Abstract
Persistent, sub-clinical inflammation predisposes to chronic disease, as well as the development of sarcopenia and disability, in frail elderly. Thus, the inflammatory pathway is a potential target for interventions to reduce aging-related disease and disability. This article highlights emerging data suggesting that increasing physical activity could be effective for reducing chronic inflammation in the elderly.
Keywords: aging, sarcopenia, inflammation, cytokines, physical activity
INTRODUCTION
As a greater proportion of the population is surviving to very old ages, the public health burden of aging-related disability, and the associated increases in utilization of medical care and need for long-term care, has become a critical concern. As a result, it has become imperative to identify risk factors for disability and establish therapies that are effective for preventing or improving these risk factors. While there is likely a common pathway of sarcopenia (loss of muscle mass and strength/contractility) underlying loss of physical function with age, little is known regarding the biological factors that are fundamental for the progression of this process. However, our work, as well as others, points to an important role for chronic/persistent, sub-clinical inflammation in the development of sarcopenia and physical disability, thus highlighting the need for novel, effective treatments against chronic inflammation in this age group.
The biological cascade of events comprising the body’s natural defenses against injury or infection is a vital part of the immune system. Typically, this process is an acute response, resulting in rapid increases in inflammatory mediators that are released into the circulation. For example, blood concentrations of the acute phase reactant, C reactive protein (CRP), can increase greater than 1000-fold in response to infection or trauma. However, several definitive studies now provide convincing data that persistent, yet very slight, elevations in systemic biomarkers of inflammation, even when within the clinically normal range, are prospective risk factors for several adverse health conditions, particularly cardiovascular disease. Importantly, in the elderly, inflammation is also a strong predictor of both disability and mortality—even in the absence of clinical disease. Most notably, chronic inflammation contributes to the loss of skeletal muscle mass and function, leading to earlier onset of disability (25). Much higher concentrations of pro-inflammatory cytokines and acute phase reactants are seen in the elderly compared to middle-aged or younger adults, and several cytokines have direct, detrimental effects on skeletal muscle that lead to loss of myofibers and disruption of contractile function per se (10). As such, we posit that the inflammatory pathway is a potential biological target for interventions to reduce risk of disability in the frail elderly.
Although chronic inflammation is now thought to be an important contributor to aging-related disability, there are no known definitive treatments for reducing this condition in the elderly. Certainly, use of anti-inflammatory medications may reduce inflammation, but side effects of these medications are likely to reduce their clinical application for the on-going treatment of persistent inflammation. As such, presently none of the pharmaceutical agents with anti-inflammatory effects are approved for the treatment of sarcopenia or aging-related loss of function. On the other hand, regular exercise is the only therapy found to consistently improve physical function in older, frail adults. However, since a single bout of exercise induces an inflammatory response that is similar to that induced by infection or trauma, there is controversy surrounding whether or not increasing physical activity may be effective for reducing chronic inflammation in the long-term, especially in the frail elderly. Yet, recent randomized, controlled trial data from our group provide promising evidence, and advances the hypothesis, that regular exercise may indeed be an effective treatment for chronic inflammation. This article highlights the findings from our studies, and those of others in this area, which provide evidence, and suggest mechanisms, for an effect of increasing physical activity on lowering chronic, sub-clinical inflammation in this vulnerable population at high risk for disability.
ASSOCIATON BETWEEN CHRONIC INFLAMMATION AND AGE
Age is a contributing factor to an elevated, yet sub-clinical, state of inflammation. Cross-sectional data show that circulating concentrations of cytokines and acute phase reactants, as well as cellular production of cytokines, are, on average, 2–4 fold higher in older persons. A majority of these studies focused on interleukin-6 (IL-6), which has been coined a “cytokine for gerontologists”, and show that blood levels and monocyte production of IL-6 are higher with advancing age (9,13). In particular, there is a dramatic increase in the number of individuals with elevated IL-6 levels in those over the age of 70 years (13), and this rise continues into the very old (>94 yrs). Other markers of inflammation are also reported to be higher in older individuals, including IL-6 soluble receptor, IL-18, IL-1 receptor antagonist , CRP, and tumor necrosis factor alpha (TNFα) (9). However, to our knowledge, there are not yet well-established longitudinal data showing an increase in inflammatory biomarkers with aging.
