Abstract
Background
Computed tomography (CT) lung cancer screening offers a unique clinical setting in which to promote smoking cessation. Focusing on outcomes related to the reporting of CT abnormality, we examined the natural history of smoking in the Pittsburgh Lung Screening Study (PLuSS).
Methods
PLuSS recruited 50 to 79 year-old current and former cigarette smokers living in the Pittsburgh area. We examined self-reported smoking outcomes one year after study entry in a subgroup that contained n=2094 active cigarette smokers without interval lung cancer diagnosis (50.7% women, median age 57 years, 40 year median duration of cigarette smoking, and 65.2% ≥ 20 cigarettes per day). Analyses compared efforts to quit in relation to physician referral for abnormal CT.
Results
Since study entry, 58.5% (95% confidence interval (CI) 56.3%, 60.6%) reported any quit attempt and 27.2% (95% CI 25.3%, 29.1%) any quit interval longer than 30 days. One year after study entry, 15.5% (95% CI 14.0%, 17.1%) reported not smoking for more than 30 days. Comparing persons referred because of CT abnormalities creating moderate or high lung cancer suspicion (n=156; 7.4%) to persons not referred for any reason (n=1145; 54.7%), propensity score-adjusted fractions with any quit attempt and with any quit interval longer than 30 days increased 18.8% (95% CI 11.1%, 26.5%) and 17.7% (95% CI 9.4%, 26.0%), respectively. The fraction quit more than 30 days at one year increased 12.2% (95% CI 4.9%, 19.5%).
Conclusions
Persons who experienced referral because of abnormal CT reported more smoking cessation.
Introduction
In 2007, according to the National Health Interview Survey, 47.9% of adult (≥18 year-old) ever smokers still smoked.(1) According to the 2003 Tobacco Use Supplement to the Current Population Survey, 64%, 36%, and 5.1% of 35-64 year-old recent (in past year) smokers seriously tried to quit, quit for at least one day, and quit for at least 6 months in the past year, respectively.(2)
Research groups internationally continue to evaluate low-dose computed tomography (CT) screening for detecting early lung cancer in at-risk current and former cigarette smokers.(3-9) CT screening may offer a unique clinical setting, that is, a teachable moment, particularly conducive to quit smoking intervention.(6, 10) In this context, investigators question the effects of CT screening on smoking behavior.(11) A negative CT screening result could dampen a smoker's motivation to quit, whereas a positive result could stimulate quitting. To explore this phenomenon, we compared the subsequent quit behaviors of smokers with and without abnormal results on an initial CT screening.
Materials and Methods
Study population
Between January 2002 and April 2005, the Pittsburgh Lung Screening Study (PLuSS), a research-based low-dose helical computed tomography (CT) lung cancer screening program, used mass media, physician referral, and mass mailings to recruit 50-79 year-old current and former cigarette smokers without a personal history of lung cancer.(12) PLuSS eligibility criteria included a history of cigarette smoking, at least one-half pack per day for at least 25 years and, if quit, quit for no more than 10 years. All subjects signed written informed consent.
PLuSS subjects completed a standardized, self-administered baseline questionnaire. In addition to screening CT, the baseline assessment included a pulmonary function test (PFT; forced expiratory spirometry conducted according to the American Thoracic Society standards and analyzed according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) categories)1. PLuSS mailed PFT and CT results to every subject and his/her personal physician. Subjects with clinically important CT findings were referred to their personal physicians. Using the most important CT finding, we classified subjects into four referral categories, including referral for moderate or high suspicion CT (greater than 5 percent predicted probability of lung cancer), referral for low suspicion CT (less than 5 percent predicted probability of lung cancer), referral for other reason (important CT finding not usually associated with lung cancer), and no referral. In addition to mailed reports, a nurse practitioner telephoned subjects placed in a physician referral category. During the PFT completed on the day of study enrollment, PLuSS encouraged active smokers to quit and recommended a hospital-based small group quit smoking program. During subsequent telephone contacts, the nurse practitioner updated smoking status and informally encouraged smoking cessation.
