Abstract
BACKGROUND
The authors evaluated the long-term outcomes of men with prostate cancer and very high (≥50 ng/mL) preoperative serum prostate-specific antigen (PSA) values that were treated with radical prostatectomy.
METHODS
This study included 236 men with preoperative serum PSA values ≥50 ng/mL who underwent radical retropubic prostatectomy between 1987 and 2004. For comparison, the study cohort was divided into 2 groups: patients with PSA levels between 50 and 99 ng/mL and patients with PSA levels ≥100 ng/mL. Biochemical recurrence was defined as a single postoperative serum PSA value of 0.4 ng/mL or greater. Systemic disease progression was defined as the development of a local recurrence or systemic metastases, and any death resulting from prostate cancer or its treatment was defined as a cancer-specific mortality.
RESULTS
Biochemical recurrence-free survival rates in the groups of patients with a PSA level 50 to 99 ng/mL and ≥100 ng/mL were 43% and 36% at 10 years, respectively. Systemic progression-free survival rates in the PSA 50 to 99 ng/mL and PSA ≥100 ng/mL groups were 83% and 74% at 10 years, respectively. Estimated overall cancer-specific survival was 87% at 10 years.
CONCLUSIONS
Patients with prostate cancer and a serum PSA level ≥50 ng/mL have very high-risk prostate cancer that carries a high likelihood of being pathologically advanced. Although the probability of realizing long-term survival in these high-risk patients is less than in patients with more favorable disease, 10-year survival outcomes remain excellent and argue for aggressive management of these cases.
Keywords: androgen deprivation therapy, external beam radiotherapy, prostate cancer, prostate-specific antigen, radical prostatectomy
Because of the widespread use of prostate-specific antigen (PSA) testing, there has been a significant prostate cancer grade and stage migration, with >90% of men diagnosed with prostate cancer in the current era having clinically localized disease.1 Given that PSA screening detects patients with prostate cancer earlier in their disease course, there has been a steady decline in the number of patients presenting with a PSA level >10 ng/mL. For example, the Partin tables published in 1993, 1997, 2001, and 2007 showed that 25%, 29%, 21%, and 12% of men, respectively, presented with PSA values >10 ng/mL.2 In addition, the randomized screening study of Hugosson et al showed that PSA levels of 20 to 99.9 ng/mL and >100 ng/mL occurred only in 0.5% and 0.04% of screened men, respectively.3 Despite these trends toward lower-risk prostate cancer, as many as 20% to 35% of patients with newly diagnosed prostate cancer are still classified as being at high risk based on either a Gleason score of 8 to 10, a PSA >20 ng/mL, or an advanced clinical stage.4 Importantly, Surveillance, Epidemiology, and End Results data indicate that the rate of pT3a prostate cancer has remained relatively constant over the past 20 years.5
To our knowledge, there is no consensus regarding the optimal treatment of men with high-risk prostate cancer. Watchful waiting (WW) with or without androgen deprivation therapy (ADT), brachy-therapy, external beam radiotherapy (EBRT), and radical prostatectomy (RP) are potential treatment options for high-risk patients, although EBRT and RP are the options most physicians who commonly treat high-risk prostate cancer would recommend.6 Despite a relative paucity of data in support of any particular treatment option, only 36% of high-risk cases in the CaPSURE dataset were treated with radical prostatectomy, and men with a serum PSA >20 ng/mL are more likely to undergo EBRT than RP (odds ratio [OR] of 2.9), ADT than RP (OR of 8.3), and WW than RP (OR of 4.6).7 This suggests a bias against treating high-risk prostate cancer with radical prostatectomy, which could be because of an under-appreciation of the distinct advantages of radical prostatectomy in high-risk prostate cancer: it eliminates the primary source of systemic metastases, it prevents the late wave of systemic disease that is typically observed with radiotherapy, and it decreases the need for future procedural pelvic intervention by providing good local cancer control.8
In the current study, we evaluated the long-term outcomes of men with prostate cancer and very high (>50 ng/mL) preoperative serum PSAs who were treated with RP as their primary treatment modality. We report 10-year results on biochemical failure, systemic progression-free survival, and cancer-specific survival. In addition, we identified key predictors of mortality in this cohort and ultimately described the contemporary surgical outcomes that are possible in patients with high-risk prostate cancer when multi-modality treatment regimens are used aggressively.