The postulated mechanisms contributing to this age-related low-grade inflammation are under debate and range from a dysregulation of the immune system response due to aging per se, to several lifestyle and pathological conditions associated with aging—such as increases in adipose tissue, presence of sub-clinical infections, chronic health conditions, poor nutrition, and/or decreases in sex steroid hormones. Whatever the mechanism, it is well established that older persons exhibit a pro-inflammatory condition that is characterized by a level of inflammation that is slightly elevated, yet far below that observed during acute infections. As discussed below, this persistent, low-grade inflammation is likely a contributing factor for aging-related loss of physical function and onset of disability.
INFLAMMATION AS A MECHANISM UNDERLYING LOSS OF FUNCTION AND DISBILITY ONSET WITH AGE
There are a number of cross-sectional observations demonstrating an inverse relationship of muscle mass, muscle strength, and other measures of physical function with inflammation in the elderly. Results from these studies are consistent in showing that high inflammatory marker concentrations are associated with less muscle mass, lower muscle strength, slower walking speed, poorer balance, and lower self-reported functional ability. We recently showed that elevated CRP and IL-6 levels are associated with poorer physical function in older adults across a variety of diseases/health conditions, indicating that chronic inflammation likely represents a common mechanism underlying age-related functional decline (3). There are also longitudinal data indicating that onset of disability is preceded by low-grade elevations in inflammatory markers. At least four cohort studies from three independent populations (the Established Populations for Epidemiologic Studies of the Elderly; the Women’s Health and Aging Study; and the Health, Aging and Body Composition (Health ABC) Study,) show that serum IL-6 levels predict the development of physical disability in older persons, independent of disease status (25).
While these epidemiological data are consistent in linking disability to an elevated inflammatory state, they do not prove a causal mechanism for this association. However, there is also evidence for a direct effect of inflammation on muscle catabolism. For example, there is a direct adverse effect of TNFα on myfobrillar contractile function. Infusion of IL-1 or TNFα induces muscle loss through increased myofibrillar protein degradation and apoptosis (20). Administration of anti-TNF or anti-IL-6 receptor antibodies to tumor-bearing rats results in reversal of muscle cachexia (34), and TNFα gene transfer results in muscle atrophy and inhibition of muscle regeneration following injury (7). TNFα also impairs myocyte force production independent of muscle wasting. In humans, there is a strong inverse correlation between in vivo protein breakdown and both TNFα production by mononuclear cells and circulating TNFα concentrations. In addition, in vivo myosin heavy chain protein synthesis rates correlate negatively with muscle protein expression of TNFα (15). Taken together, these findings point to chronic inflammation as an important biological contributor to aging-related disability.
ROLE OF PHYSICAL ACTIVITY IN THE TREATMENT OF CHRONIC INFLAMMATION
Evidence from Observational Studies
Data from observational studies show lower inflammatory biomarker concentrations in individuals who report performing more frequent and more intense physical activity. This is true whether a single inflammatory biomarker was assessed, or as we have done, whether inflammation is depicted as a summary factor derived from multiple biomarkers. First, several large population cohorts, including the British Regional Heart study, the Third National Health and Nutrition Examination Survey (NHANESIII), the Cardiovascular Health study (CHS), and the Health ABC study, provide evidence for an inverse and independent dose-response relationship between plasma CRP concentration and level of physical activity. This relationship does not seem to be altered with age since, in CHS participants, those older than 72 years show similar associations as those less than 72 yrs, and in the 70–79 yr olds enrolled in Health ABC, CRP concentration is inversely related to the amount of reported exercise in a dose-response manner.
