Abstract
Background
Human papillomavirus (HPV) is the cause of cervical cancer. To better understand the natural history of HPV, we assessed the incidence of type-specific HPV infection and examined risk factors for acquisition of high-risk (HR) HPV infection in Danish women.
Methods
A population-based prospective cohort study of women aged 20 – 29 years was conducted. Participants were interviewed and underwent two gynaecological examinations 2 years apart. Women for whom Hybrid Capture 2 results were available at both visits were included in the analysis (n = 7454).
Results
A HR HPV infection was acquired by 12.8% of the women during follow-up. The incidence decreased with increasing age. The commonest types were HPV16, HPV31 and HPV52. HPV66, HPV58 and HPV53 were mainly acquired with other HR types. Multiple HR types were acquired in 50% of the women who became HPV positive during follow-up. In initially HPV negative women age, number of sexual partners and oral contraceptive use were the main risk factors for acquisition, particularly of multiple HR HPV types.
Conclusions
HPV infections were commonly acquired. We confirmed the sexually transmitted nature of the infection. Our findings show that both the level of potential exposure and other behavioural factors increase the risk for HR HPV acquisition.
Keywords: Human papillomavirus, type-specific incidence, multiple HR HPV types, risk factors
INTRODUCTION
Some human papillomavirus (HPV) types have a carcinogenic potential, and high-risk (HR) HPV types are detected in almost all cervical cancers. HPV is also causing a proportion of other anogenital cancers, such as cancers of the vulva, vagina and anusas well as cancers of the mouth and pharynx1. HPV16 and HPV18 are detected particularly frequently in patients with cervical cancer 2. A prophylactic vaccine against these types is now available and might be an effective instrument for primary prevention in the future. Nevertheless, to optimize the prevention of cervical cancer and other anogenital cancers, studies are still needed on the natural history of HPV.
The earliest studies of the incidence of cervical HPV infection focused mainly on overall HPV infection (any HPV type) 3–5. Other studies, however, recognized differences in acquisition rate and risk factor profile between low-risk (LR) and high-risk HPV types 6–9 and infections with single and multiple HPV types 10–12.
In the study reported here, we assessed the incidence of specific HR HPV types and differences in the risk factor profiles for acquisition of a single and multiple HR HPV types in a large population-based cohort of more than 7000 Danish women.
MATERIALS AND METHODS
Study population
The study population consisted of women participating in a population-based cohort study initiated in 1991 to examine the association between HPV infection and the development of cervical neoplasia. All the participants gave written informed consent before inclusion. The study was approved by the Scientific Ethical Committee of Copenhagen and Frederiksberg Municipality, Denmark.
A detailed description of the study design was given previously 13. Briefly, 17 949 women aged 20–29 years were randomly sampled from the general female population of Copenhagen through the computerized Central Population Register.
A total of 1,643 women had moved out of the study area before they were contacted. The remaining 16,345 women were invited to participate, and 11,088 women (68%) accepted the invitation and were enrolled in the study. Two years later, the participants were invited again in the same order as that in which they were originally included, and 8656 women (78%) underwent a second examination.
At the first examination, all participants had a personal interview, in which they answered questions about sociodemographic variables, contraceptive use, sexual habits, reproductive history, and smoking habits. Furthermore, they gave blood samples and had a gynaecological examination, at which a Pap smear was taken and cell material for HPV testing was obtained from the ecto- and endocervix. At the second examination, the women underwent a new gynaecological examination and participated in a new interview. The second interview contained to a large extent the same questions as the first interview, only for sexual habits (number of sexual partners) we focused on the period between the two visits (number of sexual partners during follow-up).
HPV was detected by Hybrid Capture 2 (HC2) and LiPA V2 polymerase chain reaction (PCR) assay. We excluded from the present analyses women for whom HC2 results were not available from both examinations, either because they participated only in a telephone interview at the second examination (n = 371) or because the HPV test results were missing (due to menstruation at the time of examination (n=90) or because the sample was lost (n=411) or insufficient for HPV analysis (n=95)). In addition, we excluded women for whom the PCR result was missing at either the first (n=24) or the second examination (n = 128). Finally, we excluded women who reported that they were virgins at the second examination (n = 83). This left 7454 women for analysis.
