Abstract
BACKGROUND
Whether an oncofetal protein, IMP3, can serve as a prognostic biomarker to predict metastasis for patients with localized papillary and chromophobe subtypes of renal cell carcinomas (RCCs) was investigated.
METHODS
The expression of IMP3 in 334 patients with primary papillary and chromophobe RCC from multiple medical centers was evaluated by immunohistochemistry. The 317 patients with localized papillary and chromophobe RCCs were further evaluated for outcome analyses.
RESULTS
IMP3 was significantly increased in a subset of localized papillary and chromophobe RCCs that subsequently metastasized. Patients with localized IMP3-positive tumors (n = 33; 10%) were over 10 times more likely to metastasize (risk ratio [RR], 11.38; 95% confidence interval [CI], 5.40–23.96; P <.001) and were nearly twice as likely to die (RR, 1.91; 95% CI, 1.13–3.22; P =.016) compared with patients with localized IMP3 negative tumors. The 5-year metastasis-free and overall survival rates were 64% and 58% for patients with IMP3-positive localized papillary and chromophobe RCCs compared with 98% and 85% for patients with IMP3 negative tumors, respectively. In multivariable analysis adjusting for the TNM stage and nuclear grade, patients with IMP3-positive tumors were still over 10 times more likely to progress to distant metastasis (RR, 13.45; 95% CI, 6.00–30.14; P <.001) and were still nearly twice as likely die (RR, 1.95; 95% CI, 1.15–3.31; P =.013) compared with patients with IMP3-negative tumors.
CONCLUSIONS
IMP3 is an independent prognostic biomarker that can be used to identify a subgroup of patients with localized papillary and chromophobe RCC who are at high risk for developing distant metastasis.
Keywords: IMP3, oncofetal protein, mRNA binding protein, prognostic biomarker, papillary renal cell carcinoma, chromophobe renal cell carcinoma
Renal cell carcinoma (RCC) is the most common type of kidney cancer and accounts for about 85% of malignant kidney tumors.1,2 The incidence of RCC has been rising steadily.3 It is expected that about 51,190 new cases of kidney cancer will be diagnosed in the US in 2007 with approximately 12,890 mortalities.4
There are several subtypes of RCC according to the Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) recommendations,5 originally proposed at the Heidelberg conference.6 Of these, the most common subtypes are clear cell or conventional (70%), papillary (10%–15%) and chromophobe (5%) RCC.5 These subtypes of RCC not only exhibit distinct cytogenetic abnormalities but also affect patient prognosis differently.7–13 Clear-cell RCCs are characterized by loss of genetic material in the chromosome 3p.5 Fifty percent of clear-cell RCCs show somatic mutations in the von Hippel-Lindau (VHL) gene and an additional 10% to 20% of these tumors show inactivation of the VHL gene.5 Papillary RCCs are characterized by trisomies (chromosomes 3q, 7, 12, 16, 17, and 20) and loss of the Y chromosome.5 Chromophobe RCCs are characterized by monosomy of multiple chromosomes (1, 2, 6, 10, 13, 17, and 21) and hypodiploidy.5 Localized papillary and chromophobe RCCs exhibit favorable prognosis with less frequent metastasis relative to clear-cell RCCs.7–14 Consequently, relatively little is known about molecular expression profiles associated with metastatic progression of papillary and chromophobe tumors compared with clear-cell RCC, which has been the dominant focus of studies reported in the literature.
