Abstract
Agonists of TLR have been explored as vaccine adjuvants for tumor immunotherapy. However, their immunological consequences are not fully understood. Although TLR signaling increases the functional potential of dendritic cells (DCs) for priming T cells, coinduction of potentially negative immunoregulatory capacities may impair effector T cell generation. We examined the expression and function of B7 family costimulatory molecules on DCs after activation with the TLR3 agonist, polyinosinic:polycytidylic acid. We demonstrated that polyinosinic:polycytidylic acid consistently up-regulated both B7-2 and B7-H1 molecules on resident, migratory DCs from spleen and lymph nodes. Depletion or blockade of B7-H1 on activated DCs increased the magnitude of effector CD8 T cell expansion. DC-based or protein-based tumor vaccines, in combination with B7-H1 blockade, induced strong effector CD8 T cell responses, resulting in protective immunity against newly established tumors. Our studies suggest that TLR3 signaling has the potential to up-regulate both positive and negative coregulatory molecules on APCs. Selective blockade of negative regulatory molecules in combination with TLR3 agonist may be an effective strategy for increasing the efficacy of tumor vaccines.
Toll-like receptor signaling induces dendritic cell (DC)3 activation, bridging innate and adaptive immune responses (1). TLR ligands have been proposed as vaccine adjuvants for boosting adaptive immunity in cancer therapy. The adaptive T cell response is ultimately dictated by DCs, whose function is to acquire novel Ags and then direct the activation and expansion of Ag-specific T cells (2). TLR signaling induces DC activation, a process that is characterized by enhanced expression of costimulatory molecules and increased secretion of cytokines necessary for activation and differentiation of naive T cells (3). Different TLR pathways result in functionally distinct DCs, ultimately determining net outcomes of DC-T cell interaction. For example, DCs preferentially produce IL-10 following TLR2 signaling (4), but generate IL-12p70 and IFN-α subsequent to TLR4 ligation (5). TLR3 signaling induces the production of type I IFN and other inflammatory cytokines and chemokines (6–9) and promotes cross-priming of CD8 T cells by CD8α+ DCs (10, 11).
Polyinosinic:polycytidylic acid (poly(I:C)), a synthetic analog of dsRNA, is a ligand for TLR3 and melanoma differentiation-associated gene 5 (3, 12). Poly(I:C) signaling requires cooperative activation of both TLR3 and melanoma differentiation-associated gene 5 (13). Because of its ability to improve Ag cross-presentation and production of type I IFN and IL-12 in DCs (6, 10, 14), poly(I:C) has been proposed as a vaccine adjuvant in the treatment of cancer. Poly(I:C) promotes tumor Ag-specific T cell responses in conjunction with peptide-based vaccines and has direct antitumor effects when injected into tumor tissues (15–18). In humans, vaccination with poly(I:C)-activated DCs can induce tumor Ag-specific CD8 T cell responses in cancer patients (19). Although poly(I:C) activates both innate and adaptive immune responses, vaccine-elicited effector T cells fail to inhibit the growth of tumors (20–22). It is possible that effector T cells are compromised by negative immunoregulatory mechanisms activated by poly(I:C) (23, 24).
Up-regulation of B7-1, B7-2, and B7-H1 (PD-L1) costimulatory molecules has been observed in both human and mouse DCs following activation by poly(I:C) (25, 26). However, the impact of poly(I:C)-induced expression of coregulatory molecules on CD8 T cell responses to tumor vaccine therapy is largely unknown. In this study, we examined the expression of B7 family molecules in DC subsets isolated from different tissues following in vivo administration of poly(I:C). We report that poly(I:C) up-regulated B7-2 and B7-H1 on resident and migratory DCs. Using DC B7-H1 depletion or blockade models, we determined that B7-H1 induced on poly(I:C)-activated DCs restricted the expansion of effector CD8 T cells. Significantly, B7-H1 blockade improved the efficacy of tumor vaccines in the treatment of newly established tumors. Our study suggests that the combination of poly(I:C) and B7-H1 blockade is a novel approach to improve the efficacy of therapeutic tumor vaccines by interfering with the negative costimulatory molecules while simultaneously inducing robust antitumor immunity.
Materials and Methods
Mice, cell lines, and reagents
Female C57BL/6 mice were purchased from Taconic Farms. OT-1 TCR transgenic mice and actin promotor-driven membrane-bound chicken ovalbumin (Act-mOVA) transgenic mice were purchased from The Jackson Laboratory. B7-H1 knockout (KO) C57BL/6 mice provided by L. Chen (Johns Hopkins University, Baltimore, MD) (27) were used to produce B7-H1 KO Act-mOVA transgenic mice in our animal facility. Mice were maintained under specific pathogen-free conditions and used at 8–12 wk of age. Peptides were produced by the Mayo Clinic Proteomic Core Facility. Mouse rGM-CSF and rIL-4 were purchased from R&D Systems. OVA protein, FITC-conjugated OVA protein, and poly(I:C) were purchased from Sigma-Aldrich. Hamster anti-mouse B7-H1 mAb (10B5) was obtained from hybridoma cells (a gift of L. Chen, Johns Hopkins University, Baltimore, MD). B16-OVA murine melanoma cells provided by R. Vile (Mayo Clinic, Rochester, MN) were cultured in RPMI 1640 medium (Mediatech) with 10% FBS (Life Technologies), 1 U/ml penicillin, 1 μg/ml streptomycin, and 20 mM HEPES buffer (all from Mediatech). Mouse studies were conducted in accordance with the National Institutes of Health guidelines and monitored by the Mayo Clinic Institutional Animal Care and Use Committee.
Flow cytometry analysis Abs
Spleen cell suspensions were prepared and stained for analysis by flow cytometry using PBS containing 3% FBS and 2 mM EDTA. Class I MHC (KbOVA) tetramer and negative control tetramers were purchased from Beckman Coulter. Fluorochrome-conjugated Abs against CD8, CD3, CD44, CD62L, CD43 (1B11), CD80, CD86, B7-H1 (PD-L1, MIH5), PD-1 (RMP-1–14), I-Ab, B7-H3, B7-H4, B7-DC (PD-L2), and 25D1.16 (anti-OVA peptide in H-2Kb complex) were purchased from BD Biosciences, BioLegend, or eBioscience. Vybrant carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) Cell Tracer Kit was purchased from Invitrogen. A minimum of 100,000 viable cells was live gated on FACScan and FACSCalibur (BD Biosciences) instrumentation. Flow cytometry analysis was performed using FlowJo software (Tree Star).