Subjects eligible for the current analysis included the 2157 baseline current smokers who survived the first year after CT screening without receiving a lung cancer diagnosis. A brief one-year follow-up telephone interview of 2142 (99.3%) subjects supplied information about cigarette smoking behavior since study entry. Responses to one questionnaire item (“Are you currently smoking cigarettes?”) were used to distinguish ex-smokers (who then reported the duration of the current quit attempt) from active smokers (who then reported the duration of the longest quit attempt, if any, since study entry). Lacking information about earlier quit attempts, we excluded 48 persons reporting on the day of follow-up interview that they had not smoked for 30 or fewer days. Analyses characterized the remaining 2094 subjects according to three smoking outcomes, including any quit attempt, regardless of duration, since study entry, any 30 day or longer quit interval since study entry, and quit on one-year follow-up date for more than 30 days. The CT screening occurred a median 29 days (interquartile range (IQR) 17-39 days) after the assessment of baseline smoking status and the follow-up telephone interview occurred a median 353 days (IQR 340-366 days) after the CT screening.
To evaluate self-reports of smoking, we measured exhaled air carbon monoxide (CO) in a convenience sample of PLuSS subjects returning for follow-up CT screening between August 2005 and January 2006. CO measurements were consistent with self-reported abstinence (≤8 ppm) in 95 (88.0%) of 108 self-reported ex-smokers, suggesting reasonably reliable self-report of smoking status.
Statistical analysis
Primary analyses compared the frequency of a study outcome among subjects with physician referral for abnormal CT (moderate or high suspicion, low suspicion, and other reason) relative to subjects without referral. We used propensity scores (predicted odds of referral) to control for confounding.(13) Using subjects without physician referral as a common control group, we fit three logistic regression models (one for each referral category). Independent variables included 1) sex, 2) age, 3) race, 4) education, 5) marital status, 6) age started smoking, 7) years smoking, 8) cigarettes/day, 9) cancer family history, 10) personal cancer history, 11) number of symptoms (phlegm, wheezing, shortness of breath, ankle swelling), 12) number of physician diagnoses (chronic bronchitis, emphysema, asthma), 13) time since most recent chest x-ray before study entry, 14) time since most recent chest CT before study entry, 15) severity of airflow obstruction on study PFT, and 16) coronary calcification reported on screening CT. We used subject-level risk factor values and parameters estimated from logistic regression to calculate for each subject the expected odds (propensity score) of physician referral. We verified that control for propensity score quartile eliminated risk factor differences between subjects with and without physician referral. Using subjects not referred as a common reference, we then fit separate general linear models (14) (binomial distribution with identity link executed in PROC GENMOD, SAS System for Windows Release 9.2, Cary, NC) to estimate the effect, adjusted for propensity score quartile, of a physician referral category (e.g., moderate or high suspicion) on a smoking outcome (e.g., any quit interval longer than 30 days since study entry). We fit separate models to subgroups defined according to risk factor level (e.g., age 50-59, 60-69, and 70+ years of age) to estimate stratum-specific effects and then added a term to represent the interaction between risk factor and referral category to evaluate the statistical significance (Wald test) of different effects (effect modification) according to risk factor level.
Results
The study group included 50.7% women, median age 57 years (IQR 53-63 years), 8.5% 70 years of age and older, 8.3% minority race or ethnicity, and 34.2% college educated (Table 1). Subjects smoked cigarettes a median 40 years (IQR 36-45 years) and 65.2% smoked 20 or more cigarettes per day (Table 1). Eighty percent had baseline symptoms (phlegm, wheezing, shortness of breath, or ankle swelling) and 23.7% a physician diagnosis of lung disease (chronic bronchitis, emphysema, or asthma; Table 1). Finally, CT screening prompted physician referral for 45.3%, including 2.3% referred for reasons not related to a lung cancer suspicion, 35.5% referred for low lung cancer suspicion, and 7.4% referred for moderate to high lung cancer suspicion (Table 2). Time intervals between study entry and one-year follow were independent of physician referral category (p=0.30, Wilcoxon rank sum test).
Table 1.