While CRP is the most frequently studied marker of chronic inflammation, some studies also report an inverse association between other inflammatory biomarkers (including IL-6) and physical activity. In Health ABC, there was a linear trend for decreased IL-6 and TNFα with increasing amounts of reported exercise (6). In elderly men, both IL-6 and CRP concentrations were negatively related to the number of reported hours/year of moderate and strenuous exercise, and these relationships were not altered by adjustment for BMI (31). In addition, the lowest concentrations of both CRP and IL-6 were found in elderly persons with the highest levels of recreational activity (27).
The exact reason(s) for this inverse association between physical activity intensity/frequency and inflammation are not yet fully understood. Although adiposity is a likely contributor, the relationship between inflammatory biomarkers and physical activity is independent of differential levels of obesity (as measured by BMI) in these studies. For example, while adjustment for BMI reduced the effect of physical activity on CRP in the CHS study, the relationship was still significant. Also, in NHANESIII, engaging in physical activity more than 22 times/month resulted in a 63% reduction in risk for an elevated CRP compared to engaging in activity less than 3 times/month, and this association was independent of both BMI and waist-to-hip ratio (1). All of the studies mentioned above relied on self-report to assess physical activity status. Yet, in men from the Aerobics Center Longitudinal Study, plasma CRP was also inversely related, in a dose-response manner, to cardiorespiratory fitness measured directly on a treadmill (5). The odds ratio of having a high CRP (> 1.84 mg/L) was 3.2 (95% CI: 1.8–5.8) for men in the lowest quintile of fitness compared to men in the highest quintile of fitness. Additional adjustment for body fat percentage and/or waist girth did not alter the relationship between CRP and cardiorespiratory fitness (5). Thus, it appears that differences in adiposity alone do not account for the strong association seen between physical activity and lower chronic inflammation. As discussed below, there are training adaptations observed in skeletal muscle and immune cells that are likely to contribute to the lower levels of these systemic inflammatory biomarkers observed in physically active individuals.
Due to the lack of studies with measures of multiple inflammatory biomarkers, it is not known whether any one of these alone—or in specific combinations—is a more important indicator of the effect of physical activity on the underlying inflammatory state. Given that the inflammatory response is a complex and regulated system, it may be that a combination of biomarkers is more predictive of underlying health status. We recently used a factor analysis approach to identify multiple inflammatory factors from eight biomarkers measured in a subset (n=1269) of older (70–79 yrs) men and women enrolled in the Health ABC study. We then evaluated the associations between identified factors and self-reported measures of physical activity. Health ABC is a cohort study of 3075 well-functioning men and women aged 70–79 years. This analysis showed that five variables (TNFα, sTNFR1, sTNFR2, IL-6sR, IL-2sR) loaded highest on the first factor (TNFα-related), while three variables (CRP, IL-6, PAI-1) loaded highest on the second factor (CRP-related). After adjusting for age, gender, race, site, and BMI, the CRP-related factor (Odds Ratio=0.81, p=0.0031), but not the TNFα-related factor (Odds Ratio=0.91, p=NS), was associated with physical activity such that persons who had a higher CRP-related score were less likely to have high (≥1000 kcal/d) levels of physical activity. Thus, at least two inflammatory factors can be identified in an older population, and the CRP-related factor is more closely linked with physical activity.
Overall, data from observational studies show that the greater the volume of physical activity, the lower the risk of elevated levels of inflammatory biomarkers. Furthermore, the relationship between inflammation and physical activity is independent of total obesity as measured by BMI. However, as with all observational data, it is not possible to confirm whether inflammation and physical activity are causally related, nor to ascertain the direction of the relationship. Unanswered questions remain, including: 1) are individuals with less inflammation more likely to engage in physical activity?, 2) are a physically active lifestyle and lower inflammatory biomarkers linked through some other related health behavior, such as nutrition, or some other body parameter, such as abdominal adiposity?, and 3) will increasing physical activity result in a decrease in chronic inflammation? As summarized next, there are some data from intervention studies conducted by us and others which begin to answer the latter question.
