Abstract
Introduction
Recent Food and Drug Administration legislation enables the mandating of product performance standards for cigarette smoke and the evaluation of manufacturers’ health claims for modified tobacco products. Laboratory studies used for these evaluations, and also to understand tobacco smoke toxicology, use machines to generate smoke. The goal of this review is to critically evaluate methods to assess human smoking behavior and replicate this in the laboratory.
Methods
Smoking behavior and smoking machine studies were identified using PubMed and publically available databases for internal tobacco company documents.
Results
The smoking machine was developed to generate smoke to allow for comparing cigarette tar and nicotine yields. The intent was to infer relative human disease risk, but this concept was flawed because humans tailor their smoking to the product and chemical yields and toxicological effects change with different smoking profiles. While smoking machines also allow for mechanistic assessments of smoking-related diseases, the interpretations also are limited. However, available methods to assess how humans puff could be used to provide better laboratory assessments, but these need to be validated. Separately, the contribution of smoke mouth-holding and inhalation to dose need to be assessed, because these parts of smoking are not captured by the smoking machine. Better comparisons of cigarettes might be done by tailoring human puff profiles to the product based on human studies and comparing results across regimens.
Conclusions
There are major research gaps that limit the use of smoking machine studies for informing tobacco control regulation and mechanistic studies.
INTRODUCTION
In June 2009, the Food and Drug Administration (FDA) received regulatory authority over tobacco products. The FDA is now empowered to develop product performance standards and evaluate manufacturers’ health claims for modified tobacco products. Tobacco manufacturers have publicly focused efforts on lowering cigarette smoke emissions, and may be able to make health claims following the FDA review of their scientific data. The World Health Organization Study Group on Tobacco Product Regulation (TobReg) and others also have recognized potential benefits and pitfalls for tobacco harm reduction strategies (1-10). The Institute of Medicine (IOM) furthered this harm reduction concept by concluding that harm reduction through smoke exposure reduction was feasible (11,12). The IOM coined an overarching term, PREPs, for potential reduced exposure products. (A comprehensive list of existing PREPs can be found at Tobaccoproducts.org1.) As FDA performance standards to reduce exposure are developed and implemented, and the manufacturers develop new product designs proposed to reduce human tobacco toxicant exposure, reliable, validated methods are needed to assess changes in cigarette smoke chemical yields and toxicological effects. Critical to the laboratory evaluation of these products is the generation of cigarette smoke by smoking machines, for example, as have been used to estimate tar and nicotine yields. However, prior uses of the smoking machine have been invalidated in the context of human risk analysis for comparing different types of cigarettes because smoking machine protocols do not replicate human exposure. Thus, current methods preclude an estimation of human exposure and toxicological effects, challenging new regulatory processes.
The best example of the flawed use of the smoking machine relates to the earlier assumptions that reduced tar and nicotine yield cigarettes, the so-called “lights”, were less harmful than higher yield cigarettes (13). Two decades ago, the public health community advocated that for smokers who could not or would not quit to switch to lower tar yield cigarettes, for example as recommended by the Surgeon General (14-16). Advertising and marketing by the tobacco industry reinforced the perceptions that lower tar was less harmful. We now know that smoking machine yields were misunderstood in relation to human exposure and tobacco companies intentionally misrepresented the impact of lowering tar yields on smokers’ health (13,17,18). Development of smoking behavior measurements revealed that compensatory mechanisms for adjusting to the reduced nicotine yields of reduced yield cigarettes led smokers to increase their nicotine exposure by increasing cigarette puffing intensity and smoking more cigarettes per day (19-23). Moreover, human biomarker studies have demonstrated that smokers’ exposures were not different when smoking cigarettes with different tar yields (24-26). Separately, while early epidemiology data supported the hypothesis for reduced risk in relation to tar yields, a recent re-analysis of the data established that the early interpretation were wrong (13,27). As the realization for the limitations of smoking machine studies became clear, and how the uses for public health recommendations were based on flawed interpretations, the Federal Trade Commission (FTC) in November 2008 officially rescinded its widely used guidance for reporting smoking machine determined tar and nicotine yields2. Thus, today, there are no recommended smoking machine protocols in the United States that the FDA can use to inform their decision making processes regarding performance standards and health claims, although the World Health Organization has made recommendations (see below) (10,28).
In order to develop and validate new smoking machine methods, a better understanding of how to assess human smoking behavior is needed. Currently, smoking behavior is assessed by smoking topography devices that record puff profiles (e.g., puff volume, interpuff interval, puff duration and air flow) and methods to assess inhalation. However, there are limitations to these methods for estimating human exposure, and very few combined these research tools in the same study, so how to use this data for smoking machine puff profiles is unclear. Conceptually, these methods could be validated by human biomarker studies, and some studies have been done. The goal of this review is to critically evaluate methods to assess human behavior and how best to replicate this on smoking machines. While there will always be limitations to such studies, certain limitations can be mitigated, and the context for other limitations can be better understood. This review will summarize the state of the art in smoking machine protocols and human smoking behavior measurement. These data will be synthesized to identify research gaps related to laboratory research on cigarette smoke and regulation of tobacco products. This review is organized into three major sections, followed by a discussion. The first section provides a review of the technical aspects of machine smoking and the early development of the standardized smoking machine. This will set the stage to contrast this early work with what we know about human smoking behavior and how well we measure that. The third section reviews methods where researchers have tried to apply what we know about human smoking behavior for smoking machine studies. Last, the discussion provides an overall summary of the most important points and identifies the research gaps that lead from earlier work.
METHODS
Smoking behavior and smoking machine studies were identified using PubMed search strategies. The search keyword strings included “human smoking behavior, smoking topography, human puff profiles, smoking machines, smoke exposure, and PREPs”, and combinations of these. All identified studies were reviewed that have been published since 1980, and citation lists were cross-referenced to ensure that the most complete list of publications was identified. Articles published prior to 1980 with high relevance to the study of PREPs or low yield cigarettes also were identified and reviewed. Separately, internal tobacco company documents were reviewed, as identified by searches using TobaccoDocuments.org3 and the Legacy Tobacco Documents Library4. Studies were identified that investigated methodological, descriptive, validation and application aspects related to the assessment of human smoking behavior, human puff and respiration patterns, biomarkers of acute smoke exposure, and smoking machine regimens and yields as they relate to exposure. Research publications were compiled to examine: 1) goals of the study; 2) methods for assessing human smoking behavior or machine smoking protocols; 3) experimental designs that were used, and; 4) the effects of smoking behavior in relation to the effects of smoking machine protocols on smoke yields. The information was synthesized to provide usefulness for the study of cigarettes and identify research gaps. While others have reviewed the origins and limitations of smoking machine yield testing (29-31), the focus of this manuscript is to identify how to better replicate human smoking in the laboratory through understanding both the design of smoking machine and human behavior studies, and identify the research gaps associated with this.
RESULTS
Technical aspects of machine smoking
Smoking machines are intended to generate smoke in a systematic fashion for laboratory testing, and they have been used to compare cigarette smoke toxicant yields by puffing cigarettes according to specified settings. Cigarette smoke is a suspension of particles in a gaseous vapor, and so it can be collected and analyzed in various ways. A recent review comprehensively describes how smoke is collected for toxicology studies (32). Particles in smoke can be collected on a Cambridge filter pad, which is composed of glass fibers. The change in weight of the pad defines the total particulate matter (TPM) or wet total particulate matter (WTPM). Tar is mathematically derived value defined as TPM minus water and nicotine. The gas and vapor phase (GVP) passes through the Cambridge filter pad and can be collected or tested directly. Alternatively, smoke can be collected as a condensate [termed cigarette smoke condensate (CSC)], usually in a liquid trap or directly assayed as whole smoke (WS). TPM and CSC are typically used in studies assessing the toxicology of tobacco smoke in vitro and for animal skin painting studies, and for assessing the chemical constituents. WS is used to determine the smoke constituents and in inhalational animal studies, although it is sometimes used for in vitro toxicology studies. While smoking machines have several variables that can be adjusted, typically the programmable parameters are puff volume, puff frequency, puff duration, the length of cigarette smoked (butt length) and more recently puff shape.
The first smoking machines with high accuracy and reproducibility were developed by Pfyl and Bradford et al. in the 1930s (33,34). Today, commercially available analytical smoking machines having flexibility for controlling puffing parameters are manufactured by various companies (e.g., Borgwaldt GmbH [www.borgwaldt.de/cms] and Cerulean [www.cerulean.com]). The analytical cigarette smoking machines of today vary in the number of ports, how many cigarettes they hold, whether they are in-line or rotary, and by their ability to capture mainstream or sidestream smoke. Different smoking machine designs are suitable for different tasks. Rotary machines are ideally suited for smoking a large number of cigarettes quickly (usually the same type or brand) and the smoke is funneled into a single smoke trapping system. One major drawback of the rotary machine is that it cannot easily accommodate modification in the puff interval. Linear smoking machines, on the other hand, are ideally suited for smoking a number of replicates (same or different types) onto individual smoke trapping systems and have more flexibility for altering puff profiles.
Most smoking machines use electric lighters to ignite test cigarettes for machine smoking. However, Adam and coworkers found different yields from the first puff of a cigarette as it is lit, depending on the lighting device (35). Comparing an electric lighter, a propane/butane gas lighter, a match, a candle, and the burning zone of another cigarette, they found that the three open flame sources produced mainly unsaturated hydrocarbons, while the electric lighting device produced oxygen-containing compounds. Therefore, they suggest that the use of electric lighters in smoking machines be reconsidered, since human smokers generally use open flame lighters. Some smoking machines have sensors to determine if the cigarette is lit and they are programmed to stop smoking once the cigarette is smoke down to a specified distance from the end of the filter (e.g., by using a laser detector). Less sophisticated machines rely on a string to mark the stopping point – when the cigarette burns through the string, the puffing mechanism is deactivated.
The early development of standardized smoking regimens
The development of smoking machine regimens has been extensively reviewed elsewhere (36-38). In 1936, Bradford, et.al., who worked for the American Tobacco Company, described the need for standardized smoking parameters that would aid in the characterization and reproducibility of cigarette smoke experiments in the laboratory (34). However, machine-measured emissions were not widely publicized until the early 1950's (39,40), when studies became available linking smoking and lung cancer, and as cigarette manufacturers were racing to produce lower tar products (commonly referred to as a “tar derby”), making a multitude of inconsistent, non-comparable claims about tar yields to consumers (31). The tar derby ended in 1960 with a voluntary agreement by the FTC and the manufacturers to end tar and nicotine yield claims5. The FTC later reversed this agreement and decided to develop a standardized testing method. The initial protocol was largely based on the work of U.S. Department of Agriculture chemist C.L. Ogg in 1964 (31,41). It appears, however, that this protocol was based on one person's observations about how people smoked, was not determined with some systematic method and it actually was very similar to the 1936 method of Bradford and coworkers (34). However, the protocol was not intended to represent the typical smoker; but rather to offer a common basis for a comparison among brands.
The FTC puffing protocol prescribes drawing a 35ml puff of 2 second duration, every minute until the length of the cigarette is no less than 23mm for non-filtered cigarettes or filter overwrap plus 3mm for filtered cigarettes. Table 1 describes this protocol and others that have been developed over time. The standard, developed by Ogg et al., also consisted of conditioning of cigarettes at 23.9 °C and 60% relative humidity for 24 hours (42). At the outset, the FTC method was intended only to compare tar and nicotine yields across brands, although carbon monoxide (CO) was added to the protocol in 1980. The analysis of other smoke constituents have never been specified by the FTC, but the FTC protocol has been widely adopted in analyses of other constituents for product testing and research
Table 1.
