Abstract
Purpose of review
Significant gastrointestinal pathology occurs in progressive HIV and simian immunodeficiency virus (SIV) infections. This review will examine the relationship between the detrimental events to the gastrointestinal tract during the acute phase of infection and disease progression through the chronic phase and, ultimately, AIDS.
Recent findings
Gastrointestinal tract CD4 T cells are dramatically depleted in acutely HIV-infected humans and SIV-infected rhesus macaques, sooty mangabeys, and African green monkeys. In addition, HIV infection of humans and SIV-infection of rhesus macaques are characterized by enteropathy and increased intestinal permeability. While SIV-infected rhesus macaques and HIV-infected humans manifest chronic and systemic immune activation and microbial translocation, and progress to chronic infection and AIDS, however, SIV-infected sooty mangabeys and African green monkeys do not.
Summary
Recent studies have increased our understanding of the mechanisms that relate structural and immunological damage to the gastrointestinal tract during the acute phase of HIV/SIV infection to immune activation and disease progression in the chronic phase.
Keywords: gastrointestinal depletion, immune activation, microbial translocation, natural infection
Introduction
The mucosal surface of the gastrointestinal tract forms a unique anatomical and physiological niche, serving as a predominant structural and immunological barrier against the microorganisms of the outside world. In addition, the mucosal epithelium also absorbs water and nutrients during the digestive process critical to host survival. Hence, loss of the integrity of the mucosal surface results in multiple deleterious sequelae.
The hallmarks of HIV infection include chronic activation of the immune system and loss of CD4 T cells, which ultimately leaves affected individuals mortally susceptible to opportunistic infections. Immune activation is multifaceted, including polyclonal B-cell activation [1], increased T-cell turnover [2], increased frequencies of T-cells with an activated phenotype [3], and increased serum levels of proinflammatory cytokines and chemokines [4]. Importantly, the degree of immune activation is a better predictor of disease progression than plasma viral load [5]. HIV disease progression results in the progressive loss of CD4 T cells and ultimately renders infected individuals susceptible to a variety of opportunistic infections. Many of the opportunistic infections that ultimately plague such individuals involve infectious agents that are normally checked by the mucosal barriers. While many AIDS-defining illnesses could be attributed to loss of mucosal immunity and are only manifest years after acquisition of HIV, however, many pathological changes, both structural and immunological, occur at the mucosal surfaces from the very onset of HIV infection. Recent studies have provided a direct link between the devastation that occurs to the mucosal surfaces during acute infection, the immune activation that defines the chronic phase of infection and progression to AIDS.
The consequences of immune activation
The multifaceted systemic immune activation that is a hallmark of chronic HIV infection has several consequences. Many of the consequences of immune activation are deleterious to the immune systems of HIV-infected individuals. Firstly, high turnover of both CD4 and CD8 T cells imposes a strain on T cell homeostatic mechanisms [6] with a decrease in the overall half-life of T cells [7,8]. Additionally, clonal exhaustion of T cells may ultimately result drainage of memory T cell pools [9–11]. Secondly, damage to lymphoid tissue results in thymic dysfunction (possibly attributed to IFNα) [12–14] and TGFβ-mediated fibrosis [15,16]. Fibrosis of lymph nodes is, in turn, associated with abnormal retention of effector type T cells [17]. Indeed, multiple studies have shown that immune activation results in abnormal T cell trafficking [18–21]. Finally, immune activation results in generation of targets for the virus itself, which helps drive viral replication [22]. It should be noted, however, that not all consequences of immune activation are deleterious to the host. Indeed, immune activation results in T cell proliferation and, by inference, restoration of tissue memory CD4 T cells [23••]. Hence immune activation may help maintain a certain degree of immunocompetence. Over time, however, damage by the virus to the sources of CD4 T cell compartments and anatomical niches that maintain the CD4 T cell compartments act together with the homeostatic strains imposed by chronic immune activation to exacerbate further the progressive net loss in CD4 T cell numbers. Understanding the causes of immune activation in HIV infection could, therefore, lead to novel therapeutic interventions that might improve the prognosis of HIV-infected individuals.
