Abstract
Aflatoxin B1 (AFB1) is a carcinogenic metabolite produced by certain Aspergillus species on agricultural commodities. AFB1 biosynthesis is affected by jasmonic acid and also by its methylester (MeJA), a plant growth regulator derived from linoleic acid. This study reports the effect of MeJA on the growth of A. parasiticus and AFB1 output in yeast extract sucrose (YES) medium when added at three different concentrations; namely, 10−2 M, 10−4 M, and 10−6 M. AFB1 determination was performed by immunoaffinity and HPLC. MeJA at 10−4 and 10−6 M concentrations had no significant effect on mycelial growth but did affect AFB1 production after the 7th day of incubation; on the 12th day, AFB1 production was increased by 212.7% and 141.6% compared to the control samples (addition of 10−6 M and 10−4 M MeJA, resp.). Treatment of A. parasiticus cultures with 10−2 M MeJA inhibited mycelial growth and AFB1 production as well. These results suggest that the effect of MeJA on AFB1 biosynthesis by A. parasiticus depends on the MeJA concentration used.
1. Introduction
Aflatoxins are polyketide secondary fungal metabolites produced by the toxigenic strains of Aspergillus flavus, Aspergillus parasiticus and Aspergillus nomius [1–4], and they are known as potent carcinogenic, teratogenic as well as genotoxic mycotoxins. The most potent of the four naturally occurring aflatoxins is Aflatoxin B1 (AFB1) [5].
A variety of studies has been conducted in order to understand the process of crop contamination by aflatoxins. They all suggested that Aspergilli generally gain access to the plant seeds either through cracks generated by an environmental stress (heat or draft) or via insect damage [6, 7]. In addition, oily seeds are preferentially colonized in comparison to starchy ones [8]. Once the fungus has invaded the seed, it first destroys the lipid bodies, which are primarily composed of palmitic, oleic, and linolenic acids [9, 10]. Since there are many in vitro studies which have shown that lipid oxidation affects aflatoxin biosynthesis [11–14], it is of great interest to determine the mode of interaction between unsaturated fatty acids and their metabolites and aflatoxin production. Linoleic as well as linolenic acids can undergo a regio and stereospecific oxygenation [15] catalyzed by the widely distributed plant stress response enzyme lipoxygenase (LOX) [16] to yield 13S-hydroperoxy-cis-9-trans-11-octadecadienoic acid (13S-HPODE) and 13S-hydroperoxy-cis-9, trans-11, cis-15-octadecatrienoic acid (13S-HPOTE), respectively [17]. These fatty acid hydroperoxides are further converted through the octadecanoid pathway to jasmonates [18], a group of bioactive signaling compounds involved in different (multiple) aspects of plant response to their biotic and abiotic environment [19, 20].
In plants, jasmonates are synthesized as a response to systemic or localized signals like oligosaccharides released from fungi or plant cell walls during plant-pathogen interactions [21]. According to Pühler et al. [22], phytopathogenic bacterial species have developed specific methods to attack plant cells and to use plant molecules for their own growth; the bacterial genome research gave information on the distribution of bacterial secretion systems, which play a role in the interactions with plant cells. Weiler et al. [23] have reported that jasmonic acid levels increased rapidly in response to biotic and abiotic stress such as mechanical stress. Moreover, it has been reported that aflatoxins affect the amino acid uptake, enzymatic activities, germination as well as protein and nucleic acid synthesis in several plant systems. Recently, Ağar et al. [24] have reported that the levels of endogenous hormones (Gibberelic acid equivalents) decreased in Zea mays seeds treated with AFB1. In addition, natural elicitors were combined with methyl jasmonate (MeJA) to evaluate its effects on phytoalexin and AFB1 production in cotton plants [25].
In the case of Aspergilli, interactions between jasmonates, mycelial growth and aflatoxin production have been reported by several authors. These interactions are of great interest as they suggest that there is a mechanism involving plant LOX pathways that affect aflatoxin biosynthesis. It is interesting that both inhibition and stimulation of aflatoxin production by various LOX metabolites have been reported. Also, some hydroperoxy fatty acids may exert a stronger signalling influence on aflatoxin/sterigmatocystin (AF/ST) biosynthesis than on others. For example, aflatoxin biosynthesis by A. parasiticus was stimulated in synthetic medium containing a mixture of 30% 13S-HPODE and ~70% 13-HPODE although 13-HPODE has an inhibitory effect when tested alone. MeJA treatment at concentrations from 10−6 M to 10−3 M reduced AFB1 production by A. flavus grown on either Czapek yeast extract agar (CYA) medium or pistachios in storage [26]. On the contrary, Vergopoulou et al. [27] have reported that treatment with MeJA at a concentration of 10−4 M stimulated AFB1 production by A. parasiticus grown on yeast extract sucrose medium (YES). In a recent review, Holmes et al. [28] have underlined that lipoxygenase-generated signals, such as jasmonates, have both inhibitory and promoting effects on the AFB1 production by the aflatoxigenic Aspergilli.