Overview of smoking regimens (from (150))
| Regimen | FTC | Massachusetts | Canadian | ISO | ISO A | ISO B | ISO C |
|---|---|---|---|---|---|---|---|
| Puff volume | 35 mL ± 0.5 mL | 45 mL ± 0.5 mL | 55 mL ± 0.5 mL | 35 mL ± 0.3 mL | 55 | 60 | 45 |
| Puff duration | 2 s ± 0.05 s | 2 s ± 0.05 s | 2 s ± 0.05 s | 2 s ± 0.05 s | 2 | 2 | 2 |
| Puff frequency | 60 s ± 0.5 s | 30 s ± 0.5 s | 30 s ± 0.5 s | 60 s ± 0.5 s | 30 | 30 | 30 |
| Ventilation holes | Open | 50% blocked | 100 % blocked | Open | 50% blocked | 50% blocked | 100% blocked |
| Conditioning atmosphere | 60 % RH ± 2 % RH 23.9 °C ± 1.1 °C min 1, max 14 days | 60 % RH ± 2 % RH 23.9 °C ± 1.1 °C min 1, max 14 days | 60 % RH ± 3 % RH 22 °C ± 1 °C min 2, max 10 days | 60 % RH ± 3 % RH 22 °C ± 1 °C min 2, max 10 days | |||
| Smoking environment | 60 % RH ± 3 % RH 23.9 °C4 ± 2 °C | 60 % RH ± 3 % RH 23.9 °C ± 2 °C | 60 % RH ± 5 % RH 22 °C ± 2 °C | 60 % RH ± 5 % RH 22 °C ± 2 °C | |||
| Air flow Linear ind. Port Linear avg. & Rotary | Sufficiet to exhaust smoke – ca 120 mL/min1 | Sufficient to exhaust smoke – ca 120 mL/min | 200 ± 50 mL/min 200 ± 30 mL/min | 200 ± 50 mL/min 200 ± 30 mL/min | |||
| Butt length (whichever is the highest value) | Tipping + 3 mm or 23 mm from butt | Tipping + 3 mm or 23 mm from butt | Tipping + 3 mm or filter + 8 mm or 23 mm from butt | Tipping + 3 mm or filter + 8 mm or 23 mm from butt |
Following the work of the FTC, virtually identical standardized smoking regimens were developed by the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA), and later the International Organization for Standardization (ISO). CORESTA's initial standardized smoking method was published in 1969 (43). The ISO protocol uses the same puffing regimen as the FTC method, except that it specifies an air flow of 200mL/min. Additionally, CORESTA and ISO stipulate standards for physical components of the machine: the cigarette holders, smoke traps, ports, channels and ashtray specifications (43). It should be noted that the tobacco companies heavily influenced CORESTA to motivate ISO to set standards and generate research results in an attempt to preempt regulations (37). While ISO and CORESTA were seemingly independent, ISO essentially adopted CORESTA's recommended methods, as the ISO committees overseeing standards development for tobacco products have been composed mostly of persons affiliated with the tobacco industry (37,44).
Human Smoking Behavior
Physical Processes Involved in Smoking
In order to understand the limitations and misuse of the smoking machine measurements, it is important to understand how smokers smoke their cigarettes. The physical process of smoking a cigarette is continuous, but can be divided into three phases: puffing, mouth-holding, and inhalation. The smoking cycle is shown in a diagram reproduced from the British American Tobacco Company (BATCo) research in 1986 (Figure 1) 6. This Figure defines different parameters that can be measured during smoking. Puffing refers to the act of drawing smoke from the cigarette into the mouth. The act of puffing draws air through the burning rod that causes an increase in temperature that in turn consumes some amount of tobacco and the cigarette paper wrap. During puffing, the tongue contracts down creating a negative pressure to aid the puffing process and the soft palate contracts, essentially blocking airflow into the nasopharynx and lungs. Puffing is then followed by a period of mouth-holding before air moves into the lungs, as typically smoke not directly inhaled from the cigarette through the mouth into the lungs7. Following puffing, as reported via the BATCo documents, the smoke is either immediately inhaled via nose inhalation into the lungs, paused in the mouth prior to nose inhalation (perhaps to enhance the sensation and taste) or paused in the mouth with some exhalation of smoke prior to nose inhalation. According to BATCo, nose inhalation allows the soft palate to relax providing an easy path for the smoke to be drawn into the pharynx and nasopharynx 8. The mouth is closed so that the air pressure sucking the smoke into the lungs is the same as the pressure from air moving from the nose into the lungs. Following nose inhalation, exhalation occurs after some period of time. Puffing resumes after some interpuff interval, and in at least 80% of smokers this takes place during the exhalation phase of a breath, which can occur at any point during exhalation, e.g., at the onset, in the middle or at the end of exhalation(45). An example of the various parameters for puffing and inhalation is shown in Figure 2. Thus, it is the combination of puffing, mouth-holding, nose inhalation and inhalation time that determines a smoker's internal dose of smoke toxicants and nicotine. These studies only had a few subjects, measurement was confined to one setting and this study has not been replicated; a systematic study might show different or more accurate patterns of inhalation, e.g., mouth inhalation in addition to nose inhalation.
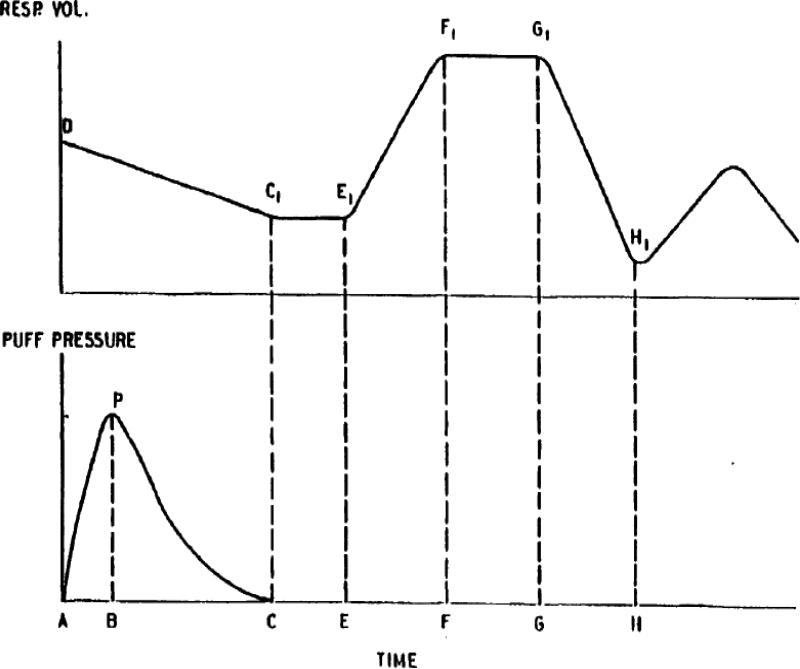
Figure 1. Schematic Representation of the Puff and Inhalation/Exhalation Pattern.
Reproduced from a British American Tobacco Company document30, simultaneous measurements were assessed for inhalation/exhalation and smoking topography. The following parameters are defined from this figure: puff volume (ml) - integration of puff pressure curve from A to C; lit draw resistance (cm H2O/ml) - the ration of integrated pressure to puff volume; puff duration - time from A to C; inhalation delay time (sec) - the time from completion of the puff to the start of inhalation from C to E; inspiratory time (sec) - the duration of time from E to F; breath hold time (sec) - the delay from the end of active inhalation to start of exhalation from F to G; expiratory time (sec) - the time for exhalation from G to H; inhalation volume (ml) - the volume difference from E1 to F1; exhalation volume (ml) - the volume difference from G1 to H1; volume change prior to inhalation (ml) - volume shift in the lungs (usually exhalation) that occurs during the puff and inhalation delay period from D to E1; volume change after puff (ml) - volume change after the puff but before the inhalation, from C1 to E1.
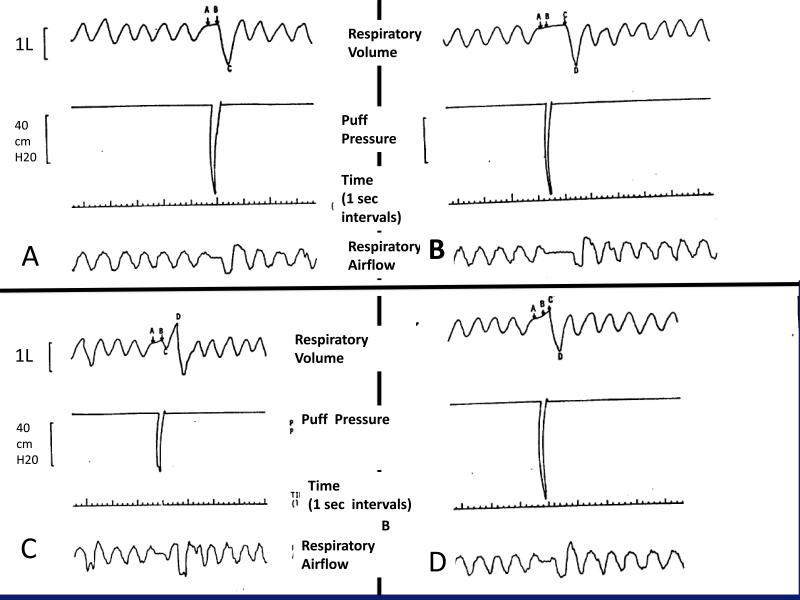
Figure 2. Puffing and inhalation patterns showing interindividual variation during the interval between puffing and inhalation31.
Puffing begins during exhalation from points A to B. For some smokers, the smoke is immediately inhaled from the mouth into the pharynx and lungs and completed at point C (A); for others, there is a mouth holding period where point C marks the beginning of the inhalation and completed at D (B); for others, there is an immediate inhalation until point C then an exhalation followed by an larger inhalation at point D (C); others have an immediate exhalation followed by an inhalation from points C to D (D).
Measuring Puff Topography
Puff profiles are measured by assessing smoking topography, namely puff volume, puff duration, interpuff interval, flow rate (sometimes also termed puff velocity), the number of puffs per cigarette, and total puff volume (46-55). These correspond to parameters that can be programmed on a smoking machine. These are typically measured by having the cigarette smoked through a small tube that can measure air flow via a transducer, and the analog signal is converted to a digital signal for recording and interpretation. Initially, various research groups employed their own puff profile recording devices, such as the ‘tobacco smoke inhalation testing system’ (TSITS) originally developed by Puustinen and coworkers in 1986, and then manufactured by the College of Engineering at the University of Kentucky (56-58). Other early techniques included flow meters (59,60), pneumotachographs (61), pressure transducers and Grass polygraphs (62), and puff analyzers (63). In parallel, tobacco industry scientists developed similar devices in the late ‘60s and early 70's, such as the cigarette-holder flow meter described by Adams and Creighton (64-66). Portable devices that can be used for at-home monitoring also have been developed (49,66-69).
Although custom-built apparatuses are still used (70), commercially available topography devices, such as the CReSS units from Plowshare Technologies, Inc.9 and the SODIM SPA/D and SPA/M smoking topography units10 have largely superseded them. To our knowledge, there are no published studies using the SODIM topography units and the great majority of studies assessing human smoking topography have used the Plowshare CReSS and CReSSmicro units (25,53,71-80). CReSS desktop topography units are capable of real time recording of individual puffs, including shape and flow rates, for later replication of human puff profiles on smoking machines (portable devices at the present time cannot do this and only provide means of the parameters). These units are not only capable of recording ad libitum smoking, but they also contain the ability to cue the smoker for controlled smoking conditions, for example cuing them when to puff, puff duration and puff volume. These systems also can integrate subjective, performance and physiological measures.
Validation of puffing topography recording devices
While there are numerous studies about puffing topography, there are few studies that have validated the available methods. Validation would be done in several ways, namely by assessing intra-individual, intra-laboratory, and inter-laboratory variation, as well as by comparing different methods to assess topography. Published validation studies for intra-individual and intra-laboratory methods are limited to the CReSS devices. These studies have conducted repeated measures on different days, which assesses both intra-laboratory and intra-individual variation measured in the laboratory, and these have generally shown good reproducibility (53,76,81). For example, Lee and coworkers found good reliability by intraclass correlation coefficients computed for puff volume (0.66), puff duration (0.75), and maximum puff velocity (0.68) (53). Hammond et al. investigated the smoking topography characteristics with the portable CReSSmicro device on 59 subjects smoking an average of 19 cigarettes per day, reporting similar measures of smoking topography for the same subject over time (82). For a biomarker assessment, in a study of 180 smokers measuring CO and nicotine boosts for 2 cigarettes one hour apart, the use of the topography device did not affect the CO or nicotine levels, because these were measured with and without the topography device in place, although the correlation coefficient with and without the device for CO was only 0.31 (p<0.001) (Shields, et. al., unpublished data). Similar results have been reported in a small study by Lee and coworkers (53). Blank and colleagues recently reported head-to-head comparisons of the desktop and portable CReSS devices versus observed smoking using a video tape (83). The authors found that measured puff duration and interpuff interval on both devices, as well as the video correlated were well correlated (r's > 0.70), though there were quantitative differences among the devices for puff volume and duration, indicating that comparisons of data across devices might not be reliable. Separately, it has been shown that topography assessments in the laboratory provide similar assessment in the naturalistic environment, e.g., at home (79,84). Thus, these studies support the reliability and validity of the CReSS devices for topography measurement.
What is known about human puffing patterns?
