Abstract
Akt substrate of 160 kDa (called AS160 or TBC1D4) and TBC1D1, Rab GTPase activating proteins that regulate glucose transport, become phosphorylated with exercise- or insulin-stimulation. Evidence suggests that this convergence may prove to be imperfect, and each stimulus will produce a unique phospho-signature, providing a plausible mechanism for their apparently unique and overlapping roles in exercise- and insulin-stimulated glucose transport.
Keywords: glucose transport, skeletal muscle, TBC1D4, AMP-activated protein kinase, protein kinase B, contraction, GLUT4
INTRODUCTION
Two members of the tre-2/USP6, BUB2, cdc16 (TBC1) domain family of proteins, Akt substrate of 160 kDa (also known as AS160 or TBC1D4) and TBC1D1, have been proposed as potential sites for the convergence of insulin and exercise signaling to stimulate glucose transport in skeletal muscle (5, 19, 28). This review will provide a summary of recent research and our speculative interpretation regarding the relationship of AS160 and TBC1D1 with skeletal muscle glucose transport under the following conditions: 1) insulin-stimulation; 2) contraction- and exercise-stimulation; and 3) insulin-stimulation after a single exercise session.
The major physiological stimuli that activate skeletal muscle glucose transport are insulin and exercise (5). Each induces the insulin responsive glucose transporter protein (GLUT4) that is stored in specialized intracellular vesicles to be rapidly recruited for insertion in the surface membranes. Although they ultimately depend on the same transporter protein, insulin and exercise do not share identical signaling pathways. Insulin initiates a signaling pathway that includes the insulin receptor, insulin receptor substrates, phosphatidylinositol 3-kinase (PI3K), and Akt. Exercise relies on the cumulative effects of multiple inputs, with adenosine monophosphate (AMP)-activated protein kinase (AMPK) and increased cytosolic calcium (Ca2+) generally considered as likely to be major factors. For many years, there were no viable candidates to link the signaling pathways for insulin and exercise with GLUT4 vesicles. As a consequence of recent research, a timely question is, do the distinct proximal signaling pathways for insulin and exercise converge at AS160 and/or TBC1D1 before recruiting intracellular GLUT4’s movement to the cell surface membranes?
AS160, TBC1D1 AND GLUCOSE TRANSPORT BY CULTURED CELLS
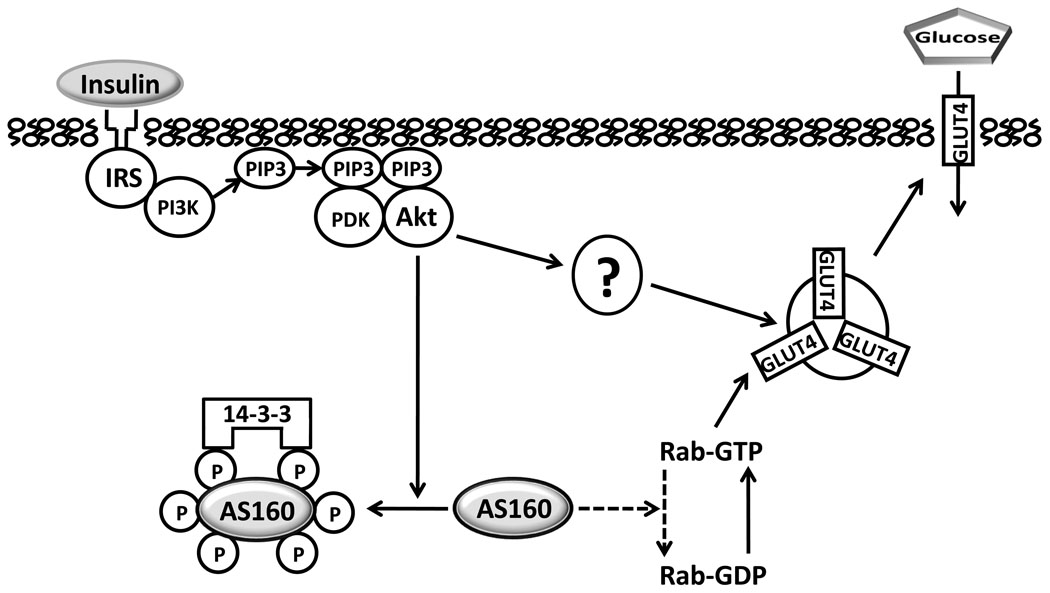
In 2002, Gus Lienhard’s group, based on experiments with 3T3-L1 adipocytes, identified AS160 as a protein that became phosphorylated in response to insulin-stimulation (17), and the following year, they demonstrated the importance of insulin-stimulated phosphorylation of AS160 for increasing cell surface GLUT4 (25). Figure 1 illustrates some key aspects of their original model for AS160’s mechanism of action along with some modifications based on subsequent publications by them and others. AS160 becomes phosphorylated on multiple Akt phosphomotifs as the result of insulin-stimulated activation of Akt. Phosphorylation is predicted to lead to inhibition of AS160’s activation of Rab-GTPase proteins associated with the GLUT4 vesicle and/or cause AS160 to disassociate from the GLUT4 vesicle. By relieving specific Rab proteins from the inhibitory effect of AS160-mediated GTP hydrolysis, accumulation of Rab-GTP would be expected (although this has not been directly demonstrated in cells or tissues), and this would favor GLUT4 vesicle recruitment to the cell’s surface. There is evidence that insulin-induced phosphorylation of specific AS160 motifs leads to its binding to one or more members of the 14-3-3 family of proteins, and this may play a role in GLUT4 translocation (22, 24). Although AS160 appears to be essential for the full insulin effect on GLUT4 translocation, AS160-independent mechanisms also contribute to insulin-stimulated GLUT4 translocation (5).