The purpose of this study was to establish the significance and the consequences from the use of different MeJA concentrations on mold growth and AFB1 output under defined conditions.
2. Materials and Methods
2.1. Apparatus
A laminar flow (Telstar Bio IIA, Madrid, Spain), an autoclave (Selecta Autester-E Dry, PBI Milano, Italy), an incubator (WTB Binder, Tuttingen, Germany), and a centrifuge (Sorvall RC-5B, Norwalk, USA) were used during this study. HPLC was performed using a Hewlett-Packard 1050 (Waldborn, Germany) liquid chromatograph equipped with a JASCO FP-920 (Japan) fluorescence detector and an HP integrator 3395. The HPLC column used was a C18 Nova-Pak (60 , 4 μm, 4.6 × 250 mm). The mobile phase for AFB1 determination [water+acetonitrile+methanol (20+4+3)] was filtered through Milipore HVLP filters (0.45 μm) before use. Detection of the AFB1 hemiacetal derivative (AFB2a) was carried out at λ ex = 365 nm and λ em = 425 nm. The flow rate was 1 mL min −1 and the retention time for AFB2a was 8 minutes.
2.2. Reagents
The AFB1 standard was purchased from Sigma-Aldrich (St. Louis, MO, USA). The filters and the C18 Nova-Pak HPLC column were from Waters (Millipore, Milford, MA, USA). The Aflaprep immunoaffinity columns were from Rhone Diagnostics (Glasgow, UK). All other reagents and HPLC solvents were of HPLC grade (LABSCAN, Dublin, Ireland). Trifluoroacetic acid was purchased from Merck (Darmstadt, Germany). The purity of MeJA used was tested by GC analysis using a Hewlett-Packard gas chromatograph (equipped with a flame ionization detector) on a BPX70-coated fused-silica capillary column [29].
2.3. Media
Aspergillus flavus parasiticus agar (AFPA) was prepared by dissolving 4 g of yeast extract (Oxoid, Basingstoke, Hampshire, England), 2 g of bacteriological peptone (Oxoid), 0.1 g of ferric ammonium citrate, 0.2 mL of Dichloran (0.2% in ethanol, Fluka, Neu-Ulm, Switzerland), 0.02 g of chloramphenicol (Oxoid), and 3 g of agar (Oxoid) in 200 mL of distilled water, final pH 6.0–6.5 [30]. Czapek Dox agar (CZA) was prepared by dissolving 0.4 g of sodium nitrite, 0.1 g of potassium chloride, 0.1 g of magnesium sulfate, 0.002 g of ferric sulfate, 0.2 g of dipotassium phosphate, 6 g of sucrose, 3 g of agar, 0.002 g of zinc sulfate, and 0.001 g of copper sulfate in 200 mL distilled water, final pH 6.0–6.5. Yeast extract sucrose (YES) broth was prepared by dissolving 2 g of yeast extract and 15 g of sucrose in 100 mL distilled water, final pH 6.0–6.5 [30].
2.4. Preparation of Spore Inoculum
The aflatoxigenic strain A. parasiticus Speare (IMI 283883) utilized throughout this study was obtained from the International Mycological Institute (Engham, Surrey, UK). An inoculum was obtained by growing the mold on a slant of stock cultures of CZA, which were maintained at 5°C [31]. Spore inoculum was prepared by growing A. parasiticus on CZA for 7 days at 30°C, and spores were harvested aseptically using 10 mL of sterile 0.01 % (v/v) Tween 80 solution [32]. AFB1 carried over from the initial growth was minimized by centrifuging the spore suspension (1000 g for 1 min) and resuspending the biomass in 10 mL of sterile Tween 80 solution twice. Dilutions (10−1, 10−2, 10−3, 10−4) from the initial spore were prepared in sterile tubes containing 10 mL of 0.05% Tween 80 (v/v) suspension. The spore concentration was determined by the spread plate surface count technique, using 0.1 mL of each dilution on four AFPA plates [30, 33] after incubation at 30°C for 2 days. The population size was estimated by counting the single colonies from their reverse intense yellow/orange coloration. In order to obtain an inoculum containing 102 conidia, plates with 10–100 colony forming units (cfu) were selected and the desired 102 spore quantity used in this study was estimated.
The quantity of 102 spores flask−1 was chosen as it was the minimum concentration found in literature producing detectable amounts of AFB1 by Aspergillus [34].
