Abstract
Purpose
Although the prognostic value of B7-H1 and B7-H4 expression by tumor cells in clear cell renal cell carcinoma (ccRCC) has been established, the role of B7-H3 is unknown. As such, we evaluated the association of B7-H3 expression with clinicopathologic outcomes in patients treated for ccRCC.
Experimental Design
Nephrectomy specimens from 743 consecutive patients treated for ccRCC at our institution from 1990 to 1999 were evaluated for B7-H3 expression by immunohistochemical staining. Associations of B7-H3 expression with clinical and pathologic features were evaluated using χ2 and Fisher's exact tests. Associations of B7-H3 expression with death from RCC were evaluated using Cox proportional hazards regression models.
Results
B7-H3 expression by tumor cells or tumor vasculature was noted in 17% and 95% of specimens, respectively. The presence of either tumor cell or diffuse tumor vasculature expression of B7-H3 was present in 46% of specimens and was associated with multiple adverse clinical and pathologic features. After multivariable adjustment, the presence of either tumor cell or diffuse tumor vasculature B7-H3 expression was significantly associated with an increased risk of death from RCC (risk ratio, 1.38; 95% confidence interval, 1.03-1.84; P = 0.029).
Conclusions
Both tumor cell and tumor vasculature B7-H3 expression convey important information to predict ccRCC outcomes. Collectively, our past and present studies pertaining to B7-H ligand expression indicate that ccRCC may use redundant mechanisms to compromise host antitumoral immunity. Future studies will focus on the effect of combined B7-H ligand expression in RCC.
Despite great advances in our understanding of the molecular biology of renal cell carcinoma (RCC), the prognosis for patients with metastatic RCC remains dismal (1, 2). Currently, immunotherapy with high-dose interleukin-2 remains the best chance for cure in patients with metastatic RCC (3, 4). However, interest in interleukin-2 therapy has waned owing to the resource-intensive nature of treatment, the toxic and limited response profile, and the introduction of new systemic treatment regimens. Although the introduction of oral multiple-tyrosine kinase and mammalian target of rapamycin inhibitors has shown a survival benefit in patients failing cytokine therapy, these agents most often fail to elicit complete or sustained disease remission (5–8). Regardless of the systemic regimen used, it remains clear that the majority of patients with advanced RCC fail current forms of systemic therapy and ultimately succumb to disease. Thus, improvements in the treatment of metastatic disease are desperately needed.
A potential barrier to a successful antitumoral immune response is the expression of costimulatory and coinhibitory molecules by tumor cells. The B7-family of T-cell costimulatory molecules play essential roles in modulating immune cell activation, function, and fate (9, 10). The prototype ligands of this family, B7-1 and B7-2, have well-defined roles as essential costimulatory molecules involved in T-cell activation. In contrast, the roles of the B7-H molecules (i.e., B7-H1, B7-H2, B7-H3, and B7-H4) remain far from clear. Although expression of B7-H molecules is principally limited to lymphoid cells, aberrant expression by a number of human malignancies has been shown. Additionally, there is increasing evidence that suggests that these B7-H molecules may serve as coinhibitors of cell-mediated immunity (9–13). As such, B7-H expression has been correlated with aggressive behavior by various tumors (11, 14). Prior reports from our group have shown that B7-H1 and B7-H4 expressions by tumor cells correlate with adverse pathologic features and predict poor survival for patients with clear cell RCC (ccRCC). In contrast, the association of B7-H3 expression with clinical outcomes in patients with ccRCC has not yet been investigated.
Translational Relevance.
Renal cell carcinoma (RCC) has long been recognized as an immunogenic tumor. As such, significant attention has been directed toward optimizing immune-based therapy. A potential barrier to a successful antitumoral immune response is expression of costimulatory and coinhibitory molecules by tumor cells. The B7-family of T-cell costimulatory molecules play essential roles in modulating immune cell activation, function, and fate. Although the prognostic value of B7-H1 and B7-H4 expression by tumor cells in ccRCC has been established, the role of B7-H3 is unknown. Herein, we report on patterns and implications of B7-H3 expression in ccRCC. Specifically, we show that the combination of tumor cell and diffuse tumor vasculature expression of B7-H3 is significantly associated with multiple adverse clinical and pathologic features and risk of disease progression and death from RCC even in a multivariable setting. These results suggest that B7-H3 expression may ultimately add to B7-H1 and B7-H4 as prognostic markers for this malignancy. Furthermore, our collective studies indicate that multiple B7-H ligands can be expressed by aggressive forms of ccRCC.Thus, simultaneous targeting of multiple immune cell inhibitory and stimulatory pathways will likely be required to promote robust ccRCC tumor regression in the clinical setting.
Herein, we report on the patterns and implications of B7-H3 expression in ccRCC. Specifically, we show that the tumor cell and diffuse tumor vasculature expressions of B7-H3 are statistically significantly associated with multiple adverse clinical and pathologic features of ccRCC. Moreover, the presence of either tumor cell or diffuse tumor vasculature B7-H3 expression is significantly associated with an increased risk of disease progression and death from RCC even in a multivariable setting. The effect of combined B7-H (B7-H1, B7-H3, and B7-H4) ligand expression may be of additional prognostic value and will be done in future studies following the development of a method of evaluating B7-H4 expression in formalin-fixed, paraffin-embedded tissue.
Materials and Methods
Patient selection
On approval from the Institutional Review Board, we identified 743 patients treated between 1990 and 1999 with radical nephrectomy or nephron-sparing surgery for unilateral, sporadic, noncystic ccRCC from the Mayo Clinic Nephrectomy Registry.
