Summary
We previously used quantitative digital image analysis to report that high immunohistochemical tumor expression levels of survivin independently predict poor outcome among patients with clear cell renal cell carcinoma. However, given the cumbersome and costly nature of digital image analysis, we evaluated simple visual assessment as an alternative to digital image analysis for assessing survivin as a predictor of clear cell renal cell carcinoma patient outcomes. We identified 310 patients treated surgically for unilateral, sporadic, clear cell renal cell carcinoma at our institution between 1990 and 1994. Survivin expression was quantified independently by digital image analysis and visual assessment in paraffin slides using a commercially available antibody. We examined the agreement between the 2 methods using the κ statistic and then used Cox regression to compare the ability of the 2 methods to predict renal cell carcinoma death. The κ statistic comparing high survivin expression determined by digital image analysis versus visual assessment was .68, indicating substantial agreement between the 2 methods. Moreover, even after multivariate adjustment, the association of high survivin expression with risk of renal cell carcinoma death was similar for both visual assessment (risk ratio = 2.01; 95% confidence interval, 1.26-3.22) and digital image analysis (risk ratio = 1.75; 95% confidence interval, 1.10-2.80). Finally, among patients with “moderate risk” (Stage, Size, Grade, and Necrosis scores 3-6) and “high risk” (Stage, Size, Grade, and Necrosis scores 7 or greater) clear cell renal cell carcinoma, high survivin expression determined by visual assessment was significantly associated with poorer survival (P = .006 and P = .017, respectively). Herein, we demonstrate substantial agreement between survivin quantification by digital image analysis and visual assessment. We further confirm that high survivin expression assessed by visual assessment remains an independent predictor of aggressive clear cell renal cell carcinoma behavior. Thus, visual assessment represents an economical, widely available, and reliable method to assess survivin as a predictor of clear cell renal cell carcinoma patient outcomes.
Keywords: Renal cell carcinoma, Survivin, Biomarker, Immunohistochemistry, Digital image analysis, Visual assessment
1. Introduction
Incidence rates for clear cell renal cell carcinoma (ccRCC) are rising steadily; and this cancer continues to represent a challenging tumor for physicians to manage, especially given its often unpredictable clinical course [1,2]. Although standard pathologic indices such as tumor stage and grade can crudely predict ccRCC patient outcomes, significant heterogeneity in ccRCC behavior can still be observed even when tumors are matched according to pathologic features [3]. As such, a vigorous search has been launched to identify ccRCC-associated biomarkers that can enhance patient outcome prediction and better facilitate the assignment of patients to stepped-up surveillance, adjuvant therapy, or stratification when accrued onto clinical trial.
As part of the overall effort to identify clinically relevant prognostic biomarkers for ccRCC, we recently reported that ccRCC patients whose tumors exhibit high levels of survivin expression based on immunohistochemical (IHC) analysis are at markedly increased risk of cancer progression and death from RCC relative to patients whose tumors express low levels of survivin [4]. More importantly, we also reported that this association remains significant even after multivariate adjustment for well-established predictors of ccRCC patient outcome, suggesting that this biomarker lends meaningful prognostic information beyond standard clinical and pathologic indices. Survivin is an antiapoptotic protein that belongs to the inhibitor of apoptosis protein family [5]. Currently, survivin is believed to function as an inhibitor of cellular apoptosis primarily through a preferential blocking of mitochondrial-dependent apoptosis by targeting of caspase 9 and SMAC/DIABLO (second mitochondria-derived activator of caspases/direct inhibitory apoptotic protein-binding protein with a low isoelectric point). In addition to its well-known antiapoptotic activity, survivin has also been shown to play a critical role in regulating mitosis and microtubule stability [6,7].
The overall significance of identifying survivin as an independent biomarker for ccRCC notwithstanding, the potential clinical impact of our finding was limited by the fact that we quantified survivin IHC expression using expensive digital image analysis (DIA). Indeed, although DIA clearly provides the ability to reproducibly quantify IHC staining levels in study tissues, notable drawbacks of this methodology are that it is labor intensive, is not entirely immune to selection bias, and requires highly specialized personnel and expensive instrumentation. As such, DIA is not readily translatable into the standard academic or community-based clinical practice setting [8,9]. Related to this, although a significant amount has been written to tout the merits of DIA, relatively little has been published pertaining to how DIA performs when compared against standard light microscopy visual assessment (VA).
Motivated by this, we conducted the current study to test whether VA by standard light microscopy represents an equally valuable and more time- and cost-effective means of evaluating survivin expression levels within ccRCC relative to the DIA approach. For this study, IHC staining for survivin was performed on paraffin-embedded tumor samples obtained from a large, consecutive-series cohort of ccRCC patients treated with nephrectomy at our institution. We then independently assessed survivin expression levels by both DIA and VA. Herein, we report a substantial level of agreement between DIA and VA for determining survivin expression levels. Moreover, we confirm that high survivin expression, as assessed by VA, independently predicts poor outcome for patients with ccRCC, even among the specific subgroups of patients classified as “moderate” and “high” risk based on the Mayo Clinic Stage, Size, Grade, and Necrosis (SSIGN) score. Based on this, we conclude that assessment of survivin within ccRCC tissues does not require DIA and, as such, can be readily conducted at any medical center by well-trained pathologists using standard light microscopy VA.