Materials and Methods
Patient Population
After approval by the Mayo Clinic Institutional Review Board, we identified all men treated with RP for adenocarcinoma of the prostate at our institution between 1987 and 2004 who had a preoperative PSA level ≥50 ng/mL. Two men who refused medical record access for research purposes were excluded from the study, leaving 236 men for the current study. All patients were staged preoperatively with digital rectal examination, an abdominopelvic computed tomography (CT) scan, and a bone scan. For comparison, the study cohort was divided into 2 groups: patients with PSA levels between 50 and 99 ng/mL and patients with PSA levels ≥100 ng/mL.
Recurrence Definitions and Patient Follow-up
Biochemical disease recurrence was defined as a single postoperative serum PSA value of ≥0.4 ng/mL or a rising serum PSA while receiving ADT.9 ADT and EBRT were defined as adjuvant treatment if administered within 3 months of surgery to patients with an undetectable PSA who were not considered treatment failures. ADT and EBRT administered >3 months after surgery were defined as salvage treatment, and such patients were considered treatment failures. Systemic disease progression was defined as the development of a biopsy-proven local recurrence or imaging-identified systemic metastases. Death resulting from prostate cancer or its treatment was defined as a cancer-specific mortality. In general, patients were observed postoperatively with serum PSA and digital rectal examination every 3 months for the first postoperative year, every 6 months for the second and third postoperative years, and annually thereafter. Patients with biochemical failures were evaluated with an abdominopelvic CT scan, a bone scan, and a chest radiograph to assess for the presence of clinically detectable prostate cancer. Transrectal ultrasound was used to biopsy any palpable abnormality on digital rectal examination, and other forms of imaging, including endorectal magnetic resonance imaging, were used in select cases to establish the location of the prostate cancer recurrence.
Statistical Analysis
Continuous variables were summarized using the mean and standard deviation if they were normally distributed and the median and interquartile range if they were not. Group comparisons were made using the Wilcoxon rank sum test for continuous covariates, the Pearson chi-square test for categoric covariates, and the log-rank test for survival endpoints. The Kaplan-Meier method was used to estimate the survivor function at various timepoints. Multivariate hazards ratios and their 95% confidence intervals were estimated using the Cox proportional hazards model. Assumptions of the Cox model were verified using the methods described by Therneau and Grambsch.7 Prespecified clinical variables considered in the Cox model included: preoperative serum PSA group (50-99 ng/mL, ≥100 ng/mL), pathologic Gleason score, organ confinement, seminal vesicle invasion, positive surgical margins, positive pelvic lymph nodes, adjuvant EBRT, and adjuvant hormonotherapy.
Results
The preoperative characteristics of the study cohort are shown in Table 1. One hundred and seventy-eight men had preoperative PSA levels between 50 ng/mL and 99 ng/mL, and 56 men had preoperative PSA levels ≥100 ng/mL. The majority of patients (88%) had tumor that was palpable on digital rectal examination and were detected early in the PSA era.
TABLE 1. Preoperative Characteristics of the Patient Cohort.
| PSA of 50-99 ng/mL (n=5178) |
PSA ≥100 ng/mL (n=56) |
Overall (n=234) |
|
|---|---|---|---|
| Mean age, y (SD) | 65 (6.9) | 65 (6.2) | 65 (6.7) |
| Median serum PSA, ng/mL (IQR) | 65 (57-76) | 134 (112-189) | 72 (59-95) |
| Biopsy Gleason score | |||
| ≤6 | 59 (34%) | 21 (38%) | 80 (35%) |
| 7 | 89 (51%) | 19 (35%) | 108 (47%) |
| 8-10 | 26 (15%) | 15 (27%) | 41 (18%) |
| 1997 TNM clinical stage | |||
| T1 | 27 (16%) | 5 (9%) | 32 (14%) |
| T2a (1 lobe) | 52 (30%) | 15 (27%) | 67 (29%) |
| T2b (2 lobes) | 32 (19%) | 14 (25%) | 46 (20%) |
| T3/4 | 62 (36%) | 22 (39%) | 84 (37%) |
| Year of surgery | |||
| <1990 | 33 (19%) | 15 (27%) | 48 (21%) |
| 1990-1995 | 106 (60%) | 32 (57%) | 138 (59%) |
| 1995-2000 | 15 (8%) | 7 (13%) | 22 (9%) |
| >2000 | 24 (14%) | 2 (4%) | 26 (11%) |
PSA indicates prostate-specific antigen; SD, standard deviation; IQR, interquartile range.