Clinical and pathologic features
Clinical features studied included age, gender, symptoms at presentation, Eastern Cooperative Oncology Group (ECOG) performance status, and tumor thrombus level. Patients with a palpable flank or abdominal mass; discomfort; gross hematuria; acute onset varicocele; or constitutional symptoms including rash, sweats, weight loss, fatigue, early satiety, and anorexia were considered symptomatic. Pathologic features studied included histologic subtype classified according to the Union Internationale Contre le Cancer, American Joint Committee on Cancer, and Heidelberg guidelines (15, 16); tumor size; the 2002 primary tumor classification; regional lymph node involvement; distant metastases; the 2002 tumor-node-metastasis (TNM) stage groupings; nuclear grade; coagulative tumor necrosis; and sarcomatoid differentiation. To obtain these features, a single urologic pathologist reviewed the microscopic slides from all specimens without knowledge of patient outcome or B7-H3 expression.
Patient outcome
Disease status for patients in the Nephrectomy Registry is updated each year. If a patient has not been seen at our institution in the previous year, the patient is sent a disease status questionnaire. If there is evidence of disease progression in this questionnaire, the date, location, and treatment are verified in writing with the patient's local physician. Patient vital status is similarly updated on a yearly basis. If a patient has died in the previous year, a death certificate is ordered to determine the cause of death. If the death certificate does not support this, the medical history is reviewed by a urologist to determine the cause of death. If a death certificate cannot be obtained, the cause of death must be verified with the patient's family or local physician.
B7-H3 immunohistochemical staining and quantitation
For each patient, a tumor block was selected for immunohistochemical analysis that was most representative of the tumor based on nuclear grade. Formalin-fixed, paraffin-embedded tissues were cut into 5-μm sections, deparaffinized, and rehydrated in a graded series of ethanols. Antigen retrieval was done by heating tissue sections in EDTA 1 mmol/L (pH 8) to 121°C using a Digital Decloing Chamber (Biocare Medical), cooling to 90°C, and incubating for 5 min. Sections were washed in Wash Buffer (Do) before being placed onto the Autostainer Plus (Do) to conduct the following protocol. Sections were blocked for endogenous peroxidase for 5 min using Endogenous Blocking Solution (Do), washed twice, and then incubated for 5 min in Serum-Free Protein Block (Do), followed by incubation for 60 min in purified goat anti-human B7-H3 antibody (R&D Systems; 100 μg/mL) diluted 1:80 with DaVinci Green antibody diluent (Biocare Medical). Sections were incubated for 15 min in probe from goat horseradish peroxidase-polymer kit (Biocare Medical #GHP516L), washed, and incubated for 15 min with polymer from goat horseradish peroxidase-polymer kit. Sections were visualized by incubating in betazoid 3,3′-diaminobenzidine (Biocare Medical) for 5 min. Sections were counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and coverslipped.
The percentages of tumor cells that stained positive for B7-H3 were quantified in 10% increments by a pathologist (Y.S.) without knowledge of patient outcome. Tumors with <10% of cells staining positive were scored as having negative expression. In addition, B7-H3 expression in the tumor vasculature and the vasculature of adjacent nontumor renal tissue was evaluated and recorded as being absent, focal, moderate, or diffuse in nature.
Flow cytometric analyses of B7-H3 expression
The human kidney cancer cell line Caki-2 (American Type Culture Collection) was examined for B7-H1, B7-H3, and B7-H4 expression essentially as described previously (17). Briefly, cells were incubated with either the goat polyclonal anti-human B7-H3 antibody used for tissue immunohistochemistry or a murine monoclonal anti-human B7-H3 antibody (R&D Systems). The goat polyclonal antibody was biotinylated using a solid-phase biotinylation kit (Pierce). Subsequently, 1 μg of antibody was incubated with 1 × 106 cells. After 30 min of incubation, cells were washed in fluorescence-activated cell sorting buffer (PBS, 3% fetal bovine serum, 2 mmol/L EDTA) and stained with streptavidin (BD PharMingen) or goat anti-mouse antibody (Biosource), both R-phycoerythrin tagged. For blocking studies, either 10 μg of recombinant B7-H3-Fc fusion protein (R&D Systems) or an irrelevant control fusion protein (P-Selectin-Fc, BD PharMingen) was combined with primary antibody before cell staining. To assess Caki-2 for expression of B7-H1 and B7-H4, cells were stained with R-phycoerythrin–labeled anti–B7-H1 (BD PharMingen) and anti–B7-H4 (eBioscience) antibodies following the manufacturer's specifications. Surface expression of B7-H ligands was quantified with a FACSCalibur fluorescent-activated cell sorter (Becton Dickinson) and analyzed with FlowJo software (Tree Star).
Statistical methods
Associations of B7-H3 expression with clinical and pathologic features were evaluated using χ2 and Fisher's exact tests. Kaplan-Meier curves were used to visualize the associations of B7-H3 expression with disease progression-free and cancer-specific survival. Associations of B7-H3 expression with disease progression and death from ccRCC were first evaluated in univariate Cox proportional hazards regression models and then in a multivariable setting, adjusting for each of the clinical and pathologic features of interest. These associations were also evaluated after simultaneous adjustment for the TNM stage groupings, nuclear grade, and ECOG performance status. We also adjusted for the Mayo Clinic PROG score (for the evaluation of disease progression) and the SSIGN score (for the evaluation of death from RCC), both of which are composite scoring systems developed specifically for patients with ccRCC based on primary tumor classification, regional lymph node involvement, distant metastases, tumor size, nuclear grade, and coagulative tumor necrosis (18, 19). Associations with outcome were summarized with risk ratios and 95% confidence intervals (95% CI). Statistical analyses were done using the SAS software package (SAS Institute). All tests were two-sided and P < 0.05 was considered statistically significant.