2.5. Inoculation
Twelve flasks for each day of observation containing 10 mL of YES medium were inoculated with 102 spores flask−1 of A. parasiticus in the appropriate volume from the selected dilution. MeJA in ethanol at final concentrations of 10−2 M, 10−4 M, and 10−6 M flask−1 was added into each of the three flasks for each day of observation. All flasks, control (simply ethanol) and treated with MeJA, were incubated under stationary conditions at 30°C. Immediately after autoclaving for 30 minutes at 115°C as it is suggested for safety reasons [35], the mycelial growth was determined and AFB1 was assayed on days 0, 3, 7, 9, 12, and 15 of incubation. The experiment was repeated in triplicate.
2.6. AFB1 Determination
The content of each flask (containing the fungus in YES medium) was mixed with 30 mL of methanol and wellshaken for 10 min. After filtration, an aliquot of 1 mL from each flask was used for AFB1 analysis. The 1 mL aliquot from the filtrate was mixed with 10 mL distilled water. The mixture was transferred onto an Aflaprep immunoaffinity column and washed twice with 10 mL of distilled water (flow rate: 6 mL min−1). The column was then allowed once more to dry by passing air through it. AFB1 was eluted with 2 mL of acetonitrile (flow rate: 0.3 mL min−1). Before derivatization, the eluate was evaporated to dryness on a water bath under a gentle steam of nitrogen [36].
2.7. Derivatization and HPLC Analysis
A derivative of AFB1 (AFB2a, hemiacetal of AFB1) was prepared by adding 200 μL of hexane and 200 μL of trifluoroacetic acid to the evaporated solution of AFB1 eluate, heating for 10 min at 40°C in a water bath, evaporating to dryness under nitrogen, redissolving in an appropriate volume of water-acetonitrile (9 : 1) to give an AFB1 concentration of <10 ng mL−1 and analyzing by HPLC (volume injected: 20 μL). AFB2a shows enhanced fluorescence compared to AFB1 [36].
2.8. Determination of Mycelial Mass
After extraction, mycelia were filtered through filters that were previously dried (24 h at 80°C) and weighed. The mycelium was washed with distilled water and allowed to dry for 24 h at 80°C. The dry weight of the mycelium was then determined [37].
2.9. Statistical Analysis
Data were analyzed by one-way and two-way analysis of variance. The mean differences which are significantly different were examined by using the Tukey test [38].
3. Results and Discussion
The analytical protocol for AFB1 determination in YES medium was previously in-house characterized in detail by Leontopoulos et al. [39]. The recovery of the method was found to be 90.9% and the detection limit, based on a signal-to-noise ratio of 3 : 1 at the retention time (8 min) of the derivatized AFB1 (AFB2a), was 0.2 ng flask−1 corresponding to 0.02 ng mL−1 YES medium.
A satisfactory linear relationship was established between different quantities (1, 2.5, 5 μg) of AFB1 spiked in 10 mL of YES medium and quantities recovered (y = 0.909x + 0.3, r = 0.999).
3.1. The Effect of MeJa on A. parasiticus Growth
YES medium is an optimum medium for A. parasiticus growth and AFB1 biosynthesis [40]. In the present study, A. parasiticus was used because AFB1 production is a more stable trait in this fungus than in A. flavus [41]. In addition, AFB1 was studied throughout this study as it is the most potent mycotoxin and it is usually produced at the highest levels by toxigenic strains [42].
We studied the effect of MeJA at final concentrations 10−6 M, 10−4 M, and 10−2 M on both mycelial growth of A. parasiticus in YES medium and AFB1 production. It must be mentioned that 10−4 M is the concentration of MeJA which has been found to be effective as a postharvest treatment for suppressing the decay caused by Botrytis cinerea on strawberries, for reducing the decay by Penicillium digitarum in grapefruit as well as for reducing microbial contamination in celery and peppers [43]. Gogala [44] has also reported that jasmonate was highly active at medium concentrations (10−6 M to 10−4 M), but inhibition of the mycorrhizal growth has been observed at lower concentrations (~10−7 M). To our knowledge, the 10−2 M concentration of MeJA has not yet been tested.
The mycelial growth of nontreated and MeJA-treated A. parasiticus in YES medium is shown in Table 1. The maximum growth of the mold was observed on the 7th day after inoculation for the control samples (352.5 mg flask−1) as well as for the samples treated with MeJA at concentrations 10−6 M (374.8 mg flask−1) and 10−4 M (359.5 mg flask−1). On the contrary, no visible mycelial growth by A. parasiticus or measurable amount of the fungus was observed in samples treated with MeJA 10−2 M during the whole period of incubation (15 days).
Table 1.