The characteristics of the pathology specimen and the adjuvant treatments applied are shown in Table 2. The majority of patients had positive surgical margins, extraprostatic extension of their malignancy, or positive pelvic lymph nodes, attesting to the aggressive nature of the disease present at prostatectomy. With a median follow-up of 12.6 years (range, 0.5-18.5 years), there were 134 PSA recurrences, 31 local recurrences of prostate cancer, 46 cases of disease progression to metastases, and 28 prostate cancer deaths. Biochemical disease recurrence-free survival rates in the patients with PSA levels of 50 to 99 ng/mL and ≥100 ng/mL were 51% and 52% at 5 years, 40% and 36% at 10 years, and 34% and 29% at 15 years, respectively (Fig. 1). Systemic progression-free survival in the groups of patients with PSA levels of 50 to 99 ng/mL and PSA ≥100 ng/mL were 89% and 86% at 5 years, 83% and 74% at 10 years, and 70% and 57% at 15 years, respectively (Fig. 2). Cancer-specific survival rates in the patients with PSA levels of 50 to 99 ng/mL at 5 years, 10 years, and 15 years were 98%, 90%, and 78% (Fig. 3). Cancer-specific survival rates in the group of patients with a PSA level of >99 ng/mL at 5 years, 10 years, and 15 years were 94%, 79%, and 72%, respectively (Fig. 3). Estimated overall local recurrence-free survival and cancer-specific survival rates were 85% and 87% at 10 years, respectively. A preoperative PSA value >100 ng/mL was not found to be a significant risk factor for biochemical disease progression, systemic progression, or death from prostate cancer when compared with the group of men with preoperative PSA values between 50 and 99 ng/mL (Table 3).
TABLE 2. Postoperative Characteristics of the Patient Cohort.
| PSA of 50-99 ng/mL (n=178) |
PSA ≥100 ng/mL (n=56) |
Overall (n=234) |
|
|---|---|---|---|
| Pathologic Gleason score | |||
| ≤6 | 42 (25%) | 16 (32%) | 58 (26%) |
| 7 | 86 (50%) | 23 (45%) | 109 (49%) |
| 8-10 | 43 (25%) | 12 (24%) | 55 (25%) |
| 1997 TNM pathologic stage | |||
| T2a | 8 (5%) | 1 (2%) | 9 (4%) |
| T2b | 27 (15%) | 4 (7%) | 31 (13%) |
| T3/4 | 143 (80%) | 51 (91%) | 194 (83%) |
| Seminal vesicles | |||
| Positive | 112 (63%) | 46 (82%) | 158 (68%) |
| Negative | 66 (37%) | 10 (18%) | 76 (33%) |
| Pelvic lymph nodes | |||
| Positive | 62 (35%) | 24 (43%) | 86 (37%) |
| Negative | 116 (65%) | 32 (57%) | 148 (63%) |
| Surgical margins | |||
| Positive | 139 (78%) | 46 (82%) | 185 (79%) |
| Negative | 39 (22%) | 10 (18%) | 49 (21%) |
| Adjuvant EBRT | |||
| Yes | 31 (17%) | 8 (14%) | 39 (17%) |
| No | 147 (83%) | 48 (86%) | 195 (83%) |
| Adjuvant ADT | |||
| Yes | 99 (56%) | 39 (70%) | 138 (59%) |
| No | 79 (44%) | 17 (30%) | 96 (41%) |
| Salvage EBRT | |||
| Yes | 34 (19%) | 9 (16%) | 43 (21%) |
| No | 144 (81%) | 47 (84%) | 191 (79%) |
| Salvage ADT | |||
| Yes | 62 (35%) | 18 (32%) | 80 (34%) |
| No | 116 (65%) | 38 (68%) | 154 (66%) |
PSA indicates prostate-specific antigen; EBRT, external beam radiotherapy; ADT, androgen deprivation therapy.