DC isolation and purification
Spleen and lymph nodes were removed, and the cells were incubated with collagenase IV (28) for 45 min. Cells were stained with anti-CD11c Ab coupled with MACS MicroBeads (Miltenyi Biotec) for 30 min at 4°C. CD11c+ cells were isolated on a magnetized MACS column (Miltenyi Biotec), according to the manufacturer's instructions. After elution, cells were centrifuged and resuspended in FACS buffer and stained for analysis. Similarly, CD8α+/− DC subsets were isolated using CD8α+ DC isolation kit (Miltenyi Biotec).
Bone marrow DC culture and immunization
B7-H1 wild-type (WT) or B7-H1 KO bone marrow (BM) was extracted, and RBC were removed. Cells were cultured in petri dishes at 2 × 106/ml containing 10 ng/ml GM-CSF and 1 ng/ml IL-4 (R&D Systems) for 5–6 days. The resulting cell populations consisted of 50–80% CD11c+ cells. Poly(I:C) (Sigma-Aldrich) was then added at 10 μg/ml for 24 h. To load Ag on DCs, OVA peptide257–264 (2 μM) was added to the DCs for 2–3 h at 37°C. Blocking B7-H1 Ab (10B5) was added at indicated concentrations at 4°C for 30 min. BM-DCs were then washed and analyzed by flow cytometry. For immunization, DCs were injected either s.c. or i.v. into WT mice.
Adjuvant administration and protein immunization
To activate skin DCs, WT mice were injected intradermally with FITC-OVA protein (20 μg) plus PBS or poly(I:C) (20 μg) in 50 μl at the base of tail. Draining lymph nodes (inguinal and caudal axillary) were removed 20–24 h later. To activate spleen DCs, WT mice were injected i.p. with 50 μg of poly(I:C). For in vivo immunization (29), OVA whole protein (0.5 mg) was mixed with 50 μg of poly(I:C) and injected i.p. into naive WT mice for CD8 T cell priming or into tumor-bearing mice for treatment. Blocking Ab (10B5) or control isotype (hamster Ig) was injected i.p. at the indicated times and doses.
Functional assay of primed CD8 T cells and in vivo CTL assay
Spleen cells from immunized mice were isolated and restimulated with OVA or control tyrosinase-related protein 2 (TRP2) peptides (2 μg/ml) in the presence of 1 μl/ml GolgiPlug (BD Biosciences) (30) for 5 h ex vivo. Following incubation, cell surface staining was performed, followed by fixation, permeabilization, and intracellular staining for IFN-γ. For the in vivo CTL assay (31), OVA257–264 peptide-pulsed or control (TRP2) peptide-pulsed WT spleen cells were labeled with a high dose of CFDA-SE or a low dose of CFDA-SE, mixed at 1:1 (2.5 × 106 of each), and injected i.v. into mice on day 7 after immunization. The CTL activity was determined 5 h after target cell transfer. Specific lysis is calculated using the following formulas: ratio = (%CFSEhigh/%CFSElow); percent specific lysis = (1 − (ratio primed/ratio unprimed)) × 100%.
Tumor treatment
Mice were inoculated s.c. in the right flank with 5 × 105 B16-OVA tumor cells in 100 μl of PBS on day 0. After tumors were established (day 3), mice were vaccinated with OVA protein and poly(I:C) or DCs. Tumor-bearing mice were injected with 200 μg of control Ab (hamster Ig) or anti-B7-H1 mAb (10B5) every other day from days 2 through 12. DC vaccine (1 × 106 DCs) was injected s.c. at the base of tail or left flank on days 3, 6, and 10. For tumor protection, B16-OVA tumor cells (1 × 106, s.c.) were injected on day 14 after OVA protein and poly(I:C) vaccination. Tumor growth was monitored by measuring the longest bisecting diameters of flank tumors. Mice were sacrificed when the tumors displayed ulceration or when one diameter measured 17 mm.
Statistical analysis
A two-sided, unpaired Student's t test or Mann-Whitney U test was used to assess statistical differences in experimental groups. A p value <0.05 was considered statistically significant.
Results
Poly(I:C) up-regulated B7-H1 and B7-2 expression on DC subsets from spleen and skin
We analyzed the expression of B7 family costimulatory molecules on two spleen DC subsets (CD8α+ and CD8α−) following poly(I:C) injection in WT mice (Fig. 1A). Twenty-four hours after poly(I:C) injection, CD8α+ DCs (potent cross-presenting Ag cells) (32) expressed elevated levels of B7-H1 and B7-2 compared with unstimulated controls, whereas B7-1 and B7-DC were only slightly increased. In contrast, poly(I:C) did not induce the expression of B7-H3 or B7-H4 on CD8α+ DC (Fig. 1A). In CD8α− DCs (less potent at cross-presenting Ag) (32), poly(I:C) selectively induced B7-2 expression (Fig. 1A). Thus, our results demonstrate that poly(I:C) selectively up-regulates the B7 family costimulatory molecules on splenic DC subsets.
FIGURE 1.
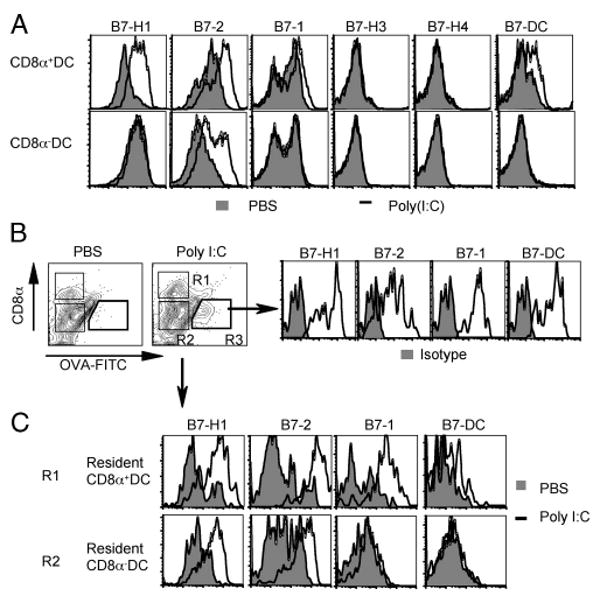
Poly(I:C) up-regulated both B7-2 and B7-H1 on DCs. A, TLR3 agonist poly(I:C) (50 μg) or PBS (control) was injected i.p. into WT mice. B and C, FITC-OVA protein (20 μg) was injected s.c. with PBS or poly(I:C) (20 μg) in WT mice. One day later, CD11c+ DCs were purified from spleen (A) or draining lymph nodes (B and C), and stained with anti-CD11c, anti-CD8α, and other Abs, as indicated. Shaded histograms represent isotype controls (B). Migratory DCs are identified as CD8α− FITC-OVA+ cells (R3), and resident CD8α+/− DCs as FITC-OVA− (R1 and R2). Data show profiles of CD11c+ CD8α+/− DCs.