Gastrointestinal damage
During the acute phase of HIV infection in humans or SIV infection in rhesus macaques the gastrointestinal tract is particularly adversely affected. Over the short period of the acute phase of infection the majority of gastrointestinal tract CD4 T cells are depleted as a result of direct viral infection [24]. Moreover, this depletion, attributed to viral infection, continues throughout the entire disease course [17,25–28]. As the majority of CD4 T cells in the body reside within the gastrointestinal tract, this represents a considerable assault to the immune system. How this assault directly affects the ability of the host to respond to subsequent immunological challenges is discussed below. In addition to the loss of CD4 T cells, other abnormalities are observed within the gastrointestinal tracts of HIV-SIV-infected individuals. Gene expression profiles of gastrointestinal tract biopsies from HIV-infected individuals reveal that genes associated with cell cycle regulation, lipid metabolism, and epithelial cell barrier and digestive functions are downregulated in HIV-infected individuals [29]. This fact may adversely influence nutrient adsorption and digestive functions, with the potential to impact on the efficacy of antiretroviral therapy. In addition, these findings are probably directly related to historical observations that HIV-infected individuals have histological abnormalities of the gastrointestinal mucosa, malabsorption and lymphocyte depletion [30]. The enteropathy that afflicts HIV-infected individuals can occur from the acute phase of the infection through to advanced disease. This disease involves diarrhea, increased gastrointestinal inflammation, increased intestinal permeability (up to five-fold higher than healthy controls) and malabsorption of bile acids and vitamin B12 [31–33]. Histologically, the enteropathy involves inflammatory infiltrates of lymphocytes and damage to the gastrointestinal epithelial layer including villous atrophy, crypt hyperplasia, and villous blunting [34–36]. Importantly these pathological changes occur in the absence of detectable bacterial, viral or fungal enteropathogens often associated with enteropathy [34].
Mechanisms of HIV enteropathy
While it is clear that both the immunological and structural barriers are compromised in HIV infection, the specific mechanisms that account for this state remain elusive. Recent studies [37] have shown increased enterocyte apoptosis in SIV-infected rhesus macaques. Increased enterocyte apoptosis could explain increased intestinal permeability, but the actual mechanisms that are directly responsible for enterocyte apoptosis are unclear. One possibility involves a virotoxic effect. Indeed, HIV gp120 can lead to increased enterocyte calcium concentrations, which are associated with tubulin depolymerization and a decreased ability of the epithelial cells to maintain ionic balances [38]. This finding was later shown to involve the surface orphan G-coupled receptor GPR15/Bob [39]. A second possibility might involve increased local concentrations of proinflammatory cytokines such as tumor necrosis factor (TNF). Proinflammatory cytokines such as TNF, IFNγ, IL-12, and IL-8 have been implicated in the pathology of inflammatory bowel disease [40] and high levels of proinflammatory mediators such as the β chemokines [41] IL-6, IL-10, and IFNγ [42] are found in the lamina propria of the colon of HIV-infected individuals. Moreover, the degree of inflammation within the gastrointestinal tract correlates with viral replication [42–44].
Progression to AIDS
With considerable damage to both the immunological and structural barriers of the gastrointestinal tract occurring during the short period of the acute phase of infection, it may seem counterintuitive that HIV/SIV disease progression is, generally, quite slow. This situation means that infected individuals do not succumb to opportunistic infections until years after the acute phase of infection. Recent studies, however, have provided direct links between the damage to the gastrointestinal tract in the acute phase of infection and disease progression. First, recent studies showed that increased levels of plasma lipopolysaccharide (LPS) occur in chronically HIV-infected individuals compared with uninfected individuals. These raised LPS levels result from microbial translocation as a consequence of damage to the gastrointestinal tract during acute HIV infection. The increased levels of LPS are associated with increased levels of soluble CD14 and lipopolysaccharide binding protein, and decreased levels of antibodies directed against LPS core antigen indicating bioactivity of LPS in vivo. Moreover, LPS levels were associated with both the frequency of activated memory CD8T cells and plasma levels of the proinflammatory cytokine IFNα. Importantly, neither of these measures of activation could be directly attributed to LPS. These findings suggest that plasma LPS, in addition to its potent immunostimulatory activity through TLR-4 is also an indicator of the translocation of additional microbial products that stimulate the immune system through other receptors.