IMP3 is a member of the insulin-like growth factor II (IGF-II) mRNA binding protein (IMP) family that consists of IMP1, IMP2, and IMP3.15 IMP family members play an important role in RNA trafficking and stabilization, cell growth, and cell migration during the early stages of embryogenesis.16 The IMP3 gene is located on chromosome 7p11.217 and is identical to the KOC (KH domain containing protein overexpressed in cancer) protein that was originally cloned from a pancreatic tumor cDNA screen.18 IMP3 is expressed in developing epithelia, muscle, and placenta during early stages of human and mouse embryogenesis, but it is expressed at low or undetectable levels in adult tissues.15,16 The expression of IMP3/KOC is also found in malignant tumors including pancreas, lung, stomach, and colon cancers, and soft tissue sarcomas but it is not detected in adjacent benign tissues.15,18–20 Moreover, recent studies have demonstrated that IMP3 is an important protein for tumor cell proliferation and invasion.21,22 These findings indicate that IMP3 is an oncofetal protein that may play a critical role in malignant transformation and tumor progression. Recently, we have found that IMP3 oncofetal protein is a new biomarker for aggressive localized RCC.23 In addition, a validation study has confirmed that IMP3 is an independent prognostic marker for the clear cell (conventional) subtype of RCCs.24 In this validation study, 163 of 629 (25.9%) localized clear-cell RCC specimens expressed tumor cell IMP3. Patients with localized clear-cell RCC and positive IMP3 expression were nearly 5 times more likely to progress to distant metastasis compared with patients with IMP3-negative tumors (risk ratio [RR] 4.71; P <.001). As the predominance of RCCs are of the clear cell subtype, it is unclear whether IMP3 plays an important role to predict metastasis for other relatively rare subtypes, such as papillary and chromophobe RCCs. In the current study we investigated the association of IMP3 expression with clinicopathologic features and outcome using a multi-institutional cohort of patients with localized papillary and chromophobe RCC subtypes to evaluate whether IMP3 could serve as an independent biomarker to predict tumor progression and metastasis.
MATERIALS AND METHODS
Patient Selection
By using the combined resources of the Mayo Clinic, the University of Massachusetts Medical Center (UMMC), Massachusetts General Hospital (MGH), and City of Hope National Medical Center (CHNMC) we identified 334 patients treated with radical nephrectomy or nephron-sparing surgery for papillary or chromophobe RCC. The Mayo Clinic patients (n = 246) were treated between 1990 and 1999, while the UMMC (n = 39), MGH (n = 38), and CHNMC (n = 11) patients were treated between 1989 and 2003. There were 254 (76%) patients with papillary RCC and 80 (24%) with chromophobe RCC.
Clinicopathologic Features
The clinicopathologic features studied included age, sex, histologic subtype classified according to the UICC, AJCC, and Heidelberg guidelines,5,6 tumor size, primary tumor classification, regional lymph node involvement, distant metastases, the TNM stage groupings, and nuclear grade.
IMP3 Immunohistochemical Staining
Immunohistochemical studies were performed by the Department of Pathology at UMMC on 5-μm sections of formalin-fixed, paraffin-embedded tissue as previously described.20 Antigen retrieval was carried out with 0.01 mol/L citrate buffer at pH 6.0 in an 800W microwave oven for 15 minutes before immunostaining. The slides were stained on the Dako Autostainer (Dako, Carpentaria, Calif) using the EnVision (Dako) staining reagents. The sections were first blocked for endogenous protein binding and peroxidase activity with an application of Dual Endogenous Block (Dako) for 10 minutes, followed by a buffer wash. The sections were then incubated with a mouse monoclonal antibody specific for IMP3 (L523S, Corixa, Seattle, Wash) at a 2.0 μg/mL concentration for 30 minutes, followed again by a buffer wash. Sections were then incubated with the Envision plus; Dual Link reagent (a polymer conjugated with goat antimouse-Ig and horseradish peroxidase) for 30 minutes. The sections were then washed and treated with diaminobenzidine (DAB) and hydrogen peroxide, which reacted with the end product. A toning solution (DAB Enhancer, Dako) was used to enrich the final color. The sections were counter-stained with hematoxylin, dehydrated, and cover-slipped with permanent media. Sections of urothelial carcinoma with known positivity of IMP3 were used as positive controls for the L523S mouse monoclonal antibody (MoAb) specific for IMP3/KOC (Corixa) staining. Negative control sections were stained by replacing the primary antibody with nonimmune mouse IgG (Vector, Burlingame, Calif) at 2.0 μg/mL.