There is wide inter-individual variation for smoking behavior, but a low intra-individual variability because smokers in general show a stable smoking pattern over time (23,54,55,82,85-88). A clear and consistent finding is that human smoking behavior differs substantially from the commonly used FTC and ISO parameters, a fact recognized early by the tobacco companies (82,89-91)11. Several factors have been documented to influence smoking behavior, such as gender, race, psychological factors and genetic background. In general, men smoke more cigarettes per day than women and have higher serum cotinine levels (92-96). Although the data is less consistent for smoking topography, men tend to have larger puffs of longer duration, but women may smoke more puffs per cigarettes (52,70,71,97,98). Differences in smoking topography have been observed between Whites and African Americans: the latter group generally reflecting greater exposure to smoke toxicants (60,70,96,99). Psychological factors, concurrent use of psychoactive drugs, time of the day and place where a cigarette is smoked also can have an effect on the smoking topography (88,100,101). Time of day also affects smoking topography (80,102).
Generally, there is a high correlation for various puff parameters, e.g., interpuff interval, puff duration, and puff volume; all of these directly impact total puff volume per cigarette (85,97,103,104). However, these parameters are not proxies for each other and so all need to be recoded when measuring smoking topography (97). Other studies indicate that topography measures are not kept constant during the course of smoking a cigarette, where puff volume decreases and inter-puff interval initially increases and then decreases (85,105,106).
The number of cigarettes smoked per day generally do not relate to puffing topography, or sometimes is positively correlated with longer inter-puff intervals (49,62,82,104,107). Published studies also are inconsistent for relating puff topography to various biomarkers such as CO and nicotine/cotinine levels, where different parameters affect these biomarkers differently (61,104,108-118). For example, puff number and to a lesser extent the puff volume and duration affect nicotine levels, while CO level are mostly influenced by puff volume and less by puff number (113). Zacny and coworkers reported that both nicotine and CO increase proportionally with an increase in puff volume (61). In a study of 180 subjects, there was a statistically significant correlation for CO boost and puff volume, but not the interpuff interval (Shields, et, al., unpublished data).
Product Design effects on smoking topography
Physical design characteristics affect puffing topography when smokers first switch, for example by changes in the draw resistance, sensation and taste (23,30,82,89). Numerous studies indicate that switching from higher to lower yield cigarettes increase topography parameters such as puff volume and puffs per cigarette (61,82,86,111,119-127), while a decrease in puffing intensity or longer time spent on smoking a cigarette takes place when smokers switch to a higher overall yield cigarette, or cigarettes with constant tar but increased nicotine content (56,111,128-133). In a 1986 British American Tobacco study12, 19 subjects who were “low” tar (<10 mg tar yields) and “middle” tar (>10 mg tar yields) smokers had similar puff topography and inhalation parameters, as shown in Table 2. The investigators found that puff volume increased when their subjects who were “middle” tar yield smokers were switched to a low tar cigarette, but decreased for the opposite test scenario. The investigators concluded that the increased puff volume was due to decreased draw resistance. In this study, inhalation parameters did not change with switching. Studies by Benowitz, et al., suggest that during short-term switching studies, smokers that switch to lower yield cigarettes tend to compensate by changing their behavior by smoking more vigorously or by increasing cigarettes per day (13,134).
Table 2.
Smoking Parameters, 1986 BAT Study27 (means +/− SD)
| Cigarette type | Puff number | Puff volume (ml) | Mouth hold (sec) | Inhaled volume (ml) | Exhaled volume (ml) | Inhalation time (sec) | Exhalation time (sec) | Breath Hold (sec) |
|---|---|---|---|---|---|---|---|---|
| >10 mg tar yield (n=11) | 9.4 +/− 2.9 | 44.9 +/− 12.3 | 0.49 +/− 0.27 | 702 +/− 437 | 577 +/− 329 | 1.19 +/− 0.29 | 2.01 +/− 0.76 | 0.45 +/− 0.48 |
| <10 mg tar yield (n=8) | 12.1 +/− 5.6 | 44.5 +/− 10.9 | 0.65 +/− 0.39 | 636 +/−138 | 655 +/−195 | 1.22 +/− 0.37 | 2.89 +/− 0.72 | 0.45 +/− 0.57 |
Similar smoking parameters were observed in subjects grouped according to cigarette tar yields with a cut-off of 10mg tar.
An important design feature of lower yield cigarettes is ventilation via holes punched on the filter paper that allow smoke to be diluted with air during puffing. However, some smokers block these ventilation holes by their fingers or lips, which would then result in yields different than predicted by a smoking machine. In a study of smokers who were trained to uniformly smoke with a particular puff profile that restricted the puffs per cigarette and puff frequency, Strasser and coworkers demonstrated that hole blocking resulted in an increase of CO boost, implying an increase of other tobacco smoke constituents (118). Puff volumes decreased for both cigarettes with 50% hole blocking. Other switching studies reported similar results but differed in the magnitude of the CO response depending on the cigarette type that was smoked, namely the effects are greatest for ultralight smokers (117,135). One explanation for the difference in results might be the lack of controlling for puff number and puff interval; in the latter two studies there were many more puffs per cigarettes that might have obscured a difference. Regardless, it is clear that smoking machine studies that compare cigarettes with different physical design characteristics using the same puffing profile fail to accommodate about what happens to smokers who switch or naturally adopt one product versus another.
Filter efficiency is affected by puffing. Increasing smoke flow through the filter, such as with greater puff volumes and decreasing filter ventilation, but not so much decreasing puff frequency, will tend to decrease filter efficiency, leading to a narrower range of yields across brands13. For example, Marlboro UltraSmooth with a novel carbon filter is much less effective in reducing toxic smoke constituents when smoked under the HC regimen compared to the FTC method (136).
For many PREPs, design features are varied and switching studies show that smoking behavior changes (summarized in Table 3). For example, smokers who switched to the Advance cigarette that has a modified filter took fewer puffs and had higher nicotine levels, while the rest of puffing characteristics remained unchanged (137,138). Two studies investigating the Accord electronic smoking system found that subjects had shorter puff intervals and fewer puffs per cigarette, because this is electronically controlled, and higher puff volume and duration compared to smoking own brand cigarettes (72,74). . Eclipse smokers, which is designed to heat tobacco rather than burn it, substantially increase their puff volumes, and decrease the interpuff interval (139-141). For Eclipse, CO levels also increase, and for some smokers the levels can be quite high (142). Acrolein also is increased. For Quest cigarettes that vary in nicotine yields, there is compensatory smoking with an increase in the total puff volume and CO boost (116). Another study reported that switching to Omni cigarettes with a modified filter results in fewer puffs compared to the usual brand, but there also is an increase in CO boost and not a significant decrease in carcinogen exposure when compared to conventional cigarettes (75). When comparing Marlboro UltraSmooth (MUS), employing a modified filter that includes charcoal particles embedded in cellulose acetate, with two conventional cigarettes (Marlboro Lights and Ultralights), investigators observed a decrease in number of puffs, but higher puff volumes (79). The overall conclusion of the study was that there is no significant change in smoking topography between the MUS and conventional cigarettes; therefore there will be no reduced exposure among smokers that switch from a conventional brand. Thus, smoking machine studies that compare PREPs to conventional products using the same puffing profile could be misleading in terms of relative effects.
Table 3.
Selected smoking topography characteristics among PREP studies
| Author/Year | Products | Participants (N) | IPI (s) | Puff numbers | puff volume (ml) | total puff volume (ml) |
|---|---|---|---|---|---|---|
| Breland AB 2003(137) | Advance | 12 (8F, 4M) | 9.6 (2.8) | |||
| Own | 11.7 (4.2) | |||||
| Breland AB 2002(138) | Advance | 20 (10F, 10M) | 34.5 (21.9) | 51.6 (9.4) | ||
| Own | 33.9 (23.6) | 56.5 (11.2) | ||||
| Sham | 17.2 (14.2) | 66.5 (43.7) | ||||
| Buchhalter R 2000(72) | Accord | 10 (7F, 3M) | 24.0 (12.1) | 7.8 (0.7) | 55.4 (17.0) | 432.12 |
| Own | 35.0 (17.9) | 10.3 (2.1) | 38.4 (11.7) | 395.52 | ||
| Breland AB 2006(139) | Eclipse | 35 (8F, 27M) | 21.38 | 17.03 | 65.01 | 1107.12 |
| Own | 30.74 | 10.03 | 50.97 | 511.22 | ||
| Slade J 2002(140) | Eclipse | 19.7 | 67 | 1371 | ||
| Own | 640 | |||||
| Breland AB 2002(74) | Eclipse | 20 (10F, 10M) | 53.3 (4.3) | |||
| Accord | 61.8 (4.8) | |||||
| Own | 49.8 (3.3) | |||||
| Strasser AA 2007(116) | Quest 0.05 | 50 | 18.6 | 10 | 59.4 | 570.5 (156.9) |
| Quest 0.3 | 19.6 | 9.9 | 55.9 | 518.1 (145.6) | ||
| Quest 0.6 | 21.6 | 9.8 | 58.1 | 540.3 (144.9) | ||
| Own | 21.6 | 14.3 | 60.5 | 832 | ||
| Rees VW 2008(79) | ML | 32 (21F, 11M) | 32.1 (11.9) | 11.4 (3) | 50.7 (19.6) | 578 |
| MUS | Tampa | 28.4 (8.4) | 10.2 (2.9) | 54.2 (19.4) | 552.84 | |
| MUL | 33.0 (14.9) | 11.3 (2.6) | 51.4 (19) | 580.82 | ||
| ML | 24.8 (11.45) | 13.1 (4.8) | 47.4 (16.9) | 620.94 | ||
| MUS | Salt Lake City | 23.9 (9.2) | 12.5 (5) | 56.7 (15.2) | 708.75 | |
| MUL | 21.8 (10.4) | 13.9 (5.6) | 50.1 (15.6) | 696.39 | ||
| Lee EM 2004(141) | Eclipse | 10 | 16.1 (2.1) | 89.3 (10.8) | 1437.73 | |
| Own | 11.5 (0.7) | 60.1 (4.0) | 691.15 | |||
| Hughes JR 2004(75) | Omni | 34 | 11.6 (0.5) | 49 (2) | 547 (25) | |
| Own | 12.7 (0.7) | 50 (2) | 612 (34) |
Data presented as mean (SD) or mean only as available in the original paper. Empty cells mean no value exists in the paper for that parameter. Calculated values are presented in italic.
N-number, s-seconds, ml-milliliters, ML-Marlboro Light, MUL-Marlboro Ultra Light, MUS-Marlboro Ultra Smooth
Measuring Inhalation and exhalation
Smoking behavior also involves not only assessing puffing behavior, but also inhalation, which more closely relates to biological dose. Several techniques have been developed for measuring times and volumes for inhalation and exhalation. Some early methods were reviewed in a report from Imperial Tobacco Ltd14. These techniques are summarized in Table 4. The main conclusion was that these devices were accurate in measuring the physical mechanics of inhalation and exhalation, but they did not permit studies in the naturalistic setting and they imposed restrictions on free smoking behavior. Chest plethysmography, combined with a cigarette holder-flow meter to assess topography, appeared to be the best. Tobin and coworkers used this method to assess the pattern of inhalation in smokers to compare with the smokers’ subjective reports for inhalation (143). They found that smokers inaccurately perceived their inhalation patterns. In another study, Tobin and Sackner used the same system to assess switching from high to low tar cigarettes, showing that there was no change in the inhalation characteristics (129).
Table 4.
Summary of inhalation/exhalation monitoring methods used for assessing human smoking behavior (excerpted from internal company documents28)
| Author/Year | Method | Variables measured | Limitations |
|---|---|---|---|
| Cinkotai F.F. 196729 |
Partial Body Plethysmography Puff volumes and duration determined with a modified cigarette holder as a flow meter |
-volume of the puff -duration of the puff -holding time of the puff in the mouth -lung volume at the beginning of the puff -time of inhalation -volume of air inhaled with the puff -volume of exhaled air -time of exhalation. |
Discomfort leading to high puff by puff variation observed in the breathing patterns of individual smokers and abnormal tidal breathing caused by stress |
| Creighton D.E. 1978(66) |
Impedance Pneumography Puff profiles and puff volumes measured with a special cigarette holder and a pressure transducer |
-puff profiles and puff volumes -semi-quantitative estimates of breathing patterns |
Needs calibration against a partial body plethysmograph before each use. Non linear response and day-to-day variations for individual and variation between subjects. |
| Guillerm R. and Radziszewski E. 1975(175) |
The Guillerm and Radziszewski Method A flow meter constructed from a classic cigarette holder with a bead placed between the two snap-in-parts of the holder connected by flexible polyvinyl tubing to a differential tr ansducer. A special infrared pyrometer used to measured the temperature variations of the combustion cone of the cigarette. |
-puff volume and duration -number of and intervals between puffs -volume of air taken between puffs -volume of air inhaled immediately after the puff -location of the puff in the ventilatory cycle - the breathing pattern was measured at the same time as the puff analysis |
The puff volume recorded did not always correspond to the true inhaled puff volume and the technique imposed some physical restrictions on the subject, particularly concerning the cigarette holder. |
| Rawbone R.G. 1978(176) |
Mercury Strain Gauge Chest Pneumography The puff parameters were obtained from measurements of the pressure drop across a small resistance inserted between the cigarette and the smoker. The depth of inhalation was measured by recording movements of the chest wall with a mercury strain gauge chest pneumogram. |
-puff volumes, -puff duration -inter-puff interval -semi-quantitative estimates of breathing patterns |
Calibration was required before each study. |
| Sackner M. A. 1980(177) Tobin M. J. 1982(129,143) |
Respiratory Inductive Plethysmography Consisting in two coils of Teflon-insulated wire, which were sown into elastic bands encircling the rib cage and the abdomen and connected to an oscillator module. Tidal volume measured by spirometry. |
-number of puffs -puff duration, -puff volumes, -integrated puff pressure. -accurate estimation of breathing patterns |
Accuracy of the results depended on the initial calibration and the stability of the calibration during changes in body positions and lung volumes. |
The most widely used device by the tobacco industry to assess smoke inhalation by inductive respiratory plethysmography has been the RespiTrace, developed for assessing respiratory function and disease (NonInvasive Monitoring Systems Inc.) (144). The system consists of insulated coils enclosed in elastic bands applied on the rib cage and abdomen of the subject, registering the changes in respiratory movements that alter the self-inductance of the coils. The device must be calibrated for tidal volume with the use of a spirometer (145). BATCo used the RespiTrace system in the studies discussed above to discern the physical process of smoking15. Research has been conducted to assess whether smoking machine tar and nicotine yields affects inhalation in two studies, but one reported no effect and the other found a positive relationship (123,146).
