Abstract
Although hepcidin, a recently discovered peptide hormone, is considered a major regulator of iron metabolism and the anemia of chronic inflammation, its role in the anemia of pregnancy has not been characterized. Our objective was to characterize the role of hepcidin in the anemia of pregnancy. We examined the relationships between urinary hepcidin, iron status indicators, hemoglobin, erythropoietin, alpha-1 acid glycoprotein, and C-reactive protein in a cross-sectional study conducted among 149 pregnant rural Bangladeshi women in biospecimens obtained during home visits. Urinary hepcidin was measured using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. Urinary hepcidin, as log(intensity per mmol/L creatinine), was correlated with log ferritin (r = 0.33, p <0.001), the transferrin receptor index (r = −0.22, p = 0.007), and log alpha-1 acid glycoprotein (r = 0.20, p = 0.01), but not hemoglobin (r = 0.07, p= 0.40), log transferrin receptor (r = −0.07, p = 0.41), log erythropoietin (r = −0.01, p = 0.88) or log C-reactive protein (r = 0.06, p = 0.48). The strength of the relationship between hepcidin and ferritin was maintained in a multiple linear regression analyses after enhancing the sample with women selected for low iron stores (n = 41). Among pregnant women in a community-based study in rural Bangladesh, urinary hepcidin levels were related to iron status and AGP but not hemoglobin, erythropoietin, or C-reactive protein.
Keywords: anemia, hepcidin, inflammation, iron, pregnancy
INTRODUCTION
Anemia is common during pregnancy and is associated with higher perinatal and maternal morbidity and mortality in developing countries.1 Iron deficiency accounts for a large proportion of the anemia among pregnant women.2 Hepcidin, a 25 amino acid peptide, is considered a major regulator of iron metabolism and the anemia of chronic inflammation.3 Hepcidin is found in human plasma and urine,4,5 and it is synthesized primarily in the liver.4–6 It regulates iron metabolism by inhibiting duodenal iron absorption at the level of intestinal epithelium,7 and by affecting mobilization of iron from liver and spleen.8 Hepcidin binds to the iron exporter, ferroportin, inducing its internalization and degradation.9 Ferroportin is the only mammalian iron exporter identified to date and is necessary for materno-fetal iron transfer and iron efflux from duodenal enterocytes, macrophages, and hepatocytes.10
Hepcidin is expressed in an iron-replete state,11 and during iron overload.5 Urinary hepcidin levels seem to change rapidly in response to changes in iron status. For example, in normal human volunteers, urinary hepcidin levels increased up to 15-fold within 24 h after 65 mg of oral iron supplementation.11 In iron deficiency anemia, urinary hepcidin levels are low to undetectable.12 Hepcidin expression is inappropriately low in most forms of hereditary hemochromatosis.13 One particularly severe form of hemochromatosis results from mutations in the gene encoding hepcidin itself.14 The signal for the iron-replete state is still unidentified.8
Despite the central role of hepcidin in the metabolism of iron, limited data are available that link hepcidin to measures of iron status, inflammation, and anemia in pregnant women. Our goal was to examine the relationship between urinary hepcidin and iron status, inflammation, and anemia. To address this goal, we measured urinary hepcidin and indicators of iron status and inflammation in a community-based study of women in Bangladesh at the time of pregnancy assessment, typically in the first trimester. Anemia is considered a population-wide public health problem in Bangladesh, and is particularly prevalent during pregnancy.15–17
MATERIAL AND METHODS
Study design
The study subjects consisted of a total of 190 pregnant women from northwestern Bangladesh from whom blood and urine samples were collected in the home following pregnancy confirmation and at the time of enrollment into a larger clinical trial of micronutrient supplementation.18 The women represented a systematic subsample of nearly 10% (n = 149 women) of over 1500 women that contributed a blood sample. Subsequent purposive sampling of women with iron deficiency (n = 41) was done to enhance the original sample with more iron deficient subjects. This was done in order to extend the range of ferritin concentrations to lower values in the sample of women, thus allowing for the elucidation of the relationship between hepcidin and iron status indicators across a wider spectrum of iron status than that observed in the original sample. Informed consent was obtained from all subjects. The study protocol was approved by the Committee on Human Research of the Johns Hopkins Bloomberg School of Public Health and the Bangladesh Medical Research Council.