Results
Clinical and pathologic features for the 743 consecutive ccRCC patients under study are summarized in Table 1. Median age at surgery was 64 years (range, 26-89 years) and median tumor size was 6.5 cm (range, 0.2-24.0 cm). At last follow-up, 419 patients had died, including 246 patients who died from RCC at a median of 2.1 years following surgery (range, 0.1-14.1 years). Among the 324 patients who were still alive at last follow-up, the median duration of follow-up was 10.1 years (range, 0.1-16.7 years); only 14 (4.3%) patients had fewer than 5 years of follow-up.
Table 1.
Summary of clinical and pathologic features for 743 ccRCC patients
| Feature | n (%) |
|---|---|
| Age at surgery (y) | |
| <65 | 378 (50.9) |
| ≥65 | 265 (49.1) |
| Gender | |
| Female | 267 (35.9) |
| Male | 476 (64.1) |
| Symptoms at presentation | |
| No | 264 (35.5) |
| Yes | 479 (64.5) |
| Constitutional symptoms at presentation | |
| No | 545 (73.4) |
| Yes | 198 (26.7) |
| ECOG performance status | |
| 0 | 672 (90.4) |
| ≥1 | 71 (9.6) |
| Tumor thrombus | |
| None | 598 (80.5) |
| Level 0 | 75 (10.1) |
| Level I-IV | 70 (9.4) |
| Primary tumor size (cm) | |
| <5 | 238 (32.0) |
| 5-<7 | 158 (21.3) |
| 7-<10 | 165 (22.2) |
| ≥10 | 182 (24.5) |
| 2002 Primary tumor classification | |
| pT1a | 179 (24.1) |
| pT1b | 202 (27.2) |
| pT2 | 150 (20.2) |
| pT3a | 63 (8.5) |
| pT3b | 128 (17.2) |
| pT3c | 12 (1.6) |
| pT4 | 9 (1.2) |
| Regional lymph node involvement | |
| pNX and pN0 | 712 (95.8) |
| pN1 and pN2 | 31 (4.2) |
| Distant Metastases | |
| pM0 | 661 (89.0) |
| pM1 | 82 (11.0) |
| 2002 TNM stage groupings | |
| I | 366 (49.3) |
| II | 123 (16.6) |
| III | 161 (21.7) |
| IV | 93 (12.5) |
| Nuclear grade | |
| 1 | 50 (6.7) |
| 2 | 333 (44.8) |
| 3 | 295 (39.7) |
| 4 | 65 (8.8) |
| Coagulative tumor necrosis | |
| No | 521 (70.1) |
| Yes | 222 (29.9) |
| Sarcomatoid differentiation | |
| No | 705 (94.9) |
| Yes | 38 (5.1) |
Overall, we observed B7-H3 expression by tumor cells within 129 (17.4%) ccRCC specimens (Fig. 1A). Although B7-H3 staining was heterogeneous within individual tumors with a median level of B7-H3 expression of 30% (range, 10-100%), the intensity of staining was similar among tumors that stained positive for B7-H3. In contrast, no evidence of B7-H3 expression was detected in tumor cells within 614 (82.6%) specimens (Fig. 1B). Moreover, B7-H3 expression was not found within the adjacent, nontumor renal parenchyma. The specificity of in situ B7-H3 staining in human specimens was further established through blocking studies using affinity-purified human B7-H3-Fc fusion protein that abrogated staining of B7-H3–positive ccRCC tumors (Fig. 1C). Of experimental interest, flow cytometric analysis of Caki-2 confirmed that this commonly studied human RCC cell line exhibits the capability to coexpress B7-H3 and B7-H1, but not the B7-H4 ligand (data not shown).
Fig. 1.
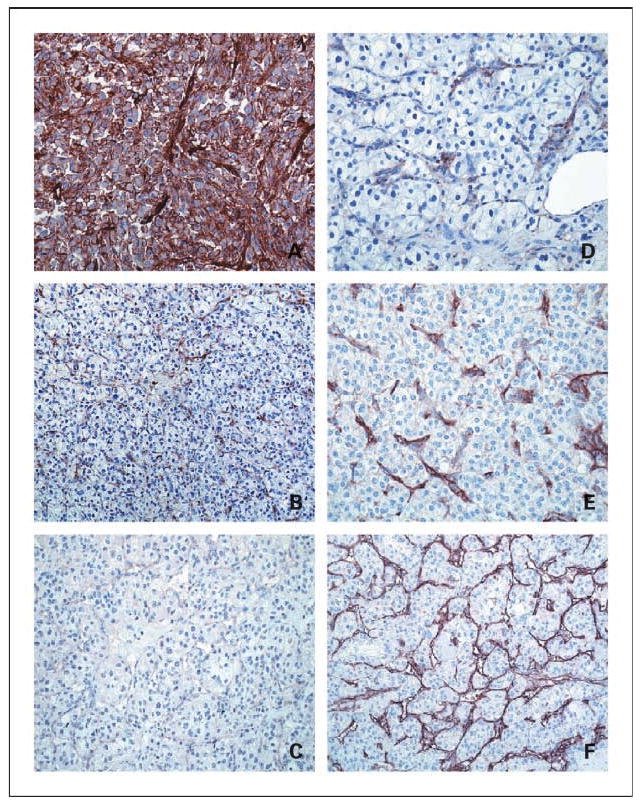
Tumor cell and vasculature B7-H3 expression within ccRCC specimens. A, representative ccRCC tumor specimen with strong tumor cell B7-H3 immunohistochemical staining. B, ccRCC tumor specimen with negative tumor cell but sporadic tumor vasculature B7-H3 staining. C, blocking with B7-H3-Fc fusion protein completely abrogates staining of B7-H3–positive ccRCC tumor. Focal (D), moderate (E), or diffuse (F) tumor vasculature staining was observed in 31.9%, 26.5%, and 36.7%, respectively, of ccRCC tumor specimens studied. All photomicrographs, ×400.