FIGURE 1.
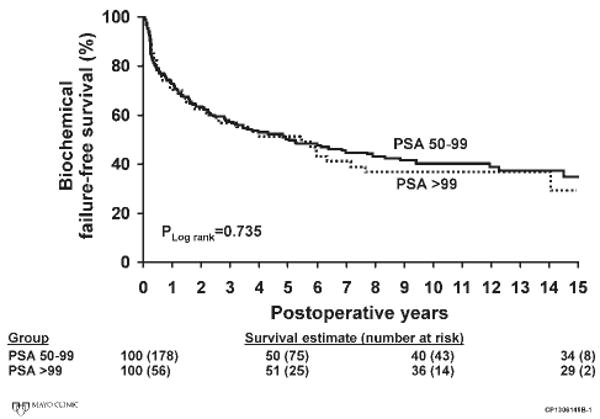
Biochemical disease recurrence-free survival is shown for men with preoperative serum prostate-specific antigen (PSA) values of 50 to 99 ng/mL and ≥100 ng/mL.
FIGURE 2.
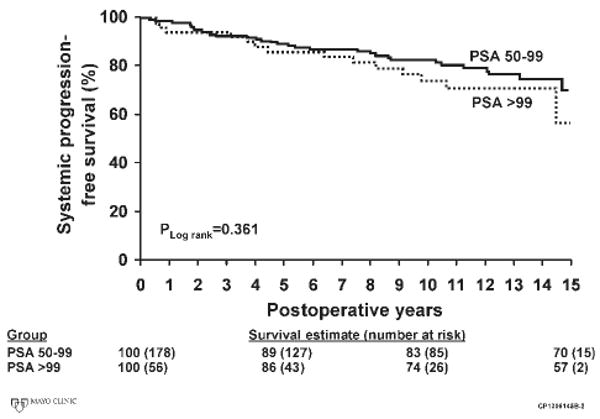
Metastasis-free survival is shown for men with preoperative serum prostate-specific antigen (PSA) values of 50 to 99 ng/mL and ≥100 ng/mL.
FIGURE 3.
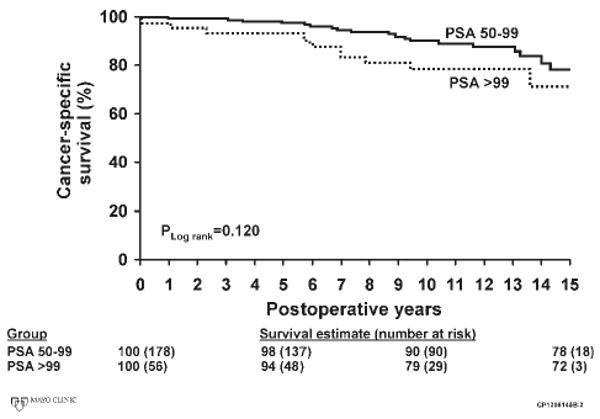
Cancer-specific survival is shown for men with preoperative serum prostate-specific antigen (PSA) values of 50 to 99 ng/mL and ≥100 ng/mL.
TABLE 3. Multivariate Cox Proportional Hazards Model.