2. Materials and methods
2.1. Patient selection
Upon approval from the Institutional Review Board, we identified 312 patients treated with radical nephrectomy or nephron-sparing surgery for unilateral, sporadic ccRCC between 1990 and 1994 from the Mayo Clinic Nephrectomy Registry. The clinical features studied included age, sex, symptomatic presentation, Eastern Cooperative Oncology Group (ECOG) performance status, tumor thrombus level, and type of surgery. Patients with a palpable flank or abdominal mass, discomfort, gross hematuria, acute onset varicocele, or constitutional symptoms including rash, sweats, weight loss, fatigue, early satiety, and anorexia were considered symptomatic at presentation. The pathologic features studied included histologic subtype classified according to the Union Internationale Contre le Cancer, American Joint Committee on Cancer, and Heidelberg guidelines [10,11]; tumor size; the 2002 primary tumor classification; perinephric fat invasion; regional lymph node involvement; distant metastases; the 2002 Tumor-Node-Metastasis (TNM) stage groupings; nuclear grade [12]; coagulative tumor necrosis [13]; and sarcomatoid differentiation [14]. To obtain these features, one study pathologist (J. C. C.) reviewed the microscopic slides from all specimens without knowledge of patient outcome or survivin expression level.
2.2. Patient outcome
Disease status for patients in the Nephrectomy Registry is updated each year. If a patient has not been seen at our institution in the previous year, the patient is sent a disease status questionnaire. If there is evidence of disease progression in this questionnaire, the date, location, and treatment are verified in writing with the patient's local physician. Patient vital status is similarly updated on a yearly basis. If a patient has died in the previous year, a death certificate is ordered to determine the cause of death. A recent visit to Mayo (within 6 months of the date of death) for metastatic disease is good documentation that RCC was the cause of death. If the death certificate does not support this, the medical history is reviewed by a Mayo urologist to determine the cause of death. If a death certificate cannot be obtained, the cause of death must be verified with the patient's family or local physician.
2.3. IHC staining of ccRCC specimens
Tissue slides prepared from paraffin-embedded tissue blocks harboring the highest grades of ccRCC tumors were selected by one pathologist (J. C. C.). Five-micrometer– sections were then cut from these blocks by our Tissue Acquisition and Cellular/Molecular Analysis facility to prepare tissue slides for our study. Slides were then immunohistochemically stained with anti-survivin (Dako, Carpenteria, CA; 1/100 dilution) as we have reported previously [4].
2.4. Evaluation of survivin levels by DIA
To conduct computer-driven assessments of survivin expression levels within ccRCC tumors, one pathologist (J. C. C.) reviewed the aforementioned survivin-stained slides and circled regions exhibiting the highest survivin staining for subsequent quantification by DIA [4]. In every case, there was at least one positive-staining cell to indicate that the staining process was successful and that the antigen was preserved in the tissue section. The staining pattern was intense and nuclear, allowing for the separation from nonspecific cytoplasmic or membrane staining. Encircled tissue regions (approximately 10-20 mm2) were then scanned using the Bacus Laboratories, Inc Slide Scanner system (Bacus Laboratories, Inc, Lombard, IL), which digitally captures images at 480 × 752 pixel resolution at ×40 magnification [15]. Multiple digital photographs were then assembled to recreate the tissue region under investigation. Computer-assisted scoring of ccRCC tissues for survivin expression was performed using the IHC Score software (Bacus Laboratories, Inc) to obtain measurements of survivin-positive area expressed as a percentage of the total area examined.
2.5. Evaluation of survivin levels by VA
For the second phase of our investigation, survivin expression levels within ccRCC tumors were independently visually scored by a separate pathologist (Y. M. S.) who was fully blinded to the results of the DIA study as well as patient outcome. Specifically, levels of survivin expression were visually estimated by counting numbers of survivin-positive (versus total) tumor cells in 5 representative high-powered fields encompassed within the previously encircled regions that were quantified by DIA. VA was performed using a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany). With a 10/25 eyepiece and a ×40 objective, the Leica DMR has an object field diameter of 0.625 mm2, resulting in a high-powered field of 0.307 mm2. As such, survivin expression could be summarized as the number of survivin-positive tumor cells per square millimeter of ccRCC tissue examined. The study pathologist discarded weak nuclear staining and quantified survivin expression only in those cells with strong and moderate nuclear staining.
2.6. Statistical methods
We previously identified a cut point of 2% for “low” and “high” survivin expression based on DIA. To do this, we used scatter plots of survivin expression against the difference in observed survival and the survival expected from a Cox proportional hazards regression model (formally known as Martingale residuals). Martingale residuals can be used to evaluate the functional form of a continuous variable in the setting of a Cox model and can therefore be helpful when selecting a logical cut point for illustrative purposes and further analysis [16]. In the current investigation, we repeated this process of identifying a cut point for survivin expression using data obtained from VA. As such, for this investigation, we use the cut point of 15 survivin-positive tumor cells per square millimeter (Fig. 1) to distinguish low and high survivin expression based on VA.
