Abstract
This article presents an overview of end-of-life care for individuals with Alzheimer’s disease (AD) and their family caregivers. We define end-stage AD, and review neuropsychological and behavioral characteristics along with concomitant issues in therapeutic assessment. We then review the literature regarding programs and treatments for end-stage AD, the need for advance care planning and family participation in medical decision-making, familial caregiving stress, and issues associated with palliative care and bereavement outcomes. Methodological issues in the extant research literature are addressed, including issues of treatment implementation, validity, and clinical significance. Translational research and demonstration projects are encouraged.
Keywords: advance care planning, caregiving stress, end of life, methodology, palliative care
Controversy surrounds current approaches to end-of-life care for individuals with Alzheimer’s disease (AD). Although Medicare reimbursement guidelines for hospice care in the context of AD attempt to characterize AD patients with a 6-month prognosis of survival, recent research has shown that these guidelines are not valid predictors of survival.1,2 The time from diagnosis with AD to death may be longer than 10 years in many cases, but median survival time is strongly dependent on age at diagnosis, with those diagnosed at age 65 living an average of 8.3 years and those diagnosed at age 90 living only 3.4 years.3 The end stage of AD may last as long as 2 to 3 years.4 This is also the estimated length of stay for residents in nursing homes.5 In a survey of 400 nursing homes completed primarily by administrative staff, over 85% of respondents reported that residents with dementia have special needs at the end of life but that these needs are difficult to address given problems in the determination of whether a resident with dementia will soon die.6
Notably, no abrupt functional changes signal the terminal phase in frail older people.1 Covinsky and colleagues1 examined data from the last 2 years of life of individuals in programs designed to maintain frail older adults who meet criteria for nursing home placement in the community. These authors found that declines in functional status were evident at least 1 year prior to death. The degree of functional impairment is greater, however, for those with cognitive impairment. Schonwetter and colleagues2 found that advanced age and impaired nutritional and functional status were associated with shortened survival in a sample of 165 individuals admitted to a community hospice with a diagnosis of dementia. Only about three fourths of individuals meeting local Medicare fiscal intermediary guidelines for hospice eligibility died within the 6-month window.
Alzheimer’s disease contributes to an estimated 7.1% of all deaths in the United States,7 which suggests that it rivals stroke as the third leading cause of death. However, AD is rarely listed as the direct cause of death on death certificates, since in end-stage AD, death results typically from pneumonia or cardiovascular disease,8 or one of many secondary impairments such as poor nutrition, decreased fluid intake, or infections related to immobility and skin breakdown. Dementia may be a far more common contributor to death in the United States than is currently acknowledged. Lunney et al9 examined Medicare data and found that there were 4 common trajectories at the end of life that accounted for nearly all deaths. The most common pattern, frailty, accounted for 47% of deaths after a history of severe impairment due to dementia, stroke, and multiple comorbid illnesses. The frequency of dementia as a contributor to mortality often necessitates involvement of caregivers in difficult medical decision-making regarding the use of life-prolonging measures such as feeding tubes and antibiotics. The process is made more difficult by a lack of prior advance care planning when the person with AD retained capacity to make treatment decisions.
The purpose of this article is to review assessment, treatment, advance care planning, palliative caregiving, and bereavement issues in individuals with AD and their families. Methodological issues in this research area will also be explored. Consistent with the approach suggested by Hurley and Volicer,10 there is no precise definition of end-stage AD. However, there is a terminal phase of dementia marked by severe declines in functional status (individuals are usually bedbound and suffer dysphagia), severe limitations in cognitive and communicative abilities (individuals are typically mute), and susceptibility to life-threatening infections (see Figure 1). Planning for the end of life in the context of AD, however, should begin when individuals are in the severe stage marked by resistiveness, incontinence, eating difficulties, and motor impairment.10 Thus, our review covers a more inclusive group of individuals with dementia than those that meet the criteria of the National Hospice and Palliative Care Organization used by Medicare to determine hospice eligibility. By these criteria, individuals must have symptoms including (a) incontinence of bowel and bladder; (b) inability to ambulate or dress without assistance; (c) inability to speak more than 6 intelligible words in an average day; and (d) progressive weight loss of 10% body weight over the preceding 6 months.12 It is our contention that these guidelines define end of life narrowly for individuals with AD and their families, who may benefit much earlier from palliative and supportive care. Given the heterogeneous disease course of AD, familial stress and uncertainty may span a long period of time A family-based approach involving assessment, treatment, and planning implemented throughout the disease process may address the wishes of the individual with AD and his or her family regarding current and future needs.13–15
Figure 1.
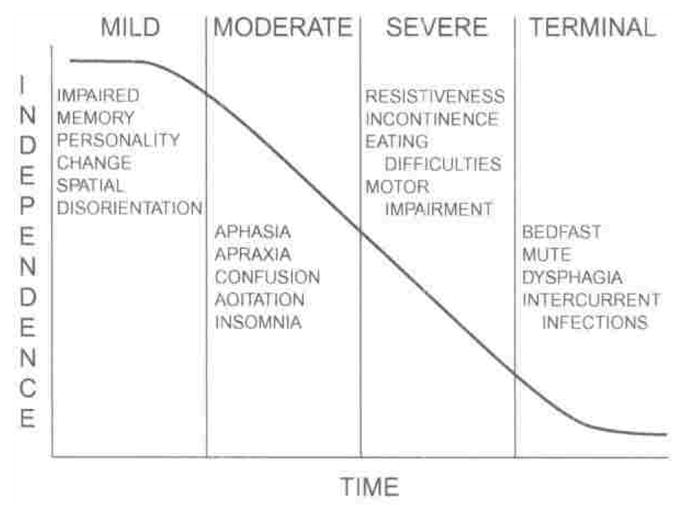
The Four stages of dementia. Reprinted from reference 11. Used by permission.
ASSESSMENT OF NEUROPSYCHOLOGICAL AND BEHAVIORAL CHARACTERISTICS
Therapeutic assessment
Careful assessment is a precursor to effective treatment, and effective treatment leads to enhanced quality of life for individuals with AD and their families late in the disease process. The importance of fine-tuned information on patient diagnoses becomes more apparent as treatment options increase. Historically, comprehensive assessment in late-stage dementia has been viewed as too time-consuming and costly, providing little benefit in directing potential interventions to ease or alleviate symptoms in advanced stages of dementia. Contemporary, alternative perspectives challenge such perceptions and advocate interventions to improve functional capacity on the basis of the assessment of symptom presentation and reserve cognitive abilities even in late-stage dementia.16 Comprehensive dementia evaluations can provide a medium for matching specific individual characteristics with appropriate therapeutic interventions. Capitalizing on individual’s cognitive strengths and minimizing reliance on cognitive deficits can allow those in the advanced stages of AD to interact more successfully with their environment.17 For example, circuits involving procedural memory processes remain relatively intact until the late stages of the disease, allowing substantial preservation of rote motor skills such as action-based memory for routine activities or previously learned musical skills.18,19 In addition, data from such assessments can provide (a) information about patient prognosis; (b) appropriate treatment and disease management options; and (c) education for family members regarding concern about their own risk and available preventative measures.
For the treatment of behavioral symptoms, it is generally suggested that a careful assessment needs to be conducted to identify possible medical sources of behavior problems before considering behavioral interventions or psychotropic medications. This is because secondary behavior problems may be caused by discomfort from a physical illness and individuals with dementia may be unable to report pain or other symptoms.11,20–23 Normalizing the disease process, in terms of typical and atypical behavioral features of end-stage dementia, may be particularly helpful for family members. Family members may associate AD with mental illness, may blame the individual with AD, become angry with the person, or feel frustrated or hurt when their loved one is unable to interact with them in meaningful ways. Many families and individuals with AD have difficulty accepting the inevitable consequences of AD and may resist engaging in interventions such as psychotherapy, support groups and respite services, making advance care plans, or seeking information about the benefits of palliative care to alleviate suffering.
One of the most critical issues for late-stage AD individuals and their families is the minimization of pain and discomfort and the facilitation of therapeutic interaction to the extent possible. Individuals with more severe cognitive impairments may not be reliable, or even capable, in self-report of pain or discomfort.22,24 Furthermore, individual pain reports may not coincide with proxy pain reports, as in the case of hospice patients with advanced cancer.25 Thus, assessment of suffering in the severely demented individual relies typically on observation and clinical assessment.4,10 Treatments for alleviation of pain and suffering have been almost exclusively biomedical. Assessment and management of pain among individuals with dementia is the focus of an article by Horgas and colleagues in this volume26; interested readers are referred to this article for consideration of this critical topic area.
Differential diagnosis
There are more than 50 causes of dementia that can occur at any age.27 Alzheimer’s disease alone accounts for approximately 65% to 72% of all dementia cases, while other progressive dementias account for approximately 15% to 17%, and reversible causes of dementia account for less than 10% of all cases of dementia.28–31 Systematic methods for differential diagnosis provide a means by which appropriate prognosis and treatment interventions can be provided. This is especially critical when the dementing illness is complicated by depression, agitation, or systemic illness, as recent research suggests a strong association between medical comorbidity, cognitive status, and optimum symptom management in the improvement of cognitive functioning.32
Many standard medical and pharmacological treatments for the management of AD may provide little benefit, or worse, may be contraindicated in other dementia presentations.33 In addition, when dementia processes co-occur, an individual’s cognitive, behavioral, and functional presentation may be greatly underestimated. Cognitive, motor, and psychiatric symptoms provide clues to differential diagnosis between the many noninfectious progressive dementias, and data obtained through a neuropsychological evaluation can provide the medium to identify and discriminate between most likely diagnoses. Ideally, a comprehensive assessment for the differential diagnosis of dementia should include (a) an examination by a physician including laboratory studies, neurological examination, and possible neuroimaging; (b) a complete medical, psychiatric, and social history, preferably done by medical record review and interviews with the individual and a knowledgeable family member; (c) a detailed evaluation of current symptoms; (d) a thorough evaluation of primary psychiatric, behavioral, and functional disturbances; (e) a comprehensive initial neuropsychological assessment to document the presence and severity of cognitive impairments: and (f) follow-up brief serial neuropsychological assessments to monitor the course of the disorder, establish cognitive strengths and weaknesses, and provide methods to enhance cognitive function.27,31
Neuropsychological characteristics
Alzheimer’s disease is characterized pathologically by the presence of neuritic plaques and neurofibrillary tangles. The plaques and tangles disproportionately affect the temporal and parietal lobes, accounting for initial symptoms of memory impairment, anomia, and impaired visuoconstructlonal ability.34 There is much empirical support for the utility of neuropsychological assessment in differentiating normal aging versus early dementia, dementia versus depression or stress, and differentiating between types of dementias at early stages of the disease process and between mild cognitive impairment and dementia.35–38 In comparison, little is documented about the neuropsychological characteristics of individuals in the end stages of dementia.