Figure 1.
Under basal conditions, the Rab GTPase activating domain of non-phosphorylated Akt Substrate of 160 kDa (AS160) catalyzes hydrolysis of Rab-bound guanosine triphosphate (GTP), producing Rab-bound guanosine diphosphate (GDP), which inhibits exocytosis of insulin responsive glucose transporter protein (GLUT4) vesicles. Insulin-stimulated, Akt-induced phosphorylation of AS160 releases this inhibition and results in more GTP-bound Rab, favoring GLUT4 translocation toward the cell surface. Proximal insulin signaling includes, insulin binding to its receptor, leading to tyrosine phosphorylation of insulin receptor substrates (IRS) which bind phosphatidylinositol-3 kinase (PI3K) and catalyze production of phosphatidylinositol 3,4,5-phosphate (PIP3) which binds phosphoinsitide-dependent kinase (PDK) and Akt, causing phosphorylation of Akt, which in turn, phosphorylates Akt Substrate of 160 kDa (AS160) on multiple Akt-phosphomotifs. Members of the 14-3-3 family of proteins bind to specific phosphomotifs of AS160, and this appears to promote AS160 release from membranes and/or to inhibit Rab GAP activity. There also appear to be unidentified Akt-dependent, but AS160 dependent mechanisms (indicated by “?”). Arrows with solid lines indicate a mechanism favoring increased GLUT4 translocation and glucose transport. Dashed lines indicate a mechanism favoring retention of intracellular GLUT4 and lower glucose transport.
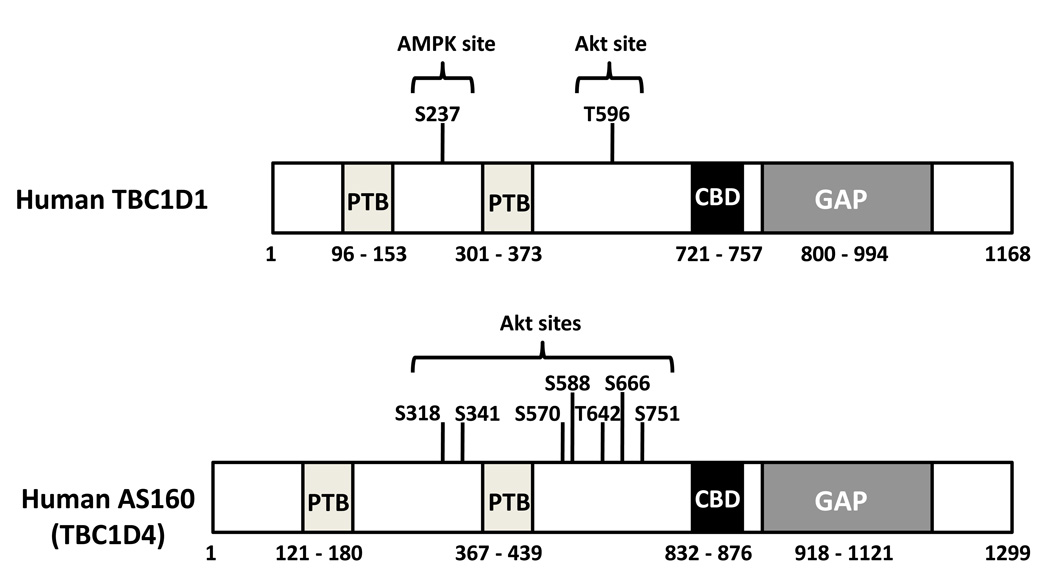
AS160 and TBC1D1 are closely related proteins that share several key structural features (7, 13, 24), most notably with regard to GLUT4 regulation, a Rab GTPase-activating protein (Rab GAP) domain (Fig. 2). Both proteins also include two phosphotyrosine binding (PTB) domains and a calmodulin binding domain. AS160 has more insulin-responsive Akt phosphomotifs than TBC1D1 (7, 23, 25). TBC1D1 includes a predicted AMPK phosphomotif (Serine 237, S237), located between the PTB domains, that is strongly phosphorylated in cultured cells incubated with AICAR (5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside), a compound that activates AMPK (7). Although AMPK can phosphorylate both AS160 (7) and TBC1D1 (13), the S237 AMPK site is only found on TBC1D1 (23). The apparently different potential for phosphorylation of AS160 and TBC1D1 via Akt and AMPK may have regulatory implications.
Figure 2.
Schematic diagrams depict some key elements of two human Rab GTPase activating proteins (GAP): Akt substrate of 160 kDa (AS160 or TBC1D4) and TBC1D1 (7, 13, 23–25, 30). Each protein includes two phosphotyrosine binding (PTB) domains, a calmodulin binding domain (CBD) and a GAP domain. AS160 includes more Akt phosphomotifs than TBC1D1. TBC1D1 includes an adenosine monophosphate (AMP)-activated protein kinase (AMPK) phosphomotif between the PTB domains that is absent from AS160. For clarity, the list of phosphomotifs identified in the figure is incomplete. TBC1D indicates tre-2/USP6, BUB2, cdc16 domain.
AS160 and TBC1D1 also have functional similarities and differences. Overexpression of wildtype AS160 does not alter insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes, indicating that, even at high AS160 levels, insulin can release GLUT4 from the inhibitory effect of AS160 (25). In contrast, overexpression of wildtype TBC1D1 essentially prevented insulin-stimulation of GLUT4 translocation in 3T3-L1 adipocytes providing evidence for insulin's inability to oppose the inhibitory effect of TBC1D1 on GLUT4 (6). In L6 myotubes, genetically knocking down expression of either AS160 or TBC1D1 resulted in a modest increase in basal cell surface GLUT4 content (16). With insulin-stimulation of L6 cells, the increase above basal GLUT4 translocation was unaffected by AS160 knockdown, but TBC1D1 knockdown increased the net gain above basal for GLUT4 translocation. These data suggest that TBC1D1 knockdown eliminates an inhibitory effect on insulin-stimulated GLUT4 translocation, and that TBC1D1's regulation of GLUT4 may not be controlled by insulin. It remains possible that TBC1D1’s effect on GLUT4 can be modulated by other stimuli (e.g., exercise) in normal individuals.