We next examined the expression of B7 family molecules on migratory and resident DCs following intradermal immunization with FITC-labeled OVA protein plus poly(I:C). In this situation, poly(I:C) induced the migration of Ag (OVA)-bearing DCs from peripheral tissues into lymph nodes. The Ag-bearing migratory DCs were CD8α− OVA-FITC+ CD11c+ cells and expressed high levels of B7-H1, B7-2, B7-1, and B7-DC (Fig. 1B). Resident CD8α+ and CD8α− DCs were identified as OVA-FITC− CD11c+ cells in the same lymph nodes. Both resident CD8α+ and CD8α− DCs expressed higher levels of B7-H1 and B7-2 (Fig. 1C) upon poly(I:C) treatment, whereas only CD8α+ DCs expressed higher levels of B7-1 and B7-DC. Thus, poly(I:C) induces and up-regulates B7-H1 and B7-2 on both migratory and resident DCs.
Immunization plus poly(I:C) induced more Ag-specific effector CD8 T cells in B7-H1-deficient mice
To investigate whether poly(I:C)-up-regulated B7-H1 impacts T cell responses following immunization, we immunized WT and B7-H1 KO mice using OVA protein with or without poly(I:C) as an adjuvant. As shown in Fig. 2, immunization with OVA protein without poly(I:C) only induced a slight and comparable increase of OVA-specific (KbOVA-tet+) and OVA-reactive (IFN-γ+) CD8 T cells in the spleens of B7-H1 WT and KO mice. With poly(I:C) as adjuvant, OVA protein immunization induced a more than 2-fold increase in OVA Ag-specific and OVA-reactive CD8 T cells in B7-H1 KO mice (0.41% Tet+, 0.28% IFN-γ+) compared with WT mice (0.18% Tet+, 0.13% IFN-γ+; p < 0.05; Fig. 2). These results suggest that the endogenous B7-H1 at its steady state does not impact T cell priming. However, once up-regulated by poly(I:C), B7-H1 may function as a negative regulator in the expansion of effector T cells.
FIGURE 2.
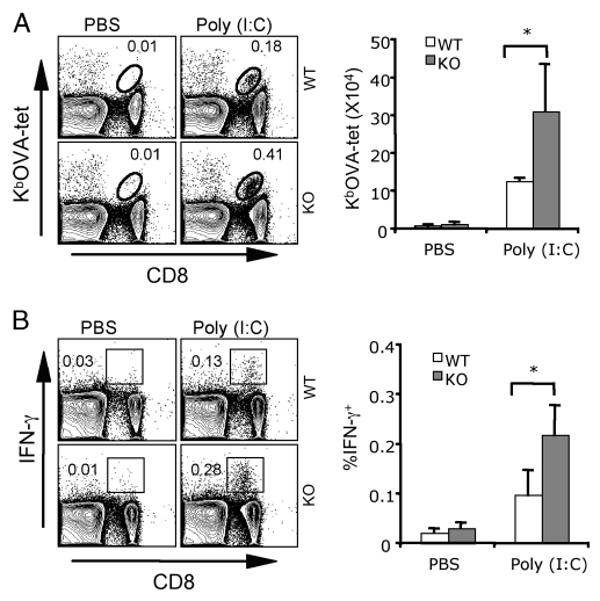
Immunization with poly(I:C) induced a more robust expansion of Ag-specific CD8 T cells in B7-H1-deficient mice. WT or B7-H1 KO mice were immunized i.p. with whole OVA protein (0.5 mg) and poly(I:C) (50 μg) or PBS. Seven days after immunization, splenocytes were isolated and analyzed for OVA-specific CD8 T cells by KbOVA tetramer staining (A). Functionality of OVA-reactive CD8 T cells was analyzed by intracellular staining for IFN-γ after 5-h restimulation with OVA peptide (B). Bar graphs show the average numbers or percentages ± SD of three mice per group. One representative of three experiments is shown; *, p < 0.05.
B7-H1 on poly(I:C)-activated CD8α+ DCs limited the expansion of Ag-specific effector CD8 T cells
Because DCs play a central role in regulating the priming of T cells and poly(I:C) has the potential to up-regulate B7-H1 in both migratory and resident DC subsets (Fig. 1), we next examined whether B7-H1 on different DC subsets will affect CD8 T cell priming. We introduced B7-H1 deficiency into Act-mOVA transgenic mice. B7-H1 KO Act-mOVA mice express membrane-bound class I-restricted OVA on the surface of all nucleated cells (33) and do not express B7-H1 molecules. We purified CD8α+/− DC subsets from the spleen of B7-H1 WT and KO Act-mOVA mice 20 h after poly(I:C) injection. Both WT and B7-H1 KO Act-mOVA DCs showed comparable levels of B7-1 and B7-2 costimulatory molecules, as well as of OVA Ag presentation in MHC class I (H-2Kb) complex (Fig. 3A). As expected, KO Act-mOVA DCs were negative for B7-H1 expression.
FIGURE 3.
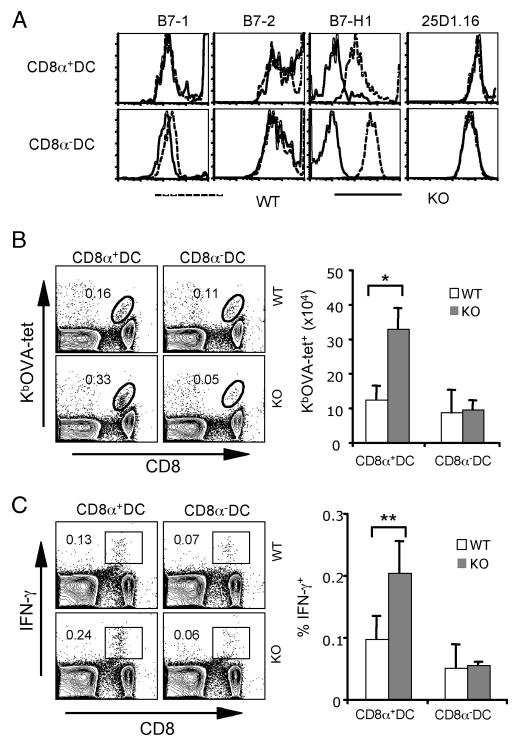
Immunization with B7-H1-deficient CD8α+ DCs increased Ag-reactive CD8 T cells. CD8α+ or CD8α− DCs were purified from spleens of WT or B7-H1 KO Act-mOVA mice 20 h after poly(I:C) (50 μg/ml) injection. A, Histograms show phenotypes of splenic DC subsets. Presentation of OVA peptide within MHC I complex was identified by anti-Kb/OVA mAb (25D1.16). B and C, Immunization of naive WT mice with CD8α+ or CD8α− DCs (1 × 105, s.c.). One week after immunization, splenocytes were isolated and stained with KbOVA tetramer and anti-CD8 Ab (B). Effector function of CD8 T cells was analyzed by intracellular staining for IFN-γ after 5-h restimulation with OVA peptide (C). One representative of three experiments is shown. Bar graphs show the average numbers or percentages ± SD of three or four mice per group; *, p < 0.01; **, p < 0.05.