Evidence from Intervention Studies
The large variability and transient nature of most biomarkers of inflammation within and among individuals, necessitates the use of a control group in order to definitively examine the effects of increasing physical activity on chronic inflammation. Besides two studies conducted by our group, there are relatively few controlled trials of an exercise intervention on inflammatory biomarkers. One of the larger studies which did incorporate a control group (but did not randomize participants) demonstrated that a phase II cardiac rehabilitation and exercise training program reduced median CRP concentrations by 41% in CHD patients, but CRP did not change in CHD patients who did not undergo rehabilitation (21). The effect of exercise training was more effective in patients with the highest CRP concentrations. Changes in CRP were not associated with changes in body weight or percent body fat, again providing compelling evidence that the exercise training effect was independent of body fat loss. During a 6-yr trial in 140 middle-aged men, CRP remained constantly lower in an aerobic exercise group, compared to a control group, but the trend was not statistically significant (P>0.20) (26). In our 18-month exercise trial in older, overweight men and women with knee osteoarthritis, we also found only a trend towards a statistically significant effect for exercise training to decrease IL-6 values relative to a non-exercise group (22). Finally, in a small number of elderly (60–85 yrs) adults, 6 months of exercise training without weight loss did not statistically alter serum CRP levels, even though the reduction in CRP was two times greater in the exercise vs. the control group (16). Yet, a smaller study of shorter duration showed a significant effect of exercise training to reduce CRP relative to a no-exercise control group (33). Most of these studies show a trend toward lowering of inflammation with exercise, but these were small and likely not adequately powered.
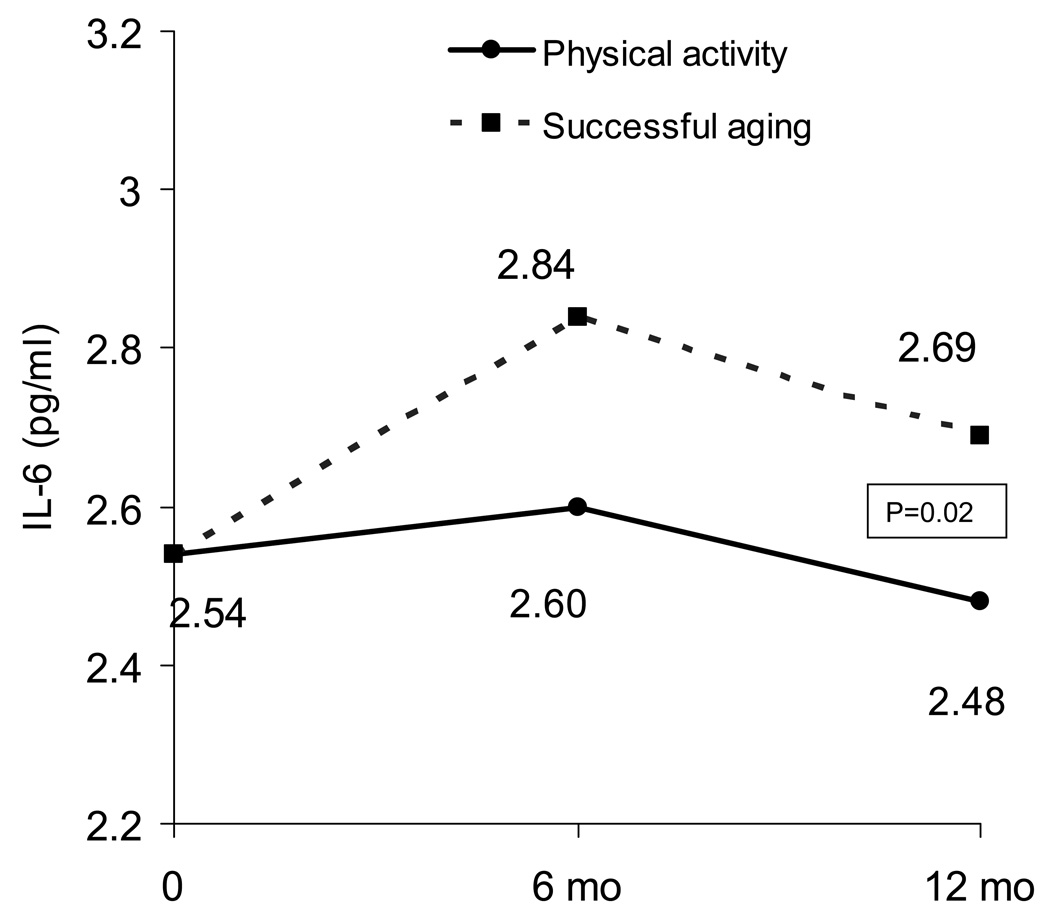
We recently completed an ancillary study to The Lifestyle Interventions and Independence for Elders (LIFE) trial to determine the effects of a long-term exercise intervention on CRP and IL-6 in elderly men and women (23). The LIFE study was a four-site, single-blind, randomized controlled trial comparing a 12-month Physical Activity (PA) intervention to a Successful Aging (SA) intervention in 424 elderly (70–89 yrs), non-disabled, community-dwelling men and women at risk for physical disability. The PA intervention consisted of a combination of aerobic, strength, balance, and flexibility exercises and did not result in a change in body weight. After adjustment for baseline IL-6, gender, clinic site, diabetes, treatment group, visit, and group by visit interaction, the PA intervention resulted in a