Neuropsychological features in moderate to late-stage AD include generalized intellectual decline, aphasia, agnosia, apraxia, and both immediate and remote memory impairment. Individuals with AD experience gradual loss of remote memories as the disease progresses.39 It is noteworthy that individuals with significant memory impairments may lack insight into their condition and that impaired insight is more frequent in the later stages of AD.40 While there are different physical, cognitive, and behavioral symptoms experienced by people with dementia throughout the disease course, persons with dementia at the end stage are often characterized with severe decline in motor skills, cognitive, communication, and self-care abilities, and decline in immune function.10,11,23 Such severe deficits present a number of unique challenges for clinicians who attempt to characterize cognition and behavior through the use of traditional assessment instruments.
Relatively little is known about the preservation of cognitive skills in severe dementia. There are studies suggesting that individuals with severe dementia who are unable to perform verbal learning tasks may retain the ability to learn motor skills or procedural memory tasks.19,41–45 Poe and Seifert45 reported evidence for intact implicit (procedural) memory on a puzzle-assembly task for probable AD individuals. Even when individuals with AD did not have explicit memory of practicing the task, they demonstrated procedural savings with practice. In a 6-year longitudinal study, Rusted and Sheppard19 examined memory for a single routine activity (tea making) in individuals with AD. Results showed substantial preservation of performed recall of the everyday task, even in the more severe phases of the disease. Clinical observations suggest that some individuals with severe dementia who apparently have lost all verbal skills may be able to recite meaningful and overlearned material, such as prayers, with prompts.46 Similarly, individuals with severe dementia may retain the ability to sing hymns or other overlearned and/or emotionally charged material. Identifying such areas of preservation of function can be very significant in planning activities for persons with severe dementia.
Assessment instruments
Assessment of end-stage dementia requires much flexibility on the part of the clinician. Sensory and motor deficits, such as poor hearing, poor eyesight, or restlessness, are often comorbid conditions in individuals with end-stage AD. Such deficits can result in communication difficulties and low tolerance for anything other than very brief testing situations. It is also difficult to assess individuals with end-stage dementia without the presence of an informant, such as a family member, who can provide useful information regarding memory and functional limitations of the person with AD. Few instruments exist that avoid floor effects and are both sensitive and specific to cognitive deficits and strengths that accompany end-stage AD.47
Cognitive and Functional Status
Commonly used instruments for the assessment of cognitive function include the Mini-Mental State Examination (MMSE),48 the Short Portable Mental Status Questionnaire,49 and the Dementia Rating Scale—2 (DRS-2).50 However, floor effects limit the usefulness of most of these instruments in the identification of preserved cognitive and functional abilities, not only among nursing home samples, but also among community samples of individuals with dementia. For example, Haley et al51 found that, in an outpatient sample of 170 individuals with AD, over 20% had MMSE scores of 0; another 20% had MMSE scores of 1 to 10.
There is some evidence to support the use of the DRS-2 to assess individuals with severe dementia.50 The DRS-2 provides a measure of general cognitive ability that is easy to administer and objectively scored. Norms extend to the age of 105, and the scale was developed in order to avoid floor effects in clinically impaired populations and is therefore sensitive to differences at the lower end of functioning. The DRS-2 measures areas such as attention, initiation, perseveration, visual construction, memory, and conceptualization.
Commonly used measures of functional status include the Katz Activities of Daily Living (ADL) scale or Instrumental Activities of Daily Living (IADL) scales.52,53 Concrete descriptive information about the characteristic symptoms of individuals with very severe dementia was reported by Haley et al.51 In a subgroup of 34 dementia patients with MMSE scores of 0, over 90% were impaired in all IADLs, such as using a telephone and managing medications. Over 80% were incontinent, and needed assistance with dressing and bathing, with 65% unable to feed themselves independently. Only 35% were reported to ask repeated questions, far less than in individuals with moderate dementia because of the severe language impairments experienced in severe dementia. Cognitive, ADL, and IADL problems increase steadily throughout the progression of dementia.54,55 Such declines are often quantified by clinicians through the use of staging instruments.
Staging Instruments
Measures of stage or severity of dementia, rated by health care professionals or other expert raters, include the Severe Impairment Battery,56 Functional Assessment Staging Test,55 the Bedford Alzheimer Nursing Scale-Severity,57 and the Washington University Clinical Dementia Rating.58 These staging instruments are reliable and relatively accurate in the assessment of individuals whose cognitive abilities are severely impaired. However, these measures do not focus on identifying areas of preserved Functioning and means of ameliorating the skills deficits caused by such cognitive and functional decline.47
Behavioral characteristics
Behavioral and psychological symptoms of dementia (BPSD) include behavioral excesses such as disruptive vocalization and aggression and behavioral deficits such as apathy, emotional blunting, decreased appetite, and sleep problems.8,59,60 In the middle stages of the disorder, benign confabulations may be replaced by delusions of persecution or infidelity. Such symptoms may occur when memory loss and perceptual distortions result in incorrect assessment of the environment (ie, mistaking a caregiver for an intruder).61 Researchers have conceptualized this as an incongruent fit between the needs of the individual with dementia and the resources available within the individual and the environment.62–64
There is little empirical research literature available about the course of specific behavioral symptoms manifested by individuals with end-stage dementia. The existing findings suggest that the relationship between behavior problems and stage of dementia is complex and depends on the specific BPSD displayed. Certain behavior problems such as delusions and hallucinations, depression, anxiety, agitation, and apathy worsen as dementia progresses,11 but some of these may improve in late dementia. McCarty and colleagues54 found both linear and curvilinear relations between specific behavior problems and dementia severity, through cross-sectional and longitudinal studies. They found that, among individuals with very severe dementia at an initial assessment, overall BPSD decreased in frequency over a 2-year follow-up as individuals became globally incapacitated.
Assessment instruments
The most common means of assessing behavioral disturbances among individuals with AD in the community or in nursing homes is through survey instruments administered to primary caregivers. The most commonly used measure of the frequency of behavior problems among individuals with dementia in the community is the Revised Memory and Behavior Problems Checklist (RMBPC).65 This instrument not only provides data on the frequency of behavior problems but also provides information on caregivers’ perceptions of burden associated with these behaviors. Behavior problems exhibited by the person with dementia have consistently been associated with perceived burden among community caregivers.65,66
In nursing homes and other long-term care facilities, the best estimates of the frequency of behavior problems likely will be obtained from certified nursing assistants (CNAs) because these staff members have the most direct contact with residents.67–69 Although several assessment instruments are available, the most commonly used survey of the frequency of resident behavior problems in nursing homes is the Cohen-Mansfield Agitation Inventory (CMAI).70 Recently, a measure of memory and behavior problems among nursing home residents (Memory and Behavior Problems Checklist—Nursing Home)71,72 has been revised to measure burden experienced by nursing home staff in response to the frequency of behavior problems exhibited by residents. However, research using the Revised Memory and Behavior Problems Checklist—Nursing Home73 has indicated that CNAs with similar knowledge of a nursing home resident’s cognitive and functional status may differ substantially in their report of the residents’ behavior problem frequency. Clearly, assessment of the burden construct among nursing home staff needs further exploration.
Instruments that rely on direct observation of behavior problems and discomfort among nursing home residents have also been developed. Use of direct observational measures may reveal different outcomes than would data from proxy informants,74,75 raising validity concerns regarding the meaning of proxy informant data. Thus, use of paper-and-pencil instruments may be inappropriate and observational measures of individuals’ behavior may be more revealing. Such measures include the Discomfort Scale for Dementia of the Alzheimer’s Type (DS-DAT),76 Scale for Observation of Agitation in Patients with Dementia of the Alzheimer Type (SOAPD),77 Resistiveness to Care (RTC-DAT).78 Pain Assessment in Advanced Dementia,79 and the Non-communicative Patients’ Pain Assessment Instrument (NOPPAIN).80 Our research group has used computer-assisted behavior observation systems to measure the frequency and duration of residents’ social and disruptive behaviors75,81,82 and is currently using the NOPPAIN in a nursing-home intervention project. While direct observational methodology provides objective information about the behavior of end-stage AD patients, it is costly and time intensive.
THERAPEUTIC PROGRAMS AND TREATMENTS FOR END-STAGE AD
Pharmacological interventions
Medications are often used to decrease the suffering of individuals in late stages of AD. However, there are several drawbacks to relying solely on medication to the exclusion of psychosocial interventions to improve quality of life. Polypharmacy is likely to be present in many individuals with end-stage dementia. Commonly used medications may have unwanted side effects of sedation, constipation, and further cognitive decline. Such side effects can also be caused by interactions between drugs and changes in the rate of absoption, distribution, metabolism, and elimination of drugs that occur with age.83
Acetylcholinesterase inhibitors (AchE-I) have been shown to be effective for treatment of cognitive symptoms in patients with mild to moderate AD through randomized, placebo-controlled clinical trials, although the impact of these changes on clinically significant outcomes such as individual quality of life and daily functioning are less clear.21 Findings on the efficacy of gingko biloba and high doses of vitamin E and selegiline are not conclusive because of problems in methodology (ie, different study designs and small sample size). Most of these pharmacological interventions have not been validated among individuals with severe AD.21 In fact, drugs designed to affect cognitive status are not routinely prescribed in the advanced stages of dementia, as the side effects and costs of such drugs may outweigh the benefits.
Antidepressant treatment has been found effective in treating depression among individuals with advanced dementia.8,84 Serotonin reuptake inhibitors such as sertraline and paroxetine were suggested to have fewer side effects than did tricyclic antidepressants.11 Although drugs such as neuroleptics and other sedatives are often prescribed to reduce symptoms of agitation, these agents may have various unwanted side effects such as sedation and, possibly, accelerated decline.85 Evidence to date shows that pharmacological and behavioral interventions for behavior problems in dementia, and for geriatric depression, are similarly effective,86 but there are too few comparative studies for severe dementia to know if this is true for these individuals as well. Given the potential complications of pharmacological interventions, environmental and caregiver/staff-facilitated interventions have been attempted on a small number of individuals to reduce agitation and improve quality of life.
