. 2007;231(4):540–556. doi: 10.2460/javma.231.4.540. [DOI] [PubMed] [Google Scholar]
- 5.Fooks A. The challenge of new and emerging lyssaviruses. Expert Rev Vaccines. 2004;3(4):333–336. doi: 10.1586/14760584.3.4.333. [DOI] [PubMed] [Google Scholar]
- 6.Arai Y.T., Kuzmin I.V., Kameoka Y., Botvinkin A.D. New lyssavirus genotype from the Lesser mouse-eared bat (Myotis blythi), Kyrghyzstan. Emerg Infect Dis. 2003;9(3):333–337. doi: 10.3201/eid0903.020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botvinkin A.D., Poleschuk E.M., Kuzmin I.V., Borisova T.I., Gazaryan S.V., Yager P. Novel lyssaviruses isolated from bats in Russia. Emerg Infect Dis. 2003;9(12):1623–1625. doi: 10.3201/eid0912.030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzmin I.V., Orciari L.A., Arai Y.T., Smith J.S., Hanlon C.A., Kameoka Y. Bat lyssaviruses (Aravan and Khujand) from Central Asia: phylogenetic relationships according to N, P and G gene sequences. Virus Res. 2003;97(2):65–79. doi: 10.1016/s0168-1702(03)00217-x. [DOI] [PubMed] [Google Scholar]
- 9.Markotter W., Kuzmin I., Rupprecht C.E., Nel L.H. Phylogeny of Lagos bat virus: challenges for lyssavirus taxonomy. Virus Res. 2008;135(1):10–21. doi: 10.1016/j.virusres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Velasco-Villa A., Messenger S.L., Orciari L.A., Niezgoda M., Blanton J.D., Fukagawa C. New rabies virus variant in Mexican immigrant. Emerg Infect Dis. 2008;14(12):1906–1908. doi: 10.3201/eid1412.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nel L.H., Rupprecht C.E. Emergence of lyssaviruses in the Old World: the case of Africa. Curr Top Microbiol Immunol. 2007;315:161–193. doi: 10.1007/978-3-540-70962-6_8. [DOI] [PubMed] [Google Scholar]
- 12.Hayman D.T., Fooks A.R., Horton D., Suu-Ire R., Breed A.C., Cunningham A.A. Antibodies against Lagos bat virus in megachiroptera from West Africa. Emerg Infect Dis. 2008;14(6):926–928. doi: 10.3201/eid1406.071421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzmin I.V., Niezgoda M., Franka R., Agwanda B., Markotter W., Beagley J.C. Lagos bat virus in Kenya. J Clin Microbiol. 2008;46(4):1451–1461. doi: 10.1128/JCM.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleaveland S. Epidemiology and control of rabies. The growing problem of rabies in Africa. Trans R Soc Trop Med Hyg. 1998;92(March–April (2)):131–134. doi: 10.1016/s0035-9203(98)90718-0. [Royal Society of Tropical Medicine and Hygiene meeting at Manson House, London, 20 March 1997] [DOI] [PubMed] [Google Scholar]
- 15.Hampson K., Dobson A., Kaare M., Dushoff J., Magoto M., Sindoya E. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis. 2008;2(11):e339. doi: 10.1371/journal.pntd.0000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampson K. The 9th SEARG meeting in Gaborone, Botswana. In: Taylor L., editor. Rabid Bytes. Alliance for Rabies Control; 2008. p. 5. [Google Scholar]
- 17.Anonymous. WHO Expert Consultation on Rabies. WHO Technical Report Series; 2005. p. 931. [PubMed]
- 18.Dodet B, Fooks AR, Muller T, Tordo N (eds). Towards the Elimination of Rabies in Eurasia. OIE Publications; 2008.