Fig. 1.
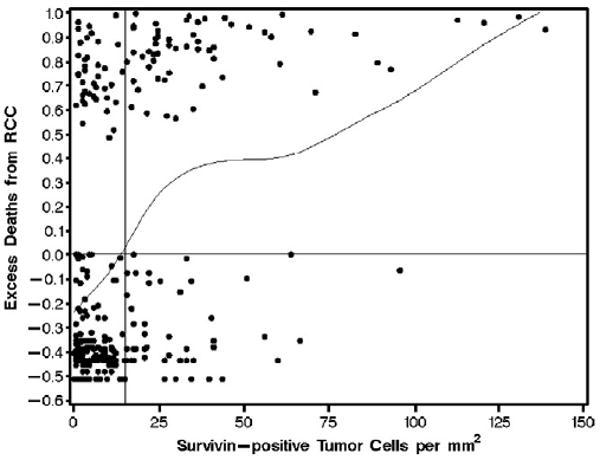
Scatter plot of the number of survivin-positive tumor cells per square millimeter from the VA versus the expected risk of death from RCC in each of 310 patients with ccRCC. Patients above the horizontal line were at increased risk for death compared with the expected risk from a Cox proportional hazards regression model. Patients below the horizontal line were at decreased risk for death compared with what we would expect. The curved line represents a scatter plot smoother. The point at which the smoother crosses the horizontal line occurs at approximately 15 survivin-positive tumor cells per square millimeter (see vertical reference line), indicating that this would be a logical cut point for illustrative purposes and further analysis.
The agreement in survivin expression between DIA and VA was measured using both the κ statistic (low versus high) and the intraclass correlation coefficient (continuous). For the analysis using intraclass correlation coefficient, we first standardized each continuous variable to have a mean of 0 and a standard deviation of 1. The interpretation of both the κ statistic and the intraclass correlation coefficient is similar: values of 0.0 to 0.2 indicate slight agreement; values of 0.2 to 0.4, 0.4 to 0.6, 0.6 to 0.8, and 0.8 to 1.0 indicate far, moderate, substantial, and almost perfect agreement, respectively [17].
For our survival analysis based on VA, we used the same methodology that we used in our previous publication using DIA [4]. Briefly, the distributions of clinical and pathologic features between survivin expression groups were evaluated using χ2 and Fisher exact tests. Similarly, we used Kaplan-Meier curves to visualize the association of survivin expression with cancer-specific survival and compared differences in outcome using a log-rank test. The duration of follow-up for each patient was calculated from the date of surgery to the date of death or last known follow-up. Univariate and multivariate Cox proportional hazards regression models were then used to estimate the magnitude of the association of survivin expression with death from RCC. The covariates considered in the multivariate modeling included ECOG performance status, the TNM stage groupings, tumor size, presence of necrosis, and nuclear grade. We also adjusted for the individual components of the University of California–Los Angeles Integrated Scoring System (UISS) [18] and the Mayo Clinic SSIGN score, a composite scoring system developed specifically for patients with ccRCC [19]. The SSIGN score combines TNM stage, tumor size, nuclear grade, and tumor necrosis into a single summary score to predict death from RCC. These associations were summarized with risk ratios and 95% confidence intervals (95% CIs).
The abilities of the univariate and multivariate models to predict death from RCC were evaluated using a c (for concordance) index [20]. The interpretation of the c index is similar to the interpretation of the area under a receiver operating characteristic curve. A value of 1.0 indicates that the features in the model perfectly separate patients with different outcomes, whereas a value of 0.5 indicates that the features contain prognostic information equal to that obtained by chance alone. All statistical analyses were performed using the SAS software package (SAS Institute, Cary, NC), and P values < .05 were considered statistically significant.
3. Results
Two patient specimens evaluated by DIA were not adequate for VA, leaving a total of 310 patients for our current study. At last follow-up, 177 patients had died, including 97 patients who died from RCC at a mean of 3.5 years after nephrectomy (median, 2.2; range, 0-13). Among the 133 patients who were still alive at last follow-up, the mean duration of follow-up was 11.1 years (median, 11.3; range, 0-15). Cancer-specific survival rates (SE, number still at risk) at 1, 5, and 10 years after nephrectomy were 90.6% (1.7%, 262), 73.9% (2.6%, 192), and 67.7% (2.9%, 129), respectively.
The average survivin expression by DIA was 2.3% (median, 1.0%; minimum, 0.01%; maximum, 35.8%). There were 95 (30.7%) patients with tumors that were classified as having high survivin expression (≥2%) using this method. The average survivin expression by VA was 16.2 positive tumor cells per square millimeter (median, 9.1; minimum, 0; maximum, 138.8). One hundred five (33.9%) patients had high survivin expression (≥15 survivin-positive tumor cells per square millimeter) by VA (Fig. 2). However, the patients classified as having high survivin expression by DIA were not necessarily the same patients with high expression by VA. There were 188 (60.7%) patients with low expression by both methods and 78 (25.1%) classified as having high expression by both methods. Conversely, there were 17 (5.5%) with high expression by DIA but low expression by VA and 27 (8.7%) with low expression by DIA but high expression by VA. Of interest, when we compared the 85.8% of cases with concordant results to the 14.2% with discordant results, we did not detect any statistically significant differences with respect to any of our clinical or pathologic covariates. The κ statistic for the comparison of low and high survivin tumor expression between the 2 methods was .68, indicating evidence of a “substantial” level of agreement. In addition, when we analyzed survivin expression as a continuously scaled variable, the intraclass correlation coefficient was 0.70, again indicating substantial agreement between the DIA and VA methods.