Hemoglobin (Hb) was measured from venous blood collected during the home visit using a B-Hemoglobin Analyzer (HemoCue Inc, Lake Forest, CA). Plasma and spot urine samples were aliquoted and stored in liquid nitrogen until analysis at the Johns Hopkins University and Medical Institutions for iron status indicators, C-reactive protein (CRP), alpha-1 acid glycoprotein (AGP), and urinary hepcidin.
Urinary hepcidin was measured in triplicate using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) using hydrophilic normal phase chips (ProteinChip® NP20, Ciphergen Biosystems, Fremont, CA),19 using alpha-cyano-4-hydroxy cinnamic acid in 0.25% (v/v) in trifluoroacetic acid and 50% (v/v) acetonitrile as sample matrix. Mass spectrometry was performed with a PBS IIc mass spectrometer (Ciphergen Biosystems). Peak annotation was conducted using Ciphergen ProteinChip Software (version 3.2.0), after calibration with hepcidin standard (Peptide Institute, Inc, Minoh-shi, Japan) baseline subtraction and adjustment. A peak was recorded for hepcidin at the characteristic m/v of 2790 when the signal-to-noise ratio was >3:1. Urinary creatinine was measured using a commercial ELISA (Quidel Corporation, San Diego, CA). Between run coefficient of variation (CV) for urinary creatinine was 3.8% and 12.8% for high and low controls, respectively. Urinary hepcidin concentrations were expressed as intensity per mmol/L creatinine.
Plasma ferritin, CRP, and erythropoietin (EPO) were assessed using an Immulite 1000 chemiluminescent immunoassay system (Diagnostic Products Corporation, Los Angeles, CA). Soluble plasma transferrin receptor (TfR) concentration was measured using a commercial immunoassay kit (Ramco Laboratories Inc., Houston, TX). Alpha-1 acid glycoprotein was assessed using a radial immunodiffusion assay (Kent Laboratories, Bellingham, WA). Within assay and between assay CV for plasma ferritin, CRP, EPO, soluble TfR and AGP were all <5%.
The distributions of variables were examined and natural log transformed data were used when distributions were skewed. Pearson’s correlation coefficients were calculated among the original sample of 149 women to examine the correlation among hepcidin and indicators of iron stores (ferritin), status (TfR and TfR index — ie. transferrin receptor/log plasma ferritin),20 and erythropoiesis (EPO, Hb), as well as CRP and AGP as indicators of inflammation. To explain hepcidin concentrations as a function of these variables as well as gestational age, regression analysis was utilized. Among the 190 women, complete data on all variables of interest and CRP were available for 179 women, and all variables of interest and AGP were available for 181 women. Multiple linear regression was used to examine the strongest determinants of hepcidin, adjusted for gestational age of pregnancy. All data were analyzed in SAS v 9.1 (SAS Institute Inc, Cary, NC) and R (v. 2.4.1).
RESULTS
Subject characteristics for the entire sample are shown in Table 1. Nearly half the women were primiparous (48%), with 37% of parity 1–2 and 15% of parity ≥3. Women in the original sample were enrolled earlier in pregnancy than those who were selected to enhance the sample (11.1 versus 15.7 weeks gestation, p < 0.001). Among the women selected to enhance the sample, all iron status indicators were consistent with greater degree of iron deficiency, but CRP, weight, and parity did not differ by group. Among all participants, 11% of women had CRP values > 3 mg/L, 13% of women had AGP values above 110 mg/L,21 and there was a moderate correlation between AGP and CRP (r = 0.17, p = 0.03).
Table 1.