Environmental and caregiver/staff-facilitated interventions
Several authors have reviewed current literature on therapeutic environmental interventions for people with dementia, but the focus of these reviews has uniformly been management of BPSD.87–89 Although use of behavioral treatments such as environmental and relaxation techniques have been shown to reduce agitation among individuals with moderate and severe dementia,90–92 almost no intervention studies have attempted to improve the quality of end of life care.
Music therapy and touch
In small sample studies, individuals in the late stage of AD have been found to respond positively to music therapy.93–95 Others, however, have concluded that the findings on the effectiveness of touch are inconclusive.11,96
Aromatherapy
Although stage of dementia was not specified, 3 placebo-controlled trials of aromatherapy have been completed, and each has reported a significant reduction of agitation in comparison with placebo.85 Individuals’ compliance with aromatherapy treatment has been high and few side effects have been identified. In comparison with neuroleptic drugs, which had a detrimental impact on quality of life, aromatherapy was associated with improvements in well-being. Lemon balm or lavender oil are the 2 agents most frequently used and are delivered by either inhalation or skin application.97–99
Cohen-Mansfield and Werner,100 however, found limited results using aromatherapy as one facet of an “enhanced environment” intervention to decrease wandering and agitation among nursing home residents. They found that, while residents spent more time and seemed to express more pleasure in areas enhanced by visual, auditory, and olfactory stimuli, there was no effect of the intervention on wandering or observed agitation among residents.
Bright light treatment
Burns and colleagues85 reviewed findings of 3 controlled trials of the use of bright light therapy on sleep disturbance and behavioral disorders in individuals with dementia. Sleep disturbances improved dramatically while more-modest benefits were reported for restlessness.101–103 Once again, however, stage of dementia was not specified. Future research is needed to clarify if treatments such as music therapy, therapeutic touch, aromatherapy, and bright light treatment have positive impact on the quality of life of individuals with end-stage AD.
Caregiver/staff-facilitated interventions
Use of staff motivational systems in nursing homes has increased staff-to-resident communication, improved the quality of this communication by increasing positive statements made to residents, and decreased resident agitation.81,82 One of the commonly experienced problems in individuals with terminal-stage dementia involves difficulties with eating. Manual or natural feeding is generally recommended by clinicians because of the risk for aspiration as well as benefit from the interaction provided during the feeding process.104 Increased length of survival for terminal-stage dementia patients who were fed by skilled staff have been reported.105,106 A promising new line of intervention research concerning this issue is reviewed by Simmons and Schnelle107 in this volume.
Under certain conditions, the possibility of prolonged survival of the individual with end-stage dementia underscores the need for ongoing assessment of multiple perspectives regarding quality of life.108 Prolongation of survival for individuals with terminal-stage dementia may have both positive and negative sequella. The need for ongoing advance care planning for the medical treatment of individuals with late-stage dementia has been identified as a critical area for intervention.6,10,109
ADVANCE CARE PLANNING AND AD
Many individual characteristics have been associated with the possession of an advance directive such as a living will or a durable power of attorney for health care (ie, health care proxy; DPAHC), the most notable of which are advanced age, not identifying oneself as a member of a racial/ethnic minority, higher income and education, and having poorer health status.110–112 More recently, having experienced more negative life events has been related to execution of a DPAHC.113 It is often challenging for families to make decisions when the individual with dementia has no written advance directive and there has been no prior communication of the health care wishes of the individual.11 Lack of knowledge or communication of the individual’s preference regarding end-of-life care in dementia caregiving can cause emotional burden and stress for family members. There are 4 main life-prolonging medical interventions that are raising ethical debates for individuals with end-stage dementia and their families: (a) cardiopulmonary resuscitation, (b) renal dialysis, (c) tube feeding, and (d) antibiotic prescription.8
Decisions regarding placement of a feeding tube are often the most difficult and most frequently debated in advanced dementia.4,8 Finucane and colleagues105 reviewed published studies between 1996 and 1999 and found that tube feeding did not improve survival, functioning, risk of pressure sores, risk of infection, or risk of aspiration. However, such treatments may be initiated because malnutrition and vitamin deficiencies may cause the development of delirium in individuals with dementia.4
A recent study investigated correlates of treatment decision-making capacity among nursing home residents with moderate dementia in the context of decisions about the future placement of a feeding tube.109 These authors found that most nursing home residents lack the decisional capacity to participate meaningfully in the process. Notably, it was found that global measures of cognitive ability such as the MMSE are poor predictors of understanding the treatment situation or appreciation of its consequences. Although no measure of treatment consent capacity was associated with advance directive possession, execution of formal advance directives by residents was associated with possession of advance directives by proxies, proxies who endorsed less religiosity, and residents who engaged in more verbal social behaviors. Allen and colleagues recommend direct assessment of treatment consent capacity and the development of advance care planning interventions for use with able residents and their proxies, usually family members.
It is precisely within the context of serious illness such as end-stage AD that families are most likely to be in distress and in need of assistance with end-of-life decision-making for their family member. A recent study by Boyd114 indicated that lack of prior verbal or written communication on advance care planning led to increased levels of stress among family members, though all family members experienced increased stress following a decision to terminate end-of-life care. Another study115 showed that family stress associated with the decision to withdraw treatment was highest in the absence of advance directives and was lower when verbal or written advance directives guided the family. Interventions to promote elder care preparations within a family context are currently being developed.13,116,117
FAMILIAL CAREGIVING STRAIN AND END-OF-LIFE CARE IN AD
Recently, there has been an increasing number of studies that examine family caregiving at the end of life. However, little is known about caregiving at the end of life for individuals with dementia. In general, caregiving is a risk factor for negative health and social outcomes. A number of studies on caregiving found that caregivers are at increased risk of developing negative mental and health outcomes such as depression, lowered immune system function, and mortality.118–122 Caregivers were also found to experience less participation in social and recreational activities, and movement out of the workforce.123,124 Although there is some evidence that caregivers of individuals with dementia experience greater adverse effects as a result of caregiving than do caregivers for individuals with cancer.125 several other studies have shown that there is little difference in terms of caregiving effects on mental and physical health of family caregivers of individuals with dementia and cancer when the severity of illness is equated, though both groups experience higher rates of psychological distress than does a noncaregiving population.126,127
Clinical reports and observations of experienced clinicians who work with families through hospice and palliative care programs have documented the psychosocial and physical challenges associated with caregiving for terminally ill individuals.128–130 However, there is less literature available on caregiving strain experienced by caregivers for individuals with dementia at the end of life. In general, family caregivers of individuals with dementia are found with higher rates of depression and poorer physical health than age-matched controls and the general population.121,131 Moreover, caregivers of terminally ill individuals are likely to experience high levels of burden and depression.126,132,133 Hospice caregivers who have high levels of social activity and social support, and who are able to find meaning and benefit in caregiving, report lower levels of depression and higher levels of life satisfaction.134
Notably, few studies address potential positive aspects of caregiving, even palliative caregiving, for persons with AD (L. L. Roff, L. D. Burgio, L. Gitlin, et al, unpublished data, 2003). Exploring the impact of religiousness and spirituality as a means of coping has been a particularly neglected area of research.135 Religiousness and spirituality may reduce the sense of loss of control and helplessness that can accompany end-stage AD caregiving. Religious and spiritual beliefs provide a cognitive framework that reduces stress and increases purpose and meaning in the face of illness. Spiritual and religious activities may reduce the sense of isolation and increase the caregivers’ sense of forgiveness, or of control over the illness. Rabins and colleagues127 found that 13% of the variance in positive adaptation after 2 years of caregiving was explained by self-reported religious faith. Even nonreligious caregivers for individuals with AD may face needs that could be identified as existential or spiritual, including (a) finding purpose and meaning, (b) forgiving and receiving forgiveness, (c) maintaining hope, (d) saying goodbye, and (e) coming to terms with expectations for what may occur after death.136
ISSUES ASSOCIATED WITH PALLIATIVE CARE
Hospices and palliative care services have only recently begun in service individuals with end-stage AD at home. Benefits of hospice programs to both individuals with end-stage dementia and their caregivers have been increasingly recognized by professionals and caregivers.131 Case examples of African American family caregivers for individuals with dementia also suggest that palliative care provided by medical staff within the home environment is perceived as beneficial for patients, while respite, education, counseling, and bereavement services are helpful for caregivers.137 Therefore, for individuals with terminal-phase dementia, hospice and palliative care may be appropriate.
Unfortunately, only 2% of individuals who receive hospice services have a primary diagnosis of dementia, 138 Studies found knowledge of hospice services and of the specific services provided by hospice care,139–141 the availability of a caregiver,142 the timing of communications about hospice,139 and being at home at the end of life140 to be simultaneously deterrent and facilitating factors to the use of hospice and palliative care.
Little is known about how families of individuals with dementia make decisions for hospice and palliative care, or how such care is viewed by experts in dementia care. Luchins and Hanrahan143 found that, when given the choice, over 90% of family and professional caregivers for individuals with dementia viewed hospice care as appropriate for the latter stages of this disease.
Many individuals in the late stages of AD live in institutions such as nursing homes. Examination of data from the Minimum Data Set has shown positive treatment outcomes for nursing home residents for whom palliative treatment options were executed.114 Decedents who received hospice care had improved pain management, decreased hospitalization, and decreased use of feeding tubes relative to those residents who died receiving standard nursing home care.144 Volicer and colleagues, through their prospective study of individuals with probable dementia of the Alzheimer type, found decreased discomfort and lower cost of medical care for the comfort-care-only group when compared with a traditional long-term care group.145 Thus, it is important to develop and implement a more accurate and multidimensional prognostic measure for determining when individuals with dementia may be eligible for hospice care.
BEREAVEMENT CARE FOR FAMILY CAREGIVERS
Most studies of palliative caregivers have been retrospective accounts of adjustment to bereavement after the individual’s death. Bereavement has been recognized as a common and important stressful life event, and clearly it is a stressor that has the potential to produce serious negative effects on both mental and physical health.146 Many bereaved individuals exhibit symptoms of grief such as sadness, withdrawal from social activities, and reminiscence or intrusive rumination about the lost loved one. While there are a number of risk factors for pathological grief, such as gender, the nature of relationship between the bereaved and the deceased, and characteristics of the death,147,148 little is known about how the experience of caregiving may affect the grieving process of families and caregivers in case of dementia.