Subjects in a lung cancer computed tomography screening program, number (N) and percentage (%) according to baseline characteristic and smoking outcome.
| Quit >30 days |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | Column % | No quit attempt n=870 Row % | Quit ≤30 days n=655 Row % | Relapsed at one year n=244 Row % | Quit at one year n=325 Row % | p-value [1] |
| Overall | 2094 | 100.0 | 41.5 | 31.3 | 11.7 | 15.5 | |
| Demographic factors | |||||||
| Sex | 0.0075 | ||||||
| Men | 1032 | 49.3 | 45.0 | 29.2 | 10.3 | 15.6 | |
| Women | 1062 | 50.7 | 38.2 | 33.3 | 13.0 | 15.4 | |
| Baseline age (years) | 0.4386 | ||||||
| 50-59 | 1292 | 61.7 | 43.0 | 30.1 | 11.8 | 15.0 | |
| 60-69 | 625 | 29.8 | 39.8 | 33.6 | 11.0 | 15.5 | |
| 70+ | 177 | 8.5 | 36.7 | 31.6 | 12.4 | 19.2 | |
| Race | 0.0028 | ||||||
| White, not Hispanic | 1918 | 91.7 | 42.4 | 30.3 | 11.4 | 15.8 | |
| Other | 174 | 8.3 | 31.6 | 42.0 | 14.4 | 12.1 | |
| Education | 0.1743 | ||||||
| High school or less | 518 | 24.7 | 40.3 | 30.9 | 13.9 | 14.9 | |
| Post high school | 859 | 41.0 | 40.0 | 33.9 | 10.7 | 15.4 | |
| College graduate | 717 | 34.2 | 44.2 | 28.5 | 11.2 | 16.2 | |
| Baseline smoking behavior | |||||||
| Cigarettes/day | 0.0411 | ||||||
| 1-19 | 729 | 34.8 | 39.1 | 31.1 | 11.4 | 18.4 | |
| 20-29 | 921 | 44.0 | 40.9 | 32.6 | 11.7 | 14.8 | |
| 30-39 | 306 | 14.6 | 49.0 | 28.8 | 9.8 | 12.4 | |
| 40+ | 138 | 6.6 | 42.0 | 29.0 | 16.7 | 12.3 | |
| Medical factors | |||||||
| Number of symptoms [2] | 0.0002 | ||||||
| None | 418 | 20.0 | 45.5 | 25.6 | 9.3 | 19.6 | |
| One | 604 | 28.8 | 41.4 | 30.1 | 10.8 | 17.7 | |
| Two | 495 | 23.6 | 42.2 | 33.3 | 10.7 | 13.7 | |
| More than two | 577 | 27.6 | 38.3 | 34.8 | 15.1 | 11.8 | |
| Number of diagnoses [3] | 0.0097 | ||||||
| None | 1599 | 76.4 | 42.8 | 30.2 | 10.8 | 16.3 | |
| One | 370 | 17.7 | 39.7 | 32.2 | 14.6 | 13.5 | |
| More than one | 125 | 6.0 | 31.2 | 42.4 | 14.4 | 12.0 | |
Chi-square test of independence between baseline characteristic and smoking outcome
Baseline symptoms, including phlegm, wheezing, shortness of breath, and ankle swelling
Baseline physician diagnoses, including chronic bronchitis, emphysema, and asthma
4. Referral outcome from screening computed tomography
Table 2.
Smoking outcomes, according to physician referral category.
| Smoking outcome | No referral n=1145 | Other referral n=49 | Low suspicion n=744 | Moderate or high suspicion n=156 |
|---|---|---|---|---|
| Quit attempt, % | 54.1 | 69.4 | 61.2 | 73.7 |
| Quit >30 days, % | 23.8 | 30.6 | 29.0 | 41.7 |
| Quit >30 days at one year, % | 13.8 | 26.5 | 15.3 | 25.6 |
Overall, 58.5% (95% confidence interval (CI) 56.3%, 60.6%) reported any quit attempt since study entry, 27.2% (95% CI 25.3%, 29.1%) any quit interval longer than 30 days, and 15.5% (95% CI 14.0%, 17.1%) quit on one-year follow-up date for more than 30 days. Baseline factors related to cigarette smoking outcomes (p < 0.05) included sex, race, cigarettes smoked per day, number of symptoms, number of physician diagnoses, and CT referral category (Table 1). Quit attempt, long quit interval, and long quit interval without relapse at one year were more frequent among persons referred for CT abnormalities, relative to persons not referred (Table 2). Propensity score-adjusted, the fraction of subjects attempting to quit increased, in absolute terms, 15.9% (95% CI 2.2%, 29.5%), 7.2% (95% CI 2.6%, 11.8%), and 18.8% (95% CI 11.1%, 26.5%) among persons with other referral, low suspicion referral, and moderate or high suspicion referral, respectively (Table 3). The fraction of subjects able to quit for more than 30 days increased 6.0% (95% CI –7.4%, 19.4%), 5.6% (95% CI 1.5%, 9.7%), and 17.7% (95% CI 9.4%, 26.0%). Finally, the fraction of subjects quit for more than 30 days without relapse at one year increased 13.7% (95% CI 1.1%, 26.4%), 1.6% (95% CI –1.7%, 4.9%), and 12.2% (95% CI 4.9%, 19.5%).