Characteristics of pregnant Bangladeshi women (n = 190)
| Mean | Standard Deviation |
Median | Inter-quartile Range | |
|---|---|---|---|---|
| Age (y) † | 21.9 | (5.9) | 20.0 | (17.5–25.0) |
| Height (cm) | 149 | (5) | 149 | (138–164) |
| Weight (kg) | 42.5 | (5.1) | 41.8 | (28.9–59.9) |
| Gestational age (wk) † | 12.0 | (8.4) | 11.0 | (8.0–14.0) |
| Hepcidin (intensity/mmol creatinine) | 4.55 | (5.63) | 2.35 | (0.51–7.22) |
| Hemoglobin (g/L) | 117 | (14) | 117 | (109–126) |
| Ferritin (µg/L) | 86 | (71) | 74 | (36–115) |
| Transferrin receptor (µg/mL) | 4.2 | (1.5) | 3.9 | (3.2–4.8) |
| Transferrin receptor index (TfR/log ferritin) | 0.12 | (0.19) | 0.06 | (0.03–0.11) |
| Erythropoietin (mIU/mL) † | 17.6 | (17.0) | 13.5 | (10.4–19.6) |
| C-reactive protein (mg/L) † | 1.60 | (3.90) | 0.35 | (0.15–1.30) |
| Alpha-1 acid glycoprotein (mg/dL) | 77.6 | (32.8) | 72.6 | (54.4–95.0) |
Missing data for n = 2, 4, 5, and 6 individuals, respectively.
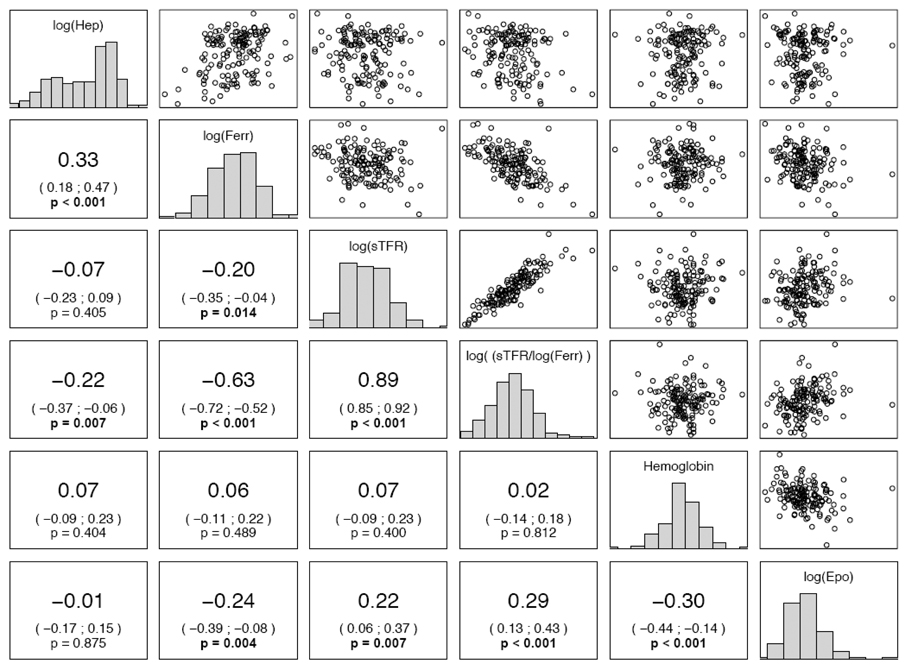
Frequency distributions for each iron status variable and relationships among variables are shown with scatterplots and correlation coefficients for the original sample of women in Figure 1. Urinary hepcidin was correlated with both ferritin (p <0.001) and TfR index (p = 0.007). There were no significant correlations between urinary hepcidin and soluble TfR, Hb, or EPO, although EPO was strongly associated with all other iron status indicators. CRP was not correlated with urinary hepcidin (r = 0.06, p = 0.48), although AGP was (r = 0.20, p = 0.01). C-reactive protein was inversely correlated with hemoglobin concentration (r = −0.21, p < 0.01) but not with other indicators of iron status. Conversely, AGP was correlated with TfR (r = 0.23, p = 0.005).
Figure 1. Pairwise scatterplots and correlations among indicators of iron metabolism among 149 Bangladeshi women.
On the diagonal, the marginal distributions are shown as histograms. In the lower left triangle of the figure, we indicate the respective correlation coefficients, confidence intervals, and p-values. P-values less than 0.05 are shown in bold face. For clarity, and since correlations are scale independent, we omitted the axis labels. Urinary hepcidin, intensity/mMol creatinine, is abbreviated Hep; ferritin, µg/L, is abbreviated ferr.