Comparisons of clinical and pathologic features by tumor cell B7-H3 expression are summarized in Table 2. B7-H3 expression in the tumor cells of ccRCC specimens was significantly associated with symptomatic presentation and the presence of aggressive tumor pathology including larger tumor size, advanced TNM stage, higher nuclear grade, coagulative tumor necrosis, and sarcomatoid differentiation.
Table 2.
Comparison of clinical and pathologic features by tumor cell B7-H3 expression for 743 ccRCC patients
| Feature | Tumor Cell B7-H3 expression, n (%) | P | |
|---|---|---|---|
| Negative, n = 614 |
Positive, n = 129 |
||
| Age at surgery (y) | |||
| <65 | 310 (50.5) | 68 (52.7) | 0.646 |
| ≥65 | 304 (49.5) | 61 (47.3) | |
| Gender | |||
| Female | 221 (36.0) | 46 (35.7) | 0.943 |
| Male | 393 (64.0) | 83 (64.3) | |
| Symptoms at presentation | |||
| No | 237 (38.6) | 27 (20.9) | <0.001 |
| Yes | 377 (61.4) | 102 (79.1) | |
| Constitutional symptoms at presentation | |||
| No | 475 (77.4) | 70 (54.3) | <0.001 |
| Yes | 139 (22.6) | 59 (45.7) | |
| ECOG performance status | |||
| 0 | 552 (89.9) | 120 (93.0) | 0.273 |
| ≥1 | 62 (10.1) | 9 (7.0) | |
| Tumor thrombus | |||
| None | 522 (85.0) | 76 (58.9) | <0.001 |
| Level 0 | 49 (8.0) | 26 (20.2) | |
| Level I-IV | 43 (7.0) | 27 (20.9) | |
| Primary tumor size (cm) | |||
| <5 | 219 (35.7) | 19 (14.7) | <0.001 |
| 5-<7 | 140 (22.8) | 18 (14.0) | |
| 7-<10 | 130 (21.2) | 35 (27.1) | |
| ≥10 | 125 (20.4) | 57 (44.2) | |
| 2002 Primary tumor classification | |||
| pT1a | 173 (28.2) | 6 (4.7) | <0.001 |
| pT1b | 177 (28.8) | 25 (19.4) | |
| pT2 | 126 (20.5) | 24 (18.6) | |
| pT3a | 44 (7.2) | 19 (14.7) | |
| pT3b | 83 (13.5) | 45 (34.9) | |
| pT3c | 6 (1.0) | 6 (4.7) | |
| pT4 | 5 (0.8) | 4 (3.1) | |
| Regional lymph node involvement | |||
| pNX and pN0 | 597 (97.2) | 115 (89.2) | <0.001 |
| pN1 and pN2 | 17 (2.8) | 14 (10.9) | |
| Distant metastases | |||
| pM0 | 560 (91.2) | 101 (78.3) | <0.001 |
| pM1 | 54 (8.8) | 28 (21.7) | |
| 2002 TNM stage groupings | |||
| I | 336 (54.7) | 30 (23.3) | <0.001 |
| II | 106 (17.3) | 17 (13.2) | |
| III | 110(17.9) | 51 (39.5) | |
| IV | 62 (10.1) | 31 (24.0) | |
| Nuclear grade | |||
| 1 | 50 (8.1) | 0 | <0.001 |
| 2 | 324 (52.8) | 9 (7.0) | |
| 3 | 218 (35.5) | 77 (59.7) | |
| 4 | 22 (3.6) | 43 (33.3) | |
| Coagulative tumor necrosis | |||
| No | 493 (80.3) | 28 (21.7) | <0.001 |
| Yes | 121 (19.7) | 101 (78.3) | |
| Sarcomatoid differentiation | |||
| No | 600 (97.7) | 105 (81.4) | <0.001 |
| Yes | 14 (2.3) | 24 (18.6) | |
Whereas less than a fifth of specimens analyzed contained B7-H3–expressing tumor cells, tumor vasculature expression of B7-H3 was apparent in 95.1% of tumors. There were 237 (31.9%), 197 (26.5%), and 273 (36.7%) specimens with focal, moderate, and diffuse tumor vasculature B7-H3 expression, respectively (Fig. 1D-F). Comparisons of clinical and pathologic features by the extent of tumor vasculature B7-H3 expression are summarized in Table 3. Increasing levels of tumor vasculature B7-H3 expression, particularly diffuse tumor vasculature expression, were significantly associated with adverse clinical and pathologic features. There were 378 specimens with nontumor renal tissue present; only 18 (4.8%) showed vasculature B7-H3 expression, all of which were focal.
Table 3.