METHODOLOGICAL AND SEMANTIC ISSUES
A commercially available antibody [phospho-(Ser/Thr) Akt substrate; PAS] has been used for most of the published research on skeletal muscle AS160 and/or TBC1D1 (5, 17). Designed to recognize Akt phosphomotif peptide sequences, the PAS antibody has high affinity for phosphothreonine 642 (pT642) of AS160, much lower affinity for pS588, and little if any detectable immunoreactivity against three other insulin-regulated Akt phosphomotifs in AS160 that were evaluated (13, 25). Thus, samples that are immunoprecipitated using anti-AS160 before immunoblotting with anti-PAS may provide insights into the levels of pT642 and possibly pS588, but there will be uncertainty about the relative effects on each of these sites. Furthermore, this approach will not reveal the phosphorylation status of the other Akt phosphomotifs, and it is possible that PAS also interacts with other phosphosites that have not been tested. Although insulin and exercise can each lead to increased PAS-AS160, it cannot be assumed that they produce an identical pattern of AS160 phosphorylation. Recently, phosphosite-specific antibodies were used to assess AS160 phosphorylation in human skeletal muscle (30). Insulin caused a significant increase in AS160 phosphorylation on all six sites tested (S318, S341, S588, T642, S666 and S751) (30). Taylor et al. (28), using mass spectrometry, found that treatment of skeletal muscle with insulin or the AMPK-activator AICAR induced distinctive phosphorylation patterns in TBC1D1. An important goal of future research should be to identify and compare the phospho-signatures for AS160 and TBC1D1 in muscle stimulated by insulin and/or exercise.
A common approach has been to subject homogenized muscle samples to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with anti-PAS. The PAS band visualized at ~160 kDa is often assumed to be phosphorylated AS160. However, without initially immunoprecipitating samples using anti-AS160 or anti-TBC1D1, there is the potential to include PAS immunoreactivity from multiple proteins that migrate on SDS-PAGE at ~160 kDa. Indeed, Taylor et al. (28) reported that in mouse skeletal muscles subjected to SDS-PAGE, both AS160 and TBC1D1 migrated at ~160 kDa. Furthermore, they reported that PAS immunoreactivity at 160 kDa included both proteins. In mice, AS160 levels varied in different muscles such that: soleus > extensor digitorum longus (EDL) > tibialis anterior (TA) (28). TBC1D1 abundance ranked: TA > EDL > soleus. Accordingly, studies that have immunoblotted with anti-PAS without initial immunoprecipitation using anti-AS160 or anti-TBC1D1 may include both proteins, and the relative contribution of the PAS signal originating from AS160 versus TBC1D1 depends on the muscle being analyzed. Studying rat epitrochlearis muscle samples, we (10, 11) found that migration of TBC1D1 on SDS-PAGE corresponded to the migration of the 150 kDa molecular mass standard, and AS160 migrated slightly more slowly than this standard at ~160–170 kDa. Treebak et al. (30) reported that in human vastus lateralis muscle subjected to SDS-PAGE, migration of TBC1D1 (apparent molecular mass of ~150 kDa) was also slightly faster than AS160 (with apparent molecular mass of ~160 kDa). Caution should be used in the interpretation of published results that lack appropriate controls.
REGULATION AND ROLES OF AS160 AND TBCID1 FOR GLUCOSE TRANSPORT BY SKELETAL MUSCLE
AS160, TBC1D1, and insulin-stimulated glucose transport by skeletal muscle
AS160 phosphorylation is important for insulin-stimulated glucose transport in adipocytes, so it was reasonable to anticipate a similar function for AS160 in skeletal muscle. A substantial amount of evidence supports this expectation. We (1) found an insulin dose-dependent increase in PAS-160 using physiologic insulin concentrations and subsequently confirmed that the phosphorylated protein was AS160 (4). For AS160 to be physiologically relevant for insulin-stimulated glucose transport, it must have kinetics consistent with insulin’s rapid effects on glucose transport, and this has been demonstrated with a rapid increase in PAS-160 of isolated muscles with insulin exposure (4), and rapid reversal upon removal of muscles from insulin (26). The PI3K-inhibitor wortmannin eliminates insulin-stimulation of both PAS-AS160 and glucose transport in isolated rat epitrochlearis muscle (4, 11). Electroporation of mouse TA muscle with plasmids including DNA for wildtype or mutated AS160 has provided valuable insights into the molecular mechanisms whereby AS160 modulates insulin-stimulated glucose uptake (18, 20). In vivo glucose uptake by TA muscle determined in mice with elevated circulating insulin was unaffected by an ~8-fold increase in wildtype AS160 compared to insulin-stimulated control muscle, but glucose uptake was reduced by ~50% in muscles with overexpression of mutant AS160 (known as 4P) which could not be phosphorylated on four of the Akt responsive phosphomotifs, suggesting that the ability to phosphorylate these sites is important for a large portion, but not all, of insulin-stimulated glucose uptake. Overexpression of a double mutant AS160, that could not be phosphorylated on these sites and also lacked a functional Rab GAP domain, rescued glucose uptake by insulin-stimulated muscle, suggesting that insulin's regulation of glucose uptake via phosphorylation of AS160 depends on inhibition of AS160's Rab GAP activity. These results were strikingly similar to the results found for insulin-stimulated GLUT4 translocation in adipocytes with overexpression of wildtype or mutant AS160 (25).