Fig. 2.
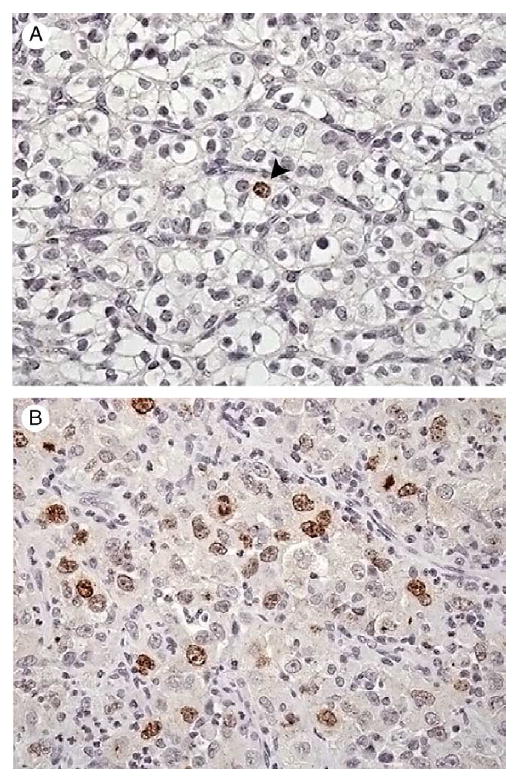
Representative photomicrographs of ccRCC showing low (A) and high (B) levels of survivin expression as determined by VA using light microscopy. Original magnification, × 400.
Table 1 provides a comparison of clinical and pathologic features between those patients classified as having low and high survivin expression by VA. Similar to our previously published data using DIA to evaluate survivin expression [4], high expression was significantly associated with several adverse pathologic features. Specifically, patients with tumors that had ≥15 survivin-positive cells per square millimeter were more likely to be symptomatic at presentation and have larger tumors that were more likely to extend into the renal vein, be of advanced stage and grade, and contain areas of necrosis and sarcomatoid differentiation compared with patients whose tumors showed low levels of survivin expression.
Table 1.
Comparison of clinical and pathologic features by VA of survivin expression for 310 patients with ccRCC
| Feature | Survivin-positive tumor cells/mm2 | P value | |
|---|---|---|---|
| <15 Low expression n = 205 |
≥15 High expression n = 105 |
||
| n (%) | |||
| Age at surgery (y) | |||
| <65 | 109 (53.2) | 46 (43.8) | .119 |
| ≥65 | 96 (46.8) | 59 (56.2) | |
| Sex | |||
| Female | 78 (38.0) | 47 (44.8) | .254 |
| Male | 127 (62.0) | 58 (55.2) | |
| Symptoms at presentation | |||
| No | 77 (37.6) | 28 (26.7) | .055 |
| Yes | 128 (62.4) | 77 (73.3) | |
| Constitutional symptoms at presentation | |||
| No | 161 (78.5) | 64 (61.0) | .001 |
| Yes | 44 (21.5) | 41 (39.0) | |
| ECOG performance status | |||
| 0 | 183 (89.3) | 97 (92.4) | .380 |
| ≥1 | 22 (10.7) | 8 (7.6) | |
| Tumor thrombus | |||
| None | 173 (84.4) | 66 (62.9) | <.001 |
| Level 0 | 20 (9.8) | 23 (21.9) | |
| Level I-IV | 12 (5.8) | 16 (15.2) | |
| Type of surgery | |||
| Nephron-sparing surgery | 31 (15.1) | 11 (10.5) | .258 |
| Radical nephrectomy | 174 (84.9) | 94 (89.5) | |
| Primary tumor size(cm) | |||
| <5 | 95 (46.3) | 28 (26.7) | .002 |
| 5 to <7 | 46 (22.4) | 22 (21.0) | |
| 7 to <10 | 31 (15.1) | 27 (25.7) | |
| ≥10 | 33 (16.1) | 28 (26.7) | |
| 2002 Primary tumor classification | |||
| pT1a | 77 (37.6) | 19 (18.1) | <.001 |
| pT1b | 65 (31.7) | 29 (27.6) | |
| pT2 | 19 (9.3) | 9 (8.6) | |
| pT3a | 11 (5.4) | 9 (8.6) | |
| pT3b | 27 (13.2) | 36 (34.3) | |
| pT3c | 4 (2.0) | 2 (1.9) | |
| pT4 | 2 (1.0) | 1 (1.0) | |
| Perinephric fat invasion | |||
| No | 175 (85.4) | 75 (71.4) | .003 |
| Yes | 30 (14.6) | 30 (28.6) | |
| Regional lymph node involvement | |||
| pNX and pN0 | 200 (97.6) | 96 (91.4) | .020 |
| pN1 and pN2 | 5 (2.4) | 9 (8.6) | |
| Distant metastases | |||
| pM0 | 193 (94.2) | 88 (83.8) | .003 |
| pM1 | 12 (5.8) | 17 (16.2) | |
| 2002 TNM stage groupings | |||
| I | 137 (66.8) | 46 (43.8) | <.001 |
| II | 18 (8.8) | 3 (2.9) | |
| III | 35 (17.1) | 36 (34.3) | |
| Nuclear grade | |||
| 1 | 34 (16.6) | 3 (2.9) | <.001 |
| 2 | 114 (55.6) | 32 (30.5) | |
| 3 | 52 (25.4) | 43 (41.0) | |
| 4 | 5 (2.4) | 27 (25.7) | |
| Coagulative tumor necrosis | |||
| No | 174 (84.9) | 43 (41.0) | <.001 |
| Yes | 31 (15.1) | 62 (59.0) | |