| Variable | Outcome | Hazards Ratio | 95% CI | P |
|---|---|---|---|---|
| PSA >100 ng/mL | PSA failure | 1.05 | 0.69-1.60 | .181 |
| Metastases | 0.97 | 0.49-1.90 | .918 | |
| Cancer survival | 0.87 | 0.37-2.06 | .749 | |
| Gleason score | PSA failure | 1.14 | 0.97-1.33 | .111 |
| Metastases | 1.04 | 0.79-1.38 | .763 | |
| Cancer survival | 1.28 | 0.90-1.83 | .168 | |
| Organ confined | PSA failure | 0.58 | 0.31-1.08 | .085 |
| Metastases | 0.32 | 0.06-1.75 | .192 | |
| Cancer survival | 0.79 | 0.07-9.43 | .854 | |
| Seminal vesicle invasion | PSA failure | 1.25 | 0.77-2.03 | .368 |
| Metastases | 2.27 | 0.88-5.82 | .089 | |
| Cancer survival | 3.26 | 0.76-14.07 | .113 | |
| Positive margins | PSA failure | 1.04 | 0.68-1.59 | .856 |
| Metastases | 1.09 | 0.51-2.31 | .833 | |
| Cancer survival | 2.57 | 0.73-9.02 | .141 | |
| Positive lymph nodes | PSA failure | 1.06 | 0.64-1.78 | .812 |
| Metastases | 1.37 | 0.62-3.05 | .440 | |
| Cancer survival | 3.20 | 1.06-9.62 | .039 | |
| Adjuvant ADT | PSA failure | 0.24 | 0.14-0.40 | <.001 |
| Metastases | 0.60 | 0.25-1.42 | .243 | |
| Cancer survival | 0.62 | 0.16-2.32 | .473 | |
| Adjuvant EBRT | PSA failure | 0.41 | 0.23-0.72 | .002 |
| Metastases | 0.55 | 0.22-1.35 | .190 | |
| Cancer survival | 0.61 | 0.22-1.73 | .345 |
95% CI indicates 95% confidence interval; PSA, prostate-specific antigen; ADT, androgen deprivation therapy; EBRT, external beam radiotherapy.
Adjuvant ADT was used in 138 (59%) patients, adjuvant EBRT was used in 39 (17%) patients, and 79 (34%) received no adjuvant treatment. Biochemical disease recurrence-free, metastasis-free, and cancer-specific survival plots by adjuvant therapy status are shown in Figure 4. These plots demonstrate improved biochemical failure-free survival in patients who received adjuvant therapy but no difference in metastasis-free or cancer-specific survival. Salvage ADT was used in 80 (34%) patients and salvage EBRT in 43 (21%) patients.
FIGURE 4.
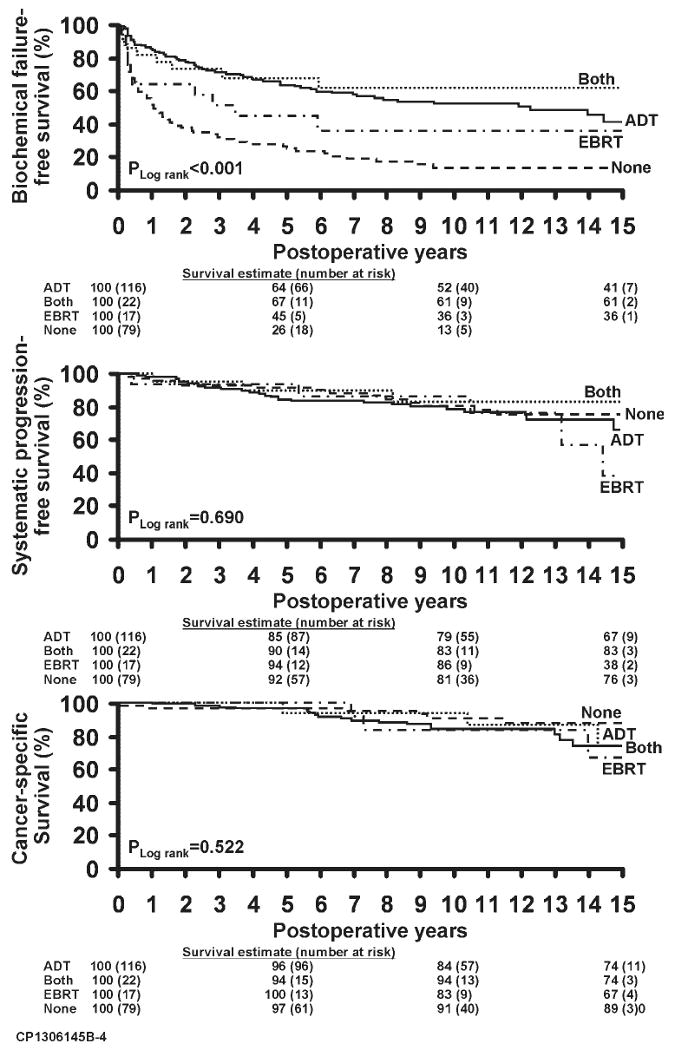
(Top) Biochemical disease recurrence-free survival is shown by immediate adjuvant treatment status. (Middle) Metastasis-free survival is shown by immediate adjuvant treatment status. (Bottom) Cancer-specific survival is shown by immediate adjuvant treatment status. ADT indicates androgen deprivation therapy; EBRT, external beam radiotherapy.