Insulin can also increase PAS-TBC1D1 in mouse TA muscle (28) and rat epitrochlearis muscle (12). The role of TBC1D1 in insulin-stimulated GLUT4 translocation and glucose transport in skeletal muscle remains to be determined, but based primarily on results in cultured cells (6, 7, 16, 23), we speculate that in skeletal muscle, as in cells, TBC1D1 will likely restrain basal and insulin-stimulated GLUT4 translocation, but TBC1D1’s inhibitory action may not be subject to regulation by insulin.
AS160, contraction, exercise, and insulin-independent glucose transport by skeletal muscle
Bruss et al. (4) found that in isolated rat epitrochlearis muscle, 5 minutes of contractile activity, in the absence of insulin, caused an increase in PAS-AS160. Endurance exercise (2 hour swim) by rats increased epitrochlearis muscle PAS-AS160 (2), and moderate intensity cycling (60 or 90 minutes at 70% peak oxygen consumption, VO2peak) by humans increased vastus lateralis (VL) muscle PAS-AS160 (8, 14, 29), although this increase was not detectable in human VL sampled immediately after exercise lasting only 1, 10, or 30 minutes (29). PAS-160 was increased in VL sampled immediately after 40 minutes, but not immediately after 10 minutes of moderate intensity (70% VO2peak) cycling performed by lean humans (27). Sprint cycling did not increase PAS-160 (29), and resistance exercise did not increase PAS-AS160 in muscle of humans (8, 9, 15) immediately post-exercise. Howlett et al. (15) found the reduced PAS-AS160 immediately after resistance exercise was accompanied by a decreased capacity for muscle AS160 to bind 14-3-3 protein, and increased PAS-AS160 evident immediately after endurance exercise by humans was accompanied by increased 14-3-3 binding capacity of AS160 (14). It would be valuable to determine if altered 14-3-3 binding capacity measured by this in vitro assay corresponds with the level of association of endogenous 14-3-3 with AS160 in the intact muscle.
We were surprised to find that PAS-AS160 was still increased in rat epitrochlearis muscles dissected out 3–4 hours after swim exercise and incubated without insulin compared to resting controls (2). We extended this finding by demonstrating insulin-independent PAS-AS160 remains increased at 27 hours after exercise (12). Elevated PAS-160 was also evident in muscle from lean, sedentary humans at 2.5 hours after moderate intensity exercise (27). Although muscle PAS-AS160 has consistently been observed to not increase immediately after resistance exercise, Dreyer et al. (9) found increased values at 1 and 2 hours after acute resistance exercise. Using antibodies specific for individual phosphosites on AS160, Treebak (30) recently provided support for increased AS160 phosphorylation in skeletal muscle several hours following acute endurance exercise. At 4 hours after one-legged knee extensor exercise by lean humans, they reported small, but significant increases for phosphorylation at S318, S341, and S751, with a trend for an increase at S588. However, no exercise effects were evident for T642, S666, or anti-PAS immunoreactivity. There is not an obvious explanation for the apparent discrepancy using anti-PAS results in this study compared to several other studies. Nonetheless, a robust finding is an increased phosphorylation of AS160 in muscles several hours after acute exercise.
Multiple lines of evidence demonstrate that increased PAS-AS160 is not essential for the insulin-independent increase in glucose uptake by skeletal muscle during exercise or after contractile activity. Wortmannin eliminated the contraction-induced increase in PAS-AS160 (4, 11) without altering contraction-stimulated glucose transport by isolated rat epitrochlearis muscle (11). Conversely, an AMPK-inhibitor markedly reduced contraction-stimulated glucose transport without reducing PAS-AS160 (11). At 3–4 hours post-exercise, rat epitrochlearis muscle PAS-AS160 in the absence of insulin remained elevated, but insulin-independent glucose transport was no longer increased (2). Resistance exercise increased glucose uptake determined immediately post-exercise, but PAS-160 was not elevated (9). Endurance exercise by humans did not increase PAS-AS160 at 10 or 30 min of endurance exercise (29), even though glucose uptake would be expected to be increased. Taken together, these results demonstrate that mechanisms other than increased PAS-AS160 account for the insulin-independent glucose transport during and after exercise or contraction.
Nonetheless, it remains possible that phosphorylation of AS160 on sites not recognized by anti-PAS is important for contraction or exercise-stimulated glucose transport. TA muscles of mice were transfected to express high levels of mutated AS160 (known as the 4P mutant) that could not be phosphorylated on 4 Akt-phosphomotifs (including two sites not recognized by anti-PAS) and stimulated to contract in situ (20). There were several notable differences in the results for contraction-stimulated compared to insulin-stimulated glucose uptake with overexpression of wildtype or mutant AS160. Overexpression of wildtype AS160 did not alter the insulin-stimulated increase in GLUT4 translocation of 3T3-L1 adipocytes (25) or insulin-stimulated glucose uptake by mouse TA muscle, but overexpression of wildtype AS160 caused a significant decline in glucose uptake by mouse TA stimulated to contract (20). In other words, contraction (unlike insulin) was unable to relieve the inhibition of glucose uptake attributable to overexpression of wildtype AS160, suggesting that contraction was relatively ineffective at preventing AS160’s restraint of glucose uptake. There are many possible interpretations of the results, but we speculate that overexpression of wildtype AS160 to 6-8-fold above endogenous AS160 might interfere with the function of another protein that regulates contraction-mediated glucose transport. For example, AS160 and TBC1D1 are similar proteins, and each may need to associate with GLUT4 vesicles to regulate the Rab protein(s) that control vesicular traffic. Perhaps AS160 and TBC1D1 compete for vesicle binding, and AS160 overexpression displaces TBC1D1. If contraction is relatively more effective at inhibiting TBC1D1-associated GAP activity compared to AS160-associated GAP activity, this may explain the effect of wildtype AS160 overexpression on contraction-stimulated glucose uptake. However, the magnitude of contraction-induced glucose uptake inhibition in muscles expressing the 4P mutant of AS160 was greater than in muscles overexpressing wildtype AS160, providing evidence that the inability to phosphorylate AS160 on these four sites eliminated ~20–25% of the contraction-stimulated glucose uptake (18, 20).