HPV detection and genotyping
The laboratory personnel were fully blinded to the cytological and clinical diagnosis at the time of the HR HPV DNA test. As described previously, the specimens were collected into a medium, not originally recommended for the HC2 test; therefore, a conversion protocol was performed (HC2; Qiagen Corporation, Gaithersburg, Maryland, USA) 14. The assay was performed with the cut-off of 1.0 pg/ml recommended by the United States Food and Drug Administration, only with the HR probe that detects at least 13 carcinogenic types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) 15. Replicate assays were performed on a number of plates, with virtually identical results.
Genotyping was performed in HC2 positive samples by use of the validated PCR method LiPAv2 (Innogenetics Inc., Ghent, Belgium) 16. Genotyping was performed on 200 μl of the remaining denatured specimen transport medium sample of positive HC2 tests, from which total nucleic acid (DNA) was isolated with the MagnaPure device (Roche Systems, Indianapolis, Indiana, USA). The efficiency of DNA extraction was tested by including 104 HPV16-positive SiHa cervical carcinoma cells. For the LiPaV2 SPF-PCR assay, 5 μl of the DNA solution were used in a final volume of 50 μl, with AmpliTaq Gold and 40 cycles of 30 s of denaturation at 95 °C, followed by 45 s at 52 °C annealing temperature and 45 s of extension at 72 °C run on a MJ Thermocycler PCT 200. The PCR product was then denatured, and a 10-μl aliquot was hybridized onto an HPV genotype detection strip at 49 °C for 60 min, followed by multiple washings. The genotype was detected in the substrate solution provided. The hybridized strips were read with a scanner and the LiRAS prototype software (Innogenetics Inc., Ghent, Belgium), which reports the band intensities in greytone values of 0.1–1.0 and allows direct data transfer onto Excel spreadsheets.
Statistical analysis
We estimated the overall acquisition rate of HR HPV for women who were HPV-negative at enrolment and became HR HPV-positive during the 2-year follow-up. In addition, we estimated the type-specific 2-year incidence of HR types of HPV (i.e. the number of women infected with a specific type at the second examination divided by the number of women who were HPV-negative for that HPV type at the first examination). Age-specific 2-year incidence was estimated overall and for each HPV type. Finally, we examined whether specific HPV types were acquired as single HPV infections or with other HR HPV types.
We also estimated the overall acquisition rate of new HR HPV types in initially HR HPV-positive women. We distinguished between acquisition by women who were HPV16-positive initially and those who were initially positive for other HR types than HPV16.
In the risk factor analysis, we distinguished between acquisition by women who were HC2 HPV-negative at enrolment and became HC2 HPV-positive for respectively, a single or multiple HR HPV types in the follow-up period.
The risk factors included were selected on the basis of the literature. In the analyses, we used variables from the baseline (first interview), but included in addition number of partners during follow-up (second interview). We first examined each variable in age-adjusted regression models (SAS STAT version viewer 8.2). The measure of association was the odds ratio (OR) with a 95% confidence interval (CI). Age was included in all the multivariate models. Further selection was made among variables that were significant at the 10% level in the age-adjusted model. Stepwise regression was performed to define the final multivariate model. In the final model we included those variables found to be significantly associated with risk of acquisition and/or those which changed the estimates of the other variables. When interaction was tested in a regression model, we found a significant interaction between marital status and number of sexual partners. The continuous variables age, number of sexual partners during follow-up and duration of oral contraceptive use were initially modelled as linear splines with knots placed according to quartiles in cases. We tested whether the model could be reduced to one with linear associations. As none of the variables deviated from linearity, they were treated as linear in the multivariate models.
RESULTS
In our study population of 7454 women, all women had a HC2 test result 1208 were HR HPV-positive at the time of the initial examination (16.2%; 95% CI: 15.4–17.0). At the second examination, 16.7% (95% CI: 15.9–17.6) were HR HPV-positive. A total of 5448 women were HPV negative at both examinations (73.1%), whereas 448 women (6.0%) were HR HPV-positive at both examinations. In all, 720 women (63%) went from HC2 HPV positive at the first examination to HC2 HPV negative at the second examination (Table 1).
Table 1.