Table 3.
Differences in smoking outcome (expressed as percent of subjects who experience the outcome) between groups with and without physician referral after lung cancer computed tomography screening, unadjusted and adjusted for the predicted odds of referral (propensity score quartile).
| Other referral vs. no referral |
Low suspicion vs. no referral |
Moderate or high suspicion vs. no referral |
|||||
|---|---|---|---|---|---|---|---|
| Smoking outcome | Δ | 95% CI | Δ | 95% CI | Δ | 95% CI | |
| Quit attempt | Unadjusted | 15.2 | 2.0, 28.5 | 7.0 | 2.5, 11.6 | 19.6 | 12.1, 27.1 |
| Adjusted | 15.9 | 2.2, 29.5 | 7.2 | 2.6, 11.8 | 18.8 | 11.1, 26.5 | |
| Quit >30 days | Unadjusted | 6.8 | –6.4, 19.9 | 5.2 | 1.1, 9.3 | 17.8 | 9.7, 25.9 |
| Adjusted | 6.0 | –7.4, 19.4 | 5.6 | 1.5, 9.7 | 17.7 | 9.4, 26.0 | |
| Quit >30 days at one year | Unadjusted | 12.7 | 0.2, 25.3 | 1.5 | –1.8, 4.8 | 11.8 | 4.7, 19.0 |
| Adjusted | 13.7 | 1.1, 26.4 | 1.6 | –1.7, 4.9 | 12.2 | 4.9, 19.5 | |
Legend: Δ – Smoking outcome in group with physician referral minus smoking outcome in group without referral
As shown in Table 4, higher suspicion CT referral categories contained higher proportions of subjects with newly discovered chronic lung disease, defined by PFT airflow obstruction in persons without history of emphysema, chronic bronchitis, or asthma. Higher suspicion CT referral categories also contained higher proportions of subjects with confirmed chronic lung disease, defined by PFT airflow obstruction in persons with history of lung disease (Table 4). Communicating PFT results plausibly stimulated smoking cessation. This consideration motivated closer evaluation of smoking outcomes associated with PFT abnormality, particularly in subjects reporting no history of chronic lung disease at baseline. In analyses restricted to subjects with no baseline history of lung disease and no CT referral, the quit attempt outcome occurred more frequently among subjects referred because of moderate-severe airflow obstruction than among subjects without airflow obstruction (Δ = 8.8%, 95% CI 0.6%, 17.0%, p-value 0.04, Table 5). Adjustments for sex, age, race, cigarettes/day, and number of symptoms partially attenuated this difference (Δ = 7.4%, 95% CI −1.2%, 16.0%, p-value 0.10). Neither the quit > 30 days nor the quit > 30 days at one year outcomes differed statistically according to PFT result (Table 5).
Table 4.
Subjects in CT referral categories [1] distributed (%, [2]) according to baseline history of lung disease (emphysema, chronic bronchitis, or asthma) and entry PFT result.
| History of lung disease | PFT result – airflow obstruction [3] | No referral n=1145 | Low suspicion n=744 | Moderate or high suspicion n=156 |
|---|---|---|---|---|
| No | None | 50.7 | 46.0 | 34.6 |
| Mild | 10.2 | 12.8 | 13.5 | |
| Moderate-severe | 15.9 | 18.0 | 25.6 | |
| Yes | None | 10.0 | 7.5 | 7.1 |
| Mild | 2.6 | 2.2 | 3.8 | |
| Moderate-severe | 10.5 | 13.6 | 15.4 |
The table excludes n=49 subjects in the other CT referral category.