To determine whether B7-H1 on poly(I:C)-activated CD8α+/− DCs limits the expansion of endogenous Ag-specific CD8 T cells, WT mice were immunized with CD8α+ or CD8α− DCs purified from poly(I:C)-treated B7-H1 WT or KO Act-mOVA mice. On day 7 after DC immunization, poly(I:C)-stimulated B7-H1 KO CD8α+ DCs induced 2- to 3-fold more OVA-specific (Tet+) CD8 T cells in immunized mice compared with those induced by WT CD8α+ DCs in both the frequency (0.33 vs 0.16%) and absolute numbers (Fig. 3B; p < 0.01). Moreover, immunization with B7-H1 KO CD8α+ DCs induced 2-fold more OVA-reactive (IFN-γ+) effector CD8 T cells (0.24% compared with 0.13%; p < 0.05; Fig. 3C). However, both B7-H1 WT and KO CD8α− DCs induced a slight and comparable increase of OVA-specific (Tet+) or OVA-reactive (IFN-γ+) CD8 T cells in the spleen. Consistent with the observed up-regulation of B7-H1 on splenic CD8α+ DC subsets after poly(I:C) treatment (Fig. 1A), our results suggest that B7-H1 up-regulated on poly(I:C)-activated CD8α+ DCs limits the expansion of effector CD8 T cells primed by DC immunization.
To further examine the influence of DC B7-H1 in regulating IFN-γ-producing CD8 T cell expansion, we cocultured CD8α+ or CD8α− DC subsets purified from B7-H1 KO or WT Act-mOVA mice with CD8 T cells purified from OT-1 mice expressing transgenic TCR specific for H-2Kb/OVA complex. We labeled OT-1 CD8 T cells with CFDA-SE to track their proliferation before coculture with splenic CD8α+ or CD8α− DCs purified from B7-H1 WT or KO Act-mOVA mice 20 h after poly(I:C) injection. After 3-day culture, B7-H1 WT or KO CD8α+ or CD8α− DCs induced similar proliferation (4–5 divisions as indicated by discrete CFSE dilution) of OT-1 CD8 T cells (Fig. 4A). However, B7-H1 KO CD8α+ or CD8α− DCs increased the accumulation of IFN-γ-producing proliferating (IFN-γ+CFSElow) OT-1 CD8 T cells (6.7 vs 3.9% for WT CD8α+ DCs; 8.4 vs 5.9% for WT CD8α− DCs; p < 0.05; Fig. 4B). Collectively, our results suggest that B7-H1 expressed by activated DCs constrains the magnitude of effector CD8 T cell expansion.
FIGURE 4.
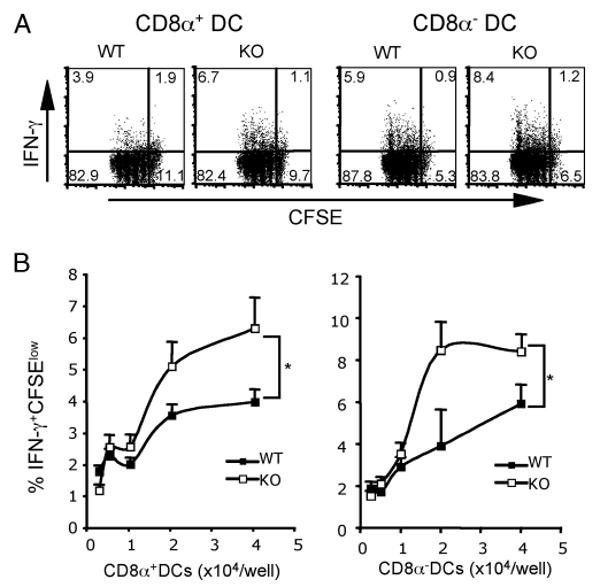
DC B7-H1 limited the expansion of effector CD8 T cells in vitro. CD8α+ or CD8α− DCs were purified from spleens of WT or B7-H1 KO Act-mOVA mice 20 h after poly(I:C) injection (50 μg). A, CFDA-SE-labeled OT-1 CD8 T cells (1 × 105/well) were incubated with WT or B7-H1 KO DC subsets (4 × 104/well) for 60 h. Proliferation of CD8 T cells was analyzed by flow cytometry based on the dilution of CFSE. Production of IFN-γ was analyzed by intracellular staining 16 h after addition of brefeldin A. One of two independent experiments is shown. B, Percentages of IFN-γ+CFSElow OT-1 CD8 T cells in culture with graded DC subsets, as indicated. *, p < 0.05.
B7-H1-deficient mature DCs as vaccine showed strong therapeutic effects
To investigate how DC B7-H1 regulates antitumor immunity, we used BM-derived DCs from WT or B7-H1 KO mice as tumor vaccine because BM-derived DCs are known to be potently expanded ex vivo. First, we tested whether B7-H1 KO BM-DCs increased the expansion of effector CD8 T cells after immunization. We i.v. immunized naive WT mice with poly(I:C)-activated, OVA peptide-pulsed BM-DCs generated from B7-H1 WT or KO mice. One week after immunization, lymphocytes from the spleens of immunized mice were recovered and analyzed for frequency of OVA Ag-specific CD8 T cells. Poly(I:C)-stimulated B7-H1 KO BM-DCs induced 3-fold more OVA-specific CD8 T cells in both frequency (Fig. 5A, upper panel) and absolute numbers (244.8 ± 41.3 × 104 compared with 85.4 ± 17.8 × 104; p < 0.05). Moreover, immunization with activated B7-H1 KO BM-DCs induced 5-fold more effector (IFN-γ+) CD8 T cells (1.6 ± 0.3% compared with 0.36 ± 0.1%; p < 0.05; Fig. 5A, lower panel). In addition, blockade of B7-H1 on poly(I:C)-activated WT OVA peptide-pulsed DCs induced 4-fold more OVA-specific CD8 T cells (2.7% compared with 0.59%), and 4-fold more effector (IFN-γ+) CD8 T cells (3.8% compared with 0.9%; p < 0.01; Fig. 5B). Collectively, our results suggest that B7-H1 on poly(I:C)-activated BM-DCs also limits the expansion of endogenous effector CD8 T cells.