In the case of dementia caregiving, in particular, progressive cognitive decline of the individual makes it difficult for individuals and caregivers to experience and resolve anticipatory grief processes together.149 However, whether the anticipatory grief process is beneficial to adaptive resolution of grief has not been supported with consistent findings. While Bass et al.150 and Mullan151 found that anticipatory grief might be helpful in post-death adjustment, Ponder and Pomeroy152 did not find a positive effect of anticipatory grief among caregivers for individuals with dementia.
Findings regarding the long-term psychosocial outcome of caregivers of individuals with AD after bereavement have also varied, Caregivers may be at risk for prolonged depression or complicated bereavement because of the long duration and challenging nature of dementia caregiving,153–155 resulting in decreased resources for coping. The end of caregiving via death may lead to some mixture of relief of the daily strains of caregiving, and feelings of loss and guilt by both adult-child and spouse-caregivers.149,156 Kiecolt-Glaser and colleagues153,154 found that bereaved caregivers remained just as depressed as they had been while caregiving up to 4 years after the death of the relative. A review by Schulz and colleagues155 found that there are improvements in social function and sense of mastery for caregivers after the death of the relative.
There is a growing appreciation of the diversity of response to illness, death, and grief by members of different cultural groups.157 Owen et al158 examined end-of-life care and reactions to death among White and African American family caregivers of individuals with AD. There were substantial racial/ethnic differences in caregivers’ reports of end-of-life care and subjective reactions to the death of the person with AD. Specifically, African American caregivers were more likely to initiate life-prolonging medical treatments, were less likely to have their relative die in a nursing home, and reported less acceptance of their relative’s death and greater perceived loss than did White caregivers. In a qualitative Study of White and African American caregivers, however, Theis and colleagues159 identified shared needs regardless of racial/ethnic group: (a) opportunities for formal religious practice; (b) social support; and (c) interactions to assist in finding meaning.
METHODOLOGICAL ISSUES IN RESEARCH WITH INDIVIDUALS WITH AD AND FAMILIES AT THE END OF LIFE
Our review of issues surrounding the end of life for individuals with AD and their families illustrates the dramatic need for clinical practice guidelines and clinically relevant research. Research regarding end-of-life issues in general is in its infancy, and targeted investigation of the needs of individuals and families within the context of late-stage AD is even less well developed. Part of the reason for this is our reluctance as a society to consider and actively address our own mortality and the mortality of those whom we love. Thus, as our society becomes more aged, the needs of individuals and families at the end of life will garner greater attention in clinical, research, and policy arenas. At this time, most of what we offer are suggestions regarding critical methodological issues to be considered in clinically relevant research in this area. Given our interest in the psychosocial issues surrounding end-of-life care, we will focus our comments on these methodological issues.
Assessment of treatment implementation
Burgio et al160 reviewed difficulties and provided suggestions in the assessment of treatment Implementation in interventions with older adults in the last issue of Alzheimer’s Care Quarterly. Treatment implementation consists of 3 components: (a) delivery, (b) receipt, and (c) enactment. Certainly, the most basic rule of well-designed and relevant clinical research is clearly conceptualizing what kind of treatment is needed and then to deliver that treatment in a consistent manner. Development of effective psychosocial interventions for individuals with AD and their families at the end of life should begin with a therapeutic needs assessment. Once the basic needs of individuals with late-stage AD and those of their families are identified, standardized protocols are needed for interventionists to accurately and consistently deliver the treatment. For example, interventionists could be trained to use standardized evaluations of treatment consent capacity and advance planning in the context of familial advance planning evaluations13,109 that are audiotaped for quality control. A predetermined percentage of these audiotaped evaluations could be monitored by clinical supervisors to ensure that all families of individuals win AD receive the same information, support, and encouragement in approaching life-prolonging medical treatment decisions (ie, feeding tube placement, use of antibiotics for infection, hospitalization for acute infection).
Delivery of an intervention in an accurate and consistent manner does not ensure that the information, support, and skill building provided by the interventionist is understood and performed accurately by the client.160,161 To assess whether a treatment works, the client must be able to link the treatment provided to their own therapeutic need. This is called treatment receipt. It is likely that therapeutic interventions to ameliorate the suffering of individuals with end-stage AD will involve the actions of caregivers such as family members, CNAs, or health care professionals. For example, family members or CNAs can be trained to assess pain that may be behaviorally expressed by the individual with AD. Then, treatment receipt can be evaluated by comparing the pain assessment rating of the family member or CNA to that of the interventionist who provided the pain assessment training. If possible, comparisons of the pain rating of caregiver can also be made to the subjective report of pain by the individual with AD. Monitoring of treatment receipt in terms of caregiver skill at pain rating of patient with AD should continue until the caregiver reaches a preset accuracy criterion of skill performance. Once this level of skill is reached, periodic monitoring of treatment receipt should continue for the length of treatment in order to ensure accurate and consistent pain assessment on the part of the caregiver.
Like treatment receipt, treatment enactment will involve proxy participants such as care providers. Treatment enactment concerns whether or not the clients use the intervention in their everyday lives. An example of treatment enactment can be drawn from an ongoing, pilot intervention involving legacy activities for family caregivers of palliative care patients,162 We define legacy activities as projects that may result in a product that can be enjoyed by the individual, family, and friends prior to the individual’s death. Scrap booking, making family photo albums or family cookbooks, and audiotaping stories of the life of the individual with AD are examples of legacy activities. Once information is provided by interventionists regarding legacy activities and treatment receipt is ensured so that it is clear that the purpose of the activity and the skills necessary to complete the activity are understood, treatment enactment can be measured by evaluating the extent to which the legacy activity is completed by the family member and the individual with AD. This may require observation of the interactions between care providers and care recipients. Notably, the individual with end-stage AD may have limited involvement in the preparation of such materials. However, individuals with moderate to severe dementia may increase their verbal interactions when materials such as memory books are provided.163 In intervention research with individuals with end-stage AD and their families, once issues regarding the independent variable of treatment have been addressed through the evaluation of treatment implementation, issues of validity, or whether or not the intended therapeutic need is being addressed, arise.
Validity issues
In the last issue of Alzheimer’s Care Quarterly, Zarit et al164 cogently reviewed considerations of several types of validity in clinical research. These authors cite the definition of validity given by Shadish et al165 as the approximate truth of an inference, proposition, or knowledge claim. In the area of end-stage AD, there are many limitations to the internal and construct validity of current intervention and nonintervention research. Limitations include the use of small samples with unspecified level of dementia severity (ie, selection issues), lack of appropriate comparison groups, and overemphasis on behavioral problems as outcome measures with relatively less focus on quality of life (ie, construct validity).
Assessment research in the context of end-stage AD is plagued by several limitations to validity, including the lack of appropriate measures. Useful assessment instruments that measure appropriate constructs are still under development. At this time, assessment instruments should be chosen with the utmost care and attention to the instruments’ psychometric properties, the availability of normative comparison data, and clinical utility.
Regarding intervention research, it is difficult to maintain internal validity when grappling with issues faced by individuals with AD at the end of their lives and the issues of loss faced by their family members. Caregivers of individuals with AD may be in such great need of assistance with issues of planning and anticipatory grief that it may be difficult for researchers to maintain protocol with control group participants, resulting in inadequate treatment implementation and a threat to internal validity. Differential attrition among intervention and control-group participants may also occur. Descriptive, ethnographic research, however, capitalizes on external validity but may reduce the ability to make causal inferences that clinical trials facilitate.
Perhaps the most notable limitation to validity in the area of end-of-life research in the context of AD is the lack of attention to construct validity. Once again, clinically relevant research must begin with accurate assessment of individual and family needs. Outcomes research in this area to date has focused on alleviation of physical symptoms within the individual at the expense of psychosocial issues that may be of great importance to individuals with AD and their families, Multimodal measurements of quality of life need to be developed that take into consideration the perspectives of all relevant stakeholders in the end-of-life care of patients with AD and their families. It is toward the issue of clinical significance in this research area that we now turn.
Clinical significance
Czaja and Schulz166 reviewed relevant methodological issues in the evaluation of clinical significance of intervention research with individuals with AD and their families. They addressed issues including symptomatology, quality of life, social validity, and social significance. To date, much of the research regarding individuals with end-stage AD has focused on alleviation of physical symptoms of pain and suffering. Focus on physical symptoms to the exclusion of psychosocial issues, however, ignores clinical experience and numerous descriptive and qualitative studies that show individuals with terminal illness who retain some capacity to make treatment decisions may choose to live with mild physical pain as long as they retain the ability to interact meaningfully with their loved ones.
Clearly, the assessment of quality of life and interventions to improve life quality within end-stage dementia must attend to the best interests of the individual as well as his or her family. At some point, the ratio of good days to bad days must be considered, from the vantage point of the individual and the individual’s family. This assessment can be facilitated by well-trained clinicians who are sensitive to racial/ethnic and cultural differences in trust of the health care system and medical treatment preferences. Decision-making style within families will also differ, with African American and Asian American families more likely to use group decision-making processes than do other families Caregiver education and communication between familial and professional caregivers is key to treatment success, and will facilitate evaluation of social validity. The social significance of this area of research and clinical service provision will gain prominence in the next decade.
SUMMARY, CLINICAL IMPLICATIONS, AND FUTURE DIRECTIONS
This review has revealed a number of important gaps in our knowledge base, and practice, for individuals with end-stage dementia and their families. Perhaps the most glaring need is for clear definition of constructs such as end-stage dementia and palliative care. Families and many health care professionals are not familiar with the differentiation between palliative care and hospice care. Despite efforts to reform the Medicare hospice benefit to allow for longer periods of care for conditions such as dementia and congestive heart failure, at present hospices must admit individuals with dementia on the basis of 6-month prognosis. Palliative care is more inclusive, as it does not require prognosis of 6 months or less to live or refusal of all life-prolonging treatments. However, there is little research regarding the optimal time for initiation of palliative care based on individual symptoms or individual and family needs. Valid indicators of short-term survival (6 months or less) in persons with dementia would enhance educational efforts targeted at health care professionals and families to inform such stakeholders of the benefits of palliative care.