Table p-value=0.0009
Mild and moderate-severe airflow obstruction defined by GOLD I and GOLD II-IV, respectively
Table 5.
Smoking outcomes according to entry PFT result, among subjects with no baseline history of lung disease (emphysema, chronic bronchitis, or emphysema) and no CT referral.
| |
|
Quit attempt |
Quit >30 days |
Quit >30 days at one year |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFT result – airflow obstruction [1] | N | N | % | Δ | 95% CI | N | % | Δ | 95% CI | N | % | Δ | 95% CI |
| None | 581 | 297 | 51.1 | Ref | 136 | 23.4 | Ref | 83 | 14.3 | Ref | |||
| Mild | 117 | 60 | 51.3 | 0.2 | −9.8, 10.1 | 26 | 22.2 | −1.2 | −9.5, 7.1 | 20 | 17.1 | 2.8 | −4.6, 10.2 |
| Moderate-severe | 182 | 109 | 59.9 | 8.8 | 0.6, 17.0 | 38 | 20.9 | −2.5 | −9.4, 4.3 | 24 | 13.2 | −1.1 | −6.8, 4.6 |
Legend: Δ – difference from percentage for reference category, CI – confidence interval, Ref – reference category
Mild and moderate-severe airflow obstruction defined by GOLD I and GOLD II-IV, respectively
Comparing persons referred for moderate or high suspicion to persons without referral, the frequency of a quit interval longer than 30 days increased in 50-59 year-old (propensity score adjusted difference 18.0%, 95% CI 7.5%, 28.5%) and in 60-69 year-old persons (propensity score adjusted difference 30.8%, 95% CI 13.8%, 47.7%), but not in ≥70 year-old persons (propensity score adjusted difference –2.7%, 95% CI –23.3%, 18.0%; pinteraction = 0.03; Figure). No other risk factor statistically modified the effect of a moderate or high suspicion referral on any smoking outcome.
Figure.
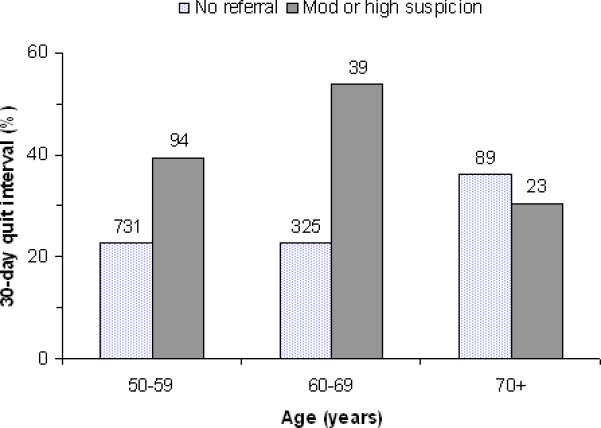
Frequency of a quit smoking interval longer the 30 days since study entry according to physician referral category and age at study entry. The number above each bar indicates the number of subjects.
Discussion
Over the one-year period after CT screening, 58.5% of PLuSS baseline smokers tried to quit and 27.2% quit for more than 30 days. At the time of the one year follow-up, 15.5% had not smoked for more than 30 days. Compared to smokers not referred, these favorable smoking-related outcomes occurred more frequently among smokers referred because of abnormalities found on screening CT (Table 2). The smoking cessation effects attributed to referral were more dramatic when the CT report indicated a moderate or high as opposed to low lung cancer suspicion (Table 3). Though consistent with the notion that knowledge of a smoking-related health problem may spur personal smoking cessation efforts, the associations between CT referral and smoking outcomes may also have occurred as a consequence of more intensive interactions with the health care system. In addition to CT, abnormal PFT could induce medical follow-up. CT referral occurred more often in subjects with abnormal PFT (Table 4). Meaningful association between CT referral and smoking outcome persisted after propensity score adjustments that included factors for severity of airflow obstruction. By comparison, only limited statistical evidence emerged for association between PFT abnormality and smoking outcome (for example, Table 5). These disparate associations involving CT and PFT suggest smoking effects specific to the CT referral process.