These findings implicate microbial translocation as a cause of immune activation in chronically HIV-infected individuals thus providing a direct link between the damage to the gastrointestinal tract during the acute phase of infection and progression to immunodeficiency. Translocated microbial products, however, are not necessarily the only cause of immune activation in HIV-infected individuals. Indeed, recent studies suggest that HIV RNA directly stimulates plasmacytoid dendritic cells to produce IFNα through TLR-7 [45]. IFNα that is produced in response to HIV RNA can, in turn, activate natural killer cells in vitro [46].
To investigate more carefully the contribution of translocated microbial products to immune activation in HIV-infected individuals, recent studies have examined HIV-infected individuals that control viral replication in the absence of antiviral therapy (elite controllers). Gene chip analysis of gastrointestinal tract biopsies from such HIV-infected individuals revealed that genes associated with cell cycle regulation, lipid metabolism, and epithelial cell barrier and digestive functions were actually down-regulated [29]. Subsequently, Hunt and colleagues [47] studied microbial translocation, immune activation and peripheral blood CD4 T cell depletion in a separate cohort of HIV-infected elite controllers and found that while individuals that control viral replication for years in the absence of antiretrovirals have significantly lower frequencies of activated T cells than HIV-infected individuals that progress to the chronic phase of infection, such elite controllers have significantly higher frequencies of activated T cells than uninfected individuals. In addition to increased frequencies of activated T cells, several of these elite controllers ultimately became depleted of CD4 T cells and had an AIDS-defining illness. Importantly, the degree of CD4 T cell depletion was closely associated with the level of T cell activation. The frequencies of activated T cells were, in turn, associated with levels of significantly raised levels of plasma LPS. As elite controllers have very low viral loads in the absence of antiviral therapy, the immune activation in these individuals cannot be attributed to the virus itself.
Another group of HIV-infected individuals with undetected viral replication where immune activation might be detrimental is individuals treated with highly active antiretroviral therapy (HAART). After initiation of HAART most HIV-infected individuals have viral loads below detection limits and reconstitute peripheral blood CD4 T cells. Many individuals, however, reconstitute peripheral blood CD4 T cells very poorly and some individuals maintain detectable viral replication. Anselmi and colleagues [48] recently investigated microbial translocation, immune activation and T cell reconstitution in HIV-infected children receiving HAART. They found that children who did not respond to HAART with significant decreases in viral loads and CD4 T cell reconstitution maintained high frequencies of activated T cells and levels of plasma LPS increased [48].
The damage that occurs to the gastrointestinal tract during the acute phase of HIV/SIV infection continues into the chronic phase of infection. Indeed, Okoye and colleagues [23••] recently demonstrated that, in the chronic phase of progressive SIV infection in rhesus macaques, there is a slow, but continuous, decline in gastrointestinal tract CD4 T cells and the degree of this continuous depletion remains a highly significant correlate of late-onset AIDS. This continuous depletion was especially evident within the short-lived effector memory CD4 T cell subset and the homeostasis of this T cell subset was critically dependent on the production of new CD4 T effector memory cells from central-memory CD4 T cell precursors. The instability of tissue CD4 T effector memory cell populations over time was not explained by increasing destruction of these cells, but rather was attributable to progressive reduction in their production from the central memory pool. The tempo of this depletion and the timing of disease onset are largely determined by destruction by the virus itself, failing production, and gradual decline of central memory CD4 T cells [23••].
Gastrointestinal damage in nonprogressive infection
While pathogenic SIV infection of rhesus macaques is also associated with microbial translocation and immune activation, nonpathogenic SIV infections of sooty mangabeys and African green monkeys, which typically lack high levels of immune activation even in the presence of high viral loads and do not progress to AIDS [49], do not show raised plasma LPS levels [50••–52••]. These data suggest that even though they are infected with SIV, these animals somehow maintain mucosal integrity and avert the deleterious consequences of microbial translocation and systemic immune activation. Recent studies, however, have shown that, even though these animals do not progress, both African green monkeys [51••] and sooty mangabeys [52••] lose a significant fraction of their gastrointestinal tract CD4 T cells following acute SIV infection. Moreover, while SIV-infected sooty mangabeys generally maintain healthy levels of peripheral blood CD4 T cells despite having viral replication comparable to HIV-infected patients, a recent study [53••] identified the emergence of a multitropic (R5/X4/R8-using) SIV 43 or 71 weeks after infection in two sooty mangabeys. These infections were associated with a persistent, and generalized loss of systemic CD4 T cells (5–80 cells/μl of blood) in the absence of clinical signs of any opportunistic infections.