significant decrease in IL-6 compared to the SA intervention such that the adjusted mean IL-6 at month 12 was 8.5% (0.21 pg/ml) higher in the SA compared to the PA group (Figure 1). However, there was only a trend for significant differences in CRP between the groups at 12 months (p=0.09). Interestingly, there was a greater effect of the PA intervention in individuals with a lower functional status at baseline. Thus, a 1-year physical activity intervention results in reduced systemic concentrations of IL-6 in elderly individuals, and this benefit is most pronounced in those individuals at the greatest risk for disability and subsequent loss of independence. However, it appears that increasing physical activity without weight loss has a small, often undetectable, effect on CRP in the elderly.
Figure 1.
The effects of a 1-year Physical Activity and Successful Aging control intervention on plasma levels of IL-6 in 369 older adults enrolled in the Lifestyle Interventions and Independence for Elders trial (LIFE), a four-site, single-blind, randomized, controlled trial in elderly (70–89 yrs), non-disabled, community-dwelling men and women at risk for physical disability. The IL-6 values are means estimated from repeated measures ANCOVA adjusted for baseline IL-6, gender, clinic site, diabetes, treatment group, visit, and treatment by visit interaction.
As summarized above, besides our LIFE study, there are very few data from fully-powered, prospective, randomized, controlled trials to definitely conclude that increasing physical activity through regular exercise training reduces chronic inflammation in older adults. Nevertheless, there are several uncontrolled studies, most of which show a favorable exercise training effect on specific inflammatory biomarkers. For instance, in older coronary heart disease (CHD) patients participating in cardiac rehabilitation, 12 weeks of aerobic exercise resulted in reductions in CRP, IL-6 and IL-1, and increases in IL-10 (14). In patients with chronic heart failure (CHF), aerobic exercise reduces TNFα and soluble TNFα receptor concentrations (19). In postmenopausal women, 14 weeks of aerobic exercise, both with and without a reduced calorie diet intervention, decreased CRP by 15% and tended to decrease IL-6 (11), and 20 weeks of aerobic exercise decreased TNFα, sTNFRI and sTNFRII (35). In one of the largest, but uncontrolled, exercise studies conducted to date (HERITAGE Family study), plasma CRP was significantly reduced with 20 weeks of training, but only in the sub-group of persons with a high (>3.0 mg/L) baseline CRP. The approximate 29% CRP decrease in this study was not mediated by changes in body weight (17). These results suggest that aerobic exercise training lowers concentrations of inflammatory biomarkers, and this effect is independent of the training-induced reductions in fat mass.