Distribution of study participants according to high risk (HR) Hybrid Capture (HC2) status at enrollment (1st examination) and at the follow-up visit (2nd examination)
| 2nd examination |
|||
|---|---|---|---|
| 1st examination | HR HPV negative | HR HPV positive | Total |
| HR HPV negative | 5448 | 798 | 6246 |
| HR HPV positive | 760 | 448 | 1208 |
| Total | 6208 | 1246 | 7454 |
Risk of HR HPV acquisition
The overall 2-year incidence of HR HPV infection in initially HPV-negative women was 12.8% (798/6246; 95% CI: 12.0–13.6) (Table 2). The age-specific incidence was significantly higher in women aged 20–23 years (17.9%; 95% CI: 16.2–19.6) than in women aged 24–26 years (12.3%; 95% CI: 10.9–13.7) and women aged 27–29 years (8.7%; 95% CI: 7.6–9.9) (Table 2). The type-specific 2-year incidence of oncogenic types was highest for HPV52 (3.0%; 23.1% of women who became HPV-positive), HPV16 (2.9%; 22.8% of women who became HPV-positive) and HPV31 (2.6%; 20.2% of women who became HPV-positive). The cumulative proportion of women positive for specific types of HPV at either the first and/or at the second examination ranged from 7.5% for HPV16 to 1.5% for HPV35 (data not shown). For all HPV types, a decreasing trend in incidence was observed with increasing age (Table 2).
Table 2.
The 2-year incidence of human papillomavirus (HPV) infection in initially HPV negative women, overall and for specific oncogenic types
| HPV type | N | % in total cohort of initially HPV negative women (95% CI) (n=6,246) | % in women who become HPV-positive(95% CI) (n=798) | % acquisition by age group (95% CI) |
||
|---|---|---|---|---|---|---|
| 20–23 years (n=1,959) | 24–26 years (n=2,032) | 27–29 years (n=2,255) | ||||
| Any HR type# | 798 | 12.8 (12.0–13.6) | 100.0 - | 17.9 (16.2–19.6) | 12.3 (10.9–13.7) | 8.7 (7.6–9.9) |
| 16 | 182 | 2.9 (2.5–3.3) | 22.8 (19.9–25.7) | 4.2 (3.3–5.1) | 2.7 (2.0–3.4) | 2.0 (1.5–2.6) |
| 18 | 73 | 1.2 (0.9–1.4) | 9.2 (7.2–11.2) | 2.3 (1.6–2.9) | 1.1 (0.7–1.6) | 0.3 (0.1–0.5) |
| 31 | 161 | 2.6 (2.2–3.0) | 20.2 (17.4–23.0) | 4.4 (3.5–5.4) | 2.3 (1.6–2.9) | 1.2 (0.8–1.7) |
| 33 | 113 | 1.8 (1.5–2.1) | 14.2 (11.7–16.6) | 2.6 (1.9–3.3) | 1.7 (1.1–2.2) | 1.3 (0.8–1.8) |
| 35 | 31 | 0.5 (0.3–0.7) | 3.9 (2.5–5.2) | 0.6 (0.3–1.0) | 0.5 (0.2–0.8) | 0.4 (0.1–0.7) |
| 39 | 95 | 1.5 (1.2–1.8) | 11.9 (9.7–14.2) | 2.1 (1.5–2.8) | 1.3 (0.8–1.8) | 1.2 (0.7–1.6) |
| 45 | 101 | 1.6 (1.3–1.9) | 12.7 (10.4–15.0) | 1.9 (1.3–2.5) | 1.7 (1.2–2.3) | 1.3 (0.8–1.8) |
| 51 | 130 | 2.1 (1.7–2.4) | 16.3 (13.7–18.9) | 3.1 (2.3–3.8) | 2.2 (1.5–2.8) | 1.2 (0.7–1.6) |