Mycelial growth (mg flask−1) of A. parasiticus in YES medium.
| MeJA addition | ||||
|---|---|---|---|---|
| 0(a) | 10−6 M(b) | 10−4 M(c) | 10−2 M(d) | |
| Dry weight of mycelium | ||||
| Days | mg flask−1 | mg flask−1 | mg flask−1 | mg flask−1 |
| (±SD) | (±SD) | (±SD) | (±SD) | |
| 0 | 0 | 0 | 0 | 0 |
| 3 | 282.7 (±17.7) | 290.8 (±5.0) | 259.2 (±5.0) | NMe |
| 7 | 352.5 (±9.9) | 374.8 (±5.1) | 359.5 (±5.8) | NM |
| 9 | 314.9 (±13.5) | 341.8 (±9.5) | 321.6 (±14.4) | NM |
| 12 | 272.4 (±4.7) | 296.3 (±1.8) | 285.4 (±17.4) | NM |
| 15 | 224.6 (±23.54) | 249.8 (±5.4) | 233.3 (±21.5) | NM |
(a)Without MeJA addition (control); (b)addition of 0.0022 mg MeJA flask−1; (c)addition of 0.227 mg MeJA flask−1; (d)addition of 22.67 mg; no visible mycelial growth was observed during the whole period of observation; (e)non measurable.
The statistical analysis by using one-way Anova applied to all groups, with or without MeJA treatment, showed that the F exp = 7.40 was higher than the F theor = 3.05 for df 3, 20. Therefore, the variation of mycelial growth between the four groups is statistically significant (P < .05). It must be added, however, that the statistically significant variation between the four groups of samples may be due to just one of the four groups, probably to the samples treated with 10−2 M MeJA. The comparison of variances of the other three groups (control, MeJA 10−6 M, MeJA 10−4 M) showed that, at 0.05 level, the differences between the mycelial growth for the three groups are not significant and that the 10−6 M and 10−4 M MeJA concentrations had no apparent effect on A. parasiticus growth.
These results are in agreement with Vergopoulou et al. [27] who only studied the 10−4 M MeJA concentration and showed that no effect on the mycelial growth of A. parasiticus was observed. Goodrich-Tanrikulu et al. [26] also reported that MeJA concentrations ranging from 10−3 M to 10−8 M had no apparent effect on the mycelial growth of A. flavus.
3.2. MeJa Effect on AFB1 Production in YES Medium
In this study, AFB1 production was measurable from day 0 of the incubation (0.002 μg AFB1 flask−1, Table 2)
Table 2.
AFB1 production (μg flask−1) by A. parasiticus in YES medium.
| MeJA addition | ||||
|---|---|---|---|---|
| 0(a) | 10−6 M(b) | 10−4 M(c) | 10−2 M(d) | |
| AFB1 production | ||||
| Days | μg flask−1(± SD) | μg flask−1(± SD) | μg flask−1(± SD) | μg flask−1(± SD) |
| 0 | 0.002 (±0) | 0.002 (±0) | 0.002 (±0) | 0.002 (±0) |
| 3 | 40.17 (±4.31) | 44.94 (±5.39) | 23.45 (±5.76) | 0.019 (±0.002) |
| 7 | 56.18 (±15.86) | 72.44 (±4.17) | 69.01 (±17.06) | 0.051 (±0.005) |
| 9 | 29.80 (± 8.11) | 60.16 (±9.26) | 48.00 (±6.30) | 0.063 (±0.006) |
| 12 | 35.14 (±9.37) | 109.91 (±10.78) | 84.91 (±7.66) | 0.061 (±0.004) |
| 15 | 70.57 (±4.69) | 89.58 (±6.82) | 58.51 (±11.45) | ND(e) |
(a)Without MeJA addition (control); (b)addition of 0.0022 mg flask−1; (c)addition of 0.227 mg flask−1; (d)addition of 22.67 mg flask−1; (e)ND: not detected.
This AFB1 occurrence was due to the inoculation of samples with 10−2 conidia of A. parasiticus per flask. In the case of cultures treated with 10−2 M MeJA, the AFB1 amounts are negligible in comparison to AFB1 production in samples treated with MeJA at concentrations 10−6 M and 10−4 M as well as in control samples during the whole period of incubation. These traces of AFB1 are probably due to the conidia of A. parasiticus, which survived in spite of the inhibition of the fungus growth by the MeJA at this concentration. Treatment with MeJA at the highest concentration (10−2 M) reduced AFB1 production against control by 99.6% to 99.9% during the incubation while, on the 15th day of observation, AFB1 was not detectable ( < D L = 0.02 ng mL−1 YES). This was due to the inhibition of A. parasiticus growth by MeJA.