The effects of inhalation on dose measured via biomarkers has received little attention. Zacny and coworkers trained smokers to smoke their cigarettes according to a controlled smoking regimen for inhalation depth and time (61). They measured CO and nicotine boosts, and showed that post-puff inhalation volume and duration under ad libitum and controlled smoking conditions had no effect on the CO and nicotine levels. Similarly, Herning and coworkers found that nicotine blood levels where not related to inhalation (103). In a third study, nicotine retention was almost complete even at low inhalation volumes (147). These studies indicate that nicotine absorption is very quick and so unrelated to inhalation, but, it may be that other tobacco smoke constituents would be affected by inhalation. This has received even less attention, but one study has reported that the retention of solanesol was related to inhalation volume (147). In a study by Philip Morris scientists, a novel method was used to measure the estimated intake into the lungs by having smokers exhale through a Cambridge filter pad (148). The difference between the estimated chemical yield, as measured by a smoking machine, and the amount of the chemical constituent on the pad was considered retained in the smoker. Under controlled smoking conditions where the smokers varied their depth of inhalation, they found similar results as above for no relation of inhalation to nicotine retention (61), but that the retention of tobacco specific nitrosamines (TSNAs) was greater with deeper breaths. For the gas vapor phase, however, depth of inhalation had little effect on retention. Thus, inhalation can be an important parameter for some smoke constituents such as TSNAs.
In summary, smoking behavior is complex and many of the individual components co-vary, so that affecting one might affect each other. These are directly affected by cigarette designs. However, the various aspects of smoking also affect smoking machine yields and smoke toxicant effects, as indicated below. Some parts of human smoking are not captured at all by the smoking machine, while some variables such as puff velocity and puff shape are usually not considered. Smokers vary their puffing behavior during the course of their cigarette, by day, and by who they are. These added variables make it impossible to replicate a typical smoker using one smoking machine regimen.
Smoking machine profiles: mimicking human smoking behavior?
As evidence accumulated that smokers’ behaviors and exposures were distinct from machine-measured yields, increased interest was placed on altering machine smoking methods to better reflect smoker practices. The 1981 Surgeon General Report, for example, acknowledged that the FTC testing method needed to account for compensatory smoking (via larger and more frequent puffs) and ventilation hole blocking (16). A National Cancer Institute ad hoc expert committee convened in 1996 came to similar conclusions (88). Research on alternative testing regimens was ongoing in the public health/regulatory community. For example, Rickert and coworkers tested smoke yields under ISO conditions and two more intensive conditions and reported that the yields of tar, nicotine, and carbon monoxide more than doubled when cigarettes were smoked under the intensive regimens compared to the standard one (149). Djordjevic, et al. determined the actual human puff profiles of 133 smokers and replicated the profiles of a randomly chosen subset of 72 on the smoking machine (110). The investigators found that the yields of tobacco-specific N’-nitrosamines and benzo(a)pyrene (BaP) increased by two fold, while the nicotine and tar levels increased more than two-fold compared to the FTC measures yields.
In 1996, the Massachusetts Department of Public Health (MDPH) Tobacco Control Program began a research project to establish a machine smoking regimen that more resemble human smoking. Initially, two sets of smoking regimens were chosen, derived from 32 studies on ad libitum smoking topography presented in the 1988 Surgeon General's report (150). One was termed the “average smoker” protocol and the other a more intense “heavy smoker” protocol. The former had a 45mL puff volume every 30 seconds, with a puff duration of 2 seconds and taping closed 50% of the ventilation holes. The MDPH 50% hole blocking in particular was recommended in the context that smokers will block ventilation holes when they smoke, for example with their fingers or lips (20,21,151-153). The initial proposal also included an “intense,” or “heavy,” smoking condition (60-ml puff every 26 seconds, 100% vent blocking), but this was dropped from the final plans. From 1997, cigarette manufacturers have been required to report results to the MDPH under the “average” protocol, along with levels of filter ventilation, tobacco nicotine content, and smoke “pH” (154). It should be noted that derivation of ‘average’ and ‘intense’ smoking for this protocol reflected topographical data available prior to 1988 and not necessarily reflective of today's products’ design and smokers’ behavior.
In the same year (1996) as the MDPH, Health Canada (HC) began work on amending its tobacco regulatory authority and convened an Expert Committee on cigarette modifications. Discussions on reducing the harmfulness of cigarettes led to a formal exploration of alternative smoking conditions (155). In this report, Rickert noted that puff volume and interpuff interval are the key variables to consider in a new machine smoking regimen. This resulted in the proposal of an HC protocol with a 56mL puff volume with a 2 second duration and a 26 second interpuff interval; the ventilation holes would be fully blocked. Other elements of the ISO protocol (conditioning, duration, butt length) were retained. The 100% hole blocking was adopted in order to directly compare the performance of cigarettes removing the strongest predictor of tar and nicotine yields. The report concluded that testing under two conditions (ISO and HC) would be sufficient to capture the range of deliveries that might be experienced by smokers (and later adopted by the World Health Organization TobReg (10)). In June 1998, the Health Protection Branch of Health Canada outlined proposed reporting requirements of 40 constituents in mainstream smoke based on the standard and extreme regimens. The Tobacco Act of 2000 made the new regimen official. During the regulatory purposes, the parameters were changed to a 55mL puff volume of 2 second duration and a 30 second puff frequency (150).
In 2004, an ISO Working Group (ISO/TC126/WG9) was convened to craft an alternative smoking regimen that more closely hewed to human smoking behaviors (150). The ISO was faced with the overwhelming evidence that the ISO/FTC regimen inadequately characterized modern cigarette exposures, that there were emerging test methods in different jurisdictions, and the prospect of impending regulations under Articles 9 and 10 of the Framework Convention on Tobacco Control (FCTC)16. The group, which included members affiliated with the tobacco industry, reviewed published literature on smoking topography from 1956 to 2004, and used 100 datasets comprising 2432 subjects (156). They derived summary statistics for puff volume, duration, interval, number of puffs per cigarette and how these vary with cigarette tar yield as determined by the ISO/FTC smoking regimen. Significant differences were noted between the experimentally-derived average human puffing profiles (HPPs) and the ISO/FTC parameters, as summarized in Table 5. Ultimately the Working Group proposed 3 different smoking machine protocols for testing, as shown in Table 1. These were determined by grouping the human puffing profiles according to machine-smoked ISO/FTC cigarette “tar” yield ranges. The work of Working Group 9 was set aside in May 2006 and Working Group 10 was established. The work of Working Group 10 is ongoing because this group was convened to serve as a forum for exchange of information between WHO (the public health sector in general) and the tobacco industry scientists. For this, and given that industry labs are precluded from participating in the validation work of TobLabNet, the WG 10 is an important forum. No tangible products have yet come out of WG10 because the purpose is for information exchange.
Table 5.
Average smoking topography parameters values of 2432 subjects compared to the ISO/FTC parameters (modified from the reviewed by the WG 9 of the ISO TC 126) (156)
| HPPs grouped by tar yield (mg) | ||||||
|---|---|---|---|---|---|---|
| Puff characteristics* | ISO/FTC | ≥14 | 8-14 | 3-8 | <3 | |
| Puff volume (ml) | 35 | 48.1(10.7) | 47.8(6.3) | 54.7(9.7) | 57.2(8.9) | |
| Puff duration (s) | 2 | 1.9(0.4) | 1.8(0.3) | 2(0.3) | 1.9(0.1) | |
| Puff interval (s) | 60 | 26.1(8.8) | 27.3(8.7) | 22.6(7.1) | 18.9(0.7) | |
mean (SD) values for HPPs
One can notice that the HPP puff volume is higher and puffs are drawn at less than half the interval of the ISO/FTC parameters. Also, the HPP puff volumes increase and the puff intervals decrease corresponding to the decrease in tar yields.
In 1997, the FTC announced plans to revise its cigarette testing method with a public comment period (Federal Register 62/177, 9/12/97). In addition to the standard method, a more intense method was being considered (a 2-sec, 55-ml puff every 30 sec). However, no action was taken at that time. Later, in 2008, the FTC proposed rescinding in its entirety their 40-year guidance for smoking machine testing, rather than recommending a second and more intense puffing regimen. The Agency stated: “Today, however, the scientific consensus is that machine-based measurements of tar and nicotine yields based on the Cambridge Filter Method do not provide meaningful information on the amounts of tar and nicotine smokers receive from cigarettes or on the relative amounts of tar and nicotine they are likely to receive from smoking different brands of cigarettes. The primary reason for this is smoker compensation – that is, smokers alter their smoking behavior in order to obtain the necessary nicotine dosage17. After a 60 day public comment period, the FTC followed-through and rescinded its guidance, drawing the era of “FTC” yields to a close.
Changes in yields by smoking regimen
Changing specific parameters of the puff profile independently can directly affect smoke yields. For example, decreasing puff volume, increasing puff frequency (decrease inter-puff interval), and increasing filter ventilation decrease tar and chemical yields on a per cigarette basis (157)18. (In smokers, though, using higher ventilated cigarettes generally results in larger puff volumes.) Toxicology studies also show the influence of puff volume, ventilation and ventilation hole blocking (158)19. The ISO/FTC, MDPH and HC methods use different puff volumes, puff frequency and ventilation hole blocking, and increases in these variables result in increased tar, nicotine and other constituent yields on a per cigarette basis (110,149,159-161).
The data indicate that the relative rankings of different products, on a per cigarette basis, will generally be preserved across regimens although the gap in toxicant emissions with more intense protocol is reduced. Counts and colleagues from Philip Morris published a large survey of emissions from international brands tested under ISO, MDPH, and HC conditions (160), showing that the ratios of constituents to total tar were dependent on the puffing profile, and mostly driven by filter ventilation. For example, when cigarettes were grouped broadly by filter ventilation, the yields of individual constituents relative to tar changed differently as the different profiles were compared. This effect was greater for vapor phase compared to particulate phase constituents. However, the effect was least for the cigarettes with lower ventilation and higher tar yields. Separately, Hammond and O'Connor examined the relationships between yields under the ISO and HC regimens for the 2004 Canadian market and showed that the increased intensity of the HC system changes the absolute concentrations of constituents, but also their concentrations relative to nicotine (159).
Both Philip Morris and RJ Reynolds Tobacco companies, as early as 1974, developed the capability to capture human topography data and mimic this on a smoking machine, and it was shown that the yields predicted for different smokers substantially varied among them, and higher than the FTC predicted yields20. In a 1982 report by RJ Reynolds’ scientists, an analysis indicated that using 5 variables within the puff for flow velocity at different times of the puff and the time to reach maximum velocity that 6 types of shapes could be described21. While, each smoker would vary their shape within a cigarette, it was reported that 12 patterns would characterize all 550 smokers.
Whether changing the shape of the puff affects yields is unclear, and there are no recently published studies, although a 1968 report from Brown and Williamson demonstrated that when air flow peaked (early versus late), different yields were obtained22. The parameters that affect the shape of the puff or the variability for the puff-by-puff profile are unknown, but it appears that filter ventilation does not affect the latter23. None of the above studies, however, measured specific chemical constituents. New commercial topography devices have the capability to record puff-by-puff data, including the change of airflow within a puff. Today, smoking machines also can be programmed with the use of specialized pumps and software to better replicate the human-type puff on a puff-by-puff basis. However, whether this new technology affects the smoke yields and is provides better replication of human smoking remains to be determined.