| 52 | 184 | 3.0 (2.5–3.4) | 23.1 (20.1–26.0) | 4.2 (3.3–5.1) | 2.8 (2.1–3.5) | 2.0 (1.4–2.6) |
| 53 | 80 | 1.3 (1.0–1.6) | 10.0 (7.9–12.1) | 2.1 (1.5–2.8) | 1.2 (0.8–1.7) | 0.6 (0.3–1.0) |
| 56 | 86 | 1.4 (1.1–1.7) | 10.8 (8.6–12.9) | 2.3 (1.6–3.0) | 1.0 (0.6–1.4) | 0.9 (0.5–1.3) |
| 58 | 46 | 0.7 (0.5–1.0) | 5.8 (4.2–7.4) | 1.1 (0.7–1.6) | 0.7 (0.3–1.1) | 0.4 (0.2–0.7) |
| 59 | 32 | 0.5 (0.3–0.7) | 4.0 (2.7–5.4) | 0.9 (0.5–1.3) | 0.4 (0.2–0.7) | 0.3 (0.1–0.5) |
| 66 | 94 | 1.5 (1.2–1.8) | 11.8 (9.5–14.0) | 2.5 (1.8–3.2) | 1.2 (0.7–4.7) | 0.9 (0.5–1.3) |
| 68 | 82 | 1.3 (1.0–1.6) | 10.3 (8.2–12.4) | 1.6 (1.1–2.2) | 1.2 (0.7–1.7) | 1.2(0.7–1.6) |
| Multiple HR types | 399 | 6.4 (5.8–7.0) | 50.0 (46.5–53.5) | 10.3 (8.9–11.6) | 5.9 (4.8–6.9) | 3.5 (2.7–4.3) |
HR=high-risk
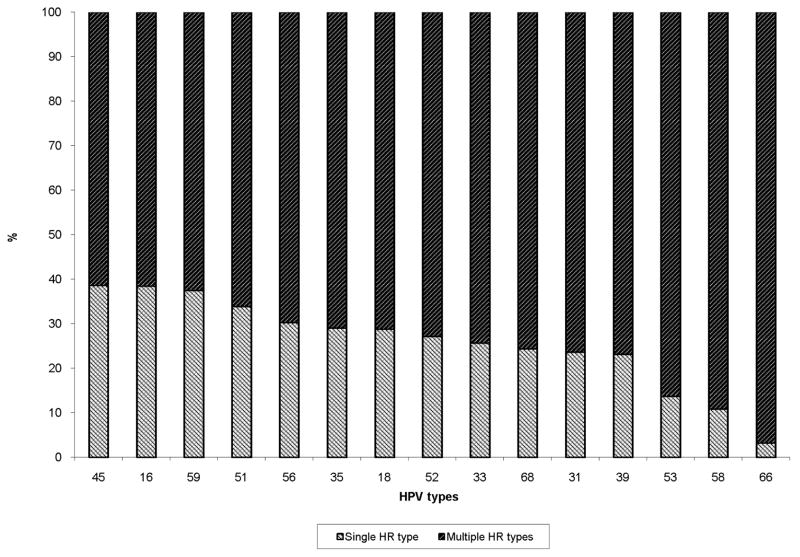
Overall, 50% of the women who became HPV-positive during follow-up acquired multiple HR HPV types (Table 2). Examination of the type-specific acquisition of single and multiple HR HPV types showed that HPV66, HPV58 and HPV53 in particular were acquired mainly with other HR HPV types (97%, 89% and 86%, respectively); HPV16 and HPV45 were also often acquired with other HR HPV types (61.4% and 61.5%, respectively) (Figure 1). However, no two types were more likely to be acquired together than others; neither did we find that HPV types from the same species groups were more likely acquired together (data not shown).
Figure 1.
Acquisition of specific human papillomavirus (HPV) types as single high- risk (HR) infections or together with other HR HPV types (multiple HR infections)
Of the initially HR HPV-positive women, 20.8% (95% CI: 18.5–23.1) acquired new HR HPV types during follow-up (data not shown). Initially HPV-positive women who did not have HPV16 at baseline were more likely to acquire a new HR HPV type during follow-up (22.8%; 95% CI: 20.0–25.6) than women who had an HPV16 infection at baseline (15.8%; 95% CI: 11.9–19.6) (data not shown).
Risk factors for acquisition of HR HPV
Table 3 shows the risk factors for incident HR HPV infection in initially HPV-negative women. We distinguished between acquisition of a single and multiple HR HPV types. In both analyses, age and number of sexual partners were the most important risk determinants, with the strongest association in women who acquired multiple HR HPV types. Younger age implied an increased risk for HR HPV acquisition, the odds ratio per younger year being 1.06 (95% CI: 1.02–1.11) for women who acquired a single HR HPV type and 1.14 (95% CI: 1.10–1.19) for women who acquired multiple HR HPV types.
Table 3.