Exercise training also reduces the expression of inflammatory mediators within skeletal muscle. For example, aerobic training reduced mRNA levels of TNFα, IL-1β, and IL-6 in skeletal muscle of elderly CHF patients (12), and combined resistance and aerobic training decreased skeletal muscle IL-6 and TNFα gene expression in frail obese elderly (18). Similarly, significant reductions in TNFα mRNA and protein expression were reported after resistance training in frail elderly men and women (15). Thus, reductions in inflammatory cytokines with exercise training are evident both locally within skeletal muscle and systemically in circulation.
TRAINING ADAPTATIONS UNDERLYING IMPROVEMENTS IN CHRONIC INFLAMMATION
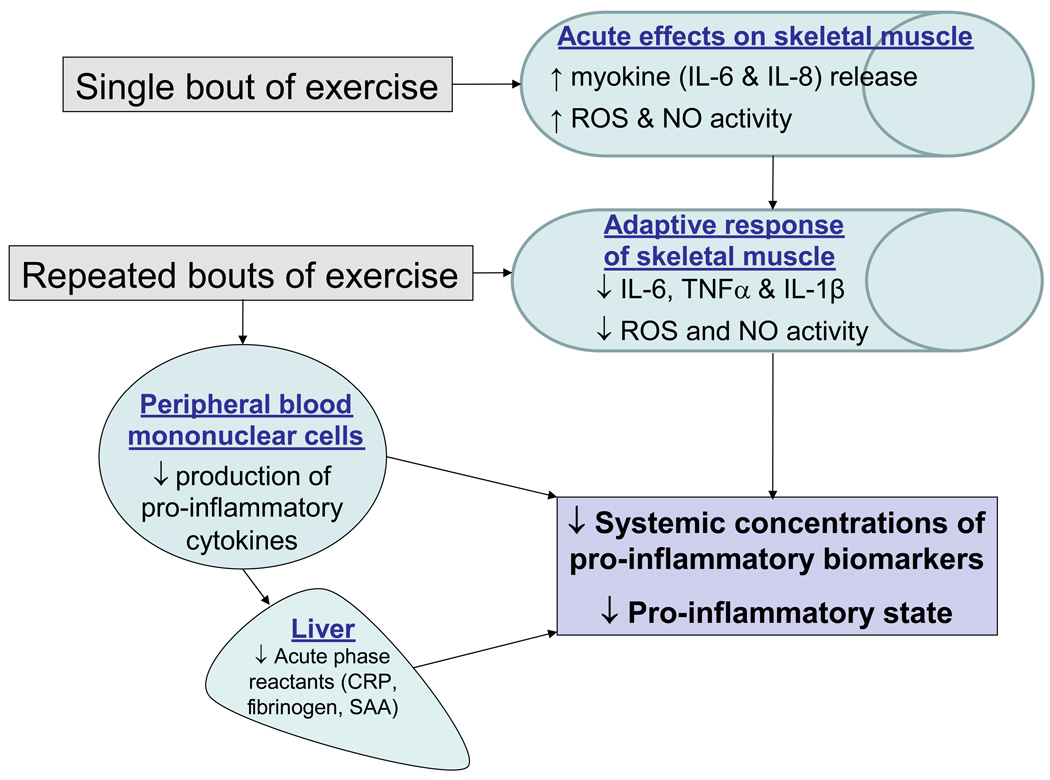
Repeated bouts of exercise lead to biochemical changes in exercised muscle that ultimately contribute to improvements in inflammatory status and muscle function over time. These improvements in inflammation in response to regular activity are likely due, in part, to reductions in systemic immune cell production of inflammatory mediators, and to adaptations that occur locally in the skeletal muscle (Figure 2). In one study, aerobic exercise training in adults at high risk for ischemic heart disease resulted in a 58% decrease in mononuclear cell production of atherogenic cytokines (interferon γ, TNFα and IL-1α), while the production of atheroprotective cytokines (IL-10, IL-4, and TGFβ1) increased by 36% (29). Exercise training also induces reductions in CD14+CD16+ monocytes and in TNFα production by monocytes (32). The mechanism through which exercise training modifies mononuclear cell production of cytokines is unknown, but, as discussed below, may be through adaptations in the immune system brought on by increases in muscle-derived cytokines (myokines) during acute exercise, which when performed on a regular basis, results in lower cytokine release from mononuclear cells.