Several studies have described changes in smoking behaviors after CT screening and the effects of positive as opposed to negative CT screen results. Contacted a median 6 months after screening, 66 (49%) of 134 baseline smokers from the Early Lung Cancer Action Program (ELCAP) stopped or decreased smoking. (9) Twenty one (62%) of 34 and 45 (45%) of 100 with positive and negative CT results, respectively, stopped or decreased smoking (p<0.10). In a Mayo Clinic study, 129 (14%) of 901 baseline smokers self reported not smoking one year after an initial CT screening. (6) Rates of smoking cessation did not vary according to the CT follow-up recommendation. However, a Mayo Clinic reanalysis examining outcomes from a sequence of three annual CT screenings showed a significant 1.37-fold (95% CI 1.12, 1.67; p=0.002) increase in the multivariable-adjusted odds of smoking cessation at a next follow-up visit among subjects who received a recommendation for additional follow-up because of an abnormal screening result.(15) In the Danish Lung Cancer Screening Trial, 174 (11.9%) of 1462 baseline smokers were not smoking (for at least four weeks, exhaled carbon monoxide-verified) one year after CT screening.(16) Quit rates differed between subjects with and without significant CT findings (17.7% vs. 11.4%; p=0.04).
In the view of most authors, (3, 6, 9, 15, 16) smoking cessation rates after CT screening are favorable when judged against the natural history of smoking in the general population (2) or the response of smokers to physicians’ advice to quit.(17) Any favorable outcome could reflect either preferential selection of smokers predisposed to quit or specific behavioral effects from CT screening (or associated quit smoking intervention). The current study adjusted findings for many covariates commonly related to quitting activity, suggesting that baseline factors were not responsible for differences in quitting. Also, suggesting a direct effect from CT screening, Taylor et al. compared responses to questionnaires administered before and one month after a second (Lung Screening Study, LSS) or first (National Lung Screening Trial, NLST) CT screening and detected a greater readiness to quit in 12 (18.2%) of 66 LSS smokers and 23 (31.5%) of 73 NLST smokers.(18) Dispelling the notion that participation in an organized CT screening program, as such, promotes smoking cessation, the Danish Lung Cancer Screening Trial observed identical one-year quit rates among smokers randomly exposed vs. not exposed to CT screening (11.9% of 1462 vs. 11.8% of 1395).(16) Whatever the explanation, evidence of favorable smoking outcomes may distinguish CT screening as a clinical opportunity, a teachable moment (10, 18) particularly conducive to quit smoking intervention.
Confirming other studies, (9, 15, 16) we observed more favorable smoking outcomes in persons informed about significant CT screen abnormality. Explanations include 1) diminished motivation to quit in screen-negative smokers, 2) intensified motivation to quit in screen-positive smokers, 3) quit smoking co-intervention targeting screen-positive smokers, and 4) greater tendency for screen-positive smokers to misrepresent quit smoking outcomes. In PLuSS, active smokers were advised to quit and referred to quit smoking programs. In order to facilitate and document diagnostic follow-up, the nurse practitioner contacted subjects with abnormal CT results specifically, thereby providing unique opportunity for informal quit smoking co-intervention. Referred subjects received more intensive diagnostic follow-up, (12) thereby providing more opportunity for co-intervention by non-study health care providers. Whatever the explanation, strong associations observed between CT results and quit smoking outcomes reinforce the need for quit smoking interventions that respond to the unique behavioral effects of participation in CT screening. However, our observation that CT referral was associated with quitting among younger (<70 years), but not older persons (Figure) suggests that older smokers resist a referral-induced motivation to quit. Our finding is consistent with Taylor et al.,(18) who reported that receiving an abnormal CT report appeared to boost readiness to quit among 55-64 year-old, but not necessarily older, smokers in the LSS study. Thus, use of CT referral as a teachable moment may be limited to smokers who perceive significant long-term health gains from quitting. Accumulated experiences with medical screening or diagnostic tests may have immunized older smokers emotionally against disturbing CT results. Other interventions may be necessary to promote quitting in older smokers.