| Sarcomatoid differentiation | |||
| No | 202 (98.5) | 90 (85.7) | <.001 |
| Yes | 3 (1.5) | 15 (14.3) | |
In a univariate setting, each increase of 10 survivin-positive tumor cells per square millimeter was associated with a 37% increase in the risk of death from RCC (risk ratio = 1.37; 95% CI, 1.28-1.46; P < .001). Patients classified as having high survivin tumor expression by VA were nearly 5 times more likely to die from RCC compared with patients with low survivin tumor expression (risk ratio = 4.73; 95% CI, 3.13-7.13; P < .001; Table 2). Visualization of the survival experience of these 2 groups confirmed the poorer survival for patients with high survivin tumor expression and showed that this difference was apparent early in the follow-up period (Fig. 3, P < .001). Cancer-specific survival rates (SE, number still at risk) at 1, 5, and 10 years after nephrectomy were 77.2% (4.2%, 74), 45.8% (5.2%, 37), and 41.8% (5.3%, 22), respectively, for patients with tumors that had ≥15 survivin-positive cells per square millimeter compared with 97.5% (1.1%, 188), 87.7% (2.4%, 155), and 80.4% (3.0%, 107), respectively, for patients with tumors containing <15 survivin-positive cells per square millimeter.
Table 2.
Association of survivin tumor expression (high versus low) with death from RCC for 310 patients with ccRCC
| High survivin tumor expression | Risk ratio (95% CI) | P value | C index |
|---|---|---|---|
| Univariate | |||
| By DIA (≥2%) | 5.16 (3.42-7.77) | <.001 | 0.693 |
| By VA (≥15/mm2) | 4.73 (3.13-7.13) | <.001 | 0.692 |
| Adjusted for ECOG | |||
| By DIA (≥2%) | 5.19 (3.44-7.83) | <.001 | 0.693 |
| By VA (≥15/mm2) | 4.74 (3.14-7.15) | <.001 | 0.693 |
| Adjusted for TNM stage | |||
| By DIA (≥2%) | 3.00 (1.95-4.62) | <.001 | 0.840 |
| By VA (≥15/mm2) | 4.14 (2.64-6.49) | <.001 | 0.850 |
| Adjusted for tumor size | |||
| By DIA (≥2%) | 4.24 (2.79-6.43) | <.001 | 0.772 |
| By VA (≥15/mm2) | 4.21 (2.77-6.40) | <.001 | 0.778 |
| Adjusted for grade | |||
| By DIA (≥2%) | 2.63 (1.68-4.12) | <.001 | 0.829 |
| By VA (≥15/mm2) | 2.74 (1.77-4.26) | <.001 | 0.831 |
| Adjusted for necrosis | |||
| By DIA (≥2%) | 2.34 (1.49-3.68) | <.001 | 0.790 |
| By VA (≥15/mm2) | 2.65 (1.71-4.11) | <.001 | 0.795 |
| Adjusted for ECOG, TNM stage, grade a | |||
| By DIA (≥2%) | 2.39 (1.51-3.77) | <.001 | 0.870 |
| By VA (≥15/mm2) | 2.82 (1.76-4.52) | <.001 | 0.872 |
| Adjusted for SSIGN score | |||
| By DIA (≥2%) | 1.75 (1.10-2.80) | .019 | 0.881 |
| By VA (≥15/mm2) | 2.01 (1.26-3.22) | .004 | 0.884 |
Represents components of the UISS as reported by Zisman et al [18].
Fig. 3.
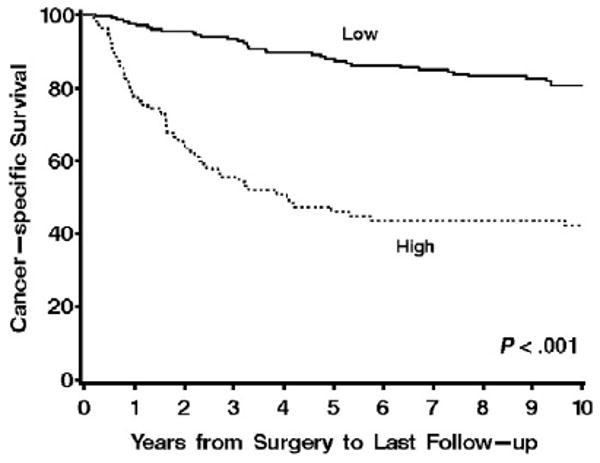
Association of survivin expression by VA with death from RCC for 310 patients with ccRCC (risk ratio = 4.73; 95% CI, 3.13-7.13; P < .001). Cancer-specific survival rates (SE, number still at risk) at 1, 5, and 10 years after nephrectomy were 77.2% (4.2%, 74), 45.8% (5.2%, 37), and 41.8% (5.3%, 22), respectively, for patients with tumors that had ≥15 survivin-positive cells per square millimeter compared with 97.5% (1.1%, 188), 87.7% (2.4%, 155), and 80.4% (3.0%, 107), respectively, for patients with tumors containing <15 survivin-positive cells per square millimeter.