Risk factors associated with high-risk (HR) human papillomavirus (HPV) acquisition among initially HPV negative women
| Acquistion of one HR HPV type |
Acquistion of multiple HR HPV types |
|||||
|---|---|---|---|---|---|---|
| Number of cases# | OR## | 95% CI | Number of cases# | OR## | 95% CI | |
| Age (years) | ||||||
| 27–29 | 118 | 1.00 | ref. | 79 | 1.00 | ref. |
| 24–26 | 131 | 1.25 | 0.96–1.63 | 119 | 1.61 | 1.19–2.18 |
| 20–23 | 150 | 1.50 | 1.15–1.97 | 201 | 2.48 | 1.85–3.32 |
| per year (linear) | 1.06 | 1.02–1.11 | 1.14 | 1.10–1.19 | ||
| Number of sexual partners during follow-up | ||||||
| Married/cohabiting: | ||||||
| 0–1 | 100 | 1.00 | ref. | 40 | 1.00 | ref. |
| 2–3 | 40 | 2.29 | 1.56–3.35 | 41 | 5.69 | 3.63–8.91 |
| ≥ 4 | 19 | 4.15 | 2.45–7.02 | 32 | 15.39 | 9.26–25.58 |
| per partner (linear) | 1.25 | 1.15–1.36 | 1.47 | 1.33–1.62 | ||
| Single: | ||||||
| 0–1 | 76 | 1.00 | ref. | 58 | 1.00 | ref. |
| 2–3 | 97 | 1.51 | 1.10–2.08 | 120 | 2.63 | 1.89–3.66 |
| ≥ 4 | 66 | 1.80 | 1.27–2.55 | 106 | 4.01 | 2.84–5.65 |
| per partner (linear) | 1.05 | 1.02–1.09 | 1.12 | 1.07–1.16 | ||
| Self-reported history of genital Chlamydia infection | ||||||
| No | 337 | 1.00 | ref. | 347 | 1.00 | ref. |
| Yes | 62 | 0.95 | 0.71–1.27 | 52 | 0.76 | 0.55–1.04 |
| Self-reported history of genital warts | ||||||
| No | 340 | 1.00 | ref. | 343 | 1.00 | ref. |
| Yes | 59 | 0.94 | 0.70–1.26 | 56 | 0.94 | 0.69–1.28 |
| Parity | ||||||
| Nulliparous | 342 | 1.00 | ref. | 375 | 1.00 | ref. |
| Parous | 57 | 1.02 | 0.75–1.40 | 24 | 0.57 | 0.37–0.90 |
| Use of oral contraceptives (years) | ||||||
| Noncurrent use | 263 | 1.00 | ref. | 230 | 1.00 | ref. |
| Current use: | ||||||
| ≤2 | 22 | 0.63 | 0.39–1.01 | 35 | 1.01 | 0.68–1.50 |
| 3–4 | 38 | 1.12 | 0.77–1.62 | 47 | 1.39 | 0.98–1.99 |
| 5–6 | 30 | 0.93 | 0.63–1.39 | 40 | 1.44 | 1.00–2.07 |
| ≥7 | 46 | 1.24 | 0.89–1.73 | 47 | 1.66 | 1.17–2.35 |
| per year (linear) | 1.07 | 1.01–1.14 | 1.04 | 0.98–1.10 | ||
| Condom use | ||||||
| Noncurrent use | 216 | 1.00 | ref. | 189 | 1.00 | ref. |
| Current use | 183 | 0.92 | 0.73–1.16 | 210 | 1.26 | 0.99–1.62 |
| Amount of smoking (cigarettes/day) | ||||||
| Noncurrent smokers | 189 | 1.00 | ref. | 214 | 1.00 | ref. |
| Current smokers: | ||||||
| 1–9 | 45 | 1.16 | 0.83–1.63 | 49 | 1.04 | 0.75–1.46 |
| ≥ 10 | 165 | 1.52 | 1.22–1.90 | 136 | 1.04 | 0.82–1.32 |
The sum will not always add up to the total because of missing values
Adjusted for age (linear), no. of sexual partners during follow-up (linear), marital status (single/married), use of oral contraceptives (current/noncurrent and years of use among current users(linear)), and interaction between marital status and no. of sexual partners during follow-up
We found a significant interaction between number of sexual partners during follow-up and marital status (single HR type: p = 0.0005; multiple HR types: p < 0.0001). The risk associated with the number of sexual partners increased more for married or cohabiting women than for women who reported that they were single. For married women the OR for acquisition of multiple HPV types was 15.39 (95% CI: 9.26–25.58) in women with ≥4 sexual partners during follow-up compared to women with ≤ 1 sexual partner, whereas the corresponding OR for single women was 4.15 (95% CI: 2.45–7.02). The number of sexual partners between the two examinations (i.e. recent partners) was a stronger determinant of acquisition of HR HPV than the lifetime number of sexual partners, e.g. in women with multiple HR HPV types the OR in married women was 1.47 (95% CI: 1.33–1.62) per each recent sexual partner, whereas the OR was 1.11 (95% CI: 1.07–1.15) per each lifetime sexual partner.