A more meaningful comparison might come from an assessment using different puff profiles tailored to the product as it might be used by smokers. For example, a method has been proposed based on nicotine yields by Kozlowski and O'Connor (153). They proposed a two-step system where the first step would use the traditional ISO/FTC yield on a per cigarette basis, while a second step would use puff parameters adjusted to yield the same nicotine levels, for example by adjusting the puff volume. Later, Hammond and colleagues revised this recommendation to propose a system whereby puffing profiles would be iteratively adjusted so that all brands yielded a specific nicotine level. In both cases, the goal would be to better simulate compensatory smoking by humans within the limitations of machines. However, little work has been done to operationalize these methods. It should be noted that the above methods adjust smoking machine parameters based on total cigarette yields and not on a per mg of tar basis and assumes that the chemical composition of tars are similar, however this is known not to be true (159,161,162)
Hammond and coworkers attempted to examine this issue by comparing the smoke yields produced under ISO, MDPH, HC and the two-stage compensatory regimen described above to the average of actual topography measures for 51 smokers of the usual brand and 21 switched to ultralights (human mimic profiles) (30). Ventilation hole blocking was 50% for the MDPH and compensatory and human mimic profiles, while it was 100% for the HC method. None of the yields for the four smoking regimens replicated the human mimic conditions. Tar, nicotine and CO yields obtained for the regular tar smokers under the mimic protocol were double of those obtained with the ISO and compensatory regimens, but lower than the HC regimen. For the ultralight switchers, the human mimic yields were three to four times greater than the ISO and MDPH regimens, but slightly lower than the HC regimen and similar to the compensatory regimen. Importantly, none of the standardized machine determined nicotine yields predicted levels of salivary cotinine, except for the human mimic regimen. Thus, it is likely that no single smoking regimen can adequately characterize smoking.
Philip Morris has proposed another method for comparing products, which is to characterize human smoking behavior on a smoking machine based on several regimens statistically modeled based on topography data and urinary nicotine metabolites (163). The method uses the determined 10th percentile, mean and the 90th percentile of the puff volumes, and the other parameters were modeled. Thus they proposed testing cigarettes with a low (25 mL puff volume, 0.8 s puff duration, 2.4/min puff frequency), a medium (48 mL puff volume, 1.3 s puff duration, 1.8/min puff frequency), and a high (65 mL puff volume, 1.6 s puff duration, 1.9/min puff frequency) puffing profile. However, we are unaware of any actual implementation of this proposal.
Data on comparative emissions for PREPs are rare. A specific example of a PREP for the utility of testing under multiple smoking machine methods is the Eclipse cigarette, which is claimed to heat rather than burn tobacco under the FTC conditions. When smoked on a machine in a way more similar what smokers do, the tobacco becomes significantly charred and the smoke chemistry differences compared to conventional cigarettes become much less24.
DISCUSSION
The need for validated laboratory methods to assess tobacco smoke for chemical constituents and toxic effects has recently been underscored by the new FDA authority to enact product performance standards and evaluate manufacturer health claims for modified tobacco products. Prior uses of smoking machine results led to misinterpretations and misunderstandings about cigarette comparisons and their relationship to human health (13,27). As a result, smoking machine data are regarded as poor indicators of health risk, leading the FTC to rescind its imprimatur from the method25. However, smoking machines will continue to be used for laboratory screening of product design changes and the assessment of performance standards, and so better methods need to be developed (10,28). Critical to the development of new smoking machine methods is a better understanding of human smoking behavior, including how interindividual variation in puffing, mouth-holding and inhalation affect exposure. These studies can then inform the use of cross-regimen comparisons, for example as previously described (30,153,163), which may better reflect the differences among human exposure for specific product comparisons about product design. This would lead to tailoring puff profiles to particular products as used by smokers. Thus, there are several research gaps that need to be addressed in order to maximally apply and interpret smoking machine studies.
Currently, almost all methods for assessing human puffing that can be extrapolated to smoking machine protocols is through commercial topography devices. Data from such studies suggest that topography may differ by gender and race (52,60,70,70,71,92-96,96-99). However, there are many other likely determinants, such as age, co-morbidities, prior smoking history, nicotine metabolism, genetics and psychological factors that have been studied even less in the context of topography and application to smoking machine studies (88,100,101,164-172). Other variables include smoking environment at time of measurement (naturalistic versus laboratory), time of day and circadian rhythms (80,102). Without a better understanding of how much these variables affect the range of human exposures, it will be difficult to know if future smoking machine regimens are sufficiently mimicking human exposure.
While there are some data demonstrating the replicability of smoking behavior using these devices (53,76,81,82), additional studies are needed to compare different commercial units and to validate them. It is unknown if these devices are measuring accurately air flow and volumes, and so a major limitation for validating topography measurements is the comparison to some “gold standard”. But, none exists. Validation of topography as an indicator of exposure requires statistically significant and consistent correlation with biomarkers that have been validated for smoking (142), but the data thus far for comparing topography to biomarkers have produced conflicting results. Biomarkers of exposure reflect not only puff topography but also mouth holding and inhalation, and so it may be that a biomarker comparison is not valid, assuming that varying mouth holding and inhalation affect the dose to smokers. Thus, additional studies are needed to assess mouth-holding and inhalation to determine how much, if any, these components of smoking affect exposure. However, methods to assess mouth-holding and inhalation are poorly developed, and so better technologies are needed that can be applied to human studies. Once developed, controlled smoking and cross-sectional studies can be conducted with biomarkers to determine how much puffing, mouth-holding and inhalation contribute to variance in human smoke exposure.
The current designs for smoking machine puffing profiles have been developed considering each parameter as independent effects, but changing one actually influences the others (85,105,106). So, a better understanding of the impact of changing one parameter on others is needed, both for topography and for smoking machine studies. It is known that many of the various topography parameters co-vary (85,97,103,104), but a systematic study has not been done to identify the extent of this. Another parameter that is only partially characterized is blocking ventilation holes (54,117,118,135). To determine how people block holes, how much and how often has been insufficiently studied. However, filter ventilation affecting smoke dilution and also impacting puff volume is critical for determining smoke yields. Thus, better technologies are needed to determine ventilation hole blocking and incorporate them into human studies that assess topography.
There is sufficient data to know that different machine puff profiles cause cigarettes to burn differently and have different chemical yields and biological activity, and that this would also result in different exposures in humans (173,174)26. Thus, smoking machines need to better mimic human smoking, including methods to replicate puff-by-puff parameters, and studies need to be done to determine if the shape of the puff significantly affects yields. How to model the diversity of human smoking behavior needs to be developed. Then, better methods to compare cigarettes and product design changes through cross-regimen comparisons are needed. Whether this is done using topography data or by standardizing for nicotine yields needs to be developed, and there is sufficient rationale to indicate that both methods might have utility.
Since the passing of the FDA legislation and the IOM report conclusion that risk reduction through PREPs is a feasible approach (11), a comprehensive framework for studying tobacco products, including PREPs, is needed. This would include studies ranging from premarket assessments using laboratory studies to population surveillance. It would use integrative approaches by examining individual smoking behavior for new products and establish their relationship with actual delivered dosages of nicotine and a select panel of toxic and carcinogenic agents. An iterative process would therefore be used, where product design changes are tested first in the laboratory for increases in smoking yields and toxicity, followed by human use in short term studies, and then replication of human use in the laboratory to confirm the yield and toxicity changes. Central to this process is the understanding of human smoking behavior and how to replicate this in the laboratory, but current knowledge and methods are insufficient to do this. Additional research, however, can fill in the research gaps to improve tobacco product assessment. Having validated methods for assessing tobacco products in the laboratory is vital for the fulfillment of the promise of regulatory oversight to protect the public health.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. William Farone (Applied Power Concepts, Inc. (Anaheim, CA 92801) for his insightful comments on this manuscript.
Funding: This study was supported by NCI N01-PC-64402 - Laboratory Assessment of Tobacco Use Behavior and Exposure to Toxins Among Users of New Tobacco Products Promoted to Reduce Harm
Abbreviations
- BATCo
British American Tobacco Company
- CO
carbon monoxide
- CORESTA
Cooperation Centre for Scientific Research Relative to Tobacco
- CSC
Cigarette smoke condensate
- EMG
Electromyographic activity
- FDA
Food and Drug Administration
- FTC
Federal Trade Commission
- GVP
gas/vapor phase
- HC
Health Canada
- HPPs
human puffing profiles
- IARC
International Agency for Research on Cancer
- IOM
Institute of Medicine
- ISO
,International Standards Organization
- MUS
Marlboro UltraSmooth
- MDPH
Massachusetts Department of Public Health
- NCI
National Cancer Institute
- PAHs
Polycyclic aromatic hydrocarbons
- PREP
Potential reduction exposure product
- RJRT
R.J. Reynolds Tobacco Company
- SGR
Surgeon General Report
- TPM
Total particulate matter
- TSNA
Tobacco-specific nitrosamines
- U.S.
United States
- WS
Whole smoke
- WTPM
wet total particulate matter
Footnotes
http://legacy.library.ucsf.edu/tid/xpt60f00; http://legacy.library.ucsf.edu/tid/czv24f00; http://legacy.library.ucsf.edu/tid/yci66a99/pdf; http://legacy.library.ucsf.edu/tid/lsn86a99/pdf; http://www.library.ucsf.edu/tobacco/batco/html/13200/13274/index.html; http://legacy.library.ucsf.edu/tid/dss00f00; http://legacy.library.ucsf.edu/tid/syj51f00: http://www.library.ucsf.edu/tobacco/batco/html/6900/6922/index.htm
http://legacy.library.ucsf.edu/tid/aob34c00; http://legacy.library.ucsf.edu/tid/cbi31d00; http://legacy.library.ucsf.edu/tid/qtp03f00; http://legacy.library.ucsf.edu/tid/rto73d00; http://legacy.library.ucsf.edu/tid/gkb11d00
http://tobaccodocuments org/rjr/508352445-2461 html; http://legacy.library.ucsf.edu/tid/cbi31d00; http://legacy.library.ucsf.edu/tid/aob34c00; http://legacy.library.ucsf.edu/tid/qtp03f00; http://legacy.library.ucsf.edu/tid/rto73d00; http://legacy.library.ucsf.edu/tid/gkb11d00; http://legacy.library.ucsf.edu/tid/mxa35d00
Reference List
- 1.Warner KE. Tobacco harm reduction: promise and perils. Nicotine Tob Res. 2002;4(Suppl 2):S61–S71. doi: 10.1080/1462220021000032825. [DOI] [PubMed] [Google Scholar]
- 2.Britton J. Smokeless tobacco: friend or foe? Addiction. 2003;98:1197–207. doi: 10.1046/j.1360-0443.2003.00480.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramstrom L. Snus: part of the problem or part of the solution? Addiction. 2003 Sep;98:1198–9. doi: 10.1046/j.1360-0443.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- 4.Fagerstrom KO, Schildt EB. Should the European Union lift the ban on snus? Evidence from the Swedish experience. Addiction. 2003;98:1191–5. doi: 10.1046/j.1360-0443.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 5.Pierce JP. Harm reduction or harm maintenance. Nicotine Tob Res. 2002;4:S53–S54. doi: 10.1080/1462220021000032834. [DOI] [PubMed] [Google Scholar]
- 6.Fox BJ, Cohen JE. Tobacco harm reduction: a call to address the ethical dilemmas. Nicotine Tob Res. 2002;4:S81–S87. doi: 10.1080/1462220021000032861. [DOI] [PubMed] [Google Scholar]
- 7.Hatsukami DK, Slade J, Benowitz NL, et al. Reducing tobacco harm: research challenges and issues. Nicotine Tob Res. 2002;4(Suppl 2):S89–101. doi: 10.1080/1462220021000032852. [DOI] [PubMed] [Google Scholar]
- 8.Gartner CE, Hall WD, Chapman S, Freeman B. Should the health community promote smokeless tobacco (snus) as a harm reduction measure? PLoS Med. 2007 Jul;4:e185. doi: 10.1371/journal.pmed.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foulds J, Kozlowski L. Snus--what should the public-health response be? Lancet. 2007 Jun 16;369:1976–8. doi: 10.1016/S0140-6736(07)60679-5. [DOI] [PubMed] [Google Scholar]
- 10.WHO Study Group on Tobacco Product Regulation Report on the Scientific Basis of Tobacco Product Regulation. 2008. WHO Technical Report Series, no. 951. Report No.: 951.