Comparison of clinical and pathologic features by tumor vasculature B7-H3 expression for 743 ccRCC patients
| Feature | Tumor vasculature B7-H3 expression, n (%) | P | |||
|---|---|---|---|---|---|
| Absent, n = 36 | Focal, n = 237 | Moderate, n = 197 | Diffuse, n = 273 | ||
| Age at surgery (y) | |||||
| <65 | 21 (58.3) | 122 (51.5) | 93 (47.2) | 142 (52.0) | 0.565 |
| ≥65 | 15 (41.7) | 115 (48.5) | 104 (52.8) | 131 (48.0) | |
| Gender | |||||
| Female | 12 (33.3) | 85 (35.9) | 78 (39.6) | 92 (33.7) | 0.605 |
| Male | 24 (66.7) | 152 (64.1) | 119 (60.4) | 181 (66.3) | |
| Symptoms at presentation | |||||
| No | 19 (52.8) | 103 (43.5) | 70 (35.5) | 72 (26.4) | <0.001 |
| Yes | 17 (47.2) | 134 (56.5) | 127 (64.5) | 201 (73.6) | |
| Constitutional symptoms at presentation | |||||
| No | 33 (91.7) | 202 (85.2) | 149 (75.6) | 161 (59.0) | <0.001 |
| Yes | 3 (8.3) | 35 (14.8) | 48 (24.4) | 112 (41.0) | |
| ECOG performance status | |||||
| 0 | 33 (91.7) | 216 (91.1) | 169 (85.8) | 254 (93.0) | 0.064 |
| ≥1 | 3 (8.3) | 21 (8.9) | 28 (14.2) | 19 (7.0) | |
| Tumor thrombus | |||||
| None | 33 (91.7) | 208 (87.8) | 156 (79.2) | 201 (73.6) | <0.001 |
| Level 0 | 2 (5.6) | 19 (8.0) | 23 (11.7) | 31 (11.4) | |
| Level I-IV | 1 (2.8) | 10(4.2) | 18 (9.1) | 41 (15.0) | |
| Primary tumor size (cm) | |||||
| <5 | 25 (69.4) | 93 (39.2) | 66 (33.5) | 54 (19.8) | <0.001 |
| 5-<7 | 2 (5.6) | 58 (24.5) | 47 (23.9) | 51 (18.7) | |
| 7-<10 | 3 (8.3) | 45 (19.0) | 41 (20.8) | 76 (27.8) | |
| ≥10 | 6 (16.7) | 41 (17.3) | 43 (21.8) | 92 (33.7) | |
| 2002 Primary tumor classification | |||||
| pT1a | 24 (66.7) | 70 (29.5) | 46 (23.4) | 39 (14.3) | <0.001 |
| pT1b | 2 (5.6) | 72 (30.4) | 69 (35.0) | 59 (21.6) | |
| pT2 | 5 (13.9) | 48 (20.3) | 28 (14.2) | 69 (25.3) | |
| pT3a | 2 (5.6) | 17 (7.2) | 12 (6.1) | 32 (11.7) | |
| pT3b | 3 (8.3) | 27 (11.4) | 38 (19.3) | 60 (22.0) | |
| pT3c | 0 | 2 (0.8) | 3 (1.5) | 7 (2.6) | |
| pT4 | 0 | 1 (0.4) | 1 (0.5) | 7 (2.6) | |
| Regional lymph node involvement | |||||
| pNX and pN0 | 36 (100.0) | 233 (98.3) | 189 (95.9) | 254 (93.0) | 0.015 |
| pN1 and pN2 | 0 | 4 (1.7) | 8 (4.1) | 19 (7.0) | |
| Distant metastases | |||||
| pM0 | 33 (91.7) | 222 (93.7) | 181 (91.9) | 225 (82.4) | <0.001 |
| pM1 | 3 (8.3) | 15 (6.3) | 16 (8.1) | 48 (17.6) | |
| 2002 TNM stage groupings | |||||
| I | 26 (72.2) | 137 (57.8) | 110 (55.8) | 93 (34.1) | <0.001 |
| II | 3 (8.3) | 42 (17.7) | 25 (12.7) | 53 (19.4) | |
| III | 4 (11.1) | 41 (17.3) | 42 (21.3) | 74 (27.1) | |
| IV | 3 (8.3) | 17 (7.2) | 20 (10.2) | 53 (19.4) | |
| Nuclear grade | |||||
| 1 | 6 (16.7) | 25 (10.6) | 14 (7.1) | 5 (1.8) | <0.001 |
| 2 | 15 (41.7) | 131 (55.3) | 88 (44.7) | 99 (36.3) | |
| 3 | 15 (41.7) | 71 (30.0) | 78 (39.6) | 131 (48.0) | |
| 4 | 0 | 10 (4.2) | 17 (8.6) | 238 (13.9) | |
| Coagulative tumor necrosis | |||||
| No | 29 (80.6) | 200 (84.4) | 141 (71.6) | 151 (55.3) | <0.001 |
| Yes | 7 (19.4) | 37 (15.6) | 56 (28.4) | 122 (44.7) | |
| Sarcomatoid differentiation | |||||
| No | 36 (100.0) | 231 (97.5) | 186 (94.4) | 252 (92.3) | 0.029 |
| Yes | 0 | 6 (2.5) | 11 (5.6) | 21 (7.7) | |
On univariate analysis, ccRCC patients with B7-H3–positive tumor cells were nearly four times more likely to die from RCC compared with patients with B7-H3–negative tumors (risk ratio, 3.98; 95% CI, 3.06-5.19; P < 0.001; Fig. 2A). After simultaneous adjustment for the TNM stage groupings, nuclear grade, and ECOG performance status, patients with B7-H3–positive tumor cells remained significantly more likely to die from RCC compared with patients with B7-H3–negative tumors (risk ratio, 1.53; 95% CI, 1.15-2.05; P = 0.004). However, the association of tumor cell B7-H3 expression with death from RCC was no longer statistically significant after adjusting for the SSIGN score (risk ratio, 1.25; 95% CI, 0.94-1.68; P = 0.129).
Fig. 2.