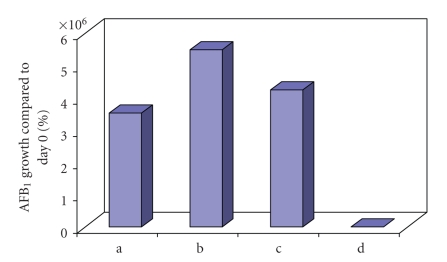
In the case of samples treated with 10−6 M and 10−4 M MeJA, treatment enhanced AFB1 biosynthesis by A. parasiticus, while maximum AFB1 production was observed on the 12th day. This production was 5.5 × 107 and 4.3 × 107 times higher compared to samples on day 0, respectively, as shown in Figure 1. AFB1 production was also observed in control samples but, in this case, maximum production (3.5 × 107) was revealed on the 15th day (Figure 1). Under the same conditions, treatment with 10−2 M MeJA resulted in only 3 × 103 (9th day) AFB1 maximum production compared to day 0.
Figure 1.
% maximum AFB1 production by A. parasiticus in YES medium compared to day 0 in: (a) control samples on day 15, (b) samples treated with MeJA (10−6 M) on day 12, (c) samples treated with MeJA (10−4 M) on day 12, and (d) samples treated with MeJA (10−2 M) on day 9.
As shown in Table 2, in the samples treated with MeJA at concentration 10−6 M, AFB1 output was stimulated after the 9th day of incubation while maximum production was observed on the 12th day (109.91 μg flask−1), and thus reaching 212.8% of the control. In the samples treated with MeJA at concentration 10−4 M, AFB1 production was also stimulated after the 9th day and reached on the 12th day 84.91 μg AFB1 flask−1, which corresponds to 141.6% of the control. Concerning 10−4 MeJA concentration, the results are in agreement with Vergopoulou et al. [27] who previously reported that this MeJA concetration stimulated AFB1 production by A. parasiticus after the 7th day of incubation, although Goodrich-Tanrikulu et al. [26] showed that aflatoxin production by A. flavus was inhibited at all MeJA concentrations tested, which ranged from 10−3 M to 10−8 M. It must be added that according to De Luca et al. [14] and Fabbri et al. [11], aflatoxin production increased 50 to 200 times after treatment of 10-day-old cultures of A. parasiticus and A. flavus with a mixture of linoleic acid and soybean LOX1. Furthermore, according to Greene-McDowelle et al. [45], although some LOX products have antifungal activity, other LOX products influence aflatoxin production while they have little influence on the fungal growth. It is obvious that the results of this study support a similar mechanism for the MeJA action.
Our results were confirmed by the two-way Anova statistical analysis.The Null Hypothesis is that there are no significant differences between the aflatoxin output at different MeJA concentrations. The value of F exper = 0.238.46 greatly exceeds that one tabulated at P = .05, namely, about 3.8 at df 3, 40. The Null Hypothesis is therefore rejected and it is concluded that different MeJA concentrations do affect the aflatoxin output. The second Null Hypothesis is that there are no significant differences between the aflatoxin outputs on different days of incubation. The value of F = 32.34 exceeds the tabulated value at P = .05 of 4.21 for df 9, 40. The Null Hypothesis is therefore rejected and it is concluded that incubation days do affect the aflatoxin output. The third Null Hypothesis is that there is no interaction between different MeJA concentrations and days of incubation, which influences the aflatoxin outputs. The calculated value of F = 11.68 exceeds the tabulated one at P = .05, namely, 2.039 at df 12, 40. We therefore reject the Null Hypothesis once more and conclude that there is an interaction between different MeJA concentrations and days of incubation, which influences aflatoxin output. These results revealed the possibility of the existence of different mechanisms, by which MeJA influences AFB1 biosynthesis when different concentrations are used.
Burow et al. [42] have already reported that treatment with 9S-HPODE increased or decreased aflatoxin production depending on the concentration tested. In addition, 9S-HPODE induced prolonged accumulation of transcripts of the aflatoxin/sterigmatocystin biosynthetic genes. Several jasmonates have also been shown to activate genes encoding antifungal proteins such as thionin [46], osmotin [47], as well as genes involved in phytoalexin biosynthesis [48]. In addition, cultures of A. parasiticus and A. flavus produce amounts of aflatoxins, which decrease during continued incubation of the cultures [49]. In this case, the authors reported that molds, which are capable of producing aflatoxin, may also degrade them. Doyle and Marth [50] observed that the ability of Aspergilli to degrade aflatoxins was dependent on the time of incubation; mycelia aging 8 to 10 days old were most effective in degrading AFB1.