The associations of survivin expression (high versus low) by both DIA and VA with death from RCC after multivariate adjustment are summarized in Table 2. Although the associations of survivin expression with death from RCC were attenuated after multivariate adjustment, high survivin expression by either method was still significantly associated with patient outcome after adjusting individually for ECOG performance status, the 2002 TNM stage groupings, tumor size, nuclear grade, and presence of necrosis (Table 2). Moreover, patients with high survivin tumor expression by VA were more than twice as likely to die from RCC compared with patients with low survivin tumor expression even after adjusting for the UISS scoring system (risk ratio = 2.82; 95% CI, 1.76-4.52; P = .004; Table 2) and the SSIGN score (risk ratio = 2.01; 95% CI, 1.26-3.22; P = .004; Table 2). Finally, based on c index values obtained from univariate and multivariate analysis, the ability of survivin expression to predict death from RCC was nearly identical whether the expression was determined by DIA or by VA (Table 2).
4. Discussion
Light microscopy review of histologic samples continues to play a major role in the management of patients with cancer, including those with ccRCC. Over the past decade, IHC staining of tissues has garnered increased attention as a means to elucidate tumor-associated protein biomarkers that correlate with cancer progression, response to therapy, and patient survival [21-24]. In parallel, the evaluation of IHC-stained tissues has shifted over the years from simple dichotomous determinations (ie, positive versus negative staining) toward more quantitative assessments of protein expression levels within tumors. Prompted by this, a number of semiautomated platforms and software programs have been engineered to specifically quantify IHC staining levels by DIA. As stated by Cregger et al in 2006 [25], a common goal of DIA is to mechanically standardize interpretation of IHC-stained tissues, thereby reducing the potential for artifact that might be introduced by intra- and interobserver variability. Thus, the obvious strengths of DIA are its increased sensitivity and reproducibility with regard to the quantitation of IHC-based biomarker expression levels. However, significant drawbacks of DIA include its requirements of costly instrumentation and software as well as expert technical personnel who are trained to perform and troubleshoot the analyses. Moreover, tissue fields to be evaluated by DIA often still require some form of preselection by an expert pathologist; as such, DIA fails to obviate the demand for various labor- and resource-intensive processes. Collectively, these issues pertaining to the conduct and evaluation of DIA limit the number of institutions that can realistically implement this technology.
Prompted by these issues, we compared DIA against standard light microscopy VA as a means of assessing survivin expression levels within ccRCC tumors after IHC staining. The impetus for our present study was spurred by our prior report demonstrating that high survivin expression in ccRCC tumor tissues, assessed using DIA, represents an independent predictor of poor outcome for patients treated by nephrectomy. However, appreciating the complexity and expense of DIA, we subsequently wished to test whether conventional light microscopy VA could be used to assess ccRCC tumor survivin levels and if this method yields data comparable to that generated by DIA. Given the relative low cost and limited resources compared with DIA, such evidence in support of VA to assess tumor survivin expression would facilitate the further translation of this biomarker into most academic and community-based medical practices.
To conduct this study, we used both DIA and VA to score survivin expression in tumor specimens obtained from 310 consecutive patients who were treated surgically for ccRCC at our institution between 1990 and 1994. The κ statistic of .68 supports substantial agreement between the DIA and VA methods for determining high survivin expression in ccRCC tumors. Similarly, when we analyzed survivin expression as a continuously scaled variable, the intraclass correlation coefficient was 0.70, again indicating substantial agreement between the 2 methods. More importantly, the association of high survivin expression with risk of cancer-specific death was similar for both VA (risk ratio = 2.01; 95% CI, 1.26-3.22) and DIA (risk ratio = 1.75; 95% CI, 1.10-2.80), even after multivariate adjustment. Based on these data, we conclude that VAyields data comparable to that generated by DIA; and further, we confirm that high survivin expression as assessed by VA remains an independent predictor of aggressive ccRCC behavior. As such, VA represents a reliable and more readily translatable approach to assess survivin expression as a predictor of ccRCC patient outcomes. We are mindful, however, that a specific limitation of this investigation is our inability to assess interreviewer variability associated with VA of survivin. As such, future investigations can and should focus on evaluating agreement for VA of survivin expression across independent reviewers.