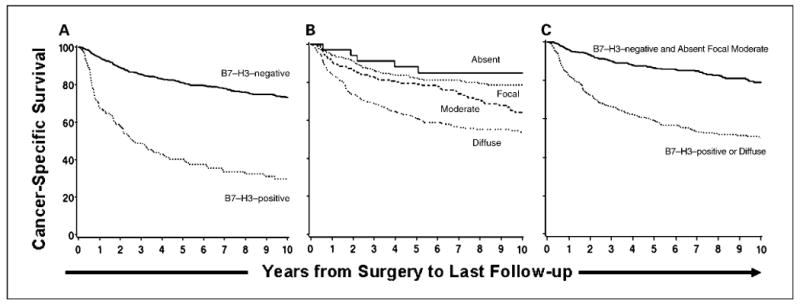
A, association of tumor cell B7-H3 expression with death from RCC. Cancer-specific survival rates (SE, number still at risk) at 5 and 10 y following surgery were 40.3% (4.5%, 44) and 29.8% (4.4%, 20), respectively, for patients with B7-H3–positive tumors compared with 81.0% (1.6%, 425) and 73.2% (2.0%, 200), respectively, for patients with B7-H3–negative tumors. B, association of tumor vasculature B7-H3 expression with death from RCC. Cancer-specific survival rates (SE, number still at risk) at 5 and 10 y following surgery were 88.2% (5.5%, 27) and 84.9% (6.2%, 9); 82.2% (2.6%, 159) and 78.6% (2.9%, 74); 79.0% (3.0%,134) and 64.2% (3.9%, 65); and 61.4% (3.0%, 149) and 53.8% (3.2%, 72) for patients with absent, focal, moderate, and diffuse tumor vasculature B7-H3 expression, respectively. C, association of either tumor cell or diffuse tumor vasculature B7-H3 expression with death from RCC. Cancer-specific survival rates (SE, number still at risk) at 5 and 10 y following surgery were 59.3% (2.7%, 177) and 50.2% (2.9%, 85), respectively, for patients with B7-H3–positive tumor cell or diffuse tumor vasculature B7-H3 expression compared with 86.5% (1.8%, 292) and 79.0% (2.3%, 135), respectively, for patients with B7-H3–negative tumor cell and absent, focal, or moderate tumor vasculature B7-H3 expression.
Focal or moderate tumor vasculature B7-H3 expression was not statistically significantly associated with an increased risk of death from RCC compared with patients with no tumor vasculature B7-H3 expression. In contrast, patients with diffuse tumor vasculature B7-H3 expression were nearly four times more likely to die from RCC compared with patients with no tumor vasculature B7-H3 expression (risk ratio, 3.90; 95% CI, 1.60-9.53; P = 0.003; Fig. 2B). After simultaneous adjustment for the TNM stage groupings, nuclear grade, and ECOG performance status, patients with diffuse tumor vasculature B7-H3 expression were 41% more likely to die from RCC compared with patients with absent, focal, or moderate tumor vasculature B7-H3 expression (risk ratio, 1.41; 95% CI, 1.09-1.83; P = 0.009). However, the association of diffuse tumor vasculature B7-H3 expression with death from RCC was no longer statistically significant after adjusting for the SSIGN score (risk ratio, 1.14; 95% CI, 0.88-1.48; P = 0.316).
Overall, 340 (45.8%) patients exhibited either positive tumor cell or diffuse tumor vasculature B7-H3 expression. The combination of these two markers was significantly associated with adverse clinical and pathologic features (data not shown). Univariately, patients with positive tumor cell or diffuse tumor vasculature B7-H3 expression were over three times more likely to die from RCC compared with patients with negative tumor cell and absent, focal, or moderate tumor vasculature B7-H3 expression (risk ratio, 3.09; 95% CI, 2.36-4.03; P < 0.001; Fig. 2C). Associations of this combined variable with death from RCC after adjusting for each of the clinical and pathologic features studied are shown in Table 4. After simultaneous adjustment for the TNM stage groupings, nuclear grade, and ECOG performance status (risk ratio, 1.68; 95% CI, 1.27-2.24; P < 0.001) and the SSIGN score (risk ratio, 1.38; 95% CI, 1.03-1.84; P = 0.029), the presence of this combined variable remained statistically significantly associated with an increased risk of death from RCC.
Table 4.
Adjusted associations of the presence of tumor cell or diffuse tumor vasculature B7-H3 expression with death from RCC for 743 ccRCC patients
| Feature | Risk ratio (95% CI)* | P |
|---|---|---|
| Age at surgery | 3.08 (2.36-4.03) | <0.001 |
| Gender | 3.10 (2.37-4.05) | <0.001 |
| Symptoms at presentation | 2.84 (2.17-3.72) | <0.001 |
| Constitutional symptoms at presentation | 2.73 (2.07-3.60) | <0.001 |
| ECOG performance status | 3.08 (2.36-4.03) | <0.001 |
| Tumor thrombus | 2.68 (2.04-3.53) | <0.001 |
| Primary tumor size | 2.32 (1.77-3.04) | <0.001 |
| 2002 Primary tumor classification | 2.17 (1.65-2.85) | <0.001 |
| Regional lymph node involvement | 3.01 (2.30-3.93) | <0.001 |
| Distant metastases | 2.72 (2.07-3.56) | <0.001 |
| 2002 TNM stage groupings | 2.25 (1.71-2.96) | <0.001 |
| Nuclear grade | 1.78 (1.34-2.36) | 0.003 |
| Coagulative tumor necrosis | 1.75 (1.31-2.34) | <0.001 |
| Sarcomatoid Differentiation | 2.74 (2.08-3.60) | <0.001 |
| TNM stage groupings, nuclear grade, ECOG | 1.68 (1.27-2.24) | <0.001 |
| SSIGN score | 1.38 (1.03-1.84) | 0.029 |
Risk ratio represents the association of the presence of positive tumor cell or diffuse tumor vasculature B7-H3 expression with death from RCC (using negative tumor cell and absent, focal, or moderate tumor vasculature B7-H3 expression as the reference group) after adjustment for the feature or combination of features listed.