Self-reported history of genital warts and genital Chlamydia infection were not associated with acquisition of HR HPV, nor did we observe an association with current genital Chlamydia infection (DNA) (data not shown). Parity was associated with a decreased risk for acquiring multiple HR HPV types (OR=0.57; 95% CI: 0.37–0.90 for parous women compared with nulliparous women).
The risk for acquiring HR HPV infection, notably the acquisition of multiple HPV types, increased with increasing years of oral contraceptive use. Current users who had taken oral contraceptives for 7 years or more had a higher risk for acquiring multiple HR HPV types than women who were not current users (OR=1.66; 95% CI: 1.17–2.35). Use of condoms was not strongly associated with acquisition of HR HPV. Current smokers of more than 10 cigarettes per day had a higher risk for acquiring a single HR HPV infection (OR=1.52; 95% CI: 1.22–1.90) than non-current smokers, but no association was seen with acquisition of multiple HR HPV types. We found no association with alcohol intake, age at first sexual intercourse or number of sexually active years (data not shown).
The analysis of the risk for acquiring HPV16 during follow-up showed similar results, age and number of sexual partners being the most important risk factors (data not shown).
DISCUSSION
The 2-year acquisition rate of HR HPV in our study was 13%, implying frequent exposure to HPV. As expected, age and number of sexual partners were the main determinants of HPV acquisition, once more emphasizing the sexually transmitted nature of the HPV infection. Beside this, we found that oral contraceptive use was significantly associated with the risk of HPV acquisition.
Although the age range of our study population was quite narrow, we observed that the incidence decreased with age, in agreement with other cohort studies 7,11,17,18. The type-specific 2-year incidence analysis showed that HPV52, HPV16 and HPV31 are acquired most frequently. Previous studies have similarly found that HPV52 4,6,7,17, HPV16 4–7,12,17–20 and HPV31 7,12 are commonly detected.
In line with previous findings for women 4,12,18,22 and men 23, we found that acquisition of multiple HPV types was common. Having multiple HPV types is recognized as a possible risk factor for HPV persistence and for cervical precancerous lesions 24. It is not yet known whether the increased risk is due to the sum of risks from all individual types acquired or whether the risk for cervical lesions accumulates with the number of HPV types acquired 24. Consistent with two previous studies in women 22 and men 23, we observed that women who were initially HR HPV-positive were particularly likely to acquire new HR HPV types. These findings may indicate that acquisition of one HPV type facilitates acquisition of other HPV types or, alternatively, that some women are more susceptible to HPV acquisition.
Our analysis indicated that the likelihood of acquiring new HPV types was lower for women who were initially HPV16-positive than for women who were HPV positive for other HR HPV types. Women without HPV16 at baseline, however, reported slightly more sexual partners, which may partly explain their increased likelihood of acquisition. Furthermore, from a statistical point of view acquisition of new HPV types may occur more frequently in women who are not HPV16-positive (HPV16 is very common and therefore more frequently acquired than other HPV types). Alternatively, it may be hypothesized that HPV16 may initiate an immune response that alters the ability to acquire other HPV types, thus making it less likely that new HPV types will be acquired by a woman who is already HPV16-positive25. However, in contrast to our finding, Rousseau et al. (22) and Liaw et al. (25) observed a slightly elevated risk for new HPV types in women who were initially HPV16- or HPV18-positive. In view of the relatively small numbers in the present analysis, however, more studies are needed to understand the implications of HPV positivity on subsequent acquisition of new HPV types.
In our analysis of whether HR HPV types were acquired mainly as a single infection or together with other types, some HR HPV types, particularly HPV66, were almost always detected in combination with others. In agreement with the results of Thomas et al. 12, however, we found no evidence of a pattern of concurrent acquisition of other HR HPV types, and no HR HPV types were acquired together more frequently than others.