Having provided data supporting VA as a viable alternative to DIA, our inclination was to then seek incremental improvement in our existing SSIGN score algorithm [19] via global integration of VA survivin expression; however, we posit that a more judicious approach would be to evaluate the ability of VA survivin expression to further refine outcome prediction for patients falling into risk groups already defined by the SSIGN score. In support of this, Fig. 4 illustrates that high survivin levels within ccRCC tumors, as determined using VA, portends a significantly (P = .006) poorer outcome than low survivin levels for patients who have been assigned a SSIGN score ranging from 3 to 6 (eg, moderate-risk patients). Similarly, high survivin levels within ccRCC tumors forecast a significantly (P = .017) poorer outcome than low survivin levels for high-risk patients assigned a SSIGN score of 7 or more. Interestingly, survivin levels failed to improve upon risk estimates for patients assigned a SSIGN score ranging from 0 to 2 (P = .848). This, however, is not particularly surprising given that the 10-year survival for these low-risk patients is greater than 95% [18,19]. Thus, we conclude that evaluation of survivin expression by VA may provide limited utility for those ccRCC patients who already have an overall excellent prognosis based on SSIGN score determination. In contrast, evaluation of survivin expression by VA may prove most useful as an ancillary test for those patients who fall into moderate- or high-risk categories based on the SSIGN score.
Fig. 4.
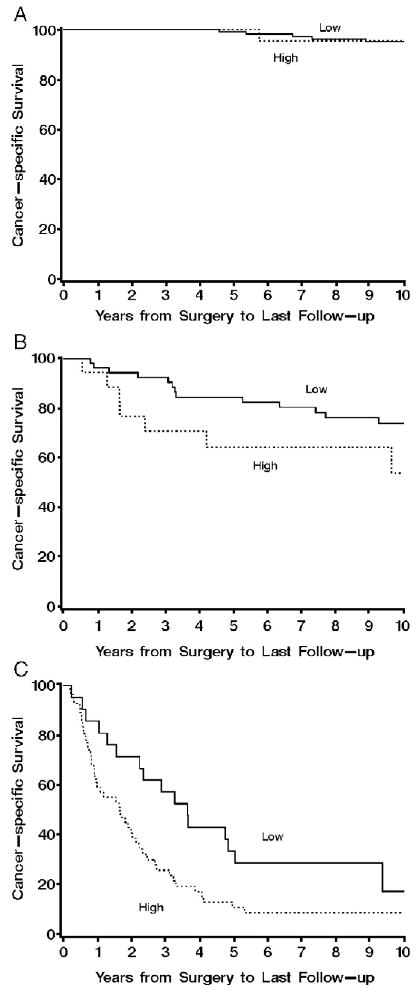
A, Association of survivin expression by VA with death from RCC for 158 patients with ccRCC determined to be low risk with SSIGN scores of 0 to 2 (risk ratio = 0.81; 95% CI, 0.10-6.75; P = .848). Cancer-specific survival rates (SE, number still at risk) at 1, 5, and 10 years after nephrectomy were 100.0% (0, 27), 100.0% (0, 22), and 95.5% (4.4%, 15), respectively, for patients with tumors that had ≥15 survivin-positive cells per square millimeter compared with 100.0% (0, 118), 99.1% (0.9%, 107), and 95.1% (2.1%, 74), respectively, for patients with tumors containing <15 survivin-positive cells per square millimeter. B, Association of survivin expression by VAwith death from RCC for 76 patients with ccRCC determined to be moderate risk with SSIGN scores of 3 to 6 (risk ratio = 3.23; 95% CI, 1.40-7.43; P = .006). Cancer-specific survival rates (SE, number still at risk) at 1, 5, and 10 years after nephrectomy were 94.4% (5.4%, 17), 64.4% (11.7%, 10), and 53.7% (13.8%, 4), respectively, for patients with tumors that had ≥15 survivin-positive cells per square millimeter compared with 96.3% (5.0%, 52), 84.6% (5.0%, 41), and 74.0% (6.3%, 30), respectively, for patients with tumors containing <15 survivin-positive cells per square millimeter. C, Association of survivin expression by VA with death from RCC for 76 patients with ccRCC determined to be high risk with SSIGN scores of 7 or greater (risk ratio = 1.99; 95% CI, 1.13-3.50; P = .017). Cancer-specific survival rates (SE, number still at risk) at 1, 5, and 10 years after nephrectomy were 59.0% (6.7%, 30), 10.7% (4.5%, 5), and 8.5% (4.1%, 3), respectively, for patients with tumors that had ≥15 survivin-positive cells per square millimeter compared with 85.7% (7.6%, 18), 33.3% (10.3%, 14), and 17.1% (8.6%, 3), respectively, for patients with tumors containing <15 survivin-positive cells per square millimeter.
4.1. Conclusion
The ultimate clinical utility of our previous observation that survivin levels independently predict ccRCC patient outcome was hampered by the requirement of DIA to evaluate survivin expression levels in tumor tissue. Herein, we report evidence of substantial agreement between survivin expression levels in ccRCC tumors quantified by DIA and VA. We also report that high survivin expression determined by the more cost-effective VA method is an independent predictor of poor ccRCC outcome and, more importantly, can be used to further refine outcome prediction among existing prognostic categories of ccRCC patients (ie, moderate- and high-risk patients). As such, this quantitation method represents a viable alternative to DIA and further enhances the translational potential of survivin as a clinical biomarker of ccRCC outcome.