The multiple linear regression including CRP demonstrated that urinary hepcidin was positively associated with ferritin (p < 0.001) and, to a lesser extent, inversely associated with soluble TfR (p = 0.04) (Table 2). However, an overall test comparing the full model with the regression of hepcidin against ferritin alone did not reject the more parsimonious model (p = 0.18), indicating that iron stores were the overwhelming predictor of urinary hepcidin concentrations. Hepcidin was not related to EPO, Hb, CRP, or gestational age to a significant degree in the multiple linear regression model that included CRP. In the regression model that included AGP, the β-coefficients relating each variable to hepcidin were similar and the strength of the assocation between ferritin and hepcidin remained. However, the association of TfR to hepcidin was strengthened (p = 0.009), and the association of AGP with hepcidin was significant (p = 0.03). A comparison of the full model with the regression of hepcidin against ferritin alone suggested that the full model including AGP better explained urinary hepcidin concentrations (p = 0.02).
Table 2.
Multiple linear regression analysis† of factors associated with hepcidin (intensity per mmol/L creatinine) among Bangladeshi women
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| Ferritin (µg/L) | 1.14 | 0.13 | <0.001 | 1.13 | 0.13 | <0.001 |
| Transferrin Receptor (µg/ML) | −0.71 | 0.34 | 0.04 | −0.93 | 0.35 | 0.009 |
| Erythropoietin (mIU/mL) | 0.37 | 0.22 | 0.09 | 0.35 | 0.21 | 0.10 |
| Hemoglobin (g/L) | 0.013 | 0.008 | 0.13 | 0.014 | 0.008 | 0.09 |
| Gestational age (wk) | −0.011 | 0.022 | 0.64 | 0.006 | 0.022 | 0.78 |
| C-reactive protein (mg/L) | 0.052 | 0.072 | 0.48 | --- | --- | --- |
| Alpha-1 acid glycoprotein (mg/dL) | --- | --- | --- | 0.503 | 0.225 | 0.03 |
Model 1: n = 179; F(6, 172) = 18.4, P < 0.001; R2 = 0.39; Model 2: n = 181, F(6, 174) = 21.1, P < 0.001, R2 = 0.42. All variables except hemoglobin and gestational age expressed as log(x)
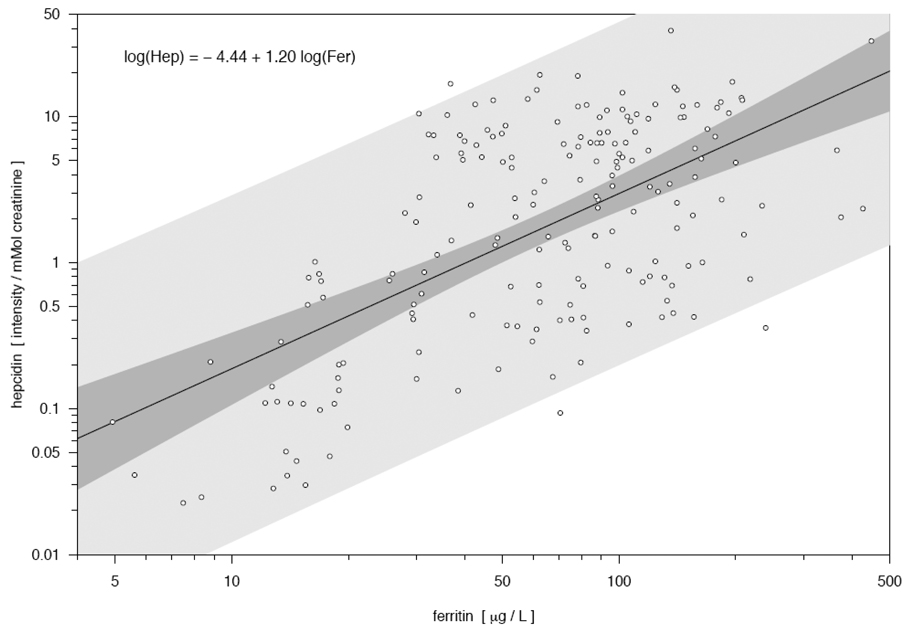
Based on the slope of the relationship between hepcidin and ferritin (Figure 2), a one unit increase in log plasma ferritin resulted in a 1.20 unit increase in log hepcidin intensity, and 36% of the variability in log hepcidin was explained by plasma ferritin values.
Figure 2. Scatterplot and linear relationship between hepcidin (intensity/mmol creatinine) and ferritin (µg/L), both expressed on a log scale, among 190 Bangladeshi women.