Broad principles of palliative care have not been sufficiently disseminated into practice in diverse settings In particular, there is a need for nursing homes to more widely utilize effective pain management strategies. Specialized AD clinics and programs, which have recently emphasized early identification of AD and mild cognitive impairment, could do a real service to individuals, families, and providers by increasing attention to severe dementia and its management. Organizations such as the Alzheimer’s Association could also do more to disseminate the effective programs that are available to manage end-stage dementia.
One of the greatest research needs is for replicable, evidence-based interventions for both individuals with severe dementia, and their family caregivers. We have argued that better assessment instruments and serial, brief assessments of cognitive, functional, and behavioral status can lead to identification not only of deficits, but also remaining abilities, in persons with severe dementia. Innovative clinical practices, such as use of sensory stimulation with end-stage dementia, have not been widely studied. We have not found a study of intervention to treat depression or symptoms of pathological or complicated bereavement in bereaved former caregivers. Given that many such caregivers have sustained distress, this is an important area for further research.
In a related vein, little is known about the impact of intervention, such as ongoing familial advance planning for the end of life while caregiving is ongoing, on the subsequent bereavement process, While it is possible that treatment of caregiver depression or anxiety over treatment-related decisions (ie, insertion of feeding tubes) could prevent problems after bereavement, we do not know of any such published research. It is possible that educational and supportive interventions aimed at family caregivers’ treatment decisions and the development of meaning during the experience of palliative caregiving could improve bereavement outcomes.
Finally, a primary barrier to the provision of quality end-of-life care across disease categories, and in particular in the context of end-stage dementia, is the lack of standard policy regarding reimbursement of palliative care services. There is no standard mechanism for reimbursement of palliative care services on the national level. This policy issue, more than any other, serves as a deterrent to provision of quality end-of-life care to individuals with end-stage AD and their families.
IMPLICATIONS FOR CLINICAL PRACTICE
There is no clear definition for end stage AD. However, useful indicators for consideration of end-stage dementia include age, functional and nutritional status, cognitive and communicative abilities, and susceptibility to life-threatening infections.
Clinicians may enhance the quality of life of the individual by focusing on the preservation and support of remaining cognitive abilities and functions.
Characteristics of comprehensive assessment include but are not limited to (a) differential diagnosis based on multiple areas of symptom assessment (eg, biomedical, psychiatric, behavioral, cognitive, and social);(b) identification of medical sources of behavioral problems; (c) education of family members on the disease process; and (d) use of appropriate, validated, and up to date assessment and staging instruments.
Family members are effective in assisting clinicians in identifying the individual’s needs and appropriate care. However, they are also at risk of negative mental and physical health conditions and may need supportive services and interventions to cope with stress, burden, and grief from caregiving.
Few interventions have been examined and shown to be effective with this special population. Thus, clinicians should evaluate the strengths and weaknesses of any intervention through rigorous examination of treatment implementation.
Therapeutic programs and treatments for this population include pharmacological, behavioral, and environmental interventions. Side effects and costs of pharmacological interventions should be carefully considered as little evidence exists for the efficacy of these treatments for individuals with severe dementia.
Ongoing assessment of the individual’s quality of life and, potentially, wishes concerning end-of-life treatment options may help alleviate-family members’ burden and stress. Advance care planning must be ongoing and occur within a family context so that treatment consent capacity expressed by the family unit may be maximized in decisions regarding life-prolonging measures at the end of life.
Clinicians should recognize that family members experience grief over many losses that occur throughout the disease course and even after the individual’s death. Family members’ symptoms of grief, particularly complicated bereavement or prolonged depression, should be monitored and treated appropriately.
Acknowledgments
Funding from the National Institute on Aging (K01AG00943) to R. S. Allen supported preparation of this manuscript. Special thanks are extended to Laura Lee Phillips and the staff of the Center for Mental Health and Aging for assistance in manuscript preparation.
Biographies
Rebecca S. Allen, PhD, is an Associate Professor of Psychology with tenure and a member of the executive committee of the Center for Mental Health and Aging at the University of Alabama in Tuscaloosa. Dr Allen is the recipient of a Mentored Research Scientist Development Award from the National Institute on Aging. Her research focuses on interventions to improve quality of life at the end of life in the community and in long-term care settings and on the process of health care decision-making among older adults and their families.
Jung Kwak, MSW, is a doctoral student in the Aging Studies program at the University of South Florida. Her current research interest is in cultural diversity and end of life decision-making among ethnically diverse groups of older people and their families. Her work experience includes working in both clinical and policy settings. Currently she is the principal investigator on pilot project examining knowledge and preferences for end-of-life care and hospice among Korean American elders and their caregivers.
Kristine L. Lokken, PhD, is an Assistant Professor of Psychology at the University of Alabama in Tuscaloosa. Dr Lokken’s primary research and clinical interests are in the area of clinical neuropsychology. Her research to date has an emphasis on women’s health issues, focusing on neuropsychological aspects of hormonal status and the neuropsychology of eating disorders and obesity. Her clinical interests include neuropsychological evaluation of geriatric and other adult populations with wide-ranging clinical diagnoses.
William E. Haley, PhD, is Professor and Chair in the Department of Gerontology at the University of South Florida. He is also Chair-elect of the Behavioral and Social Sciences Section of the Gerontological Society of America, and a Past President of the Section on Clinical Geropsychology of the American Psychological Association. Dr Haley’s research interests include family caregiving, end-of-life issues, and health psychology.
References
- 1.Covinsky KE, Eng C, Lui LY, Sands LP, Yaffe K. The last 2 years of life: functional trajectories of frail older people. J Am Geriatr Soc. 2003;51:492–498. doi: 10.1046/j.1532-5415.2003.51157.x. [DOI] [PubMed] [Google Scholar]
- 2.Schonwetter RS, Han B, Small BJ, Martin B, Tope K, Haley WE. Predictors of six-month survival among patients with dementia: An evaluation of hospice Medicare guidelines. Am J Hosp Palliat Care. 2003;2:105–115. doi: 10.1177/104990910302000208. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol. 2002;59:1764–1767. doi: 10.1001/archneur.59.11.1764. [DOI] [PubMed] [Google Scholar]
- 4.Shuster JL. Palliative care for advanced dementia. Clin Geriatr Med. 2000;16:373–386. doi: 10.1016/s0749-0690(05)70062-8. [DOI] [PubMed] [Google Scholar]
- 5.Dey AN. Characteristics of elderly nursing home residents: data from the 1995 National Nursing Home Survey. Adv Data. 1997;2:1–8. [PubMed] [Google Scholar]
- 6.Moss MS, Braunshweig H, Rubinstein RL. Terminal care for nursing home residents with dementia. Alzheimer’s Care Q. 2002;3:233–246. [Google Scholar]
- 7.Ewbank DC. Deaths attributable to Alzheimer’s disease in the United States. Am J Public Health. 1999;89:90–92. doi: 10.2105/ajph.89.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel JP, Pautex S, Zekry D, Zulian G, Gold G. End of life care of persons with dementia. J Gerontol A Biol Sci Med Sci. 2002;57:M640–M644. doi: 10.1093/gerona/57.10.m640. [DOI] [PubMed] [Google Scholar]
- 9.Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J Am Geriatr Soc. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 10.Hurley AC, Volicer BJ. Alzheimer disease: “It’s okay, Mama, if you want to go, it’s okay”. JAMA. 2002;288:2324–2331. doi: 10.1001/jama.288.18.2324. [DOI] [PubMed] [Google Scholar]
- 11.Voilcer L, Hurley AC, editors. Hospice Care for Patients With Advanced Progressive Dementia. New York: Springer Publishing Co Inc; 1998. [Google Scholar]
- 12.Standards and Accreditation Committee: Medical Guidelines Task Force of the National Hospice Organization. Medical Guidelines for Determining prognosis in selected Non Selected Non cancer Diseases. 2. Arlington, Va: National Hospice Organization; 1996. [DOI] [PubMed] [Google Scholar]
- 13.Allen RS, Shuster JL. The role of proxies in treatment decisions: evaluating functional capacity to consent to end of life treatments within a family context. Bebav Sci Law. 2002;20:235–252. doi: 10.1002/bs1.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haley WE, Allen RS, Reynolds S, Chen H, Burton A, Gallagher-Thompson D. Family issues in end of life decision making and end of life care. Am Bebav Sci. 2002;46:284–297. [Google Scholar]