- 11.Institute of Medicine Committee to Assess the Science Base for Tobacco Harm Reduction and Board on Health Promotion and Disease Prevention . Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. National Academy Press; Washington, DC: 2001. [Google Scholar]
- 12.Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. National Academy Press (Institute of Medicine); 2001. Products for tobacco exposure reduction. pp. 82–92. [PubMed] [Google Scholar]
- 13.National Cancer Institute . Smoking and Tobacco Control Monograph No. 13. U.S.Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 2001. Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. NIH Pub.No.02-5074. [Google Scholar]
- 14.Cohen JB. Smokers’ knowledge and understanding of advertised tar numbers: health policy implications. Am J Public Health. 1996 Jan;86:18–24. doi: 10.2105/ajph.86.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S.Department of Health and Human Services Reducing the Health Cunsequences of Smoking: 25 Years of Progress. A Report of the Surgeon General. Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 1989. Report No.: DHHS Publication No. (CDC) 89-8411.
- 16.U.S.Department of Health and Human Services Public Health Service Office on Smoking and Health The Health Consequences of Smoking: the Changing Cigarette, a report of the Surgeon General. 1981.
- 17.United States District Court for the District of Columbia Final Opinion on U.S. Department of Justice v. Philip Morris Inc, et al. 2006. Civil Action No. 99-2496.
- 18.United States Court of Appeals Unanimous Opinion on U.S. Department of Justice v. Philip Morris Inc et al., No. 99cv02496. Appeals from the United States District Court for the District of Columbia. 2009.
- 19.Stellman SD, Muscat JE, Hoffmann D, Wynder EL. Impact of filter cigarette smoking on lung cancer histology. Prev Med. 1997 Jul;26:451–6. doi: 10.1006/pmed.1997.0212. [DOI] [PubMed] [Google Scholar]
- 20.Kozlowski LT, Mehta NY, Sweeney CT, et al. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;7:369–75. doi: 10.1136/tc.7.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozlowski LT, Rickert WS, Robinson JC, Grunberg NE. Have tar and nicotine yields of cigarettes changed? Science. 1980 Sep 26;209:1550–1. doi: 10.1126/science.7433979. [DOI] [PubMed] [Google Scholar]
- 22.Benowitz NL, Hall SM, Herning RI, et al. Smokers of low-yield cigarettes do not consume less nicotine. N Engl J Med. 1983 Jul 21;309:139–42. doi: 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. U.S. Department of Health and Human Services, NIH, National Cancer Institute; Bethesda MD: 2001. Compensatory smoking of low-yield cigarettes. U.S.Department of Health and Human Services NNCI, editor. Smoking and tobacco control monograph no. 13. pp. 39–63. [Google Scholar]
- 24.Scherer G, Engl J, Urban M, et al. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007 Mar;47:171–83. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Mendes P, Kapur S, Wang J, Feng S, Roethig H. A randomized, controlled exposure study in adult smokers of full flavor Marlboro cigarettes switching to Marlboro Lights or Marlboro Ultra Lights cigarettes. Regul Toxicol Pharmacol. 2008 Aug;51:295–305. doi: 10.1016/j.yrtph.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Hecht SS, Murphy SE, Carmella SG, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005 Mar;14:693–8. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- 27.Thun MJ, Burns DM. Health impact of “reduced yield” cigarettes: a critical assessment of the epidemiological evidence. Tob Control. 2001;10(Suppl 1):i4–11. doi: 10.1136/tc.10.suppl_1.i4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns DM, Dybing E, Gray N, et al. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob Control. 2008 Apr;17:132–41. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond D, Wiebel F, Kozlowski LT, et al. Revising the machine smoking regime for cigarette emissions: implications for tobacco control policy. Tob Control. 2007 Feb;16:8–14. doi: 10.1136/tc.2005.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond D, Fong G, Cummings KM, et al. Cigarette yields and human exposure: a comparison of alternative testing regimens. Cancer Epidemiol Biomark Prev. 2006;15:1495–501. doi: 10.1158/1055-9965.EPI-06-0047. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute . Smoking and Tobacco Control Monograph 7. U.S.Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 1996. The FTC Cigarette Test Method for Detemining Tar, Nicotine, and Carbon Monoxide Yields of U.S. Cigarettes. NIH Pub.No.96-4028. [Google Scholar]
- 32.Wan J, Johnson M, Schilz J, et al. Evaluation of In Vitro Assays For Assessing the Toxicity of Cigarette Smoke and Smokeless Tobacco. Cancer Epidemiology Biomarkers and Prevention. 2009;XX doi: 10.1158/1055-9965.EPI-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfyl B. The determination of nicotine in tobacco smoke II. Z Unters Lebensm. 1933;6:501–9. [Google Scholar]
- 34.Bradford JA, Harlan WR, Hanmer HR. Nature of cigarette smoke. Technic of experimental smoking. Industrial and Engineering Chemistry. 1936;28:836–9. [Google Scholar]
- 35.Adam T, Baker RR, Zimmermann R. Investigation, by single photon ionisation (SPI)-time-of-flight mass spectrometry (TOFMS), of the effect of different cigarette-lighting devices on the chemical composition of the first cigarette puff. Anal Bioanal Chem. 2007 Jan;387:575–84. doi: 10.1007/s00216-006-0945-9. [DOI] [PubMed] [Google Scholar]
- 36.Peeler CL, Butters GR. Re: It's time for a change: cigarette smokers deserve meaningful information about their cigarettes. J Natl Cancer Inst. 2000 May 17;92:842–3. doi: 10.1093/jnci/92.10.842. [DOI] [PubMed] [Google Scholar]
- 37.Bialous SA, Yach D. Whose standard is it, anyway? How the tobacco industry determines the International Organization for Standardization (ISO) standards for tobacco and tobacco products. Tob Control. 2001 Jun;10:96–104. doi: 10.1136/tc.10.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker RR, Massey ED, Smith G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem Toxicol. 2004;42(Suppl):S53–S83. doi: 10.1016/j.fct.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchogenic carcinoma. J A M A. 1950;143:329–36. doi: 10.1001/jama.1950.02910390001001. [DOI] [PubMed] [Google Scholar]
- 40.Doll R, Hill AB. Smoking and carcinoma of the lung: Preliminary report. Br Med J. 1950;2:739–48. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Federal Trade Commission News Release. FTC to begin cigarette testing. Legacy Tobacco Documents Library. 1967.
- 42.Ogg CL. Determination of Particulate Matter and Alkaloids (as Nicotine) in Cigarette Smoke. Journal of the Association of Official Agricultural Chemists. 1964;47:356–62. [Google Scholar]
- 43.CORESTA Recommended Method No 22. Routine Analytical Cigarette-Smoking Machine Specifications, Definitions and Standard Conditions. CORESTA Information Bulletin. 1991:124–40. [Google Scholar]
- 44.International Organisation for Standardisation . Specification for the Machine and Auxiliary Equipment. First Edition. 1977. Routine Analytical Cigarette Smoking Machine. ISO 3308. [Google Scholar]
- 45.Guillerm R, Radziszewski E. Analysis of smoking pattern including intake of carbon monoxide and influences of changes in cigarette design. In: Thornton RE, editor. Smoking behaviour. Churchill Livingstone; Edinburgh, London and New York: 1978. [Google Scholar]
- 46.International Agency for Research on Cancer IARC Monographs on the Evaluation of the Carcinogenic Risks of Chemicals to Humans: Tobacco Smoking and Involuntary Smoking. [83] 2004. [PMC free article] [PubMed]
- 47.Frederiksen LW, Frazier M. Temporal distribution of smoking. Addict Behav. 1977;2:187–94. doi: 10.1016/0306-4603(77)90016-8. [DOI] [PubMed] [Google Scholar]
- 48.Frederiksen LW, Miller PM, Peterson GL. Topographical components of smoking behavior. Addict Behav. 1977;2:55–61. doi: 10.1016/0306-4603(77)90009-0. [DOI] [PubMed] [Google Scholar]
- 49.Hatsukami D, Morgan SF, Pickens RW, Hughes JR. Smoking topography in a nonlaboratory environment. Int J Addict. 1987 Aug;22:719–25. doi: 10.3109/10826088709027453. [DOI] [PubMed] [Google Scholar]
- 50.Hatsukami DK, Pickens RW, Svikis DS, Hughes JR. Smoking topography and nicotine blood levels. Addict Behav. 1988;13:91–5. doi: 10.1016/0306-4603(88)90031-7. [DOI] [PubMed] [Google Scholar]
- 51.Nemeth-Coslett R, Griffiths RR. Determinants of puff duration in cigarette smokers: I. Pharmacol Biochem Behav. 1984;20:965–71. doi: 10.1016/0091-3057(84)90024-8. [DOI] [PubMed] [Google Scholar]
- 52.Battig K, Buzzi R, Nil R. Smoke yield of cigarettes and puffing behavior in men and women. Psychopharmacology (Berl) 1982;76:139–48. doi: 10.1007/BF00435268. [DOI] [PubMed] [Google Scholar]
- 53.Lee E, Malson J, Waters A, Moolchan E, Pickworth W. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003 Oct;5:673–9. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 54.Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000 Jan 19;92:106–11. doi: 10.1093/jnci/92.2.106. [see comments] [DOI] [PubMed] [Google Scholar]
- 55.Melikian AA, Djordjevic MV, Chen S, Richie J, Jr., Stellman SD. Effect of delivered dosage of cigarette smoke toxins on the levels of urinary biomarkers of exposure. Cancer Epidemiol Biomarkers Prev. 2007 Jul;16:1408–15. doi: 10.1158/1055-9965.EPI-06-1097. [DOI] [PubMed] [Google Scholar]
- 56.Puustinen P, Olkkonen H, Kolonen S, Tuomisto J. Microcomputer-aided measurement of puff parameters during smoking of low- and medium-tar cigarettes. Scand J Clin Lab Invest. 1987 Nov;47:655–60. doi: 10.1080/00365518709168925. [DOI] [PubMed] [Google Scholar]
- 57.Puustinen P, Olkkonen H, Kolonen S, Tuomisto J. Microcomputer assisted measurement of inhalation parameters during smoking. Arch Toxicol Suppl. 1986;9:111–4. doi: 10.1007/978-3-642-71248-7_12. [DOI] [PubMed] [Google Scholar]
- 58.Djordjevic MV, Fan J, Ferguson S, Hoffmann D. Self-regulation of smoking intensity. Smoke yields of the low-nicotine, low-‘tar’ cigarettes. Carcinogenesis. 1995 Sep;16:2015–21. doi: 10.1093/carcin/16.9.2015. [DOI] [PubMed] [Google Scholar]
- 59.Hofer I, Nil R, Battig K. Nicotine yield as determinant of smoke exposure indicators and puffing behavior. Pharmacol Biochem Behav. 1991 Sep;40:139–49. doi: 10.1016/0091-3057(91)90335-y. [DOI] [PubMed] [Google Scholar]
- 60.Ahijevych K, Gillespie J, Demirci M, Jagadeesh J. Menthol and nonmenthol cigarettes and smoke exposure in black and white women. Pharmacol Biochem Behav. 1996 Feb;53:355–60. doi: 10.1016/0091-3057(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 61.Zacny JP, Stitzer ML, Brown FJ, Yingling JE, Griffiths RR. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther. 1987 Feb;240:554–64. [PubMed] [Google Scholar]
- 62.Ossip-Klein DJ, Martin JE, Lomax BD, Prue DM, Davis CJ. Assessment of smoking topography generalization across laboratory, clinical, and naturalistic settings. Addict Behav. 1983;8:11–7. doi: 10.1016/0306-4603(83)90049-7. [DOI] [PubMed] [Google Scholar]
- 63.Sutton SR, Russell MA, Iyer R, Feyerabend C, Saloojee Y. Relationship between cigarette yields, puffing patterns, and smoke intake: evidence for tar compensation? Br Med J (Clin Res Ed) 1982 Aug 28;285:600–3. doi: 10.1136/bmj.285.6342.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams PI. Measurements on puffs taken by human smokers.. 20th Tobacco Chemists Research Conference.; Winston Salem, N.C.. 1966. [Google Scholar]
- 65.Adams PI. Changes in Personal Smoking Habits brought about by changes in cigarette smoke yields. Japan Tobacco and Salt Public Corporation; Tokyo: 1976. pp. 102–8. [Google Scholar]
- 66.Creighton DE, Noble MJ, Whewell RT. Instruments to measure, record, and duplicate human smoking patterns. In: Thornton RE, editor. Smoking Behaviour - Physiological and Psychological Influences. Churchill Livingstone; Edinburgh, London and New York: 1978. pp. 277–88. [Google Scholar]