FIGURE 5.
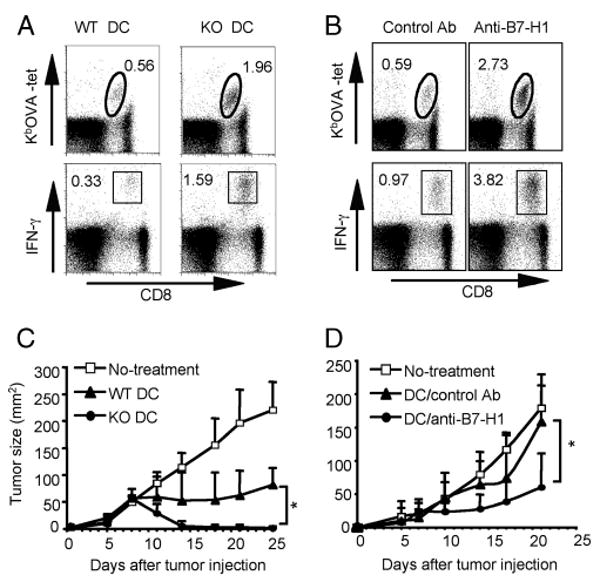
Immunization with B7-H1-deficient or blocked DCs increased Ag-specific effector CD8 T cells and suppressed tumor growth. WT mice were immunized i.v. with A, poly(I:C)-activated OVA peptide-pulsed WT or B7-H1 KO BM-derived DCs, or with B, poly(I:C)-activated OVA peptide-pulsed WT DCs preincubated with anti-B7-H1 blocking or control Ab. One week later, splenocytes were isolated and stained with KbOVA tetramer and anti-CD8 Ab (upper panels in A and B). Effector function of CD8 T cells was analyzed by intracellular staining for IFN-γ after 5-h restimulation with OVA peptide (lower panels in A and B). One representative of three experiments is shown. C and D, Immunotherapy with poly(I:C)-activated DC vaccines. B16-OVA tumor cells (5 × 105) were injected s.c. into WT mice on day 0. Mice were treated with C, WT or B7-H1 KO DC vaccines (s.c.) on days 3, 6, and 10, or with D, WT DC vaccines combined with B7-H1 blocking Ab or control Ab (s.c.) on days 3, 6, and 10. Data show tumor sizes as mean ± SD of four or five mice per group. *, p < 0.05. One of two independent experiments is shown.
To test whether B7-H1 deficiency or B7-H1 blockade on activated DCs leads to effective antitumoral immunity, we used DC vaccines to treat mice on day 3 after injection of B16-OVA melanoma tumor cells (5 × 105, s.c. on the right flank) (34–36). Mice were treated (s.c. at the base of tail or left flank) with OVA peptide-pulsed poly(I:C)-activated WT or B7-H1 KO DCs. Fig. 5C shows that B16-OVA tumors progressed in untreated mice and regressed in WT DC vaccine-treated mice. Of note, B7-H1 KO DC vaccination dramatically inhibited the B16-OVA tumor growth (tumor size 2.5 ± 3.5 mm2) compared with WT DC vaccine (81.3 ± 32.1 mm2) on day 25 after tumor injection (p < 0.05; Fig. 5C). We next determined whether B7-H1-blocked DCs could improve antitumoral immunity. Poly(I:C)-activated OVA-peptide-pulsed WT DCs were incubated with or without anti-B7-H1 Ab. The DC vaccine was then injected s.c. into B16-OVA tumor-bearing mice. Injection of DCs without B7-H1 blockade only slightly inhibited the growth of established (day 3) B16-OVA tumors (158.6 ± 50.8 mm2 on day 21 after tumor injection). However, injection of B7-H1-blocked DCs dramatically delayed the growth of established B16-OVA tumors (tumor size 60.3 ± 55.2 mm2; p < 0.05; Fig. 5D). In summary, these studies collectively confirm that B7-H1 expressed by activated DCs functions to inhibit the expansion of Ag-specific effector CD8 T cells capable of rejecting tumor cells.
Combined B7-H1 blockade and poly(I:C) increased the efficacy of protein vaccination
Given that CD8α+ DCs mediate the cross-priming of CD8 T cells (37, 38), B7-H1 up-regulation on CD8α+ DCs following poly(I:C) stimulation may be a harbinger of heretofore unappreciated immunoregulating interactions. To test this possibility, we immunized WT mice with whole OVA protein plus poly(I:C) in combination with B7-H1 blockade. In this model, endogenous CD8 T cells are primed by CD8α+ DCs that cross-present soluble OVA protein (39). Eight days after immunization, blockade of B7-H1 generated a 1.5-fold increase in the frequency of OVA-specific CD8 T cells (0.28 vs 0.17%; p < 0.05), and twice as many OVA-specific CD8 T cells in the spleen (30.2 ± 11.8 × 104 vs 14.1 ± 4.0 × 104; p < 0.05; Fig. 6A). B7-H1 blockade also increased the frequency of effector (IFN-γ+) CD8 T cells (0.27 vs 0.19%; p < 0.05; Fig. 6B). In vivo cytotoxicity assays showed significantly increased cytotoxicity of primed OVA-specific CD8 T cells against OVA-peptide-pulsed target cells in mice immunized with OVA/poly(I:C) plus B7-H1 blockade compared with those without B7-H1 blockade (64.2 ± 5.5% vs 15.1 ± 5.5% specific lysis; p < 0.01; Fig. 6C).
FIGURE 6.