The results of multivariate Cox modeling predicting cancer-specific survival are shown in Table 3. Immediate adjuvant radiotherapy reduced the risk of a PSA recurrence by 2.4–fold (P <.001), and immediate adjuvant ADT reduced the risk of PSA failure by 4.2–fold (P = .002) (Fig. 4 top). Because of a paucity of metastases and deaths, neither of these treatments was found to be a significant predictor of metastasis-free and prostate cancer-specific survival (Fig. 4 top and middle). In fact, the only significant predictor of prostate cancer death was the presence of positive pelvic lymph nodes, which more than tripled the probability of dying of prostate cancer.
Discussion
Of the various clinicopathologic features that characterize high-risk prostate cancer, serum PSA is arguably the most objective, and men with preoperative serum PSA levels >50 ng/mL are undoubtedly a high-risk cohort. Although high-risk prostate cancer has been studied by several groups, to our knowledge the outcomes of men with very high PSAs treated with radical prostatectomy are not well known. In fact, D'Amico et al did not include any men with pretherapy PSA values ≥50 ng/mL in their landmark study that defined prostate cancer risk groups based on PSA level, biopsy Gleason score, and clinical stage.9 The current study demonstrates that patients with PSA values ≥50 ng/mL can be expected to present with Gleason grade 8 or higher tumors roughly 25% of the time. In addition, nearly half of such men will have extraprostatic extension of their cancer, and approximately one–third will have pelvic lymph node involvement. These data are somewhat different from those of Partin et al, who found that patients with serum PSA levels ≥50 ng/mL had extraprostatic disease in >90% of cases.10 Regardless, patients with serum PSA levels ≥50 ng/mL might be expected to have poor outcomes after attempted curative treatment. The current study had the goal of demonstrating that the outcomes of radical prostatectomy in this very high-risk cohort may not be as poor as initially suspected when used as the cornerstone of an aggressive multimodal treatment regimen.
At 10 years, we observed a PSA recurrence-free survival rate of 40% and 36% for men with serum PSA levels of 50 to 99 mg/dL and ≥100 ng/mL, respectively. Although they did not include men with PSA values >50 ng/mL, D'Amico et al found that men with PSA levels >20 ng/mL had a >50% risk of PSA failure at 5 years, an estimate that is worse than that noted in the current study.9 In the study of Han et al, 120 men with palpable disease (cT2a to cT3a) and preoperative PSA values >15 ng/mL demonstrated overall PSA-free survival rates at 5 years and 10 years of 56% and 40%, respectively.11 These results are similar to ours, although their cohort did have somewhat lower PSA values. Lastly, May et al found a higher PSA failure rate, with only 27% of their patients having PSA values of 50.1 ng/mL to 100 ng/mL remaining PSA-free at 5 years.12 Differing usage of adjuvant and salvage therapies may be confounding these studies.
What these previous studies did not address are the more clinically meaningful endpoints of metastasis-free and cancer-specific survival. In the current study, patients with PSA values between 50 and 99 mg/dL experienced 10-year metastasis-free and cancer-specific survival rates of 83% and 90%, respectively. For patients with PSA levels >100 ng/mL, the 10-year metastasis-free and cancer-specific survival rates were 74% and 79%, respectively. Recent surgical series have demonstrated 10-year cancer-specific survival rates between 72% and 92% for men with high-risk disease. This indicates that a majority of men with high-risk prostate cancer, including those with very high PSA levels, stand to benefit from radical prostatectomy.13-15 In fact, when one considers that in 2004 the average life expectancy for an American aged 65 years was roughly 17 years,16 it becomes apparent that the high-risk patients in the current series have a surprisingly modest reduction in survival when compared with their normal peers. At 15 years of follow-up, the median survival of the current cohort (mean age at treatment was 65 years) had still not been met, clear evidence that with appropriate treatment many men with high-risk prostate cancer can have very prolonged survival.