Figure 2.
Schematic of adaptations to exercise training potentially underlying improvements in chronic inflammation. Adaptive responses in inflammatory and redox-sensitive pathways in skeletal muscle, as well as potential adaptive responses in innate immune cells, may serve to protect against chronic systemic low-grade inflammation.
CRP=C Reactive protein, SAA=serum amyloid a, ROS=reactive oxygen species; NO=nitric oxide
The well-known activation of inflammatory pathways invoked by a single bout of exercise makes it almost counterintuitive that regular physical activity would serve to reduce chronic inflammation. However, it is now evident that an acute inflammatory response plays a major role in the training adaptations observed in exercised muscles. Contracting skeletal muscle produces and secretes several cytokines (myokines), most notably IL-6, which mediate metabolic changes during exercise (8). IL-6 release from muscle increases up to 100-fold during contractile exercise and its production results in increased anti-inflammatory cytokines (IL-1 receptor antagonist and IL-10), but decreased TNFα and IL-1β production (24). The release of IL-6 may mediate increases in free fatty acid concentrations during exercise via its lipolytic activities and may also help regulate glucose homeostasis by affecting hepatic glucose production and/or muscle glucose uptake. There are also data to suggest that the exercise-induced increase in IL-6 inhibits TNFα production in the presence of low-grade inflammation (30). Furthermore, skeletal muscle IL-8 expression also increases with acute exercise, and this increase may play a role in stimulating angiogenesis in response to physical activity (2). Thus, acute exercise activates an immune response, but the effects are primarily anti-inflammatory and serve to enhance lipid and glucose metabolism. In turn, regular/chronic exercise not only leads to lower levels of circulating inflammatory markers, but also reduces the inflammatory response to acute exercise.
Exercise training-induced improvements in inflammatory status may also result from the modulation of intracellular signaling pathways and cellular function that are mediated by nitric oxide (NO) and reactive oxygen species (ROS). While ROS and NO are generated at low rates under resting conditions, the production of these molecules increases transiently during exercise and play a role in inducing anti-inflammatory defense mechanisms (28). ROS and NO have acute effects on contractile regulation, and exert chronic effects on muscle gene expression. In particular, the adaptive process involves the upregulation of genes encoding antioxidant enzymes and heat shock proteins. For example, treadmill training in rats reduced the release of ROS and NO from contracting muscles and increased skeletal muscle antioxidant content (4). These data indicate that the increase in antioxidant enzymes and heat shock proteins with exercise training helps to protect skeletal muscle against subsequent exposure to exercise-induced increases in ROS generation. Given that ROS mediates some of the catabolic effects of TNFα on skeletal muscle, reductions in ROS generation may lead to attenuation of the inflammatory response with resultant reductions in protein degradation. A significant reduction in skeletal muscle NO synthase expression has also been reported after exercise training, and these changes are accompanied by reductions in skeletal muscle nitrotyrosine content, a marker of NO-derived protein damage (12). Thus, similar to the anti-inflammatory effects of acute cytokine production and release during muscular contraction, these adaptive responses in redox-sensitive pathways may also serve to protect against chronic systemic low-grade inflammation.
CONCLUSION/SUMMARY
Persistent, low-grade inflammation is as an important contributor to the pathophysiology of several chronic health conditions, including aging-related sarcopenia and disability. Given the widespread deleterious health effects of an augmented inflammatory state, identification of therapies that reduce inflammation is critical. Yet, to date, there is little definitive evidence for therapies that can effectively treat individuals with elevated markers of inflammation that are within the clinically normal range, such as are found in the frail elderly. We are beginning to generate promising data, including those from randomized, controlled trials, showing that increasing physical activity could be effective for reducing chronic inflammation in this age group. However, the mechanisms by which increased physical activity may reduce persistent inflammation have not been fully elucidated. If regular exercise emerges as an effective treatment for reducing inflammation, the magnitude of the effect, and the amount of exercise necessary to produce clinically meaningful reductions, should also be delineated.