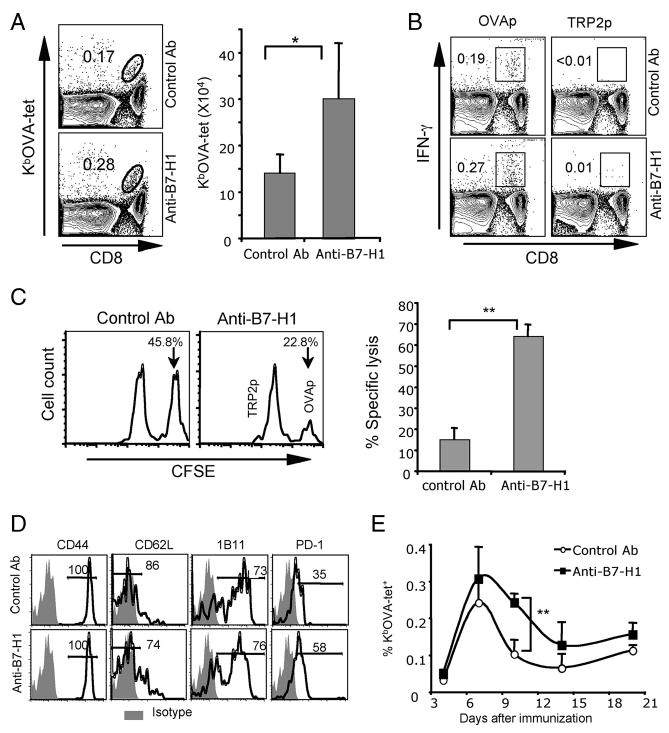
Immunization with protein/poly(I:C) combined with B7-H1 blockade increased Ag-specific effector CD8 T cells. WT mice were immunized i.p. with whole OVA protein (0.5 mg) and poly(I:C) (50 μg) on day 0. Anti-B7-H1 (10B5) or control Abs (hamster Ig) (200 μg) were injected (i.p.) on days 0 and 3. Eight days after immunization, splenocytes were isolated and analyzed for OVA-specific CD8 T cells by KbOVA tetramer staining (A). Bar graphs show the average numbers ± SD of three mice per group; *, p < 0.05. Functionality of OVA-reactive CD8 T cells was analyzed by intracellular staining for IFN-γ after 5-h restimulation with OVA peptide or control TRP2 peptide (B). One representative of three experiments is shown. C, In vivo cytotoxicity was measured in mice on day 7 after immunization with OVA/poly(I:C) combined with control Ab or B7-H1 blocking Ab. Histogram plots show the remaining target cells in spleen. The bar graph shows the average percentages of specific lysis in three mice; **, p < 0.01. D, Phenotype of OVA-specific CD8 T cells on day 8 after immunization. E, Kinetics of OVA-specific (KbOVA-tetramer+) CD8 T cell response to OVA/poly(I:C) with or without B7-H1 blockade. Data show the average percentages ± SD of three mice at each time point. One representative of two experiments is shown; **, p < 0.01.
Vaccine-induced Ag-specific CD8 T cells expressed similar levels of effector phenotype markers (CD44high, CD62Llow, and CD43/1B11high) in mice on day 8 postimmunization with or without B7-H1 blockade (Fig. 6D). Of note, B7-H1 blockade increased the percentage of PD-1-positive cells among the OVA-specific CD8 T cells (58 vs 35%). Although there was no difference in the timing of peak accumulation of Ag-specific CD8 T cells after immunization, B7-H1 blockade increased the expansion and delayed the contraction of Ag-specific CD8 T cells in the spleen on day 10 after immunization (0.24 ± 0.03% vs 0.1 ± 0.04%; p < 0.01; Fig. 6E). Collectively, these results suggest that B7-H1 blockade increases the expansion of effector CD8 T cells, especially of PD-1+ effector cells, that are generated by protein immunization when poly(I:C) is used as an adjuvant.
To determine whether the increase in effector CD8 T cells generates clinically meaningful antitumor immunity, we first assessed whether B7-H1 blockade would enhance the protective immunity against tumor challenge. Naive WT mice were immunized with OVA protein and poly(I:C) with or without B7-H1 blocking Abs. On day 14 after immunization, lethal doses of B16-OVA tumor cells were injected (1 × 106, s.c.). Vaccination without B7-H1 blockade only slightly inhibited the growth of B16-OVA tumors compared with mice without any treatment. However, immunization with B7-H1 blockade significantly delayed the tumor growth (p < 0.05; Fig. 7A). Even though B16-OVA tumor cells expressed low levels of B7-H1 (Fig. 7B, inset), injection of anti-B7-H1 Ab alone did not inhibit the growth of tumors in vivo (Fig. 7B), suggesting the improved antitumor effects of B7-H1 blockade are not due to direct cytotoxicity mediated by the anti-B7-H1 Ab. We then evaluated the therapeutic effects of vaccine with B7-H1 blockade. B16-OVA tumors were treated with a combined immunization strategy consisting of B7-H1 blocking Abs given 1 day before OVA protein and poly(I:C) injections. The growth of B16-OVA tumors was not suppressed in mice immunized with OVA/poly(I:C) and control Abs. However, tumor growth was dramatically suppressed in mice treated with vaccine plus B7-H1 blockade (tumor size 115 ± 34.5 mm2 compared with 238.6 ± 17.1 mm2 on day 25 after tumor injection; p < 0.05; Fig. 7C). Nearly 40% of the mice treated with B7-H1 blockade survived up to 50 days (Fig. 7D). Collectively, our results support the concept that B7-H1 blockade with immunization increases the effector CD8 T cell pool capable of limiting the growth of newly established tumors.
FIGURE 7.
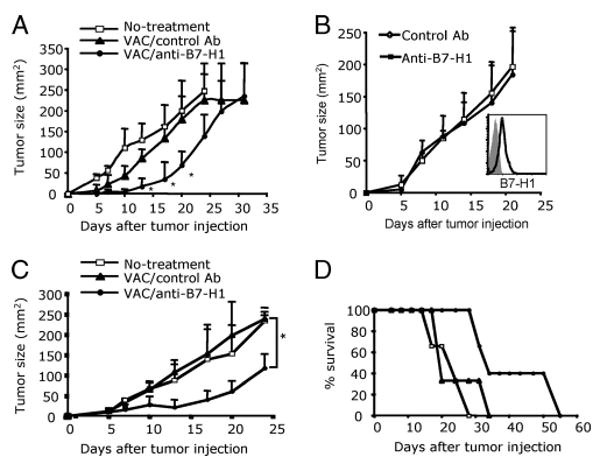
Vaccination with B7-H1 blockade improved preventive and therapeutic antitumor immunity. A, B16-OVA tumor cells (1 × 106) were injected s.c. into WT mice 14 days after immunization with OVA/poly(I:C) with or without B7-H1 blockade. Data show the average tumor size ± SD of five mice per group; *, p < 0.05, compared with control Ab groups. B, B16-OVA tumors were treated with anti-B7-H1 blocking Ab (10B5) or control Ab (200 μg, i.p.) on days 3, 6, 9, and 12 after tumor inoculation. Expression of B7-H1 by cultured B16-OVA tumor cells (inset, isotype staining is shown with shaded histogram). C and D, B16-OVA tumor cells (5 × 105/mouse) were injected s.c. into WT mice on day 0. Mice were immunized i.p. with OVA/poly(I:C) and anti-B7-H1 (10B5) or control Ab (hamster Ig) (200 μg) every 2 days for a total of five times beginning on day 2 (A). C, Data show the average tumor size ± SD of five mice per group; *, p < 0.05. D, The survival of mice treated with vaccine combined with B7-H1 blockade; p < 0.05, compared with vaccine without B7-H1 blockade. One of two independent experiments is shown.