- 15.King UA, Kim SY, Conwell Y. Family matters: a social system perspective on physician-assisted suicide and the older adult. Psychol Public Policy Law. 2000;6:434–451. [PubMed] [Google Scholar]
- 16.Kitwood T. Dementia Reconsidered. Buckingham. UK: Open University Press; 1997. [Google Scholar]
- 17.Weaverdyck SE. Intervention-based neuropsychological assessment. In: Mace NL, editor. Dementia Care: Patient, Family, and Community. Baltimore. Md: Johns Hopkins University Press; 1990. pp. 205–223. [Google Scholar]
- 18.Beatty WW, Rogers CL, Rogers RL, et al. Piano playing in Alzheimer’s disease: longitudinal study of a single case. Neurocase. 1999;5:459–469. [Google Scholar]
- 19.Rusted J, Sheppard L. Action-based memory in Alzheimer’s disease: a longitudinal look at tea making. Neurocase. 2002;8:111–126. doi: 10.1093/neucas/8.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Allen RS, Thorn BE, Fisher SE, et al. Prescription and dosage of analgesic medication in relation to resident behaviors in the nursing home. J Am Geriatr Soc. 2003;51:534–538. doi: 10.1046/j.1532-5415.2003.51164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emre M, Hanagasi HA. Evidence-based pharmacological treatment of dementia. Eur J Neurol. 2007;7:247–253. doi: 10.1046/j.1468-1331.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 22.Fisher SE, Burgio LD, Thorn BE, et al. Pain assessment and management among cognitively impaired nursing home residents: association of certified nursing assistant pain report, MDS pain report, and analgesic medication use. J Am Geriatr Soc. 2002;50:152–156. doi: 10.1046/j.1532-5415.2002.50021.x. [DOI] [PubMed] [Google Scholar]
- 23.Kovach CR, editor. Late Stage Dementia Care: A Basic Guide. Washington, DC: Taylor & Francis; 1997. [Google Scholar]
- 24.Fisher SE, Burgio LD, Thorn BE. Evaluating self-report of pain and cognitive status in the nursing home. Poster presented at: The Fourteenth Annual South-eastern Regional Student Convention in Gerontology and Geriatrics: The Cultural Diversity of Aging; March 28–29, 2003; Tybee Island, Ga.. [Google Scholar]
- 25.Allen RS, Haley WE, Small BJ, McMillan SC. Pain reports by older hospice cancer patients and family caregivers: the role of cognitive functioning. Gerontologist. 2002;42:507–514. doi: 10.1093/geront/42.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horgas AL, McLennon SM, Floetke AL. Pain management in persons with dementia. Alzheimer’s Care Q. 2003;4(4):297–311. [Google Scholar]
- 27.Nussbaum P. General assessment issues for a geriatric population. In: Snyder PJ, Nussbaum PD, editors. Clinical Neuropsychology: A pocket Handbook for Assessment. Washington, DC: American Psychological Association; 1998. pp. 173–191. [Google Scholar]
- 28.Katzman R, Rowe JW. Principles of Geriatric Neurology. Philidephia: Davis; 1992. [Google Scholar]
- 29.Kokmen E, Beard CM, O’Brien PC, Offord KP, Kurland LT. Is the incidence of dementing illness changing? A 25-year time trend study in Rochester, Minnesota (1960–1984) Neurology. 1993;43:1887–1892. doi: 10.1212/wnl.43.10.1887. [DOI] [PubMed] [Google Scholar]
- 30.Ritchie K, Lovestone S. The dementias. Lancet. 2002;360:1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [DOI] [PubMed] [Google Scholar]
- 31.Small GW, Rabins PV, Barry PP. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- 32.Doraiswamy PM, Leon J, Cummings JL, Marin D, Neumann PJ. Prevalence and impact of medical of medical comorbidity in Alzeheimer’s disease. J Gerontol A Biol Sci Med Sci. 2002;57:M173–Ml77. doi: 10.1093/gerona/57.3.m173. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstein LD. Differential diagnosis of the major progressive dementias and depression in middle and late adulthood: a summary of the literature of the early 1990s. Neuropsychol Rev. 1998;8:109–167. doi: 10.1023/a:1025628925796. [DOI] [PubMed] [Google Scholar]
- 34.Boler F, Duyckaerts C. Alzheimer disease: clinical and. anatomic aspects. In: Feinberg TE, Farah MJ, editors. Behavioral Neurology and Neuropsychology. New York: McGraw-Hill; 1997. pp. 521–544. [Google Scholar]
- 35.Butters MA, Salmon DP, Butters N. Neuropsychological assessment of dementia. In: Storandt M, Vanden-Bos GR, editors. Neuropsychological Assessment of Dementia and Depression in Older Adults: A Clinicians Guide. Washington, DC: American Psychological Association; 1994. pp. 33–59. [Google Scholar]
- 36.Pasquier F. Early diagnosis of dementia: neuropsychology. J Neurol. 1999;246:6–15. doi: 10.1007/s004150050299. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC. Aging, mild cognitive impairment, and Alzheimer’s disease. Neurol Clin. 2002;23:S145. doi: 10.1016/s0733-8619(05)70226-7. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 39.Nebes RD. Alzheimer’s disease: cognitive and neuropsychological aspects. In: Feinberg TE, Farah MJ, editors. Behavioral Neurology and Neuropsychology. New York: McGraw-Hill; 1997. pp. 545–550. [Google Scholar]
- 40.Harwood DG, Sultzer DL, Wheatly MV. Impaired insight in Alzheimer disease:association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Neuropsychiatry Neuropsychol Bebav Neurol. 2000;13:83–88. [PubMed] [Google Scholar]
- 41.Eslinger PJ, Damasio AR. Preserved motor learning in Alzheimer’s disease: implications of anatomy and behavior. J Neurosci. 1986;6:3006–3009. doi: 10.1523/JNEUROSCI.06-10-03006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deweer B, Pillon B, Michon A, Dubois B. Mirror reading in Alzheimer’s disease: normal skill learning and acquisition of item-specific information. J Clin Exp Neuropsychol. 1993;15:789–804. doi: 10.1080/01688639308402596. [DOI] [PubMed] [Google Scholar]
- 43.Knopman D. Long-term retention of implicitly acquired learning in patients with Alzheimer’s disease. J Clin Exp Neuropsychol. 1991;13:880–894. doi: 10.1080/01688639108405105. [DOI] [PubMed] [Google Scholar]
- 44.Grafman J, Weingartner H, Newhouse PA, et al. Implicit learning in patients with Alzheimer’s disease. Pharmacopsychiatry. 1990;23:94–101. doi: 10.1055/s-2007-1014490. [DOI] [PubMed] [Google Scholar]
- 45.Poe MK, Seifert LS. Implicit and explicit tests: evidence for dissociable motor skills in probable Alzheimer’s dementia. Percept Mot Skills. 1997;85:631–634. doi: 10.1177/003151259708500201. [DOI] [PubMed] [Google Scholar]
- 46.Snowdon M. Aging With Grace: What the Nun Study Teaches Us About Leading Longer, Healthier, and More Meaningful Lives. New York: Bantam Doubleday Dell; 2001. [Google Scholar]
- 47.Camp CJ, Koss E, Judge KS. Cognitive assessment in late-stage dementia. In: Lichtenberg PA, editor. Handbook of Assessment in Clinical Gerontology. New York: Wiley; 1999. pp. 442–467. [Google Scholar]
- 48.Folstein MF, Folstein SE, McHugh PR. “Mini Mental State” a practical method for grading the cognitive state of patients for the clinician. J Psychiater Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 50.Jurica PJ, Leitten C, Mattis S. Dementia Rating Scale—2 (DRS-2) Flutz, Fla: Psychological Assessment Resources Inc; 2001. [Google Scholar]
- 51.Haley WE, Wadley VG, West CC, Vetzel LL. How care-giving stressors change with severity of dementia. Semin Speech Lang. 1994;15:195–205. [Google Scholar]
- 52.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffee MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. J Am Med Assoc. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 53.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 54.McCarty HJ, Roth DL, Goode KT, et al. Longitudinal course of behavioral problems during Alzheimer’s disease: linear versus curvilinear patterns of decline. J Gerontol. 2000;55A:M200–M206. doi: 10.1093/gerona/55.4.m200. [DOI] [PubMed] [Google Scholar]
- 55.Reisberg B. Functional assessment staging (FAST) Psychopharmacol Bull. 1988;24:653–659. [PubMed] [Google Scholar]
- 56.Saxton J, McGonicle-Gibson K, Swihart A, Miller M, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. psychological assessment. J Consult Clin psychol. 1990;2:298–303. [Google Scholar]
- 57.Volicer L, Hurley AC, Lathi DC, Kowall NW. Measurement of severity in advanced Alzheimer’s disease. J Gerontol. 1994;49:M223–M226. doi: 10.1093/geronj/49.5.m223. [DOI] [PubMed] [Google Scholar]
- 58.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 59.Burgio LD, Fisher SE. Application of psyehosocial interventions for treating behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2000;12 (suppl l):351–358. [Google Scholar]
- 60.Finkel SI, Burns A. Behavior and psychological symptoms of dementia (BPSD): a clinical and research up-date. Int Psychogeriatr. 2000;12:9–12. [Google Scholar]
- 61.Fields RB. The dementias. In: Snyder PJ, Nussbaum PD, editors. Clinical Neuropsychology:A Pocket Handbook for Assessment. Washington, DC: American Psychological Association; 1998. pp. 211–239. [Google Scholar]
- 62.Algase D, Beck C, Kolanowski A, et al. Need-driven dementia-compromised behavior: An alternative view of disruptive behavior. Am J Alzheimers Dis Other Demen. 1996;11:10–19. [Google Scholar]
- 63.Beck CK, Vogelpohl TS. Problematic vocalizations in institutionalized individuals with dementia. J Gerontol Nurs. 1999;25:17–26. doi: 10.3928/0098-9134-19990901-07. quiz 48, 51. [DOI] [PubMed] [Google Scholar]
- 64.Lawton MP. Behavior-relevant ecological factors. In: Schaie KW, Schooler C, editors. Social Structure and Aging: Psychological Processes. Hillsdale, NJ: Erlbaum; 1989. pp. 57–58. [Google Scholar]
- 65.Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems check-list. Psychol Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 66.Vitaliano PP, Russo J, Young HM, Teri L, Maiuro RD. Predictors of burden in spouse caregivers of individuals with Alzheimer’s disease. Psychol Aging. 1991;6:392–402. doi: 10.1037//0882-7974.6.3.392. [DOI] [PubMed] [Google Scholar]