A great deal of evidence suggests that increased cytosolic Ca2+ is important for a portion of the contraction-stimulated increase in glucose transport, so it is interesting that both AS160 and TBC1D1 have a calmodulin binding domain (CBD). After accounting for the reduction in contraction-stimulated glucose uptake found in controls overexpressing wildtype AS160, mouse TA muscles that expressed AS160 with a mutation of the CBD had a further ~30–35% reduction in contraction-stimulated glucose uptake (18). Because expression of the CBD mutant AS160 did not alter insulin-stimulated glucose uptake by mouse TA muscle, the effect appears to be specific to contraction-stimulated glucose uptake. Surprisingly, muscles expressing a CBD+4P double mutant AS160 had a similar reduction in contraction-stimulated glucose uptake as muscles expressing either CBD or 4P mutants, i.e., there was not an additive effect. One possible explanation for this result would be if Ca2+-calmodulin binding and AS160 phosphorylation are part of a cooperative mechanism such that each event is essential, but neither is sufficient for regulating contraction-mediated GLUT4 translocation.
TBC1D1, contraction, exercise, and insulin-independent glucose transport by skeletal muscle
The results of experiments with AS160 beg the question, if a large portion of the contraction-stimulated increase in glucose transport is not attributable to AS160, what is the mechanism for this increase? TBC1D1 is a candidate because PAS-TBC1D1 was increased in mouse TA muscle after in situ contraction (28). In vitro contraction also increased PAS-TBC1D1 in the rat epitrochlearis (11). Wortmannin did not reduce the contraction-stimulated increases in glucose transport or PAS-TBC1D1. PAS-TBC1D1 was increased immediately after in vivo exercise, but unlike PAS-AS160, this effect was lost by 3–4 hours post-exercise, at which time, the insulin-independent increase in glucose transport was also reversed (11). In human VL muscle, PAS-150 was increased immediately after resistance or moderate intensity exercise (8). However, it is unclear if this 150 kDa band was TBC1D1.
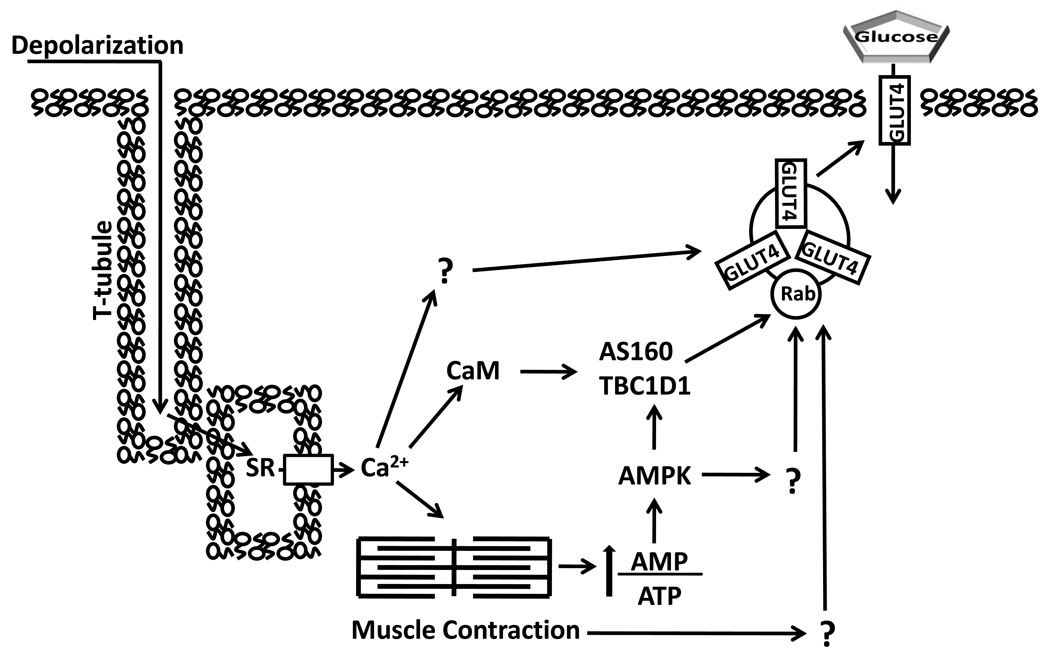
Two different inhibitors that reduced contraction-stimulated AMPK activation also eliminated the increase in PAS-TBC1D1 concomitant with a substantial decrease in glucose transport. Compound C, which effectively inhibited AMPK as evidenced by complete elimination of the contraction-stimulated increase in phosphorylation of acetyl CoA carboxylase (an AMPK substrate), also eliminated the contraction-stimulated increase in PAS-TBC1D1 and reduced contraction-stimulated glucose transport by 65% (11). It did not alter the contraction-stimulated increase in phosphorylation of Akt’s substrate glycogen synthase kinase-3, nor did it affect the contraction-stimulated increase in pCaMKII. Importantly, it did not attenuate tension development, and the inhibition of glucose transport appeared to be specific for stimuli that activated AMPK, as indicated by no effect on insulin-stimulated glucose transport and complete elimination of the glucose transport induced by the chemical AMPK-activator AICAR. Because AMPK has been shown to phosphorylate TBC1D1 in cell free assays (7), and Compound C effectively inhibited AMPK, it seems reasonable to suspect that AMPK inhibition was important for the attenuated TBC1D1 phosphorylation with Compound C. However, it is possible that Compound C also inhibited other contraction-stimulated kinases and that one or more of these unidentified kinases may also be able to phosphorylate TBC1D1. Regardless of this caveat, other evidence supports the idea that AMPK is important for the contraction-stimulated increase in TBC1D1 phosphorylation. A highly specific myosin type II inhibitor which reduced AMPK activation (presumably as an indirect consequence of attenuated tension-associated ATPase cycling) also eliminated the increase in PAS-TBC1D1 concomitant with a 57% decrease in contraction-mediated glucose transport (3). These data do not definitively establish a contraction-stimulated AMPK-TBC1D1-glucose transport pathway, but they support the idea. It would be valuable to determine the effect of contraction on TBC1D1 phosphorylation in muscles that were genetically deficient in AMPK. Our working hypothesis is that TBC1D1, in concert with AS160-dependent and TBC1D1/AS160-independent mechanisms, is required for contraction-stimulated glucose transport (Fig. 3).