According to Sweeney and Dobson [4], AFB1 biosynthesis is regulated at the level of transcription of genes involved in the aflatoxin biosynthetic pathway. These genes include two fatty acid synthase genes, a polyacetide synthase gene as well as the ord-1-gene, which encodes a cytochrome P-450 type monooxygenase, putatively responsible for the conversion of O-methylsterigmatocystin to aflatoxin. This monooxygenase is also involved in the degradative activity of A. flavus [51]. Thus, when jasmonates are exogenously applied to plant tissues, they exert either inhibitory or promoting effects in growth and developmental processes [19] and this finding concerns both the Aspergilli growth on plants as well as AFB1 biosynthesis.
The effectiveness of MeJA suggests a potential use in the postharvesting control of aflatoxin production in susceptible commodities like pistachios [26]. Moline et al. [52] also reported that MeJA can be applied effectively as a postharvest treatment to supress grey mold rot caused by Bacillus cinerea in strawberry. Markaki et al. [53], however, showed that when olives were treated with MeJA at different concentrations, AFB1 production was concentration-dependent (AFB1 either decreased or increased) in both olives inoculated with A. parasiticus and in noninoculated samples. It should be mentioned, in this case, that olives are not a suitable substrate for AFB1 biosynthesis [39].
In conclusion, in this study, it is shown that the plant growth regulator MeJA at a concentration of 10−2 M inhibits A. parasiticus growth on YES medium, and consequently, AFB1 production is insignificant. As far as lower concentrations are concerned, although in this study stimulation is reported, there are conflict results in literature concerning the effect of MeJA on AFB1 production. Therefore, it appears to be very important to identify the conditions under which the use of MeJA could be effective in preventing the biosynthesis of AFB1 mainly in products destined for long storage.
Acknowledgment
This work was supported in part by the University of Athens, Special Account for Research Grants (70/4/8786).
References
- 1.Kurtzman CP, Horn BW, Hesseltine CW. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamari . Antonie van Leeuwenhoek International Journal of General and Molecular Microbiology. 1987;53:147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
- 2.Dutton MF. Enzymes and aflatoxin biosynthesis. Microbiological Reviews. 1988;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton DL, Groopman JD. The Toxicology of Aflatoxins, Human Health, Veterinary and Agricultural Significance. San Diego, Calif, USA: Academic Press; 1994. [Google Scholar]
- 4.Sweeney MJ, Dobson ADW. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. International Journal of Food Microbiology. 1998;43(3):141–158. doi: 10.1016/s0168-1605(98)00112-3. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JW, Christensen SB. New perspectives on aflatoxin biosynthesis. Advances in Applied Microbiology. 1983;29:53–92. doi: 10.1016/s0065-2164(08)70354-x. [DOI] [PubMed] [Google Scholar]
- 6.Diener UL, Cole RJ, Sanders TH, Payne GA, Lee LS, Klich MA. Epidemiology of aflatoxin formation by Aspergillus flavus . Annual Review of Phytopathology. 1987;25:249–270. [Google Scholar]
- 7.Payne GA. Aflatoxin in maize. Critical Reviews in Plant Sciences. 1992;10:423–440. [Google Scholar]
- 8.Fanelli C, Fabbri AA. Growth requirements and lipid metabolism of Aspergillus flavus . Transactions of the British Mycological Society. 1980;75:371–375. [Google Scholar]
- 9.Bewley JD, Black M. Seeds: Physiology of Development and Germination. New York, NY, USA: Plenum Press; 1985. [Google Scholar]
- 10.Smart MG, Wicklow DT, Caldwell RW. Pathogenesis in Aspergillus ear rot of maize: light microscopy of fungal spread from wounds. Phytopathology. 1990;80:1287–1294. [Google Scholar]
- 11.Fabbri AA, Fanelli C, Panfili G, Passi S, Fasella P. Lipoperoxidation and aflatoxin biosynthesis by Aspergillus parasiticus and Aspergillus flavus . Journal of General Microbiology. 1983;129(11):3447–3452. [Google Scholar]
- 12.Fanelli C, Fabbri AA. Relationship between lipids and aflatoxin biosynthesis. Mycopathologia. 1989;107(2-3):115–120. doi: 10.1007/BF00707547. [DOI] [PubMed] [Google Scholar]