- 67.Burgio LD, Engel BT, Hawkins A, McCormick K, Scheve A. A descriptive analysis of nursing staff behaviors in a teaching nursing home: differences among NAs, LPNs, and RNs. Gerontologist. 1990;30:107–121. doi: 10.1093/geront/30.1.107. [DOI] [PubMed] [Google Scholar]
- 68.Chappell NL, Novak M. Caring for institutionalized elders: stress among nursing assistants. J Appl Gerontol. 1994;13:299–315. [Google Scholar]
- 69.Tellis-Nayak V, Tellis-Nayak M. Quality of care and the burden of two cultures: when the world of the nurse’s aide enters the world of the nursing home. Gerontologist. 1989;29:307–313. doi: 10.1093/geront/29.3.307. [DOI] [PubMed] [Google Scholar]
- 70.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44:M77–M84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 71.Wagner AW, Teri L, Orr-Rainey N. Behavior problems among dementia residents in special care units: changes over time. J Am Geriatr Soc. 1995;43:784–787. doi: 10.1111/j.1532-5415.1995.tb07051.x. [DOI] [PubMed] [Google Scholar]
- 72.Wagner AW, Teri L, Orr-Rainey N. Behavior problems of residents with dementia in special care units. Alzheimer Dis Assoc Disord. 1995;9:121–127. [PubMed] [Google Scholar]
- 73.Allen RS, Burgio LD, Roth DL, et al. The revised memory and behavior problems checklist—nursing home: instrument development and measurement of burden among certified nursing assistants. Psychol Aging. doi: 10.1037/0882-7974.18.4.886. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCann JJ, Gilley DW, Hebert LE, Beckett LA, Evans DA. Concordance between direct observation and staff rating of behavior in nursing home residents with Alzheimer’s disease. J Gerntol B Psychol Sci Soc Sci. 1997;52:P63–P72. doi: 10.1093/geronb/52b.2.p63. [DOI] [PubMed] [Google Scholar]
- 75.Vance DE, Burgio LD, Roth DL, Stevens AB, Fairchild JK, Yurick A. Predictors of agitation in nursing home residents. J Gerontol B Psychol Sci Soc Sci. 2003;58:129–137. doi: 10.1093/geronb/58.2.p129. [DOI] [PubMed] [Google Scholar]
- 76.Hurley AC, Volicer BJ, Hanrahan P, Houde S, Volicer L. Assessment of discomfort in advanced Alzheimer patient. Res Nurs Health. 1992;15:369–377. doi: 10.1002/nur.4770150506. [DOI] [PubMed] [Google Scholar]
- 77.Hurley A, Volicer L, Camberg L, et al. Measurement of observed agitation in patients with dementia of the Alzheimer type. J Ment Health Aging. 1999;5:117–133. [Google Scholar]
- 78.Mahoney EK, Hurley AC, Volicer L, et al. Development and testing of the Resistiveness to Care Scale. Res Nurs Health. 1999;22:27–38. doi: 10.1002/(sici)1098-240x(199902)22:1<27::aid-nur4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 79.Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4:9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 80.Snow AL, Hovanec I, Passano J, Brandt J. Development of a pain assessment instrument for use with severely demented patients. Poster presented at: The Annual Meeting of the American Psychological Association; August 2001; Washington, DC. [Google Scholar]
- 81.Burgio LD, Allen-Burge R, Roth DL, et al. Come talk with me: improving communication between nursing assistants and nursing home residents during care routines. Gerontologist. 2001;41:449–460. doi: 10.1093/geront/41.4.449. [DOI] [PubMed] [Google Scholar]
- 82.Burgio LD, Stevens A, Burgio KL, Roth DL, Paul P, Gerstle J. Teaching and maintaining behavior management skills in the nursing home. Gerontologist. 2002;42:487–496. doi: 10.1093/geront/42.4.487. [DOI] [PubMed] [Google Scholar]
- 83.Beizer JL. Medications and the aging body: alteration as a function of age. Generations. 1994;18:13–17. [Google Scholar]
- 84.Volicer L, Rheaume Y, Cyr D. Treatment of depression in advanced Alzheimer’s disease using sertraline. J Geriatr Psychiatry Neurol. 1994;7:227–229. doi: 10.1177/089198879400700406. [DOI] [PubMed] [Google Scholar]
- 85.Burns A, Byrne J, Ballard C, Holmes C. Sensory stimulation in dementia. BMJ. 2002;325:1312–1313. doi: 10.1136/bmj.325.7376.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bartels SJ, Haley WE, Dumas AR. Implementing evidence-based practices in geriatric mental health. Generations. 2002;26:90–98. [Google Scholar]
- 87.Allen-Burge R, Stevens AB, Burgio LD. Effective behavioral interventions for decreasing dementia-related challenging behavior in nursing homes. Int J Geriatr Psychiatry. 1999;14:213–232. [PubMed] [Google Scholar]
- 88.Carlson DL, Fleming KC, Smith GE, Evans JM. Management of dementia-related behavioral disturbances: a nonpharmacologic approach. Mayo Clin proc. 1995;70:1108–1115. doi: 10.4065/70.11.1108. [DOI] [PubMed] [Google Scholar]
- 89.Day K, Carreon D, Stump C. The therapeutic design of environments for people with dementia: a review of the empirical research. Gerontologist. 2000;40:397–416. doi: 10.1093/geront/40.4.397. [DOI] [PubMed] [Google Scholar]
- 90.Burgio L, Scilley K, Hardin JM, Hsu C, Yancey J. Environmental “white noise”: an intervention for verbally agitated nursing home residents. J Gerontol B Psycho Sci Soc Sci. 1996;51:P364–P373. doi: 10.1093/geronb/51b.6.p364. [DOI] [PubMed] [Google Scholar]
- 91.Snyder M, Egan EC, Burns KR. Efficacy of hand massage in decreasing agitated behaviors associated with care activities in persons with dementia. Geriatr Nurs. 1995;16:60–63. doi: 10.1016/s0197-4572(05)80005-9. [DOI] [PubMed] [Google Scholar]
- 92.Tabloski PA, McKinnon-Howe L, Remington R. Effect of calming music on level of agitation in cognitively impaired nursing home residents. Am J Alzheimer’s Care Relat Disord Res. 1995;10:10–15. [Google Scholar]
- 93.Norberg A, Melin E, Asplund K. Reactions to music, touch, and object presentation in the final stage of dementia: an exploratory study. Int J Nurs Stud. 1986;23:315–323. doi: 10.1016/0020-7489(86)90054-4. [DOI] [PubMed] [Google Scholar]
- 94.Jansson L, Norberg A, Sandman PO, Athlin E, Asplund K. Interpreting facial expressions in patients in the terminal stage of the Alzheimer’s disease. Omega. 1993;26:309–324. [Google Scholar]
- 95.Smith S. The unique power of music therapy benefits in Alzheimer’s patients. Act Adapt Aging. 1990;14:59–63. [Google Scholar]
- 96.Burgio LD, Butler FR, Roth DL, Hardin JM, Hsu CC, Ung K. Agitation in nursing home residents: the role of gender and social context. Int Psychogeriatr. 2000;12:495–511. doi: 10.1017/s104161020000661x. [DOI] [PubMed] [Google Scholar]
- 97.Ballard CG, O’Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double blind, placebo-controlled trial with Melissa. J Clin Psychiatry. 2002;63:553–558. doi: 10.4088/jcp.v63n0703. [DOI] [PubMed] [Google Scholar]
- 98.Holmes C, Hopkins V, Hensford C, MacLaughlin V, Wilkinson D, Rosenvinge H. Lavender oil as a treatment for agitated behaviour in severe dementia: a placebo controlled study. Int J Geriatr Psychiatry. 2002;17:305–308. doi: 10.1002/gps.593. [DOI] [PubMed] [Google Scholar]
- 99.Smaliwood J, Brown R, Coulter F, Irvine E, Copland C. Aromatherapy and behaviour disturbances in dementia: a randomized controlled trial. Int J Geriatr Psychiatry. 2001;16:1010–1013. doi: 10.1002/gps.473. [DOI] [PubMed] [Google Scholar]
- 100.Cohen-Mansfield J, Werner P. The effects of an enhanced environment on nursing home residents who pace. Gerontologist. 1998;38:199–208. doi: 10.1093/geront/38.2.199. [DOI] [PubMed] [Google Scholar]
- 101.Graf A, Wallner C, Schubert V, et al. The effects of light therapy on mini-mental state examination scores in demented patients. Biol Psychiatry. 2001;50:725–727. doi: 10.1016/s0006-3223(01)01178-7. [DOI] [PubMed] [Google Scholar]
- 102.Haffmans PM, Sival RC, Lucius SA, Cats Q, van Gelder L. Bright light therapy and melatonin in motor restless behaviour in dementia: a placebo-controlled study. Int J Geriatr Psychiatry. 2001;16:106–110. doi: 10.1002/1099-1166(200101)16:1<106::aid-gps288>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 103.Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- 104.Morris J, Volicer L. Nutritional management of individuals with Alzheimer’s disease and other progressive dementias. Nutr Clin Care. 2001;4:148–155. [Google Scholar]
- 105.Finucane TE, Chrismas C, Travis K. Tube feeding in patients with advanced dementia. JAMA. 1999;282:1365–1370. doi: 10.1001/jama.282.14.1365. [DOI] [PubMed] [Google Scholar]
- 106.Volicer L, Seltzer B, Rheaume Y, et al. Eating difficulties in patients with probable dementia of the Alzheimer type. J Geriatr Psychiatry Neurol. 1988;2:188–195. doi: 10.1177/089198878900200404. [DOI] [PubMed] [Google Scholar]
- 107.Simmons SF, Schnelle JF. Implementation of nutritional interventions in long term care. Alzheimer’s Care Q. 2003;4(4):286–296. [Google Scholar]
- 108.Gillick MR, Mitchell SL. Facing eating difficulties in end stage dementia. Alzheimer’s Care Q. 2002;3:227–232. [Google Scholar]
- 109.Allen RS, DeLaine S, Chaplin WF, et al. Advance care planning in nursing homes: correlates of capacity and possession of advance directives. Gerontologist. 2003;43:309–317. doi: 10.1093/geront/43.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eleazer GP, Hornung CA, Egbert CB, et al. The relationship between ethnicity and advance directives in a frail older population. J Am Geriatr Soc. 1996;44:938–943. doi: 10.1111/j.1532-5415.1996.tb01864.x. [DOI] [PubMed] [Google Scholar]
- 111.High DM. Advance Directives and the elderly: a study of intervention strategies to increase use. Gerontologist. 1993;33:342–349. doi: 10.1093/geront/33.3.342. [DOI] [PubMed] [Google Scholar]
- 112.Hopp FP, Duffy SA. Racial variations in end of life care. J Am Geriatr Soc. 2000;48:658–663. doi: 10.1111/j.1532-5415.2000.tb04724.x. [DOI] [PubMed] [Google Scholar]
- 113.Rosnick CB, Reynolds SL. Thinking ahead: factors associated with executing advance directives. J Aging Health. 2003;15:409–429. doi: 10.1177/0898264303015002005. [DOI] [PubMed] [Google Scholar]
- 114.Boyd L. Advance directives can reduce family stress with end of life decisions. RN. 2001;64:18. [Google Scholar]