A central point of the current study is that radical prostatectomy is not usually adequate as mono-therapy for patients with high-risk prostate cancer. Rather, a multimodality treatment approach with adjuvant and ADT is often required to achieve the long-term success rates described in the current study. Radiation oncologists and medical oncologists are therefore critical components of treatment success. Our center has previously shown that immediate androgen deprivation after RP can improve survival in patients with locally advanced disease.17 Similarly, the International Early Prostate Cancer study demonstrated that 150 mg of bicalutamide may be beneficial when given after RP to patients with high-risk prostate cancer.18 Messing et al also showed that adjuvant ADT is effective in patients with positive lymph nodes.19 Adjuvant radiotherapy is also reported to be of benefit in patients with extraprostatic disease and positive margins, whose risk of local failure is higher.20 Early adjuvant radiation and early ADT can offer significant benefit to the high-risk prostate cancer patient.
Opponents of surgery in high-risk patients have argued for a lack of benefit if the prostate cancer is not completely excised (ie, if there are micrometastases). This argument does not consider the considerable importance of local pelvic control, despite micrometastatic disease. Our experience has been that RP provides excellent local control in high-risk prostate cancer, evidenced by a local recurrence rate of only 13% in the current series. The same cannot be said of primary radiotherapy. Zagars et al found that radiotherapy provided 10-year metastasis-free and local recurrence-free survival rates of only 41% and 64%, respectively.21 Furthermore, biopsy studies after radiotherapy have demonstrated persistent prostate cancer in 14% to 91% of patients.22 Coen et al found an independent association between delayed metastasis and the local persistence of the cancer on biopsy, as well as a temporarily increasing hazards rate in men treated with radiotherapy for prostate cancer.23 The researchers postulated that a biologically altered prostate cancer after radiotherapy resulted in a late wave of metastatic seeding. In favor of this, D'Amico et al reported significantly higher 10-year prostate cancer-specific survival rates in high-risk men treated with surgery (90%) compared with radiation (75%).24 There are also data from a large population study and a randomized trial suggesting that radiotherapy is inferior to surgery as the primary therapeutic modality in patients with high-risk prostate cancer.25,26 Larger randomized trials are certainly needed to clarify this issue.
Although the low number of patients developing metastases and dying of prostate cancer in the current study is a testament to the value of an aggressive multimodality approach to treating high-risk prostate cancer, it also presents limitations for our statistical modeling. Specifically, because the number of metastases and deaths is low, our multivariate Cox model may be overfitted for these endpoints, despite having been prespecified at the design phase of the study. Although our median follow-up is nearly 13 years, it is probable that more patients will develop metastases and die over the next 5 to 10 years, and that our model estimates will change somewhat. We look forward to representing our series when another 5 years of follow-up has been achieved.
Conclusions
Patients with prostate cancer and a serum PSA ≥50 ng/mL have very high-risk prostate cancer that carries a high likelihood of being pathologically advanced. Although the probability of realizing long-term survival in these high-risk patients is blunted when compared with those with more favorable disease, high-risk patients should definitely not be classified as incurable. Rather, with aggressive surgical resection and appropriate multimodal adjuvant treatment, survival beyond 10 years is achievable in >85% of patients with prostate cancer and very elevated serum PSA levels.