To probe deeper into the associations between B7-H3 expression and disease progression, a subset analysis was conducted for 661 patients with pM0 ccRCC at nephrectomy. Progression to metastatic disease was observed in 190 of these patients at a median of 1.6 years (range, 0.1-14.6 years) following nephrectomy. There were 101 (15.3%) specimens that exhibited positive tumor cell B7-H3 expression, 225 (34.0%) with diffuse tumor vasculature B7-H3 expression, and 281 (42.5%) specimens that exhibited either tumor cell or diffuse tumor vasculature B7-H3 expression. In the univariate setting, both B7-H3 expression by tumor cells (risk ratio, 3.41; 95% CI, 2.48-4.69) as well as diffuse B7-H3 tumor vasculature expression (risk ratio, 2.09; 95% CI, 1.57-2.77) functioned as predictors of ccRCC progression (P < 0.001). In multivariable analysis after adjustment for the PROG score, positive tumor cell B7-H3 expression alone was no longer significant (risk ratio, 1.24; 95% CI, 0.88-1.75; P = 0.213), whereas diffuse tumor vascular B7-H3 expression remained a significant predictor of disease progression (risk ratio, 1.44; 95% CI, 1.08-1.92; P = 0.014). Likewise, the presence of either tumor cell or diffuse tumor vasculature B7-H3 expression among pM0 ccRCC patients was significantly associated with an increased risk of disease progression both univariately (risk ratio, 2.85; 95% CI, 2.12-3.83; P < 0.001) and after adjusting for the PROG score (risk ratio, 1.58; 95% CI, 1.16-2.15; P = 0.004).
Discussion
RCC has long been recognized as an immunogenic tumor. Prompted by this, significant attention has been directed toward optimizing immune-based approaches to treat RCC, principally by attempting to force immune cell activation through the use of stimulatory cytokines such as interleukin-2 and IFN-α or via tumor vaccine approaches. In contrast, efforts to elucidate and then circumvent mechanisms of RCC immune evasion have evolved relatively slowly. Related to this, we previously reported that B7-H1 and B7-H4, two ligands with immune cell-inhibitory potential, are often expressed by aggressive forms of ccRCC, supporting the possibility that these ligands neutralize host immunity to promote ccRCC progression. In this report, we show that a third B7-H family ligand, B7-H3, is expressed by both ccRCC tumor cells and tumor vasculature. In addition, we show that increased expression of B7-H3 correlates with adverse clinical and pathologic features of ccRCC and predicts disease progression and cancer-specific death.
Given its relatively recent discovery, mechanisms regulating B7-H3 expression are poorly understood. B7-H3 mRNA expression has been detected in multiple human tissues and cell lines, whereas protein expression in human dendritic cells and monocytes can be induced by treatment with phorbol 12-myristate 13-acetate and ionomycin. In mice, B7-H3 is constitutively expressed by all resting and activated B cells, macrophages, dendritic cells, and minor subsets of CD4+ and CD8+ T cells (9). As with other B7-H family molecules, the physiologic function of B7-H3 remains somewhat ambiguous. In one early study, human B7-H3 was reported to function as a costimulator of CD4+ and CD8+ T cells, promoting T-cell proliferation and cytokine production in vitro (20). Most recently, B7-H3 has been implicated as a potent inhibitor of T-cell activity (21). Specifically, single or duplicate constructs of the immunoglobulin-V-like and immunoglobulin-C-like domains of human B7-H3 have been shown to down-regulate both T-cell proliferation and cytokine production in response to CD3/CD28–mediated costimulatory activation (21). Furthermore, it has been reported that B7-H3–deficient mice develop accelerated forms of induced airway inflammation and experimental autoimmune encephalitis, thus implicating B7-H3 as an inhibitor of TH1-mediated immunity (22). In a separate study, plate-immobilized murine B7-H3 in the presence of anti-CD3 was shown to inhibit murine CD4+ T-cell activation and interleukin-2 production, an effect that was completely abrogated by antibody-mediated B7-H3 blockade (20). Additionally, B7-H3 has been noted to be expressed by dendritic cells and associated with suppressive activity following contact with CD4+CD25+ regulatory T cells (23). Although the above-cited studies have investigated the potential functional significance of cell-surface expression of B7-H3, a recent report by Zhang et al. (24) described a soluble form of B7-H3. In this report, soluble B7-H3 was noted to be released by monocytes, dendritic cells, activated T cells, and B7-H3–positive tumor cells, but not by B7-H3–negative tumor cells. The functional significance of soluble B7-H3 has yet to be explored. Despite the initial investigations that have proposed the functional significance of B7-H3, the specific cognate receptors for B7-H3 have not been elucidated. It also seems that B7-H3 ligand expression may be regulated by host factors or tumor microenvironment, as is supported by differential protein expression of B7-H3 according to tumor type or location within a given tumor (25).
Although future studies will certainly be required to better understand the precise events that govern B7-H3 expression and functionality, our present study suggests that the B7-H3 ligand may help to render ccRCC tumors more aggressive by imbuing such tumors with a capability to neutralize local antitumoral immune responses or to facilitate nascent blood vessel formation. As such, targeting of B7-H3 could prove useful in potentiating responses to current forms of systemic treatment for advanced ccRCC, treatments that are primarily immunotherapeutic or antiangiogenic in nature and that, in their present form, yield relatively low rates of durable disease remission. Additionally, our data indicate that B7-H3 may prove useful as a prognostic marker to identify patients with localized tumors who are at high risk for postsurgical ccRCC progression and who might benefit from adjuvant therapy in the clinical trial setting.