Discussion
T cell activation is a precisely regulated process. The nature and magnitude of the CD8 T cell response are determined by the balance of a stimuli-dependent spectrum of costimulatory and coinhibitory molecules induced on DCs. We show that poly(I:C) up-regulated coexpression of B7-2 and B7-H1 molecules on resident and migratory DCs in vivo. Given the potential for delivering opposing costimulatory (B7-2) and coinhibitory (B7-H1) signals to T cells, selective interference with these molecules on activated DCs may alter the qualitative and quantitative characteristics of T cell responses. Using B7-H1 KO mice or B7-H1 mAb in tumor models involving poly(I:C)-activated DCs, we determined that B7-H1 expressed by activated DCs does not affect the initiation of a CD8 T cell response, but does limit the expansion of tumor-eliminating effector CD8 T cells.
We previously noticed that spleen DCs expressed higher levels of B7-H1 compared with bone marrow DCs, suggesting a negative regulatory function of B7-H1 on the recall response of tumor-specific memory CD8 T cells (40). In this study, we show that the tumor vaccine adjuvant poly(I:C), a TLR3 agonist (15, 16, 18), is capable of up-regulating B7-H1 on spleen CD8α+ DCs, but not on CD8α− DCs (Fig. 1A). This difference could be due to selective expression of TLR3 by CD8α+ DCs (10). Because CD8α+ DCs have a crucial role in cross-priming CTLs in vivo (37, 38, 41), selective up-regulation of B7-H1 by TLR3 ligand may add a negative regulator, which determines the extent of CD8 T cell responses. In fact, DCs treated with TLR3 ligand have been found to promote the cross-priming of CD8 T cells (10). However, our findings suggest that primed CD8 T cell expansion may be negatively controlled by up-regulated B7-H1 on the same DCs. Therefore, TLR3-stimulated B7-2 and B7-H1 expression in DCs may demonstrate dual regulatory mechanisms (costimulatory and coinhibitory signaling) in determining the final outcome of a T cell response that has been primed by DCs. This dual regulatory feature of TLR3 may help to prevent immunopathology caused by unlimited expansion of CD8 T cells primed by DCs activated by TLR3 ligands, such as viral dsRNA. However, this feature may also reduce the efficacy of antitumoral T cell responses when TLR3 ligands are used as adjuvant in tumor vaccines. We and others have found that Ag protein plus poly(I:C) could not achieve a protective immunity even though they can trigger Ag-specific CD8 T cell responses (Fig. 7) (14, 20, 21). Our new findings indicate that selective B7-H1 blockade on activated DCs will be able to improve the antitumoral efficacy of DC- or protein-based tumor vaccine.
B7-H1 blockade on poly(I:C)-activated DCs enhanced expansion of effector CD8 T cells. Similarly, Groschel et al. (25) reported that B7-H1 blockade and poly(I:C) promote proliferation of alloreactive human CD4 T cells in vitro. However, our study addressed a largely unexplored area, namely, the influence of B7-H1 expression on activated DCs in affecting Ag-specific CD8 T cell responses. CD8 T cell priming depends upon the interplay between TCR, CD28, and PD-1 (42, 43). Therefore, B7-H1 may also play an important role (44). Although Probst et al. (45) reported that resting DCs can induce peripheral tolerance via PD-1 signaling, the question of whether B7-H1 blockade on activated DCs results in breaking tolerance has not been addressed. Given the strong immunostimulatory role of activated DCs (2), it is odd that they dramatically up-regulate B7-H1, a negative regulator, on their surface. Using B7-H1 depletion and Ab-blocking strategies, we assessed the effects of B7-H1 on Ag-specific CD8 T cell responses induced by activated DCs. We report that B7-H1 expressed by activated DCs did not affect the Ag-initiated proliferation of CD8 T cells, but did limit the expansion of effector CD8 T cells and, therefore, the magnitude of the CD8 T cell response.
Expression of B7-H1 by tumors has been associated with impaired host antitumor responses (46, 47). However, whether DC B7-H1 also negatively impacts antitumor T cell responses has not been well characterized. It has been reported that human ovarian tumors harbor populations of myeloid-derived B7-H1-expressing DCs, and blockade of B7-H1 results in increased activation of Ag-specific T cells (48). In tumor-bearing mice that harbor DCs expressing high levels of B7-H1 in tumor-draining lymph nodes, B7-H1 blockade increased the effector cell populations as well as reducing the lung tumor metastases (49). Those studies largely reiterate the generally accepted dogma of B7-H1 functioning as a coinhibitor at the effector phase of antitumor T cell responses (46, 47), however, the role of DC B7-H1 in affecting priming and expansion of tumor-reactive CD8 T cells has not been rigorously addressed. Our studies indicate that activated DCs from B7-H1 KO mice are capable of inducing expansion of effector CD8 T cells, resulting in rejection of newly established tumors. In addition, whereas DCs from WT mice were less effective in inducing curative effector CD8 T cell populations, incorporating B7-H1 blockade during vaccination resulted in protection from tumors. These results suggest that the application of B7-H1 blockade during immunotherapeutic DC tumor vaccination may be more clinically effective than currently administered DC-based therapies (50).
In conclusion, we identified a poly(I:C)/TLR3 pathway that up-regulated both positive and negative B7 costimulatory molecules on Ag-presenting DCs. Poly(I:C)-activated DCs were capable of initiating T cell responses. Selective blockade of B7-H1 on DCs enhanced the expansion of effector cells, resulting in rejection of newly established tumors. Due to the nature of the majority of tumor Ags, antitumor immunity is dependent upon breaking self tolerance (51), and incorporating B7-H1 blockade into DC-based vaccination therapies, especially those using poly(I:C) as an adjuvant, may induce clinically valuable effector populations.
Acknowledgments
We gratefully thank Lieping Chen and Richard Vile for providing us with B7-H1-deficient mice, anti-B7-H1 Ab, and B16-OVA tumor cells, and Kathryn Jensen for secretarial assistance.
Footnotes
This work was supported by Mayo Foundation Career Development Award (to H.D.) and in part by a generous donation from the Richard M. Schulze Family Foundation (to E.D.K.), as well as by National Institutes of Health/National Cancer Institute Grant R01 CA134345.