References
- 1.Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossfeld GD, Latini DM, Lubeck DP, Mehta SS, Carroll PR. Predicting recurrence after radical prostatectomy for patients with high risk prostate cancer. J Urol. 2003;169:157–163. doi: 10.1016/S0022-5347(05)64058-X. [DOI] [PubMed] [Google Scholar]
- 3.Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100:1397–1405. doi: 10.1002/cncr.20126. [DOI] [PubMed] [Google Scholar]
- 4.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 2007. pp. 1975–2004. [Google Scholar]
- 5.Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR. Treatment of patients with high risk localized prostate cancer: results from cancer of the prostate strategic urological research endeavor (CaPSURE) J Urol. 2005;173:1557–1561. doi: 10.1097/01.ju.0000154610.81916.81. [DOI] [PubMed] [Google Scholar]
- 6.Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165:1146–1151. [PubMed] [Google Scholar]
- 7.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 8.Schenk GS, Blute ML, Zincke H. Surgical management of clinical T3 (cT3) and node positive (pN+) adenocarcinoma of the prostate. In: Vogelzang NJ, Scardino PT, Shipley WU, Debruyne FMJ, Linehan WM, editors. Comprehensive Textbook of Genitourinary Oncology. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 246–254. [Google Scholar]
- 9.D'Amico AV, Whittington R, Malkowicz SB, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999;17:168–172. doi: 10.1200/JCO.1999.17.1.168. [DOI] [PubMed] [Google Scholar]
- 10.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- 11.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 12.May M, Gunia S, Helke C, et al. How far is the preoperative Kattan nomogram applicable for the prediction of recurrence after prostatectomy in patients presenting with PSA levels of more than 20 ng/ml? A validation study. Urol Int. 2006;77:222–226. doi: 10.1159/000094813. [DOI] [PubMed] [Google Scholar]
- 13.Freedland SJ, Partin AW, Humphreys EB, Mangold LA, Walsh PC. Radical prostatectomy for clinical stage T3a disease. Cancer. 2007;109:1273–1278. doi: 10.1002/cncr.22544. [DOI] [PubMed] [Google Scholar]
- 14.Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005;95:751–756. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 15.Carver BS, Bianco FJ, Jr, Scardino PT, Eastham JA. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006;176:564–568. doi: 10.1016/j.juro.2006.03.093. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Heath Statistics. Chartbook on Trends in the Health of Americans. Hyattsville, MD: National Center for Heath Statistics; 2007. Health, United States, 2007. [Google Scholar]
- 17.Zincke H, Lau W, Bergstralh E, Blute ML. Role of early adjuvant hormonal therapy after radical prostatectomy for prostate cancer. J Urol. 2001;166:2208–2215. [PubMed] [Google Scholar]
- 18.See W, Iversen P, Wirth M, McLeod D, Garside L, Morris T. Immediate treatment with bicalutamide 150mg as adjuvant therapy significantly reduces the risk of PSA progression in early prostate cancer. Eur Urol. 2003;44:512–517. doi: 10.1016/s0302-2838(03)00366-x. discussion 517-518. [DOI] [PubMed] [Google Scholar]
- 19.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 20.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 21.Zagars GK, von Eschenbach AC, Ayala AG, Schultheiss TE, Sherman NE. The influence of local control on metastatic dissemination of prostate cancer treated by external beam megavoltage radiation therapy. Cancer. 1991;68:2370–2377. doi: 10.1002/1097-0142(19911201)68:11<2370::aid-cncr2820681107>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 22.Crook JM, Perry GA, Robertson S, Esche BA. Routine prostate biopsies following radiotherapy for prostate cancer: results for 226 patients. Urology. 1995;45:624–631. doi: 10.1016/S0090-4295(99)80054-5. discussion 631-622. [DOI] [PubMed] [Google Scholar]
- 23.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 24.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21:2163–2172. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 25.Akakura K, Isaka S, Akimoto S, et al. Long-term results of a randomized trial for the treatment of Stages B2 and C prostate cancer: radical prostatectomy versus external beam radiation therapy with a common endocrine therapy in both modalities. Urology. 1999;54:313–318. doi: 10.1016/s0090-4295(99)00106-5. [DOI] [PubMed] [Google Scholar]
- 26.Lu-Yao GL, Yao SL. Population-based study of long-term survival in patients with clinically localised prostate cancer. Lancet. 1997;349:906–910. doi: 10.1016/S0140-6736(96)09380-4. [DOI] [PubMed] [Google Scholar]