In general, the expression profile and prognostic ability of B7-H3, as it pertains to ccRCC, are reminiscent of what we have previously reported for B7-H1 and B7-H4. Similar to B7-H1 and B7-H4, B7-H3 can be directly expressed by ccRCC tumor cells (11, 14, 26). In addition, like B7-H4, B7-H3 is widely expressed by ccRCC tumor vasculature. Lastly, as is the case with B7-H1 and B7-H4, enhanced tumor expression of B7-H3 correlates with adverse clinical and pathologic features of ccRCC and independently predicts disease progression and cancer-specific death. At present, however, several key questions pertaining to ccRCC B7-H ligand expression remain unanswered. Specifically, whether B7-H3 adds to the prognostic capabilities of B7-H1 and B7-H4 remains unknown. In the previous report by Krambeck et al. (11), combined B7-H1 and B7-H4 expression was associated with significantly diminished cancer-specific survival compared with patients whose tumors were negative for both or singly positive for B7-H1 or B7-H4. The significance of combined B7-H1 and B7-H4 expression on cancer-specific survival remained after multivariable adjustment. At present, our further evaluation of the effect of combined B7-H (B7-H1, B7-H3, and B7-H4) ligand expression is pending optimization of methods to stain for B7-H4 in formalin-fixed, paraffin-embedded tissue. However, our observation that the human renal carcinoma cell line Caki-2 expresses both B7-H1 and B7-H3 supports the likelihood that ccRCC tumor cells within clinical specimens will express multiple B7-H ligands simultaneously. Whether such cells encompass a particularly aggressive subpopulation of ccRCC tumor cells has not yet been tested in functional studies. However, our studies of B7-H1, B7-H3, and B7-H4 primarily reveal that the expression patterns of these three ligands within ccRCC are not entirely overlapping. In addition, irrespective of whether these three ligands collaborate together as independent predictors of ccRCC outcomes, that all three B7-H ligands can be expressed by ccRCC tumors may have ominous implications for ccRCC immunotherapy. Specifically, it seems that aggressive ccRCC tumors possess redundant mechanisms to mitigate antitumoral immunity, a critical point that we believe is severely underestimated, or often ignored, in studies pertaining to tumor immunobiology and immunotherapy. Thus, future studies will be required to better understand the full clinical and functional potential of B7-H ligands in modulating the behavior of ccRCC.
There are several limitations of the presented data, including the inherent limitations of any single institutional retrospective review and the use of a single pathologist in the interpretation of immunohistochemical staining. As with all initial series that propose the prognostic significance of molecular biomarkers, our current findings will require independent external validation. Concurrently, observations that increased B7-H ligand expression diminishes ccRCC patient response to immunotherapy and that B7-H ligand blockades facilitate antitumoral responses may provide additional evidence that these molecules function as local inhibitors of antitumoral immunity.
In summary, we report that the combined variable of tumor cell or diffuse tumor vasculature B7-H3 expression is an independent predictor of outcomes for ccRCC patients and may ultimately add to B7-H1 and B7-H4 as prognostic markers for this form of malignancy. More importantly, our collective studies indicate that multiple B7-H ligands can be expressed by aggressive forms of ccRCC, thus suggesting the sobering possibility that single-modality approaches to antitumoral immunotherapy may be overly simplistic and, ultimately, only able to yield incremental responses. From this, we surmise that simultaneous targeting of multiple immune cell inhibitory and stimulatory pathways will likely be required to promote robust ccRCC tumor regression in the clinical setting.
Acknowledgments
Grant support: The Richard M. Schulze Family Foundation, The Commonwealth Foundation for Cancer Research, and The Helen and Martin Kimmel Foundation (E.D. Kwon) and Fraternal Order of Eagles Cancer Research Fund and Mayo Junior Faculty Development Award (H.D.)
Footnotes
Disclosure of Potential Conflicts of Interest
Y. Sheinin,T.J. Roth, C.M. Lohse, and E.D. Kwon have filed a patent on B7-H3 prognostic marker.
References
- 1.Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101–6. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 3.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6 1:S55–7. [PubMed] [Google Scholar]
- 5.Hudes G, Carducci M, Tomcz P, et al. Temsirolimus, interferon α, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomcz P, et al. Sunitinib versus interferon α in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 10.Inman BA, Frigola X, Dong H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer DrugTargets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 11.Krambeck AE, Thompson RH, Dong HD, et al. B7-4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–6. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong HD, Strome SE, Salomao DR, et al. Tumor-associated B7-1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 13.Zang XX, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–92. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson RH, Gillettt MD, Cheville JC, et al. Costimulatory B7-1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–3. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Storkel S, Eble JN, Adlha K, et al. Classification of renal cell carcinoma—workgroup no 1. Cancer. 1997;80:987–9. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Roth TJ, Sheinin Y, Lohse CM, et al. B7-3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 18.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 19.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 20.Prasad DV, Nguyen T, Li Z, et al. Murine B7-3 is a negative regulator of T cells. J Immunol. 2004;173:2500–6. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 21.Ling V, Wu PW, Spaulding V, et al. Duplication of primate and rodent B7-3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365–77. doi: 10.1016/s0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 22.Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7-3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 23.Mahnke K, Ring S, Johnson TS, et al. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7-3 expression and antigen presentation. Eur J Immunol. 2007;37:2117–26. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–46. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–9. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory molecule B7-1 on primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084–91. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]