Figure 3.
Contraction stimulation of skeletal muscle glucose transport involves multiple inputs that lead to increased cell surface insulin responsive glucose transporter protein (GLUT4). T-tubule depolarization causes calcium (Ca2+) release from the sarcoplasmic reticulum (SR) which triggers actin and myosin interaction. The energy demand of contraction increases the ratio of adenosine monophosphate (AMP)/ adenosine triphosphate (ATP) which stimulates AMP-associated protein kinase (AMPK). AMPK can phosphorylate both AS160 and TBC1D1, as well as other unknown substrates, which potentially contribute to increased glucose transport. Both Akt substrate of 160 kDa (AS160) and TBC1D1 have a calmodulin (CaM) binding domain (CBD). The CBD of AS160 has been implicated in contraction-stimulated glucose transport, but the role of TBC1D1’s CBD is unknown. Increased glucose transport with contraction may also involve other Ca2+-dependent and Ca2+-independent processes (indicated by “?”).TBC1D indicates tre-2/USP6, BUB2, cdc16 domain.
It may appear inconsistent to propose that increased TBC1D1 phosphorylation is important for contraction-stimulated, but not for insulin-mediated glucose transport. This apparent paradox might be reconciled if insulin (via Akt) and contraction (via AMPK) induce different phosphorylation patterns on TBC1D1 that are not discerned using anti-PAS, as is suggested by research with cultured cells and purified recombinant enzymes in cell-free assays (7, 23). Insulin-mediated phosphorylation of TBC1D1 recognized by anti-PAS appears to be on pT596 (23), but it is uncertain which TBC1D1 phosphosite(s) is(are) recognized by anti-PAS in skeletal muscle in response to insulin or contraction. In L6 muscle cells, insulin did not stimulate increased pS237 or 14-3-3 binding to TBC1D1 (7). In contrast, pS237 and 14-3-3-bound TBC1D1 were increased in L6 myotubes stimulated with an AMPK activator (7). One possibility is that AMPK, but not Akt, triggers phosphorylation of S237 and/or other sites of TBC1D1, leading to increased 14-3-3-bound TBC1D1 which attenuates TBC1D1’s Rab GAP activity and/or causes TBC1D1 to be released from the GLUT4 vesicle. Such an outcome would be predicted to favor GLUT4’s redistribution to cell surface membranes.
In vitro electrical stimulation resulted in a transient increase in PAS-150 (apparently TBC1D1 based on control experiments) of rat epitrochlearis such that it was greater than resting controls with 20 minutes of contraction, but had returned to baseline by 60 minutes (10). Glucose transport increased, reaching a peak value by 20 minutes, and this rate was sustained for the remainder of the 60 minute stimulation period. These data suggest that although increased PAS-TBC1D1 may participate in triggering the contraction-stimulated increase in glucose transport, it is not required for the sustained increase in glucose transport. It will be important to determine if contraction induces a sustained phosphorylation of TBC1D1 on S237 and/or other sites that correspond with contraction-stimulated glucose transport.
AS160, TBC1D1and the post-exercise increase in insulin-stimulated glucose transport
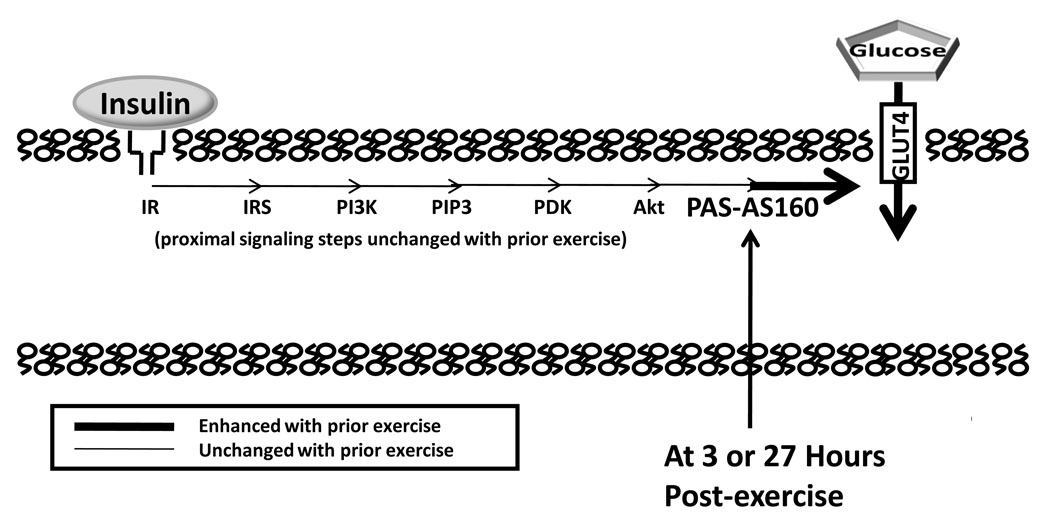
The insulin-independent effect of exercise begins to reverse minutes after exercise cessation with most or all of the increase lost within 1–4 hours. A much more persistent effect is improved insulin-sensitivity that is often found ~2–4 hours and as long as 1–2 days after acute exercise (5). The improved insulin sensitivity is the result of greater insulin-mediated GLUT4 translocation, but the cellular events responsible for this are poorly understood. Acute exercise does not appear to alter the proximal insulin signaling steps, including insulin receptor binding, insulin receptor tyrosine kinase, tyrosine phosphorylation of insulin receptor or insulin receptor substrate-1 (IRS-1), IRS-1-associated PI3K, or serine phosphorylation of Akt (Fig. 4). With the identification of AS160 and TBC1D1 as insulin regulated proteins, we wondered if prior exercise improves insulin sensitivity via these downstream steps.