We found that lower age, increasing number of sexual partners and the use of oral contraceptives were the main determinants of HPV acquisition, and in particular of the acquisition of multiple HR HPV types even after mutual adjustment and adjustment for other potential confounders. This indicates that both the level of potential exposure to HPV as well as behavioural factors are strong predictors of whether women will acquire single or multiple HR HPV types. Like Winer et al. 5, we observed that the number of recent partners was a stronger predictor of HPV acquisition than the lifetime number of sexual partners. In addition, we observed that only four of the 112 women who were initially HPV-negative and who reported not having had a sexual partner during follow-up acquired an HR HPV infection, which further supports the role of sexual transmission in the acquisition of HR HPV infections. The four cases might have been due to transmission of HPV during sexual activity not involving intercourse; however, as we did not obtain information on sexual activity other than sexual intercourse, we cannot confirm this theory. Alternatively, it may be due to misinformation about sexual activity among the four women. We also found an interaction between marital status and number of sexual partners. Our finding can be explained if this group of married or cohabiting women who have new sexual partners have more risky sexual behaviour, resulting in increased exposure to HPV, e.g. sexual intercourse without barrier contraceptives or sexual intercourse with men who are more likely to be infected. Women who reported that they were married at baseline and had new sexual partners during the follow-up had in some cases changed their marital status and were separated or divorced at the second examination, however this cannot explain the entire interaction found.
Oral contraceptive use increased the risk for acquisition significantly, even after adjustment for age and sexual partners. The results of existing studies are inconsistent; one study reported an increased risk with use of oral contraceptives 5, another found a decreased risk 3 whereas other studies observed no association 4,7,9. Our results support the hypothesis that use of oral contraceptives increases the risk for acquisition of HPV. It is possible that oral contraceptive use increases the occurrence of cervical ectropion, thus exposing the squamo-columnar junction more to potential carcinogens 26; alternatively, oestrogen and progestogen may increase cell proliferation and stimulate transcription of HPV allowing the establishment and maintenance of the viral infection 26,27 or oral contraceptives may alter the host immune response making the women more susceptible 28.
In women who acquired multiple HR HPV types we observed an increased risk with condom use, which is in agreement with several previous studies 29. Condoms may not seem effective in protecting against HPV because the virus can be transmitted through contact with areas of unprotected genital skin or more likely because the condom is not used consistently during the entire intercourse 5,23,30.
In women who acquired a single HR HPV type we observed an increased risk of acquisition with current smoking of ten or more cigarettes per day, but no such association was found in women who acquired multiple HR HPV types. Some previous studies have found smoking to be a risk factor for acquisition of HPV 5,10 whereas others have not 2,3,9. We have no explanation for the different effect of smoking for acquisition of single or multiple HPV types. The association could be due to chance, or alternatively, it has been suggested that smoking may increase susceptibility to infection 28. We found no significant association with a history of genital Chlamydia infection. Underreported information about previous genital Chlamydia infection may have occurred, as routine testing for genital Chlamydia infection was not widespread during this period.
The main strength of our study is the large number of participants, thus yielding stable estimates, even in the type-specific analyses. Most previous studies have had significantly fewer participants. As our follow-up visit took place after 24 months, we might have underestimated the acquisition rate. The clearance rate in our study was 63%, Ho et al. 4 found that 70% of all women had cleared their infections after 12 months and 80% after 18 months, a finding that is also supported by later studies 18,19. Therefore, some women in our study might have acquired an infection and cleared it again in the time between the two examinations. Most recent studies have used selected groups of participants, such as students 4,5,20 and patients at family planning clinics or practices 3,6,7,9,11,17,19. Our use of randomly sampled women from the general population will potentially allow us to generalize our results to a wider range of women.
Acquisition of respectively a single or multiple HR HPV types has been assessed separately in only a few previous studies, so the present population-based study is important for understanding the natural history of HPV. In summary, we found that the acquisition rate of HR HPV infections was high overall, and for multiple HR HPV types. Distinguishing between acquisition of single and multiple HR HPV types showed that age, sexual habits and oral contraceptive use were even stronger determinants of acquisition for women who acquired multiple HR HPV types.
Acknowledgments
We thank Betti Schopp, Nazife Kilic, Nurgul Duzenli, Anette Rothe and Barbara Holz for technical assistance with HPV testing and Kirsten Frederiksen for valuable comments on the statistical analyses.
The study was supported by the National Cancer Institute, Bethesda, Maryland, USA (RO1 CA47812), and by funding under the Sixth Research Framework Programme of the European Union, project INCA (LSHC-CT-2005–018704). Innogenetics supported this study through a grant for the LiPa kits.