Study strengths included large sample size (n=2094), nearly complete (99.3%) follow-up for smoking outcomes at one year, ability to examine smoking outcomes according to level (low vs. moderate or high lung cancer suspicion) of CT abnormality, and use of propensity score methods to control for 16 demographic, smoking-related, and health-related factors that could possibly confound associations between CT abnormality and smoking behavior. Single addition of any one of these 16 risk factors to propensity quartile-adjusted general linear models did not materially change estimated associations between physician referral category and smoking outcome (data not shown). Study weaknesses included lack of information about smoking cessation treatments used by subjects in the year after lung cancer screening and reliance on self-report measures of smoking behavior, although the concordance of self-reported quitting with biochemical validation of abstinence was reasonably high.
In conclusion, referral because of an abnormal CT affected smoking cessation. Quit smoking interventions coupled to CT screening should accommodate, anticipate, or leverage effects of CT results on smoking behavior.
Acknowledgments
Funded by University of Pittsburgh Cancer Institute's Specialized Program of Research Excellence in Lung Cancer; Grant Number: NCI P50 CA90440
Footnotes
Global Initiative for Chronic Obstructive Pulmonary Disease: Pocket guide to COPD diagnosis, management, and prevention. Medical Communications Resources, Inc., 2008. (Accessed August 29, 2009, at http://www.goldcopd.com/download.asp?intId=505.)
References
- 1.Centers for Disease Control and Prevention Cigarette smoking among adults--United States, 2007. MMWR Morb Mortal. 2008;57:1221–6. [PubMed] [Google Scholar]
- 2.Messer K, Trinidad DR, Al-Delaimy WK, Pierce JP. Smoking cessation rates in the United States: a comparison of young adult and older smokers. Am J Public Health. 2008;98:317–22. doi: 10.2105/AJPH.2007.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacRedmond R, McVey G, Lee M, et al. Screening for lung cancer using low dose CT scanning: results of 2 year follow up. Thorax. 2006;61:54–6. doi: 10.1136/thx.2004.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manser R. Screening for lung cancer: a review. Curr Opin Pulm Med. 2004;10:266–71. doi: 10.1097/01.mcp.0000128432.79891.85. [DOI] [PubMed] [Google Scholar]
- 5.Diederich S, Wormanns D. Impact of low-dose CT on lung cancer screening. Lung Cancer. 2004;45(Suppl 2):S13–9. doi: 10.1016/j.lungcan.2004.07.997. [DOI] [PubMed] [Google Scholar]
- 6.Cox LS, Clark MM, Jett JR, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer. 2003;98:2495–501. doi: 10.1002/cncr.11813. [DOI] [PubMed] [Google Scholar]
- 7.Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest. 2002;122:15–20. doi: 10.1378/chest.122.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Garg K, Keith RL, Byers T, et al. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology. 2002;225:506–10. doi: 10.1148/radiol.2252011851. [DOI] [PubMed] [Google Scholar]
- 9.Ostroff JS, Buckshee N, Mancuso CA, Yankelevitz DF, Henschke CI. Smoking cessation following CT screening for early detection of lung cancer. Prev Med. 2001;33:613–21. doi: 10.1006/pmed.2001.0935. [DOI] [PubMed] [Google Scholar]
- 10.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18:156–70. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 11.Clark MM, Jett JR. Change in smoking status after low-dose spiral chest CT screening for lung cancer: opportunity for smoking intervention. Thorax. 2009;64:371–2. doi: 10.1136/thx.2008.111039. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care. 2008;178:956–61. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol. 2005;58:550–9. doi: 10.1016/j.jclinepi.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160:301–5. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 15.Townsend CO, Clark MM, Jett JR, et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer. 2005;103:2154–62. doi: 10.1002/cncr.21045. [DOI] [PubMed] [Google Scholar]
- 16.Ashraf H, Tonnesen P, Holst Pedersen J, Dirksen A, Thorsen H, Dossing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax. 2009;64:388–92. doi: 10.1136/thx.2008.102475. [DOI] [PubMed] [Google Scholar]
- 17.Fiore MC, Bailey WC, Cohen SJ, et al. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: Jun, 2000. Treating Tobacco Use and Dependence. [Google Scholar]
- 18.Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56:125–34. doi: 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]