- 67.O'Connor RJ, Ashare RL, Cummings KM, Hawk LW., Jr Comparing smoking behaviors and exposures from flavored and unflavored cigarettes. Addict Behav. 2007 Apr;32:869–74. doi: 10.1016/j.addbeh.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Pickens R, Gust S, Catchings P, Svikis D. Measurement of some topographical aspects of smoking in the natural environment. In: Grabowski J, Bell C, editors. Monograph 48: Measurement in the Analysis and Treatment of Smoking Behavior. National Institute on Drug Abuse Research; Washington DC: 1983. pp. 62–73. [PubMed] [Google Scholar]
- 69.Henningfield JE, Yingling J, Griffiths RR, Pickens R. An inexpensive portable device for measuring puffing behavior by cigarette smokers. Pharmacology Biochemistry and Behavior. 1980;12:811–3. doi: 10.1016/0091-3057(80)90171-9. [DOI] [PubMed] [Google Scholar]
- 70.Melikian AA, Djordjevic MV, Hosey J, et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res. 2007 Mar;9:377–87. doi: 10.1080/14622200701188836. [DOI] [PubMed] [Google Scholar]
- 71.Eissenberg T, Adams C, Riggins EC, III, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999 Dec;1:317–24. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- 72.Buchhalter AR, Eissenberg T. Preliminary evaluation of a novel smoking system: effects on subjective and physiological measures and on smoking behavior. Nicotine Tob Res. 2000 Feb;2:39–43. doi: 10.1080/14622200050011286. [DOI] [PubMed] [Google Scholar]
- 73.Buchhalter AR, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: comparison with own brand, not own brand, and denicotinized cigarettes. Nicotine Tob Res. 2001 May;3:111–8. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- 74.Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced-exposure products for smokers: clinical laboratory methodology. Nicotine Tob Res. 2002;4(Suppl 2):S131–S140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- 75.Hughes JR, Hecht SS, Carmella SG, Murphy SE, Callas P. Smoking behaviour and toxin exposure during six weeks use of a potential reduced exposure product: Omni. Tob Control. 2004 Jun;13:175–9. doi: 10.1136/tc.2003.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend. 2005 Nov 1;80:259–65. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2006 Jan;15:154–7. doi: 10.1158/1055-9965.EPI-05-0167. [DOI] [PubMed] [Google Scholar]
- 78.Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007 Apr;9:511–8. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- 79.Rees VW, Wayne GF, Connolly GN. Puffing style and human exposure minimally altered by switching to a carbon-filtered cigarette. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17:2995–3003. doi: 10.1158/1055-9965.EPI-07-2533. [DOI] [PubMed] [Google Scholar]
- 80.Grainge M, Shahab L, Hammond D, O'Connor R, McNeill A. First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009 Mar 3; doi: 10.1016/j.drugalcdep.2009.01.013. -PM:19264427. [DOI] [PubMed] [Google Scholar]
- 81.Djordjevic MV, Hoffmann D, Hoffmann I. Nicotine regulates smoking patterns. Prev Med. 1997;26:435–40. doi: 10.1006/pmed.1997.0184. [DOI] [PubMed] [Google Scholar]
- 82.Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005 Jun;14:1370–5. doi: 10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- 83.Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009 Jul;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Evans SE, Buchhalter AR, Breland A, Kleykamp BA, Eissenberg T. Ambulatory puff topography measurement: A validation study. New Orleans, LA: 2003. [Google Scholar]
- 85.Gust SW, Pickens RW, Pechacek TF. Relation of puff volume to other topographical measures of smoking. Addict Behav. 1983;8:115–9. doi: 10.1016/0306-4603(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 86.Guyatt AR, Kirkham AJ, Baldry AG, Dixon M, Cumming G. How does puffing behavior alter during the smoking of a single cigarette? Pharmacol Biochem Behav. 1989 May;33:189–95. doi: 10.1016/0091-3057(89)90449-8. [DOI] [PubMed] [Google Scholar]
- 87.Ahijevych K, Gillespie J. Nicotine dependence and smoking topography among black and white women. Res Nurs Health. 1997 Dec;20:505–14. doi: 10.1002/(sici)1098-240x(199712)20:6<505::aid-nur5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 88.National Cancer Institute Expert Committee . The FTC cigarette test method for determining, tar, nicotine and carbon monoxide yields of U.S. cigarettes. [Monograph No. 7.] U.S.Department of Health and Human Services National Cancer Institute.; 1996. [Google Scholar]
- 89.Hammond D, Collishaw NE, Callard C. Secret science: tobacco industry research on smoking behaviour and cigarette toxicity. Lancet. 2006 Mar 4;367:781–7. doi: 10.1016/S0140-6736(06)68077-X. [DOI] [PubMed] [Google Scholar]
- 90.Kozlowski LT, O'Connor RJ. Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control. 2002 Mar;11(Suppl 1):I40–I50. doi: 10.1136/tc.11.suppl_1.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kozlowski LT, O'Connor RJ. Official cigarette tar tests are misleading: use a two-stage, compensating test. Lancet. 2000 Jun 17;355:2159–61. doi: 10.1016/S0140-6736(00)02390-4. [DOI] [PubMed] [Google Scholar]
- 92.Assaf AR, Parker D, Lapane KL, McKenney JL, Carleton RA. Are there gender differences in self-reported smoking practices? Correlation with thiocyanate and cotinine levels in smokers and nonsmokers from the Pawtucket Heart Health Program. J Womens Health (Larchmt ) 2002 Dec;11:899–906. doi: 10.1089/154099902762203731. [DOI] [PubMed] [Google Scholar]
- 93.Thun MJ, Heath CW., Jr Changes in mortality from smoking in two American Cancer Society prospective studies since 1959. Prev Med. 1997 Jul;26:422–6. doi: 10.1006/pmed.1997.0182. [DOI] [PubMed] [Google Scholar]
- 94.Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991-1993. Nicotine Tob Res. 2000 Aug;2:263–74. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- 95.Etter JF, Vu DT, Perneger TV. Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol. 2000 Feb 1;151:251–8. doi: 10.1093/oxfordjournals.aje.a010200. [DOI] [PubMed] [Google Scholar]
- 96.Mustonen TK, Spencer SM, Hoskinson RA, Sachs DP, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob Res. 2005 Aug;7:581–90. doi: 10.1080/14622200500185199. [DOI] [PubMed] [Google Scholar]
- 97.Epstein LH, Dickson BE, Ossip DJ, et al. Relationships among measures of smoking topography. Addict Behav. 1982;7:307–10. doi: 10.1016/0306-4603(82)90061-2. [DOI] [PubMed] [Google Scholar]
- 98.Wood T, Wewers ME, Groner J, Ahijevych K. Smoke constituent exposure and smoking topography of adolescent daily cigarette smokers. Nicotine Tob Res. 2004 Oct;6:853–62. doi: 10.1080/1462220042000282537. [DOI] [PubMed] [Google Scholar]
- 99.Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999 Jan;24:115–20. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 100.Lombardo T, Carreno L. Relationship of type A behavior pattern in smokers to carbon monoxide exposure and smoking topography. Health Psychol. 1987;6:445–52. doi: 10.1037//0278-6133.6.5.445. [DOI] [PubMed] [Google Scholar]
- 101.Hatsukami DK, Morgan SF, Pickens RW, Champagne SE. Situational factors in cigarette smoking. Addict Behav. 1990;15:1–12. doi: 10.1016/0306-4603(90)90002-f. [DOI] [PubMed] [Google Scholar]
- 102.Morgan SF, Gust SW, Pickens RW, Champagne SE, Hughes JR. Temporal patterns of smoking topography in the natural environment. Int J Addict. 1985 Apr;20:613–21. doi: 10.3109/10826088509044940. [DOI] [PubMed] [Google Scholar]
- 103.Herning RI, Jones RT, Benowitz NL, Mines AH. How a cigarette is smoked determines blood nicotine levels. Clin Pharmacol Ther. 1983 Jan;33:84–90. doi: 10.1038/clpt.1983.12. [DOI] [PubMed] [Google Scholar]
- 104.Bridges RB, Combs JG, Humble JW, et al. Puffing topography as a determinant of smoke exposure. Pharmacol Biochem Behav. 1990 Sep;37:29–39. doi: 10.1016/0091-3057(90)90037-i. [DOI] [PubMed] [Google Scholar]
- 105.Kolonen S, Tuomisto J, Puustinen P, Airaksinen MM. Puffing behavior during the smoking of a single cigarette in a naturalistic environment. Pharmacol Biochem Behav. 1992 Apr;41:701–6. doi: 10.1016/0091-3057(92)90215-2. [DOI] [PubMed] [Google Scholar]
- 106.Djordjevic MV, Fan J, Ferguson S, Hoffmann D. Self-regulation of smoking intensity. Smoke yields of the low-nicotine, low ‘tar’ cigarettes. Carcinogenesis. 1995;16:2015–21. doi: 10.1093/carcin/16.9.2015. [DOI] [PubMed] [Google Scholar]
- 107.Lichtenstein E, Antonuccio DO. Dimensions of smoking behavior. Addict Behav. 1981;6:365–7. [Google Scholar]
- 108.Burling TA, Stitzer ML, Bigelow GE, Mead AM. Smoking topography and carbon monoxide levels in smokers. Addict Behav. 1985;10:319–23. doi: 10.1016/0306-4603(85)90014-0. [DOI] [PubMed] [Google Scholar]
- 109.Bridges RB, Humble JW, Turbek JA, Rehm SR. Smoking history, cigarette yield and smoking behavior as determinants of smoke exposure. Eur J Respir Dis Suppl. 1986;146:129–37. [PubMed] [Google Scholar]
- 110.Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000 Jan 19;92:106–11. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- 111.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999 Jul;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- 112.Buzzi R, Nil R, Battig K. Development of puffing behavior along burning time of a cigarette--no relation to alveolar inhalation or nicotine delivery of the cigarettes? Psychopharmacology (Berl) 1985;86:102–7. doi: 10.1007/BF00431692. [DOI] [PubMed] [Google Scholar]
- 113.Rieben FW. Smoking behaviour and increase in nicotine and carboxyhaemoglobin in venous blood. Clin Investig. 1992 Mar;70:335–42. doi: 10.1007/BF00184670. [DOI] [PubMed] [Google Scholar]
- 114.Zacny JP, Stitzer ML, Yingling JE. Cigarette filter vent blocking: effects on smoking topography and carbon monoxide exposure. Pharmacol Biochem Behav. 1986 Dec;25:1245–52. doi: 10.1016/0091-3057(86)90119-x. [DOI] [PubMed] [Google Scholar]
- 115.Weinhold LL, Stitzer ML. Effects of puff number and puff spacing on carbon monoxide exposure from commercial brand cigarettes. Pharmacol Biochem Behav. 1989 Aug;33:853–8. doi: 10.1016/0091-3057(89)90482-6. [DOI] [PubMed] [Google Scholar]
- 116.Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007 Jan 12;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 117.Sweeney CT, Kozlowski LT. Blocking filter vents increases carbon monoxide levels from ultralight, but not light cigarettes. Pharmacol Biochem Behav. 1998 Mar;59:767–73. doi: 10.1016/s0091-3057(97)00567-4. [DOI] [PubMed] [Google Scholar]
- 118.Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 2005 Oct;82:320–9. doi: 10.1016/j.pbb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 119.Kolonen S, Tuomisto J, Puustinen P, Airaksinen MM. Smoking behavior in low-yield cigarette smokers and switchers in the natural environment. Pharmacol Biochem Behav. 1991 Sep;40:177–80. doi: 10.1016/0091-3057(91)90341-x. [DOI] [PubMed] [Google Scholar]
- 120.Stepney R. Would a medium-nicotine, low-tar cigarette be less hazardous to health? Br Med J (Clin Res Ed) 1981 Nov 14;283:1292–6. doi: 10.1136/bmj.283.6302.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Russell MA, Sutton SR, Iyer R, Feyerabend C, Vesey CJ. Long-term switching to low-tar low-nicotine cigarettes. Br J Addict. 1982 Jun;77:145–58. doi: 10.1111/j.1360-0443.1982.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 122.Ossip-Klein DJ, Epstein LH, Winter MK, et al. Does switching to low tar/nicotine/carbon monoxide-yield cigarettes decrease alveolar carbon monoxide measures? A randomized controlled trial. J Consult Clin Psychol. 1983 Apr;51:234–41. [PubMed] [Google Scholar]