Acknowledgments
Funding: NIH grants 1R01 AG027529 and R01 AG027529-S1; and the WFU Claude D. Pepper Older Americans Independence Center (P30 AG21332)
REFERENCES
- 1.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162(11):1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK. Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol. 2005;563(Pt 2):507–516. doi: 10.1113/jphysiol.2004.077610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64(4):455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks SV, Vasilaki A, Larkin LM, McArdle A, Jackson MJ. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kappaB activation. J Physiol. 2008;586(16):3979–3990. doi: 10.1113/jphysiol.2008.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22(11):1869–1876. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 6.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition study. J Am Geriatr Soc. 2004;52(7):1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 7.Coletti D, Moresi V, Adamo S, Molinaro M, Sassoon D. Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis. 2005;43(3):119–127. doi: 10.1002/gene.20160. [DOI] [PubMed] [Google Scholar]
- 8.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16(11):1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related pro-inflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. 244–254. [DOI] [PubMed] [Google Scholar]
- 11.Giannopoulou I, Fernhall B, Carhart R, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54(7):866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Gielen S, Adams V, Mobius-Winkler S, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(5):861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 13.Giuliani N, Sansoni P, Girasole G, et al. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol. 2001;36(3):547–557. doi: 10.1016/s0531-5565(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 14.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100(1):93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 15.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15(2):475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- 16.Hammett CJ, Oxenham HC, Baldi JC, et al. Effect of six months' exercise training on C-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol. 2004;44(12):2411–2413. doi: 10.1016/j.jacc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Lakka TA, Lakka HM, Rankinen T, Leon AS, Rao DC, Skinner JS, et al. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. Eur Heart J. 2005;26(19):2018–2025. doi: 10.1093/eurheartj/ehi394. [DOI] [PubMed] [Google Scholar]
- 18.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly. J Appl Physiol. 2008;105(2):473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeMaitre JP, Harris S, Fox KA, Denvir M. Change in circulating cytokines after 2 forms of exercise training in chronic stable heart failure. Am Heart J. 2004;147(1):100–105. doi: 10.1016/j.ahj.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Llovera M, Lopez-Soriano FJ, Argiles JM. Effects of tumor necrosis factor-α on muscle-protein turnover in female Wistar rats. J Natl Cancer Inst. 1993;85:1334–1339. doi: 10.1093/jnci/85.16.1334. [DOI] [PubMed] [Google Scholar]
- 21.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43(6):1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 22.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79(4):544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 23.Nicklas BJ, Hsu FC, Brinkley TJ, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103(3):1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 25.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52(7):1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 26.Rauramaa R, Halonen P, Vaisanen SB, et al. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: the DNASCO Study: a six-year randomized, controlled trial. Ann Intern Med. 2004;140(12):1007–1014. doi: 10.7326/0003-4819-140-12-200406150-00010. [DOI] [PubMed] [Google Scholar]
- 27.Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51(8):1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- 28.Scheele C, Nielsen S, Pedersen BK. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab. 2009;20(3):95–99. doi: 10.1016/j.tem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281(18):1722–1727. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 30.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 31.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of Interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol. 2000;55(12):M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 32.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84(5):1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 33.Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise-induced inflammatory response? Eur J Vasc Endovasc Surg. 1997;14(5):344–350. doi: 10.1016/s1078-5884(97)80283-3. [DOI] [PubMed] [Google Scholar]
- 34.Tsujinaka T, Fujita J, Ebisui C, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996;97(1):244–249. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukui S, Kanda T, Nara M, Nishino M, Kondo T, Kobayashi I. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int J Obes Relat Metab Disord. 2000;24(9):1207–1211. doi: 10.1038/sj.ijo.0801373. [DOI] [PubMed] [Google Scholar]