- 19.Cliquet F., Aubert M., Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998;212(1):79–87. doi: 10.1016/s0022-1759(97)00212-3. [DOI] [PubMed] [Google Scholar]
- 20.Smith J.S., Yager P.A., Baer G.M. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ. 1973;48(5):535–541. [PMC free article] [PubMed] [Google Scholar]
- 21.Cliquet F., McElhinney L.M., Servat A., Boucher J.M., Lowings J.P., Goddard T. Development of a qualitative indirect ELISA for the measurement of rabies virus-specific antibodies from vaccinated dogs and cats. J Virol Methods. 2004;117(1):1–8. doi: 10.1016/j.jviromet.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Brookes S.M., Parsons G., Johnson N., McElhinney L.M., Fooks A.R. Rabies human diploid cell vaccine elicits cross-neutralising and cross-protecting immune responses against European and Australian bat lyssaviruses. Vaccine. 2005;23(32):4101–4109. doi: 10.1016/j.vaccine.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Khawplod P., Inoue K., Shoji Y., Wilde H., Ubol S., Nishizono A. A novel rapid fluorescent focus inhibition test for rabies virus using a recombinant rabies virus visualizing a green fluorescent protein. J Virol Methods. 2005;125(1):35–40. doi: 10.1016/j.jviromet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Wright E., Temperton N.J., Marston D.A., McElhinney L.M., Fooks A.R., Weiss R.A. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a cross-species comparison. J Gen Virol. 2008;89(Pt 9):2204–2213. doi: 10.1099/vir.0.2008/000349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DuBridge R.B., Tang P., Hsia H.C., Leong P.M., Miller J.H., Calos M.P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7(1):379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoker M., Macpherson I. Syrian hamster fibroblast cell line BHK21 and its derivatives. Nature. 1964;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- 27.Soda Y., Shimizu N., Jinno A., Liu H.Y., Kanbe K., Kitamura T. Establishment of a new system for determination of coreceptor usages of HIV based on the human glioma NP-2 cell line. Biochem Biophys Res Commun. 1999;258(2):313–321. doi: 10.1006/bbrc.1999.0633. [DOI] [PubMed] [Google Scholar]
- 28.Helseth E., Kowalski M., Gabuzda D., Olshevsky U., Haseltine W., Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64(5):2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page R.D. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12(4):357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 31.Ferry N., Duplessis O., Houssin D., Danos O., Heard J.M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci USA. 1991;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupprecht C.E., Hanlon C.A., Slate D. Control and prevention of rabies in animals: paradigm shifts. Dev Biol (Basel) 2006;125:103–111. [PubMed] [Google Scholar]
- 34.Windiyaningsih C., Wilde H., Meslin F.X., Suroso T., Widarso H.S. The rabies epidemic on Flores Island, Indonesia (1998–2003) J Med Assoc Thai. 2004;87(11):1389–1393. [PubMed] [Google Scholar]
- 35.Belotto A.J. The Pan American Health Organization (PAHO) role in the control of rabies in Latin America. Dev Biol (Basel) 2004;119:213–216. [PubMed] [Google Scholar]
- 36.Cleaveland S., Kaare M., Tiringa P., Mlengeya T., Barrat J. A dog rabies vaccination campaign in rural Africa: impact on the incidence of dog rabies and human dog-bite injuries. Vaccine. 2003;21(17–18):1965–1973. doi: 10.1016/s0264-410x(02)00778-8. [DOI] [PubMed] [Google Scholar]
- 37.Kaare M., Lembo T., Hampson K., Ernest E., Estes A., Mentzel C. Rabies control in rural Africa: evaluating strategies for effective domestic dog vaccination. Vaccine. 2009;27(1):152–160. doi: 10.1016/j.vaccine.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu M., Zhang J., Flint M., Logvinoff C., Cheng-Mayer C., Rice C.M. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100(12):7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temperton N.J., Chan P.K., Simmons G., Zambon M.C., Tedder R.S., Takeuchi Y. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg Infect Dis. 2005;11(3):411–416. doi: 10.3201/eid1103.040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temperton N.J., Hoschler K., Major D., Nicolson C., Manvell R., Hien V.M. A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies. Influenza Resp Viruses. 2007;1(3):105–112. doi: 10.1111/j.1750-2659.2007.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wool-Lewis R.J., Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998;72(4):3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