Figure 4.
Increased insulin-stimulated glucose transport, as the result of greater insulin responsive glucose transporter protein (GLUT4) translocation, can occur a few or many hours after acute exercise. A number of proximal insulin signaling steps (insulin receptor, IR, binding; tyrosine phosphorylation of IR and insulin receptor substrates, IRS; IRS-associated phosphatidylinositol-3 kinase, PI3K, activity; and Akt serine phosphorylation) are not enhanced a few hours after acute exercise. At either 3 or 27 hours after acute exercise, Akt Substrate of 160 kDa (AS160) phosphorylation on sites recognized by anti-PAS (PAS-AS160) was found to be increased in rat skeletal muscle in the absence of insulin (4, 12). We hypothesize that the persistent increase in AS160 phosphorylation plays a role in the increased insulin-stimulated glucose transport after acute exercise. PIP3 indicates phosphatidylinositol 3,4,5-phosphate; PDK, phosphoinsitide-dependent kinase; PAS, phospho-(Ser/Thr) Akt substrate.
We originally hypothesized that 3–4 hours after acute exercise, the insulin-stimulated increase in PAS-AS160 (calculated by subtracting basal PAS-AS160 from the value in paired insulin-treated muscles, called the delta insulin for PAS-AS160) would be elevated (2). However, our original hypothesis was not supported: the PAS-AS160 in insulin-treated muscle was greater for post-exercise versus sedentary controls, but prior exercise did not alter the delta insulin for PAS-AS160. Rather, we found that PAS-AS160 in the absence of insulin was greater at 3–4 hours post-exercise versus sedentary controls, and this elevated baseline value accounted for the greater PAS-AS160 when muscle was incubated with insulin. We subsequently found an increase in PAS-AS160 without insulin in muscles from rats persisted at 27 hours post-exercise (12). Insulin-stimulated glucose transport (delta insulin for glucose transport) was increased above time-matched sedentary groups at both 3–4 and 27 hours post-exercise. Furthermore, when rats were allowed to eat a high carbohydrate chow for 3–4 hours after exercise, the increased PAS-AS160 and the increased insulin-stimulated glucose transport were both reversed to levels found in sedentary controls. Several studies in humans after acute exercise have also reported an elevated PAS-AS160 or PAS-160 in muscles without insulin infusion (9, 27). Interestingly, Howlett et al. (14) did not find an increase in PAS-AS160 at 3 hours after acute endurance exercise, and they also did not observe a significant increase in insulin sensitivity at that time, supporting the idea that increased PAS-AS160 is related to the post-exercise improvement in insulin sensitivity. At 240 minutes post-exercise, Treebak et al. (30) observed an increase in phosphorylation of AS160 on several sites (S318, S341 and S751) in the absence of insulin infusion, and with insulin infusion at 340 minutes post-exercise, there was a higher level for phosphorylation of these sites for post-exercised compared with resting controls. They also found increased insulin-stimulated glucose uptake at 340 minutes post exercise. Thus, an increase in AS160 phosphorylation post-exercise appears to be a robust finding in rodents and humans when insulin sensitivity is improved and absent under conditions without improved insulin action.
The association between a persistent increase in AS160 phosphorylation and increased insulin sensitivity post-exercise is not proof of a causal relationship. However, it raises the question, by what mechanism could a persistent insulin-independent increase in AS160 phosphorylation contribute to increased insulin sensitivity? AS160 phosphorylation on appropriate sites appears to induce AS160 to disassociate from intracellular membranes (21). Presumably AS160’s restraint of GLUT4 requires AS160’s proximity to its target Rab proteins associated with GLUT4 vesicles. In this context, increased PAS-AS160 in the absence of insulin, although insufficient to fully activate the redistribution of GLUT4 to the surface membranes, may eliminate AS160’s inhibitory effect and render GLUT4 vesicles more susceptible to subsequent insulin-triggered translocation. Insulin-stimulated glucose transport is the consequence of insulin’s regulation of both AS160-dependent and AS160-independent mechanisms. A simple analogy for the regulation of GLUT4 exocytosis by these two mechanisms is that opening a door can be prevented by both a deadbolt and chain lock. If the deadbolt is disengaged (AS160 becomes phosphorylated), the door still cannot be opened enough to permit entry, but now only the chain must be disconnected (insulin-induced AS160-independent mechanisms) for the door to be fully opened, allowing passage through the door.
There was not a persistent increase in PAS-TBC1D1 at 3–4 or 27 hours after exercise when insulin sensitivity was increased (12). These findings provide evidence that TBC1D1 phosphorylation as recognized by anti-PAS is not part of the mechanism for enhanced insulin sensitivity after exercise.