- 13.Aziz NH, Abushady MR, Elfouly MZ, Moussa LA. Bioregulation of aflatoxin biosynthesis by unirradiated conidia of A.flavus . Microbios. 1995;84:29–39. [Google Scholar]
- 14.De Luca C, Passi S, Fabbri AA, Fanelli C. Ergosterol oxidation may be considered a signal for fungal growth and aflatoxin production in Aspergillus parasiticus . Food Additives and Contaminants. 1995;12(3):445–450. doi: 10.1080/02652039509374328. [DOI] [PubMed] [Google Scholar]
- 15.Schaller F, Schaller A, Stintzi A. Biosynthesis and metabolism of jasmonates. Journal of Plant Growth Regulation. 2004;23(3):179–199. [Google Scholar]
- 16.Siedow JN. Plant lipoxygenase: structure and function. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42(1):145–188. [Google Scholar]
- 17.Gardner HW. Recent investigations into the lipoxygenase pathway of plants. Biochimica et Biophysica Acta. 1991;1084(3):221–239. doi: 10.1016/0005-2760(91)90063-n. [DOI] [PubMed] [Google Scholar]
- 18.Vick BA, Zimmerman DC. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochemical and Biophysical Research Communications. 1983;111(2):470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]
- 19.Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44(1):569–589. [Google Scholar]
- 20.Wasternack C, Parthier B. Jasmonate-signalled plant gene expression. Trends in Plant Science. 1997;2(8):302–307. [Google Scholar]
- 21.John M, Röhrig H, Schmidt J, Walden R, Shell J. Cell signaling by oligosaccharides. Trends in Plant Science. 1997;2:111–115. [Google Scholar]
- 22.Pühler A, Arlat M, Becker A, Gottfert M, Morrissey JP, O'Gara F. What can bacterial genome research teach us about bacteria-plant interactions? Current Opinion in Plant Biology. 2004;7(2):137–147. doi: 10.1016/j.pbi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Weiler EW, Albrecht T, Groth B, et al. Evidence for the involvement of jasmonates and other octadecanoid precursors in the tendril coiling response of Bryona dioica. Phytochemistry. 1193;32:591–600. [Google Scholar]
- 24.Ağar G, Türker M, Batal P, Erez ME. Phytoormone levels in germinating seeds of Zea mays L. exposed to selenium and aflatoxins. Ecotoxicology. 2006;15:443–450. doi: 10.1007/s10646-006-0079-z. [DOI] [PubMed] [Google Scholar]
- 25.Zeringue HJ. Effects of methyljasmonate on phytoalexin production and aflatoxin control in the developing cotton boll. Biochemical Systematics and Ecology. 2002;30:497–503. [Google Scholar]
- 26.Goodrich-Tanrikulu M, Mahoney NE, Rodriguez SB. The plant growth regulator methyl jasmonate inhibits aflatoxin production by Aspergillus flavus . Microbiology. 1995;141(11):2831–2837. doi: 10.1099/13500872-141-11-2831. [DOI] [PubMed] [Google Scholar]
- 27.Vergopoulou S, Galanopoulou D, Markaki P. Methyl jasmonate stimulates aflatoxin B1 biosynthesis by Aspergillus parasiticus . Journal of Agricultural and Food Chemistry. 2001;49(7):3494–3498. doi: 10.1021/jf010074+. [DOI] [PubMed] [Google Scholar]
- 28.Holmes RA, Boston RS, Payne GA. Diverse inhibitors of aflatoxin biosynthesis. Applied Microbiology and Biotechnology. 2008;78(4):559–572. doi: 10.1007/s00253-008-1362-0. [DOI] [PubMed] [Google Scholar]
- 29.Leondaritis G, Galanopoulou D. Characterization of inositol phospholipids and identification of a mastoparan-induced polyphosphoinositide response in Tetrahymena pyriformis . Lipids. 2000;35(5):525–532. doi: 10.1007/s11745-000-552-8. [DOI] [PubMed] [Google Scholar]
- 30.Pitt JJ. Methods for the Mycological Examination of Food. New York, NY, USA: Plenum Press; 1986. (NATO ASI Series, no.122). [Google Scholar]
- 31.Ellis WO, Smith JP, Simpson BK, Ramaswamy H, Doyon G. Growth and aflatoxin production by Aspergillus flavus in peanuts stored under modified atmosphere packaging (MAP) conditions. International Journal of Food Microbiology. 1994;22:173–187. doi: 10.1016/0168-1605(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 32.Eltem R. Growth and aflatoxin B1 production on olives and olive paste by molds isolated from Turkish-style natural black olives in brine. International Journal of Food Microbiology. 1996;32:217–223. doi: 10.1016/0168-1605(96)01115-4. [DOI] [PubMed] [Google Scholar]
- 33.El-Refai IM, Awadalla OA, Abou Zeid AM. Effects of some insect growth regulators on growth of Aspergillus flavus and its productivity of aflatoxin B1 and lipids. Food Additives and Contaminants. 1995;12(4):585–590. doi: 10.1080/02652039509374346. [DOI] [PubMed] [Google Scholar]