Abbreviations used in this paper: DC, dendritic cell; Act-mOVA, actin promotor-driven membrane-bound chicken ovalbumin; KO, knockout; poly(I:C), polyinosinic: polycytidylic acid; WT, wild type; CFDA-SE, carboxyfluorescein diacetate succinimidyl ester; TRP2, tyrosinase-related protein 2.
Disclosures: Both E. D. Kwon and the Mayo Clinic have received royalties greater than the federal threshold for significant financial interest from the licensing to Medarex of technology related to B7-H1. Additionally, E. D. Kwon and the Mayo Clinic have contractual rights to receive future royalties from the licensing of this technology. In addition, some of the authors (E. D. Kwon, X. Frigola, and H. Dong) have filed patents for the potential use of B7-H1, B7-H3, and B7-H4 as prognostic markers for assessment of cancer.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 4.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi M, Kweon MN, Kuwata H, Schreiber RD, Kiyono H, Takeda K, Akira S. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 7.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, Al-Shamkhani A, Flavell R, Borrow P, Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Delivery Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 10.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 11.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, DiDonato J, Dziarski R, Akira S, Schoenberger SP, Raz E. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008;180:683–687. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- 14.Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, Goulmy E. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 15.McBride S, Hoebe K, Georgel P, Janssen E. Cell-associated double-stranded RNA enhances antitumor activity through the production of type I IFN. J Immunol. 2006;177:6122–6128. doi: 10.4049/jimmunol.177.9.6122. [DOI] [PubMed] [Google Scholar]
- 16.Currie AJ, van der Most RG, Broomfield SA, Prosser AC, Tovey MG, Robinson BW. Targeting the effector site with IFN-αβ-inducing TLR ligands reactivates tumor-resident CD8 T cell responses to eradicate established solid tumors. J Immunol. 2008;180:1535–1544. doi: 10.4049/jimmunol.180.3.1535. [DOI] [PubMed] [Google Scholar]
- 17.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28:220–228. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 18.Celis E. Toll-like receptor ligands energize peptide vaccines through multiple paths. Cancer Res. 2007;67:7945–7947. doi: 10.1158/0008-5472.CAN-07-1652. [DOI] [PubMed] [Google Scholar]
- 19.Trakatelli M, Toungouz M, Blocklet D, Dodoo Y, Gordower L, Laporte M, Vereecken P, Sales F, Mortier L, Mazouz N, et al. A new dendritic cell vaccine generated with interleukin-3 and interferon-β induces CD8+ T cell responses against NA17-A2 tumor peptide in melanoma patients. Cancer Immunol Immunother. 2006;55:469–474. doi: 10.1007/s00262-005-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Den Boer AT, Diehl L, van Mierlo GJ, van der Voort EI, Fransen MF, Krimpenfort P, Melief CJ, Offringa R, Toes RE. Longevity of antigen presentation and activation status of APC are decisive factors in the balance between CTL immunity versus tolerance. J Immunol. 2001;167:2522–2528. doi: 10.4049/jimmunol.167.5.2522. [DOI] [PubMed] [Google Scholar]
- 21.Llopiz D, Dotor J, Zabaleta A, Lasarte JJ, Prieto J, Borras-Cuesta F, Sarobe P. Combined immunization with adjuvant molecules poly(I:C) and anti-CD40 plus a tumor antigen has potent prophylactic and therapeutic antitumor effects. Cancer Immunol Immunother. 2008;57:19–29. doi: 10.1007/s00262-007-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc Natl Acad Sci USA. 2008;105:16683–16688. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngoi SM, Tovey MG, Vella AT. Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-α/β. J Immunol. 2008;181:7670–7680. doi: 10.4049/jimmunol.181.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, Schlesinger SJ, Colonna M, Steinman RM. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groschel S, Piggott KD, Vaglio A, Ma-Krupa W, Singh K, Goronzy JJ, Weyand CM. TLR-mediated induction of negative regulatory ligands on dendritic cells. J Mol Med. 2008;86:443–455. doi: 10.1007/s00109-008-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bochtler P, Kroger A, Schirmbeck R, Reimann J. Type I IFN-induced, NKT cell-mediated negative control of CD8 T cell priming by dendritic cells. J Immunol. 2008;181:1633–1643. doi: 10.4049/jimmunol.181.3.1633. [DOI] [PubMed] [Google Scholar]
- 27.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 29.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansari MJ, Abdi R. Emerging immunomodulatory therapies targeting the co-stimulatory pathways for the prevention of transplant rejection. I Drugs. 2003;6:964–969. [PubMed] [Google Scholar]
- 31.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 32.Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86:353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 33.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 34.Bellone M, Cantarella D, Castiglioni P, Crosti MC, Ronchetti A, Moro M, Garancini MP, Casorati G, Dellabona P. Relevance of the tumor antigen in the validation of three vaccination strategies for melanoma. J Immunol. 2000;165:2651–2656. doi: 10.4049/jimmunol.165.5.2651. [DOI] [PubMed] [Google Scholar]
- 35.Hokey DA, Larregina AT, Erdos G, Watkins SC, Falo LD., Jr Tumor cell loaded type-1 polarized dendritic cells induce Th1-mediated tumor immunity. Cancer Res. 2005;65:10059–10067. doi: 10.1158/0008-5472.CAN-05-1692. [DOI] [PubMed] [Google Scholar]
- 36.Van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, Overwijk WW, Restifo NP, Melief CJ, Offringa R, Allison JP. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin ML, Zhan Y, Proietto AI, Prato S, Wu L, Heath WR, Villadangos JA, Lew AM. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci USA. 2008;105:3029–3034. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 40.Webster WS, Thompson RH, Harris KJ, Frigola X, Kuntz S, Inman BA, Dong H. Targeting molecular and cellular inhibitory mechanisms for improvement of antitumor memory responses reactivated by tumor cell vaccine. J Immunol. 2007;179:2860–2869. doi: 10.4049/jimmunol.179.5.2860. [DOI] [PubMed] [Google Scholar]
- 41.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 42.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 46.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 47.Zou W, Chen L. Inhibitory B7-family molecules in the tumor microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 48.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 49.Wei S, Shreiner AB, Takeshita N, Chen L, Zou W, Chang AE. Tumor-induced immune suppression of in vivo effector T-cell priming is mediated by the B7-H1/PD-1 axis and transforming growth factor β. Cancer Res. 2008;68:5432–5438. doi: 10.1158/0008-5472.CAN-07-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 51.Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]