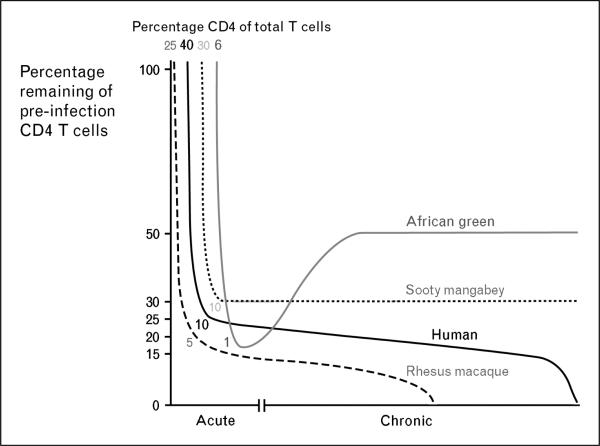
These data suggest that the generalized CD4 T cell depletion from the blood and mucosal tissues is not sufficient to induce AIDS in natural host species, which may have evolved immunological mechanisms for control of pathogens that are less dependent upon CD4 T cells. Indeed, African green monkeys have significantly fewer CD4 T cells in all anatomical locations than either humans or rhesus macaques [54••]. Surprisingly, of peripheral blood T cells in African green monkeys approximately 10% are of the CD4 lineage and a high frequency of T cells belong to the CD4–CD8– phenotype. Following acute SIV-infection, African green monkeys lose approximately 80% of their gastrointestinal tract CD4 T cells. This depletion, however, is not great in absolute terms as these animals’ gastrointestinal tract CD4 T cells constitute only 5% of total gastrointestinal T cells before infection and then become depleted to 1% following SIV infection (Fig. 1). Moreover, following the acute phase of infection African green monkeys are able to reconstitute gastrointestinal tract CD4 T cells while HIV-infected individuals only poorly reconstitute gastrointestinal tract CD4 T cells even when treated with long-term HAART [55,56••]. Moreover, SIV-uninfected sooty mangabeys also have significantly lower frequencies of CD4 T cells and high frequencies of CD4–CD8– T cells compared to either humans or rhesus macaques [52••]. Hence, neither African green monkeys nor sooty mangabeys become equally depleted of their preinfection levels of gastrointestinal tract CD4 T cells compared to either SIV-infected rhesus macaques or HIV-infected humans. An understanding of the provenance and function of the CD4–CD8– T cells in these animals may elucidate how these natural hosts for SIV are able to avoid microbial translocation, generalized immune activation and opportunistic infections after infection.
Figure 1. Relative depletion of initial gastrointestinal tract CD4 T cells in progressive and nonprogressive lentiviral infection.
Depletion of gastrointestinal tract CD4 T cells, relative to the preinfection frequency, during the acute and chronic phases of HIV in humans (black line) and SIV infection in Rhesus macaques (dashed line), sooty mangabeys (dotted line line), and African green monkeys (gray line). Frequencies of T cells that are CD4+ prior to infection and after the acute phase are listed. Graphs are based upon published data. Neither African green monkeys nor sooty mangabeys (naturally infected animals that do not progress to AIDS) are as dramatically depleted of their initial gastrointestinal tract CD4 T cell populations during the acute phase of infection as SIV-infected rhesus macaques or HIV-infected humans. Moreover, progressive depletion of gastrointestinal tract CD4 T cells does not seem to occur in SIV-infected sooty mangabeys during the chronic phase of infection and SIV-infected African green monkeys actually reconstitute CD4 T cells during the chronic phase.
Conclusion
Pathological changes to the gastrointestinal tract have long been known to be a characteristic feature of HIV infection. Recent studies have provided mechanistic insights into the underlying causes of HIV enteropathy and CD4 T cell depletion. While the structural and immunological damage to the mucosae occurs very rapidly during the acute phase of infection, HIV-infected individuals do not succumb to opportunistic infections for years, until peripheral blood CD4 T cells become depleted below 200 CD4 T cells/μl of blood and mucosal CD4 T cells below as a yet undefined threshold [57]. While recent data have shown that the devastation to the gastrointestinal tract leads to microbial translocation, which is associated with immune activation [50••] and by inference disease progression [5], the relative contribution of microbial translocation and other factors to immune activation is not completely understood. Moreover the possibility of modulating TLR-mediated immune activation therapeutically is a possible avenue to pursue. Finally, the gastrointestinal tract is progressively depleted of CD4 T cells and renewal mechanisms ultimately fail, coincident with onset of AIDS [23••].