The relationship between ferritin and hepcidin was statistically significant (P < 0.001). The darker band is a confidence band for the regression line, while the lighter gray band represents pointwise prediction intervals for future observations, based on the relationship observed in this study.
DISCUSSION
This study shows that iron status is the primary determinant of urinary hepcidin concentrations among pregnant women dwelling in a rural community of Bangladesh. To our knowledge, this is the first study to report urinary hepcidin levels in pregnancy, and it provides insights into factors involved in the regulation of iron metabolism during pregnancy. The four main factors that are thought to be involved in the upregulation of hepcidin are iron status, erythropoiesis, hypoxia, and inflammation.5,7,12 Elevated hepcidin limits the availability of iron for erythropoiesis by reducing iron absorption in the gut and limiting iron release from splenic macrophages and the liver.3 Therefore, hepcidin levels are generally higher in iron-replete individuals, during accelerated erythropoiesis, in non-hypoxic states, and in individuals with evidence of inflammation. We chose indicators that would allow us to examine, as closely as possible, these aspects of iron metabolism and their relationships with hepcidin in a free-living population at risk of iron deficiency and anemia. Among pregnant women in Bangladesh, urinary hepcidin levels were linearly related with iron stores over a wide range of serum ferritin concentrations. To a lesser degree, tissue iron deficiency (elevated TfR) was associated with lower hepcidin concentrations. However, urinary hepcidin levels were not associated with Hb concentrations and the signal for erythropoietic activity (EPO), and the association of hepcidin with inflammatory markers was modest.
It is noteworthy that women in this study were in the first trimester of pregnancy, when the demand of the body for iron is relatively low due to the cessation of menstruation. Iron requirements increase through the second and third trimesters of pregnancy to support the expansion of the red blood cell mass and tissue development of the placenta and fetus. Future work is needed to characterize changes in hepcidin in relation to iron status as pregnancy progresses and iron deficiency becomes more acute.
It is also notable that, while mild infections are common during pregnancy in this area of Bangladesh, among these community-dwelling pregnant women, CRP and AGP were not commonly elevated. Thus, the ranges of values in inflammatory markers against which to relate hepcidin were limited compared to other studies. Interleukin-6 (IL-6), which was not measured here but which is involved in the acute phase response, is thought to be directly involved in the upregulation of hepcidin.3 However, the relationship between IL-6 and hepcidin has mostly been studied previously in subjects with more severe inflammation than was apparent here; that is, in subjects with acute sepsis,19 and in healthy volunteers challenged with IL-6 or lipopolysaccharide.22,23 Although hepcidin is thought to be a major regulator of the anemia of chronic inflammation, such a role had yet to be shown definitively in a population-based study of community-dwelling adults. In this setting, AGP but not CRP showed a modest association with urinary hepcidin. The differential findings between AGP and CRP suggest that the choice of inflammatory marker is crucial for the interpretation of iron status indicators in population-based studies and may be explained by the fact that CRP rises and falls more rapidly in response to infection and inflammation than AGP.21
The findings from this study are consistent with observations of Nemeth and colleagues in which serum ferritin was significantly correlated with urinary hepcidin in a mixed sample of study subjects that consisted of patients with anemia of inflammation, compensated hereditary hemochromatosis, iron overload, iron deficiency anemia, and healthy donors.23 Other studies of hepcidin specific to pregnancy include a rat model in which liver hepcidin expression declined throughout pregnancy as iron stores declined. The decline in liver hepcidin expression was associated with increases in duodenal iron transport proteins DMT1, dcytb, and Ireg1, implying an association of declining hepcidin with increased iron requirements and enhanced iron absorption.24
Although there is considerable interest in evaluating the role of hepcidin in iron metabolism in a variety of population groups, currently the techniques for assessing bioactive hepcidin are not widely available and appear to be limited to mass spectrometric methods such as that utilized in this study. The relationship between plasma prohepcidin hormone has been explored using a commercial assay, and no association of iron or anemia status with plasma prohepcidin was demonstrated, suggesting that the commercial assay for plasma prohepcidin may not identify the active form of the hormone.25
This study provides insight into the role of hepcidin in iron deficiency associated with pregnancy. Hepcidin is likely to be a key regulator of iron metabolism during pregnancy, and this study provides strong evidence that iron status in particular influences hepcidin concentrations among pregnant women of Bangladesh. Future studies are needed with larger sample sizes and longitudinal study designs to characterize changes in hepcidin over time, and a wider panel of indicators to capture the multifactorial etiologies of iron deficiency and anemia during pregnancy and their as-yet unidentified associations with hepcidin concentrations.