- 123.Nil R, Buzzi R, Battig K. Effects of different cigarette smoke yields on puffing and inhalation: is the measurement of inhalation volumes relevant for smoke absorption? Pharmacol Biochem Behav. 1986 Mar;24:587–95. doi: 10.1016/0091-3057(86)90563-0. [DOI] [PubMed] [Google Scholar]
- 124.Armitage AK, Alexander J, Hopkins R, Ward C. Evaluation of a low to middle tar/medium nicotine cigarette designed to maintain nicotine delivery to the smoker. Psychopharmacology (Berl) 1988;96:447–53. doi: 10.1007/BF02180022. [DOI] [PubMed] [Google Scholar]
- 125.Zacny JP, Stitzer ML. Cigarette brand-switching: effects on smoke exposure and smoking behavior. J Pharmacol Exp Ther. 1988 Aug;246:619–27. [PubMed] [Google Scholar]
- 126.Nil R, Battig K. Separate effects of cigarette smoke yield and smoke taste on smoking behavior. Psychopharmacology (Berl) 1989;99:54–9. doi: 10.1007/BF00634452. [DOI] [PubMed] [Google Scholar]
- 127.Baldinger B, Hasenfratz M, Battig K. Switching to ultralow nicotine cigarettes: effects of different tar yields and blocking of olfactory cues. Pharmacol Biochem Behav. 1995 Feb;50:233–9. doi: 10.1016/0091-3057(94)00302-y. [DOI] [PubMed] [Google Scholar]
- 128.Herning RI, Jones RT, Bachman J, Mines AH. Puff volume increases when low-nicotine cigarettes are smoked. Br Med J (Clin Res Ed) 1981 Jul 18;283:187–9. doi: 10.1136/bmj.283.6285.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tobin MJ, Sackner MA. Monitoring smoking patterns of low and high tar cigarettes with inductive plethysmography. Am Rev Respir Dis. 1982 Aug;126:258–64. doi: 10.1164/arrd.1982.126.2.258. [DOI] [PubMed] [Google Scholar]
- 130.McBride MJ, Guyatt AR, Kirkham AJ, Cumming G. Assessment of smoking behaviour and ventilation with cigarettes of differing nicotine yields. Clin Sci (Lond) 1984 Dec;67:619–31. doi: 10.1042/cs0670619. [DOI] [PubMed] [Google Scholar]
- 131.Gust SW, Pickens RW. Does cigarette nicotine yield affect puff volume? Clin Pharmacol Ther. 1982 Oct;32:418–22. doi: 10.1038/clpt.1982.182. [DOI] [PubMed] [Google Scholar]
- 132.Woodman G, Newman SP, Pavia D, Clarke SW. The separate effects of tar and nicotine on the cigarette smoking manoeuvre. Eur J Respir Dis. 1987 May;70:316–21. [PubMed] [Google Scholar]
- 133.Woodman G, Newman SP, Pavia D, Clarke SW. Inhaled smoke volume and puff indices with cigarettes of different tar and nicotine levels. Eur J Respir Dis. 1987 Mar;70:187–92. [PubMed] [Google Scholar]
- 134.Benowitz NL, Jacob P, III, Bernert JT, et al. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev. 2005 Jun;14:1376–83. doi: 10.1158/1055-9965.EPI-04-0667. [DOI] [PubMed] [Google Scholar]
- 135.Sweeney CT, Kozlowski LT, Parsa P. Effect of filter vent blocking on carbon monoxide exposure from selected lower tar cigarette brands. Pharmacol Biochem Behav. 1999 May;63:167–73. doi: 10.1016/s0091-3057(98)00250-0. [DOI] [PubMed] [Google Scholar]
- 136.Rees VW, Wayne GF, Thomas BF, Connolly GN. Physical design analysis and mainstream smoke constituent yields of the new potential reduced exposure product, Marlboro UltraSmooth. Nicotine Tob Res. 2007 Nov;9:1197–206. doi: 10.1080/14622200701648375. [DOI] [PubMed] [Google Scholar]
- 137.Breland AB, Acosta MC, Eissenberg T. Tobacco specific nitrosamines and potential reduced exposure products for smokers: a preliminary evaluation of Advance. Tob Control. 2003 Sep;12:317–21. doi: 10.1136/tc.12.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Breland AB, Evans SE, Buchhalter AR, Eissenberg T. Acute effects of Advance: a potential reduced exposure product for smokers. Tob Control. 2002 Dec;11:376–8. doi: 10.1136/tc.11.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006 Dec;8:727–38. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- 140.Slade J, Connolly GN, Lymperis D. Eclipse: does it live up to its health claims? Tob Control. 2002 Jun;11(Suppl 2):ii64–ii70. doi: 10.1136/tc.11.suppl_2.ii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee EM, Malson JL, Moolchan ET, Pickworth WB. Quantitative comparisons between a nicotine delivery device (Eclipse) and conventional cigarette smoking. Nicotine Tob Res. 2004 Feb;6:95–102. doi: 10.1080/14622200310001656911. [DOI] [PubMed] [Google Scholar]
- 142.Hatsukami DK, Benowitz NL, Rennard SI, Oncken C, Hecht SS. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine Tob Res. 2006 Aug;8:600–22. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tobin MJ, Jenouri G, Sackner MA. Subjective and objective measurement of cigarette smoke inhalation. Chest. 1982 Dec;82:696–700. doi: 10.1378/chest.82.6.696. [DOI] [PubMed] [Google Scholar]
- 144.Sackner J, Nixon A, Davis B, Atkins N, Sackner M. Non-invasive measurement of ventilation during exercise using a respiratory inductive plethysmograph. I. Am Rev Respir Dis. 1980 Dec;122:867–71. doi: 10.1164/arrd.1980.122.6.867. [DOI] [PubMed] [Google Scholar]
- 145.Chadha TS, Watson H, Birch S, et al. Validation of respiratory inductive plethysmography using different calibration procedures. Am Rev Respir Dis. 1982 Jun;125:644–9. doi: 10.1164/arrd.1982.125.6.644. [DOI] [PubMed] [Google Scholar]
- 146.Charles FK, Krautter GR, Mariner DC. Post-puff respiration measures on smokers of different tar yield cigarettes. Inhal Toxicol. 2009 Feb 19;:1–7. doi: 10.1080/08958370802353443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Armitage AK, Dixon M, Frost DE, Mariner DC, Sinclair NM. The Effect of Inhalation Volume and Breath-Hold Duration on the Retention of Nicotine and Solanesol in the Human Respiratory Tract and on Subsequent Plasma Nicotine Concentrations During Cigarette Smoking. Contributions to Tobacco Research. 2004;21:240–9. doi: 10.1021/tx0340753. [DOI] [PubMed] [Google Scholar]
- 148.Feng S, Plunkett SE, Lam K, et al. A new method for estimating the retention of selected smoke constituents in the respiratory tract of smokers during cigarette smoking. Inhal Toxicol. 2007 Feb;19:169–79. doi: 10.1080/08958370601052022. [DOI] [PubMed] [Google Scholar]
- 149.Rickert WS, Robinson JC, Collishaw NE, Bray DF. Estimating the hazards of “less hazardous” cigarettes. III. A study of the effect of various smoking conditions on yields of hydrogen cyanide and cigarette tar. J Toxicol Environ Health. 1983 Jul;12:39–54. doi: 10.1080/15287398309530406. [DOI] [PubMed] [Google Scholar]
- 150.International Organization for Standardization - Ad Hoc Smoking Behavior Review Team Working Group 9 of the ISO TC 126. A review of human smoking behavior data and recommendations for a new ISO standard for the machine smoking of cigarettes. 2005.
- 151.Kozlowski LT. Application of some physical indicators of cigarette smoking. Addict Behav. 1981;6:213–9. doi: 10.1016/0306-4603(81)90019-8. [DOI] [PubMed] [Google Scholar]
- 152.Kozlowski LTOR, Sweeney C. Cigarette design. Smoking and Tobacco Control Monograph No. 13: Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. National Cancer Institute; Bethesda MD: 2001. pp. 13–37. [Google Scholar]
- 153.Kozlowski LT, O'Connor RJ. Official cigarette tar tests are misleading: use a two-stage, compensating test. Lancet. 2000;355:2159–61. doi: 10.1016/S0140-6736(00)02390-4. [DOI] [PubMed] [Google Scholar]
- 154.Harris JE. Smoke yields of tobacco-specific nitrosamines in relation to FTC tar level and cigarette manufacturer: analysis of the Massachusetts Benchmark Study. Public Health Rep. 2001 Jul;116:336–43. doi: 10.1093/phr/116.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rickert WS. Determination of Cigarette Yields under Realistic Conditions. Prepared for Health Canada by Labstat Inc. 1996.
- 156.Schorp MK. A review of human smoking behavior data and recommendations for a new ISO standard for the machine smoking of cigarettes. International Organization for Standardization; 2005. Summary of Literature Data on Smoking Topography. Report of the AD HOC ISO/TC126/WG9. [Google Scholar]
- 157.Hammond D, O'Connor RJ. Constituents in tobacco and smoke emissions from Canadian cigarettes. Tob Control. 2008 Sep;17(Suppl 1):i24–i31. doi: 10.1136/tc.2008.024778. [DOI] [PubMed] [Google Scholar]
- 158.Rickert WS, Trivedi AH, Momin RA, Wright WG, Lauterbach JH. Effect of smoking conditions and methods of collection on the mutagenicity and cytotoxicity of cigarette mainstream smoke. Toxicol Sci. 2007 Apr;96:285–93. doi: 10.1093/toxsci/kfl195. [DOI] [PubMed] [Google Scholar]
- 159.Hammond D, O'Connor RJ. Constituents in tobacco and smoke emissions from Canadian cigarettes. Tob Control. 2008 Sep;17(Suppl 1):i24–i31. doi: 10.1136/tc.2008.024778. [DOI] [PubMed] [Google Scholar]
- 160.Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005 Apr;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 161.Roemer E, Stabbert R, Rustemeier K, et al. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004 Jan 15;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 162.Harris JE. Incomplete compensation does not imply reduced harm: yields of 40 smoke toxicants per milligram nicotine in regular filter versus low-tar cigarettes in the 1999 Massachusetts Benchmark Study. Nicotine Tob Res. 2004 Oct;6:797–807. doi: 10.1080/1462220042000274266. [DOI] [PubMed] [Google Scholar]
- 163.Urban HJ, Gomm W, Schorp M. A Modelling Approach to Develop Machine Smoking Protocols Reflecting Human Puffing Behaviour for Conventional Cigarettes. Contributions to Tobacco Research. 2008;23:8–18. [Google Scholar]
- 164.Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2004 Nov;13:1800–4. [PubMed] [Google Scholar]
- 165.Lerman C, Wileyto EP, Patterson F, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J. 2004;4:184–92. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- 166.Lerman C, Shields PG, Wileyto EP, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogen. 2002 Nov;12:627–34. doi: 10.1097/00008571-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 167.Lerman C, Berrettini W. Elucidating the role of genetic factors in smoking behavior and nicotine dependence. Am J Med Genet. 2003 Apr 1;118B:48–54. doi: 10.1002/ajmg.b.10003. [DOI] [PubMed] [Google Scholar]
- 168.Lerman C, Caporaso NE, Main D, et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- 169.Ring HZ, Valdes AM, Nishita DM, et al. Gene-gene interactions between CYP2B6 and CYP2A6 in nicotine metabolism. Pharmacogenet Genomics. 2007 Dec;17:1007–15. doi: 10.1097/01.fpc.0000220560.59972.33. [DOI] [PubMed] [Google Scholar]
- 170.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007 Oct;8:1385–402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 171.Benowitz NL, Swan GE, Jacob P, III, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006 Nov;80:457–67. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 172.Malaiyandi V, Goodz SD, Sellers EM, Tyndale RF. CYP2A6 genotype, phenotype, and the use of nicotine metabolites as biomarkers during ad libitum smoking. Cancer Epidemiol Biomarkers Prev. 2006 Oct;15:1812–9. doi: 10.1158/1055-9965.EPI-05-0723. [DOI] [PubMed] [Google Scholar]
- 173.Rickert WS, Trivedi AH, Momin RA, Wright WG, Lauterbach JH. Effect of smoking conditions and methods of collection on the mutagenicity and cytotoxicity of cigarette mainstream smoke. Toxicol Sci. 2007 Apr;96:285–93. doi: 10.1093/toxsci/kfl195. [DOI] [PubMed] [Google Scholar]
- 174.Hammond D, O'Connor RJ. Constituents in tobacco and smoke emissions from Canadian cigarettes. Tob Control. 2008 Sep;17(Suppl 1):i24–i31. doi: 10.1136/tc.2008.024778. [DOI] [PubMed] [Google Scholar]
- 175.Guillerm R, Radziszewski E. A new method of analyzing the act of smoking. Ann Tabac Etudes L'Equiment. 1975;13:101–10. [Google Scholar]
- 176.Rawbone RC, Murphy K, Tate ME. The analysis of smoking patterns. In: Thornton RE, editor. Smoking Behaviour. Churchill Livingstone; London: 1978. pp. 171–94. [Google Scholar]
- 177.Sackner MA. Monitoring of ventilation without a physical connection to the airway. In: Sackner MA, editor. Diagnostic techniques in pulmonary disease. Marcel Dekker; New-York: 1980. pp. 503–37. Pat 1. [Google Scholar]