Footnotes
Several of the authors have disclosed that applications for patent have been filed for each technology listed here, although neither has been licensed. (1) Combined B7-H1 and Survivin as Prognostic Indicators of Survival and Potential Therapeutic Target for Patients With Renal Cell Carcinoma (case number 2005-262): John C. Cheville, Eugene D. Kwon, Christine M. Lohse, Alexander S. Parker. (2) Combination of Pathologic Consultation Using the Mayo Clinic SSIGN Score and Tumor B7-H1, Survivin, and Ki-67 Expression for Prognostic Assessment of Patients With Clear Cell Renal Cell Carcinoma (case number 2007-070): John C. Cheville, Eugene D. Kwon, Christine M. Lohse, Bradley C. Leibovich.
References
- 1.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176(6 Pt 1):2397–400. doi: 10.1016/j.juro.2006.07.144. [DOI] [PubMed] [Google Scholar]
- 2.Atkins MB, Ernstoff MS, Figlin RA, et al. Innovations and challenges in renal cell carcinoma: summary statement from the Second Cambridge Conference. Clin Cancer Res. 2007;13(2 Pt 2):667s–70s. doi: 10.1158/1078-0432.CCR-06-2231. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen CT, Campbell SC. Staging of renal cell carcinoma: past, present, and future. Clin Genitourin Cancer. 2006;5:190–7. doi: 10.3816/CGC.2006.n.036. [DOI] [PubMed] [Google Scholar]
- 4.Parker AS, Kosari F, Lohse CM, et al. High expression levels of survivin protein independently predict a poor outcome for patients who undergo surgery for clear cell renal cell carcinoma. Cancer. 2006;107:37–45. doi: 10.1002/cncr.21952. [DOI] [PubMed] [Google Scholar]
- 5.Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2:1–10. doi: 10.1186/gb-2001-2-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer. 2005;92:212–6. doi: 10.1038/sj.bjc.6602340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 8.Walker RA. Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment I. Histopathology. 2006;49:406–410. doi: 10.1111/j.1365-2559.2006.02514.x. [DOI] [PubMed] [Google Scholar]
- 9.Bast RC, Jr, Lilja H, Urban N, et al. Translational crossroads for biomarkers. Clin Cancer Res. 2005;11:6103–8. doi: 10.1158/1078-0432.CCR-04-2213. [DOI] [PubMed] [Google Scholar]
- 10.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:987–9. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumors. J Pathol. 1997;183:131–3. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Lohse CM, Blute ML, Zincke H, et al. Comparison of standardized and non-standardized nuclear grade of renal cell carcinoma to predict outcome among 2,042 patients. Am J Clin Pathol. 2003;118:877–86. doi: 10.1309/VLV6-BRTR-HY5B-H485. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta S, Lohse CM, Leibovich BC, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104:511–20. doi: 10.1002/cncr.21206. [DOI] [PubMed] [Google Scholar]
- 14.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28:435–41. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg DM, Ali SZ. Application of virtual microscopy in clinical cytopathology. Diagn Cytopathol. 2001;25:389–96. doi: 10.1002/dc.10021. [DOI] [PubMed] [Google Scholar]
- 16.Therneau T, Grambsch P. Modeling survival data: extending the Cox model. 1. Springer-Verlag; Ann Arbor: 2000. pp. 87–92. [Google Scholar]
- 17.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–3. [PubMed] [Google Scholar]
- 18.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–57. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 19.Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade, and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE., Jr . Regression modeling strategies. New York: Springer; 2001. p. 493. [Google Scholar]
- 21.Wang S, Saboorian MH, Frenkel EP, et al. Assessment of HER-2/neu status in breast cancer. Automated Cellular Imaging System (ACIS)– assisted quantitation of immunohistochemical assay achieves high accuracy in comparison with fluorescence in situ hybridization assay as the standard. Am J Clin Pathol. 2001;116:495–503. doi: 10.1309/TMUW-G4WB-LXJ2-FUDN. [DOI] [PubMed] [Google Scholar]
- 22.Charpin C, Dales JP, Garcia S, et al. Tumor neoangiogenesis by CD31 and CD105 expression evaluation in breast carcinoma tissue microarrays. Clin Cancer Res. 2004;10:5815–9. doi: 10.1158/1078-0432.CCR-04-0021. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Z, Wu CL, Woda BA, et al. Alpha-methylacyl-CoA racemase: a multi-institutional study of a new prostate cancer marker. Histopathology. 2004;45:218–25. doi: 10.1111/j.1365-2559.2004.01930.x. [DOI] [PubMed] [Google Scholar]
- 24.Rimm DL. What brown can not do for you. Nat Biotechnol. 2006;24:914–6. doi: 10.1038/nbt0806-914. [DOI] [PubMed] [Google Scholar]
- 25.Cregger M, Berger AJ, Rimm DL. Immunohistochemistry and quantitative analysis of protein expression. Arch Pathol Lab Med. 2006;130:1026–30. doi: 10.5858/2006-130-1026-IAQAOP. [DOI] [PubMed] [Google Scholar]