ACKNOWLEDGEMENTS
We thank the principal investigator of the field project, Dr. Keith P. West, Jr., and Dr. Mahbabur Rashid, Mr. Abu Ahmed Shamim, Mr. Alain Labrique, Dr. Rolf Klemm, and the project teams, staff, and participants involved in the study. This study was conducted under the National Integrated Population and Health Program of the Government of Bangladesh Ministry of Health and Family Welfare with support from the United States Agency for International Development (GHS-A-00-03-00019-00), the Bill and Melinda Gates Foundation, the Sight and Life Research Institute, Baltimore, MD, the National Institutes of Health (R01 AG027012), and the EHS center grant from the NIEHS (Johns Hopkins Center in Urban Environmental Health).
Footnotes
None of the authors have a conflict of interest to disclose.
Contributor Information
Kerry J. Schulze, Email: kschulze@jhsph.edu.
Parul Christian, Email: pchristi@jhsph.edu.
Ingo Ruczinski, Email: iruczins@jhsph.edu.
Amanda L. Ray, Email: aray5@jhmi.edu.
Avindra Nath, Email: anath1@jhmi.edu.
Lee S.-F. Wu, Email: lwu@jhsph.edu.
Richard D. Semba, Email: rdsemba@jhmi.edu.
REFERENCES
- 1.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2001;71 suppl:1280S–1284S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva: World Health Organization; The Prevalence of Anaemia in Women: A Tabulation of Available Information. (2nd edition) 1992
- 3.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 5.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O, Ilyin G. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 6.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Loréal O, Ilyin G. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz T. Hepcidin – a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 10.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 13.Pietrangelo A. Hereditary hemochromatosis – a new look at an old disease. N Engl J Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 14.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 15.Hyder SMZ, Persson LÅ, Chowdhury M, Lönnerdal B, Ekström EC. Anaemia and iron deficiency during pregnancy in rural Bangladesh. Public Health Nutr. 2004;7:1065–1070. doi: 10.1079/PHN2004645. [DOI] [PubMed] [Google Scholar]
- 16.Helen Keller International/Institute of Public Health Nutrition. Iron Deficiency Anaemia throughout the Lifecycle in Rural Bangladesh. Dhaka: Helen Keller International/Institute Public Health Nutriton; 1999. [Google Scholar]
- 17.Helen Keller International/Institute of Public Health Nutrition. Anemia is a severe public health problem in pre-school children and pregnant women in rural Bangladesh. Nutrition Surveillance Project Bulletin. 2002;10:1–4. [Google Scholar]
- 18.West KP, Jr, Christian P, Klemm R, Labrique A, Rashid M, Shamim AA, Katz J, Sommer A for the JiVitA Bangladesh Project. The JiVitA Bangladesh Project: Research to improve nutrition and health among mothers and infants in rural South Asia. Sight and Life Newsletter. 2006;1:10–14. [Google Scholar]
- 19.Kemna E, Tjalsma H, Laarakkers C, Nemeth E, Willems H, Swinkels D. Novel urine hepcidin assay by mass spectrometry. Blood. 2005;106:3268–3270. doi: 10.1182/blood-2005-05-1873. [DOI] [PubMed] [Google Scholar]
- 20.Markovic M, Majkic-Singh N, Subota V. Usefulness of soluble transferrin receptor and ferritin in iron deficiency and chronic disease. Scand J Clin Lab Invest. 2005;65:571–576. doi: 10.1080/00365510500206542. [DOI] [PubMed] [Google Scholar]
- 21.Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr. 2006;84:1498–1505. doi: 10.1093/ajcn/84.6.1498. [DOI] [PubMed] [Google Scholar]
- 22.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 24.Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut. 2004;53:655–660. doi: 10.1136/gut.2003.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaskowska-Klita T, Chelchowska M, Ambroszkiewicz J. Serum pro-hepcidin and iron markers during uncomplicated pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;130:273–274. doi: 10.1016/j.ejogrb.2006.03.011. [DOI] [PubMed] [Google Scholar]