CONCLUSIONS
Although current understanding is far from complete, recent research has revealed valuable insights about the roles of AS160 and TBC1D1 in regulating skeletal muscle glucose transport. Insulin’s restraint of AS160, but perhaps not TBC1D1 (based on data from cultured cells), plays a major role in insulin-mediated GLUT4 translocation. Increased phosphorylation of AS160 on sites recognized by anti-PAS is not essential for contraction-stimulated glucose transport. However, experiments using mice expressing mutant AS160 suggest that AS160-dependent mechanisms (Ca2+-calmodulin binding and/or phosphorylation on sites not recognized by anti-PAS) may contribute to contraction-induced glucose transport (18, 20). Based on studies using cultured cells and cell-free assays together with a small number of studies on rodent skeletal muscle, TBC1D1 phosphorylation by AMPK is attractive as a putative mechanism for at least a portion of the AS160-independent, contraction-stimulated glucose transport. We also hypothesize that the persistent increase in insulin-independent phosphorylation of AS160, but not TBC1D1, plays a role in the enhanced insulin-stimulated glucose transport after exercise. Further clarification of the influence of insulin and exercise on AS160 and TBC1D1 function depends on identifying each protein’s site-specific phosphoryation, subcellular localization, and 14-3-3 binding. It will be important to consider the large differences that can exist among muscles in their relative expression of AS160 and TBC1D1.
Do exercise and insulin converge or diverge at AS160 and TBC1D1? We believe the answer is, “both.” Convergence is indicated by the increased phosphorylation of both Rab GAP proteins in response to either exercise or insulin. However, several lines of evidence suggest that the convergence will likely prove to be imperfect and each stimulus will produce a unique phospho-signature. A distinctive phosphorylation pattern for exercise compared to insulin would provide a plausible mechanism for functional divergence, including the complementary roles that AS160 and TBC1D1 appear to play in regulating insulin- and exercise-stimulated glucose transport.
Acknowledgments
Funding: This work was supported by research grants from the National Institutes of Health (AG10026 and DK071771 to GDC).
REFERENCES
- 1.Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930. doi: 10.2337/diabetes.53.4.921. [DOI] [PubMed] [Google Scholar]
- 2.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E1191–E1200. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- 3.Blair DR, Funai K, Schweitzer GG, Cartee GD. A Myosin II ATPase Inhibitor Reduces Force Production, Glucose Transport and Phosphorylation of AMPK and TBC1D1 in Electrically Stimulated Rat Skeletal Muscle. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.91003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- 5.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007;32:557–566. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- 6.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem. 2008;283:9187–9195. doi: 10.1074/jbc.M708934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J. 2008;409:449–459. doi: 10.1042/BJ20071114. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes. 2006;55:1776–1782. doi: 10.2337/db05-1419. [DOI] [PubMed] [Google Scholar]
- 9.Dreyer HC, Drummond MJ, Glynn EL, et al. Resistance exercise increases human skeletal muscle AS160/TBC1D4 phosphorylation in association with enhanced leg glucose uptake during post-exercise recovery. J Appl Physiol. 2008;105:1967–1974. doi: 10.1152/japplphysiol.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funai K, Cartee GD. Contraction-stimulated glucose transport in rat skeletal muscle is sustained despite reversal of increased PAS-phosphorylation of AS160 and TBC1D1. J Appl Physiol. 2008;105:1788–1795. doi: 10.1152/japplphysiol.90838.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes. 2009;58:1096–1104. doi: 10.2337/db08-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 Phosphorylation, but Not TBC1D1 Phosphorylation, with Increased Post-exercise Insulin Sensitivity in Rat Skeletal Muscle. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geraghty KM, Chen S, Harthill JE, et al. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J. 2007;407:231–241. doi: 10.1042/BJ20070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howlett KF, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14-3-3 binding capacity in human skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E401–E407. doi: 10.1152/ajpendo.00542.2007. [DOI] [PubMed] [Google Scholar]
- 15.Howlett KF, Sakamoto K, Garnham A, Cameron-Smith D, Hargreaves M. Resistance exercise and insulin regulate AS160 and interaction with 14-3-3 in human skeletal muscle. Diabetes. 2007;56:1608–1614. doi: 10.2337/db06-1398. [DOI] [PubMed] [Google Scholar]
- 16.Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol. 2008;295:C1016–C1025. doi: 10.1152/ajpcell.00277.2008. [DOI] [PubMed] [Google Scholar]
- 17.Kane S, Sano H, Liu SC, et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 18.Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes. 2007;56:2854–2862. doi: 10.2337/db07-0681. [DOI] [PubMed] [Google Scholar]
- 19.Kramer HF, Witczak CA, Fujii N, et al. Distinct Signals Regulate AS160 Phosphorylation in Response to Insulin, AICAR, and Contraction in Mouse Skeletal Muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 20.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 21.Larance M, Ramm G, Stockli J, et al. Characterization of the Role of the Rab GTPase-activating Protein AS160 in Insulin-regulated GLUT4 Trafficking. J Biol Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 22.Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem. 2006;281:29174–29180. doi: 10.1074/jbc.M603274200. [DOI] [PubMed] [Google Scholar]
- 23.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295:E29–E37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sano H, Kane S, Sano E, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 26.Sharma N, Arias EB, Cartee GD. Rapid reversal of insulin-stimulated AS160 phosphorylation in rat skeletal muscle after insulin exposure. Physiol Res. 2009 doi: 10.33549/physiolres.931707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sriwijitkamol A, Coletta DK, Wajcberg E, et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56:836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor EB, An D, Kramer HF, et al. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008;283:9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:E715–E722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 30.Treebak JT, Frosig C, Pehmoller C, et al. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia. 2009 doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]