References
- 1.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24S3:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de SS, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285(23):2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 4.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338(7):423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 5.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 6.Giuliano AR, Harris R, Sedjo RL, Baldwin S, Roe D, Papenfuss MR, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women’s Health Study. J Infect Dis. 2002;186(4):462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 7.Munoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 8.Syrjanen S, Shabalova I, Petrovichev N, Kozachenko V, Zakharova T, Pajanidi J, et al. Acquisition of high-risk human papillomavirus infections and pap smear abnormalities among women in the New Independent States of the Former Soviet Union. J Clin Microbiol. 2004;42(2):505–511. doi: 10.1128/JCM.42.2.505-511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellors JW, Karwalajtys TL, Kaczorowski J, Mahony JB, Lytwyn A, Chong S, et al. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168(4):421–425. [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseau MC, Abrahamowicz M, Villa LL, Costa MC, Rohan TE, Franco EL. Predictors of cervical coinfection with multiple human papillomavirus types. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1029–1037. [PubMed] [Google Scholar]
- 11.Rousseau MC, Villa LL, Costa MC, Abrahamowicz M, Rohan TE, Franco E. Occurrence of cervical infection with multiple human papillomavirus types is associated with age and cytologic abnormalities. Sex Transm Dis. 2003;30(7):581–587. doi: 10.1097/00007435-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Thomas KK, Hughes JP, Kuypers JM, Kiviat NB, Lee SK, Adam DE, et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. 2000;182(4):1097–1102. doi: 10.1086/315805. [DOI] [PubMed] [Google Scholar]
- 13.Kjaer SK, van den Brule AJ, Bock JE, Poll PA, Engholm G, Sherman ME, et al. Human papillomavirus--the most significant risk determinant of cervical intraepithelial neoplasia. Int J Cancer. 1996;65(5):601–606. doi: 10.1002/(SICI)1097-0215(19960301)65:5<601::AID-IJC8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Kjaer SK, Hogdall E, Frederiksen K, Munk C, Van den BA, Svare E, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66(21):10630–10636. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 15.Iftner T, Villa LL. Chapter 12: Human papillomavirus technologies. J Natl Cancer Inst Monogr. 2003;(31):80–88. doi: 10.1093/oxfordjournals.jncimonographs.a003487. [DOI] [PubMed] [Google Scholar]
- 16.Klug SJ, Molijn A, Schopp B, Holz B, Iftner A, Quint W, et al. Comparison of the performance of different HPV genotyping methods for detecting genital HPV types. J Med Virol. 2008;80(7):1264–74. doi: 10.1002/jmv.21191. [DOI] [PubMed] [Google Scholar]
- 17.Syrjanen S, Shabalova I, Petrovichev N, Podistov J, Ivanchenko O, Zakharenko S, et al. Age-specific incidence and clearance of high-risk human papillomavirus infections in women in the former Soviet Union. Int J STD AIDS. 2005;16(3):217–223. doi: 10.1258/0956462053420211. [DOI] [PubMed] [Google Scholar]
- 18.Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180(5):1415–1423. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 19.Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357(9271):1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 20.Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, et al. The natural history of type-specific human papillomavirus infections in femaleuniversity students. Cancer Epidemiol Biomarkers Prev. 2003;12(6):485–490. [PubMed] [Google Scholar]
- 21.Xi LF, Carter JJ, Galloway DA, Kuypers J, Hughes JP, Lee SK, et al. Acquisition and natural history of human papillomavirus type 16 variant infection among a cohort of female university students. Cancer Epidemiol Biomarkers Prev. 2002;11(4):343–351. [PubMed] [Google Scholar]
- 22.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184(12):1508–1517. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 23.Kjaer SK, Munk C, Winther JF, Jorgensen HO, Meijer CJ, van den Brule AJ. Acquisition and persistence of human papillomavirus infection in younger men: a prospective follow-up study among Danish soldiers. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1528–1533. doi: 10.1158/1055-9965.EPI-04-0754. [DOI] [PubMed] [Google Scholar]
- 24.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 25.Liaw KL, Hildesheim A, Burk RD, Gravitt P, Wacholder S, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183(1):8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 26.Green J, Berrington de GA, Smith JS, Franceschi S, Appleby P, Plummer M, et al. Human papillomavirus infection and use of oral contraceptives. Br J Cancer. 2003;88(11):1713–1720. doi: 10.1038/sj.bjc.6600971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubenrauch F, Iftner T. Molecular basis of cervical neoplasia. In: Jordan JA, Singer A, editors. The cervix. Oxford, Victoria: Blackwell publishing Massachusetts; 2006. p. 277. [Google Scholar]
- 28.Burchell AN, Winer RL, de SS, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(3):52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia. Sex Transm Dis. 2002;29(11):725–735. doi: 10.1097/00007435-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Winer RL, Hughes JP, Feng Q, O’Reilly S, Kiviat NB, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354(25):2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]