- 34.Abramson D, Clear RM. A convenient method for assessing mycotoxin production in cultures of Aspergilli and Penicillia . Journal of Food Protection. 1996;59(6):642–644. doi: 10.4315/0362-028X-59.6.642. [DOI] [PubMed] [Google Scholar]
- 35.Sinha KK, Sinha AK, Prasad G. The effect of clove and cinnamon oils on growth of an aflatoxin production by Aspergillus flavus . Letters in Applied Microbiology. 1993;16(3):114–117. [Google Scholar]
- 36.Daradimos E, Marcaki P, Koupparis M. Evaluation and validation of two fluorometric HPLC methods for the determination of aflatoxin B1 in olive oil. Food Additives and Contaminants. 2000;17(1):65–73. doi: 10.1080/026520300283603. [DOI] [PubMed] [Google Scholar]
- 37.Abarca ML, Bragulat MR, Castella G, Cabanes FJ. Mycoflora and aflatoxin-producing strains in animal mixed feeds. Journal of Food Protection. 1994;57(3):256–258. doi: 10.4315/0362-028X-57.3.256. [DOI] [PubMed] [Google Scholar]
- 38.Fowler J, Cohen L. Practical Statistics for Field Biology. Chichester, UK: John Wiley & Sons; 1997. [Google Scholar]
- 39.Leontopoulos D, Siafaka A, Markaki P. Black olives as substrate for Aspergillus parasiticus growth and aflatoxin B1 production. Food Microbiology. 2003;20(1):119–126. [Google Scholar]
- 40.Luchese RH, Harrigan WF. Biosynthesis of aflatoxin—the role of nutritional factors. Journal of Applied Bacteriology. 1993;74(1):5–14. doi: 10.1111/j.1365-2672.1993.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Chang P-K, Cary JW, et al. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus . Applied and Environmental Microbiology. 1995;61(6):2365–2371. doi: 10.1128/aem.61.6.2365-2371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burow GB, Nesbitt TC, Dunlap J, Keller NP. Seed lipoxygenase products modulate Aspergillus mycotoxin biosynthesis. Molecular Plant-Microbe Interactions. 1997;10(3):380–387. [Google Scholar]
- 43.González-Aguilar GA, Buta JG, Wang CY. Methyl jasmonate reduces chilling injury symptoms and enhances colour development of “Kent” mangoes. Journal of the Science of Food and Agriculture. 2001;81(13):1244–1249. [Google Scholar]
- 44.Gogala N. Regulation of mycorrhizal infection by hormonal factors produced by hosts and fungi. Experientia. 1991;47(4):331–340. [Google Scholar]
- 45.Greene-Mcdowelle DM, Ingber B, Wright MS, Zeringue HJ, Jr., Bhatnagar D, Cleveland TE. The effects of selected cotton-leaf volatiles on growth, development and aflatoxin production of Aspergillus parasiticus . Toxicon. 1999;37(6):883–893. doi: 10.1016/s0041-0101(98)00209-8. [DOI] [PubMed] [Google Scholar]
- 46.Andresen I, Becker W, Schluter K, Burges J, Parthier B, Apel K. The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum vulgare) Plant Molecular Biology. 1992;19(2):193–204. doi: 10.1007/BF00027341. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Chang PFL, Liu D, et al. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell. 1994;6(8):1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gundlach H, Muller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shih CN, Marth EH. Aflatoxin formation , lipid synthesis,and glucose metabolism by Aspergillus parasiticus during icubatiobn with and without agitation. Biochimica et Biophysica Acta. 1974;338:286–296. [Google Scholar]
- 50.Doyle MP, Marth EH. Peroxidase activity in mycelia of Aspergillus parasiticus that degrade aflatoxin. European Journal of Applied Microbiology and Biotechnology. 1979;7(2):211–217. [Google Scholar]
- 51.Hamid AB, Smith JE. Degradation of aflatoxin by Aspergillus flavus . Journal of General Microbiology. 1987;133(8):2023–2029. doi: 10.1099/00221287-133-8-2023. [DOI] [PubMed] [Google Scholar]
- 52.Moline HE, Buta JG, Saftner RA, Maas JL. Comparison of three volatile natural products for the reduction of postharvest decay in strawberries. Advances in Strawberry Research. 1997;16:43–48. [Google Scholar]
- 53.Markaki P, Velivassaki K, Giannitsis D, Galanopoulou D. Methyljasmonate effect on AFB1 production by olives inoculated with A. parasiticus is concentration dependent. In: Njapau H, Trujillo S, van Egmond HP, Park DL, editors. Mycotoxins and Phycotoxins, Advances in Determination, Toxicology and Exposure Management. Wageningen, The Netherlands: Wageningen Academic; 2006. pp. 259–264. [Google Scholar]