In summary, in HIV and pathogenic SIV infection the gastrointestinal tract is a site of massive CD4 T cell depletion, viral infection, enterocyte apoptosis and disruption of tight epithelial junctions. Hence HIV infection could quite reasonably be considered a disease of the gastrointestinal tract. Importantly, our new understandings have pointed to new therapeutic directions; the aim would be to prevent or reduce the propagation of HIV at mucosal surfaces [58,59] and to restore the immunological and epithelial integrity of the mucosal barrier [58] thereby circumventing the associated immune activation and disease progression.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 410–411).
- 1.Lane HC, Masur H, Edgar LC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 2.Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 3.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 4.Valdez H, Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997–1998:187–228. [PubMed] [Google Scholar]
- 5.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs JA, Lempicki RA, Sidorov IA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCune JM, Hanley MB, Cesar D, et al. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Invest. 2000;105:R1–R8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellerstein M, McCune J. T cell turnover in HIV-1 disease. Immunity. 1997;7:583–589. doi: 10.1016/s1074-7613(00)80379-9. [DOI] [PubMed] [Google Scholar]
- 9.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 11.Grossman Z, Meier-Schellersheim M, Sousa AE, et al. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 12.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 13.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Hatzakis A, Touloumi G, Karanicolas R, et al. Effect of recent thymic emigrants on progression of HIV-1 disease. Lancet. 2000;355:599–604. doi: 10.1016/S0140-6736(99)10311-8. [DOI] [PubMed] [Google Scholar]
- 15.Estes JD, Wietgrefe S, Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 16.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hengel RL, Jones BM, Kennedy MS, et al. CD4+ T cells programmed to traffic to lymph nodes account for increases in numbers of CD4+ T cells up to 1 year after the initiation of highly active antiretroviral therapy for human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184:93–97. doi: 10.1086/320997. [DOI] [PubMed] [Google Scholar]
- 19.Hengel RL, Jones BM, Kennedy MS, et al. Markers of lymphocyte homing distinguish CD4 T cell subsets that turn over in response to HIV-1 infection in humans. J Immunol. 1999;163:3539–3548. [PubMed] [Google Scholar]
- 20.Rosenberg Y, Anderson A, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking? Immunology Today. 1998;19:10–17. doi: 10.1016/s0167-5699(97)01183-3. [DOI] [PubMed] [Google Scholar]
- 21.Grossman Z, Herberman R. T-cell homeostasis in HIV infection is neither failing nor blind: Modified cell counts reflect an adaptive response of the host. Nature Medicine. 1997;3:486–490. doi: 10.1038/nm0597-486. [DOI] [PubMed] [Google Scholar]
- 22.Grossman Z, Feinberg MB, Paul WE. Multiple modes of cellular activation and virus transmission in HIV infection: a role for chronically and latently infected cells in sustaining viral replication. Proc Natl Acad Sci U S A. 1998;95:6314–6319. doi: 10.1073/pnas.95.11.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [This paper shows that SIV-infected rhesus macaques progress to full blown AIDS when the supply of effector memory CD4 T cells from the central memory pool fails.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattapallil JJ, Douek DC, Hill B, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 25.Brenchley JM, Knox KK, Asher AI, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunology. doi: 10.1038/mi.2007.5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehandru S, Poles MA, Tenner-Racz K, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sankaran S, Guadalupe M, Reay E, et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A. 2005;102:9860–9865. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotler DP, Gaetz HP, Lange M, et al. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 31.Kapembwa MS, Fleming SC, Sewankambo N, et al. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991;81:327–334. doi: 10.1042/cs0810327. [DOI] [PubMed] [Google Scholar]
- 32.Bjarnason I, Sharpstone DR, Francis N, et al. Intestinal inflammation, ileal structure and function in HIV. AIDS. 1996;10:1385–1391. doi: 10.1097/00002030-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Sharpstone D, Neild P, Crane R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45:70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batman PA, Miller AR, Forster SM, et al. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. J Clin Pathol. 1989;42:275–281. doi: 10.1136/jcp.42.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heise C, Miller CJ, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 36.Batman PA, Kotler DP, Kapembwa MS, et al. HIV enteropathy: crypt stem and transit cell hyperproliferation induces villous atrophy in HIV/microsporidia-infected jejunal mucosa. AIDS. 2007;21:433–439. doi: 10.1097/QAD.0b013e3280142ee8. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Estes JD, Duan L, et al. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenative enteropathy of early infection. J Infect Dis. doi: 10.1086/525046. (in press) [DOI] [PubMed] [Google Scholar]