- 115.Tilden VP, Tolle SW, Nelson CA, Fields J. Family decision-making to withdraw life sustaining treatments from hospitalized patients. Nurs Res. 2001;50:105–115. doi: 10.1097/00006199-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 116.Parker MW, Call VR, Dunkle R, Vaitkus M. “Out of Sight” but not “Out of Mind:” Parent care contact and worry among military officers who live long distances from parents. Mil Psychol. 2002;14:257–277. [Google Scholar]
- 117.Parker MW, Roff L, Toseland R, Klemmack D. The Hartford militarv parent care project: a psychosocial educational intervention with long distance parent care providers. Poster presented at: First National Gerontological Social Work Conference, held in conjunction with Council Social Work Education Annual Conference; March 1, 2003; Atlanta, Ga. [Google Scholar]
- 118.Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 119.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci USA. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haley WE, West CA, Wadley VG, Ford GR, White FA, Barrett JJ. Psychological, social, and health impact of caregiving: a comparison of Black and White dementia family caregivers and noncaregivers. Psychol Aging. 1995;10:540–552. [PubMed] [Google Scholar]
- 121.Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 122.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 123.Brody EM. Effects of caregiving on the caregivers. In: Brody EM, editor. Women in the Middle, Their Parent-Care Years. New York: Springer; 1990. [Google Scholar]
- 124.Brody EE. Women as unpaid caregivers: the price they pay. In: Friedman E, editor. An Unfinished Revolution: Women and Health Care in America. New York: United Hospital Fund; 1994. [Google Scholar]
- 125.Clipp EC, George LK. Dementia and cancer: a comparison of spouse caregivers. Gerontologist. 1993;31:534–541. doi: 10.1093/geront/33.4.534. [DOI] [PubMed] [Google Scholar]
- 126.Haley WE, LaMonde LA, Han B, Narramore S, Schonwetter RS. Family caregiving in hospice: effects on psychological and health functioning among spousal caregivers of hospice patients with lung cancer or dementia. Hosp J. 2001;15:1–18. doi: 10.1080/0742-969x.2000.11882959. [DOI] [PubMed] [Google Scholar]
- 127.Rabins PV, Fitting MD, Eastham J, Fitting J. The emotional impact of caring for the chronically ill. Psychosomatics. 1990;31:331–336. doi: 10.1016/S0033-3182(90)72171-8. [DOI] [PubMed] [Google Scholar]
- 128.Doka KJ, editor. Living With Grief When Illness Is Prolonged. Bristol Pa: Taylor & Francis; 1997. [Google Scholar]
- 129.Lynn J, Harrold J. Handbook for Mortals: Guidance for People Facing Serious Illness. New York: Oxford Press; 1999. [Google Scholar]
- 130.Nuland SB. How We Die: Reflections on Life’s Final Chapter. New York: Alfred A Knopf; 1994. [Google Scholar]
- 131.Haley WE, Bailey S. Research on family caregiving in Alzheimer’s disease: implications for practice and policy. In: Vellas B, Fitten JL, editors. Research and Practice in Alzheimer’s Disease. Vol. 2. Paris: Serdi Publisher; 1999. pp. 321–332. [Google Scholar]
- 132.Emanuel EJ, Fairclough DL, Slutsman J, Alpert H, Baldwin D, Emanuel LL. assistance from family members, friends, paid caregivers, and volunteers in the care of terminally ill patients. N Engl J Med. 1999;341:956–963. doi: 10.1056/NEJM199909233411306. [DOI] [PubMed] [Google Scholar]
- 133.Emanuel EJ, Fairclough DL, Slutsman BA, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132:451–459. doi: 10.7326/0003-4819-132-6-200003210-00005. [DOI] [PubMed] [Google Scholar]
- 134.Haley WE, LaMonde LA, Han B, Burton AM, Schonwetter R. Predictors of depression and life satisfaction among spousal caregivers in hospice:application of a stress process model. J Palliat Med. 2003;6:215–224. doi: 10.1089/109662103764978461. [DOI] [PubMed] [Google Scholar]
- 135.Michael S, Crowther M, Schmid B, Allen RS. Widowhood and spirituality: coping responses to bereavement. J Women Aging. doi: 10.1300/J074v15n02_09. In press. [DOI] [PubMed] [Google Scholar]
- 136.Koenig HG. A commentary: the role of religion and spirituality at the end of life. Gerontologist. 2002;42:20–23. doi: 10.1093/geront/42.suppl_3.20. [DOI] [PubMed] [Google Scholar]
- 137.Luchins DJ, Hanrahan P, Litzenberg K. In: Hospice Care for Patients With Advanced Progressive Dementia. Volicer L, Hurley AC, editors. New York: Springer; 1998. [Google Scholar]
- 138.National Hospice Organization. Hospice Fact Sheet. National Hospice Organization; Arlington, Va: 1999. [Google Scholar]
- 139.Gochman DS, Bonham GS. The social structure of the hospice decision. Hosp J. 1990;6:15–36. doi: 10.1080/0742-969x.1990.11882663. [DOI] [PubMed] [Google Scholar]
- 140.Hays JC, Gold DT, Flint EP, Winer EP. Patient preference for place of death: a qualitative approach. In: de Vries B, editor. End of Life Issues:Interdisciplinary and Multidisciplinary Perspectives. New York: Springer; 1999. pp. 3–21. [Google Scholar]
- 141.Leloudis D, Pole L. Reasons for choosing hospice care. Am J Hosp Care. 1985;2:30–34. doi: 10.1177/104990918500200607. [DOI] [PubMed] [Google Scholar]
- 142.Labus JG, Dambrot FH. A comparative study of terminally ill hospice and hospital patients. Omega. 1985;16:225–232. [Google Scholar]
- 143.Luchins DJ, Hanrahan P. What is the appropriate level of health care for end stage dementia patients? J Am Geriatr Soc. 1993;41:25–30. doi: 10.1111/j.1532-5415.1993.tb05943.x. [DOI] [PubMed] [Google Scholar]
- 144.Miller SC, Gozalo P, Mor V. Hospice enrollment and hospitalization of dying nursing home patients. Am J Mad. 2001;111:38–44. doi: 10.1016/s0002-9343(01)00747-1. [DOI] [PubMed] [Google Scholar]
- 145.Volicer L, Collard A, Hurley A, Bishop C, Kern D, Karon S. Impact of special care unit for patients with advanced Alzheimer’s disease on patient’s discomfort and cost. J Am Geriater Soc. 1994;42:597–603. doi: 10.1111/j.1532-5415.1994.tb06856.x. [DOI] [PubMed] [Google Scholar]
- 146.Osterweis M, Solomon F, Green M. Bereavement: Reactions, Consequences and Care. Washington, DC: National Academy Press; 1984. [PubMed] [Google Scholar]
- 147.Hays JC, Kasl S, Jacobs S. Past personal history of dysphoria, social support, and psychological distress following conjugal bereavement. J Am Geriater Soc. 1994;42:712–718. doi: 10.1111/j.1532-5415.1994.tb06529.x. [DOI] [PubMed] [Google Scholar]
- 148.Prigerson HG, Frank E, Kasl SV, et al. Complicated grief and bereavement-related depression as distinct disorders: preliminary empirical validation in elderly bereaved spouses. Am J Psychiatry. 1995;152:22–30. doi: 10.1176/ajp.152.1.22. [DOI] [PubMed] [Google Scholar]
- 149.Meuser TM, Marwit SJ. A comprehensive, stage-sensitive model of grief in dementia caregiving. Gerontologist. 2001;41:658–670. doi: 10.1093/geront/41.5.658. [DOI] [PubMed] [Google Scholar]
- 150.Bass DM, Bowman K, Noelker LS. The influence of caregiving and bereavement support on adjusting to an older relative’s death. Gerontologist. 1991;31:32–42. doi: 10.1093/geront/31.1.32. [DOI] [PubMed] [Google Scholar]
- 151.Mullan J. The bereaved caregiver: a prospective study of changes in well-being. Gerontologisl. 1992;32:673–683. doi: 10.1093/geront/32.5.673. [DOI] [PubMed] [Google Scholar]
- 152.Ponder RJ, Pomeroy EC. The grief of caregivers: how pervasive is it? J Gerontol Soc Work. 1996;27:3–21. [Google Scholar]
- 153.Bodnar JC, Kiecolt-Glaser JK. Caregiver depression after bereavement: chronic stress isn’t over when it’s over. Psychol Aging. 1994;9:372–380. doi: 10.1037//0882-7974.9.3.372. [DOI] [PubMed] [Google Scholar]
- 154.Robinson-Whelen S, Tada Y, MacCallum RC, McGuire L, Kiecolt-Glaser JK. Long-term caregiving: what happens when it ends? J Abnorm Psychol. 2001;110:573–584. doi: 10.1037//0021-843x.110.4.573. [DOI] [PubMed] [Google Scholar]
- 155.Schulz R, Newsom JT, Fleissner K, DeCamp AR, Nieboer AP. The effects of bereavement after family caregiving. Aging Ment Health. 1997;1:269–282. [Google Scholar]
- 156.Skaff MM, Pearlin LI, Mullin JT. Transitions in the caregiving career: effects of sense of mastery. Psychol Aging. 1996;11:247–257. doi: 10.1037//0882-7974.11.2.247. [DOI] [PubMed] [Google Scholar]
- 157.Neimeyer RA, Prigerson HG, Davies B. Mourning and meaning. Am Behav Sci. 2002;46:235–251. [Google Scholar]
- 158.Owen JE, Goode KT, Haley WE. End of life care and reactions to death in African-American and white family caregivers of relatives with Alzheimer’s disease. Omega. 2001;43:349–361. doi: 10.2190/YH2B-8VVE-LA5A-02R2. [DOI] [PubMed] [Google Scholar]
- 159.Theis SI, Biordi DL, Coeling H, Nalepka C, Miller B. Spirituality in caregiving and care receiving. Holist Nurs Pract. 2003;17:48–55. doi: 10.1097/00004650-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 160.Burgio LD, Fisher SE, Phillips LL, Allen RS. Establishing treatment integrity in clinical research. Alzheimer’s Care Q. 2003;4(3):204–215. [Google Scholar]
- 161.Burgio LD, Corcoran M, Lichstein KL, et al. Judging outcomes in psychosocial interventions for dementia caregivers: the problem of treatment implementation. Gerontologist. 2001;41:481–489. doi: 10.1093/geront/41.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Allen RS. Palliative and Existentially Augmented Care at the End of life (PEACE): Examining the Role of Legacy Activities on Caregiver and Care Recipient Distress; 2003. SAMHSA 1H79S M54569. [Google Scholar]
- 163.Allen-Burge R, Burgio LD, Bourgeois MS, Sims R, Nunnikhoven J. Increasing communication among nursing home residents. J Clin Geropsychol. 2001;7:213–230. [Google Scholar]
- 164.Zarit SH, Stephens-Parris MA, Femia EE. The validities of research findings: the case of interventions with caregivers. ACQ. 2003;4(3):216–228. [Google Scholar]
- 165.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs For Generalized Causal Inference. Boston: Houghton Mifflin; 2001. [Google Scholar]
- 166.Czaja SJ, Schulz R. Does the treatment make a real difference? The measurement of clinical significance. ACQ. 2003;4(3):229–248. [Google Scholar]