- 38.Maresca M, Mahfoud R, Garmy N, et al. The virotoxin model of HIV-1 enteropathy: involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29-D4 intestinal cell line. J Biomed Sci. 2003;10:156–166. doi: 10.1007/BF02256007. [DOI] [PubMed] [Google Scholar]
- 39.Clayton F, Kotler DP, Kuwada SK, et al. Gp120-induced Bob/GPR15 activation: a possible cause of human immunodeficiency virus enteropathy. Am J Pathol. 2001;159:1933–1939. doi: 10.1016/S0002-9440(10)63040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kam LY, Targan SR. Cytokine-based therapies in inflammatory bowel disease. Curr Opin Gastroenterol. 1999;15:302. doi: 10.1097/00001574-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Olsson J, Poles M, Spetz AL, et al. Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4, and beta-chemokines. J Infect Dis. 2000;182:1625–1635. doi: 10.1086/317625. [DOI] [PubMed] [Google Scholar]
- 42.McGowan I, Elliott J, Fuerst M, et al. Increased HIV-1 Mucosal Replication Is Associated With Generalized Mucosal Cytokine Activation. J Acquir Immune Defic Syndr. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 43.Clayton F, Reka S, Cronin WJ, et al. Rectal mucosal pathology varies with human immunodeficiency virus antigen content and disease stage. Gastroenterology. 1992;103:919–933. doi: 10.1016/0016-5085(92)90026-u. [DOI] [PubMed] [Google Scholar]
- 44.Kotler DP, Reka S, Clayton F. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig Dis Sci. 1993;38:1119–1127. doi: 10.1007/BF01295730. [DOI] [PubMed] [Google Scholar]
- 45.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alter G, Suscovich TJ, Teigen N, et al. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 47.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anselmi A, Vendrame D, Rampon O, et al. Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to antiretroviral therapy. Clin Exp Immunol. 2007;150:442–450. doi: 10.1111/j.1365-2249.2007.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silvestri G, Sodora DL, Koup RA, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 50••.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [This paper shows that the damage to the gastrointestinal tract that occurs during the acute phase of HIV infection allows microbial products to translocate into circulation and these microbial products directly stimulate the immune system and are a cause of immune activation.] [DOI] [PubMed] [Google Scholar]
- 51••.Pandrea IV, Gautam R, Ribeiro RM, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [This paper shows that acutely SIV-infected African green monkeys are depleted of gastrointestinal tract CD4 T cells, but these animals do not progress to AIDS or have microbial translocation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Gordon SN, Klatt NR, Bosinger SE, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [This paper shows that SIV-infected sooty mangabeys also have significant gastrointestinal tract CD4 T cell depletion in the acute phase of HIV infection. It is important to note, however, that SIV-infected sooty mangabeys do not have microbial translocation or increased systemic immune activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Milush JM, Reeves JD, Gordon SN, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol. 2007;179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [This paper describes two SIV-infected sooty mangabeys that become systemically depleted of CD4 T cells, but these animals do not develop opportunistic infections.] [DOI] [PubMed] [Google Scholar]
- 54••.Pandrea I, Apetrei C, Dufour J, et al. Simian immunodeficiency virus SIVagm. sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J Virol. 2006;80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [This paper shows that SIV-infected African green monkeys also have significant gastrointestinal tract CD4 T cell depletion in the acute phase of HIV infection. It is important to note, however, that SIV-infected African green monkeys do not have microbial translocation or increased systemic immune activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehandru S, Poles MA, Tenner-Racz K, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [This paper shows that only if HIV-infected individuals commence antiretroviral therapy during the acute phase of infection do genes associated with repair become upregulated in the gastrointestinal tract.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Picker LJ, Hagen SI, Lum R, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.George MD, Reay E, Sankaran S, Dandekar S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]