IMP3 expression was recorded as negative or positive after visual assessment by a genitourinary pathologist (Z.J.) without knowledge of patient outcome. One slide per case for immunohistochemistry was evaluated. Positive staining of IMP3 was defined as dark brown cytoplasmic staining pattern in the tumor epithelial cells, which can be easily observed at low-power magnification (×40). Scant fine granular background staining of epithelial cells, which cannot be seen at low-power magnification, or no staining at all was considered negative.
Statistical Methods
Associations of IMP3 expression with clinicopathologic features were evaluated using Wilcoxon rank sum, chi-square, and Fisher exact tests. Kaplan-Meier curves were used to observe the associations of IMP3 expression with outcome. The magnitudes of these associations were evaluated using Cox proportional hazards regression models and summarized with RRs and 95% confidence intervals (CI). Statistical analyses were performed using the SAS software package (SAS Institute, Cary, NC). All tests were 2-sided and P-values <.05 were considered statistically significant.
RESULTS
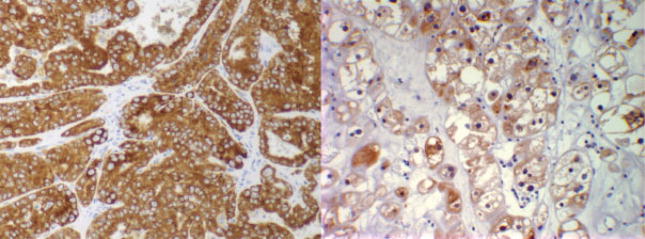
IMP3 protein was observed to be expressed within the cytoplasm of both papillary as well as chromophobe tumor cells (Fig. 1). There were 40 (12%) papillary or chromophobe RCC tumors with positive IMP3 expression and 294 (88%) with negative IMP3 expression. Comparisons of clinicopathologic features by IMP3 expression are summarized in Table 1. Positive IMP3 tumor expression was significantly associated with later tumor stage and higher tumor grade. For example, 70% of the IMP3-positive tumors were high-grade (ie, grade 3 or 4) compared with only 37% of the IMP3-negative tumors (P <.001).
FIGURE 1.
Immunohistochemical stains for IMP3 showing that papillary renal cell carcinoma (RCC) (left) and chromophobe RCC (right) were positive for IMP3.
TABLE 1.
Comparison of Clinicopathologic Features by IMP3 Tumor Expression for 334 Patients With Papillary and Chromophobe RCC
| Tumor IMP3 expression |
|||
|---|---|---|---|
| Feature | Negative n = 294 | Positive n = 40 | P |
| Age at surgery, y, median [range] | 65 [21–89] | 63 [44–80] | .883 |
| Tumor size, cm, median [range] | 4.1 [0.3–15.0] | 5.5 [0.7–25.0] | .090 |
| No. (%) | |||
| Sex | .929 | ||
| Women | 68 (23.1) | 9 (22.5) | |
| Men | 226 (76.9) | 31 (77.5) | |
| RCC histologic subtype | .081 | ||
| Papillary | 228 (77.6) | 26 (65.0) | |
| Chromophobe | 66 (22.5) | 14 (35.0) | |
| 2002 Primary tumor classification | .008 | ||
| pT1 | 213 (72.5) | 21 (52.5) | |
| pT2 | 49 (16.7) | 7 (17.5) | |
| pT3 | 31 (10.5) | 12 (30.0) | |
| pT4 | 1 (0.3) | 0 | |
| Regional lymph node involvement | .003 | ||
| pNX and pN0 | 289 (98.3) | 37 (92.5) | |
| pN1 and pN2 | 5 (1.7) | 5 (12.5) | |
| Distant metastases at presentation | .058 | ||
| pM0 | 289 (98.3) | 37 (92.5) | |
| pM1 | 5 (1.7) | 3 (7.5) | |
| 2002 TNM stage groupings | .002 | ||
| I | 211 (71.8) | 21 (52.5) | |
| II | 48 (16.3) | 6 (15.0) | |
| III | 28 (9.5) | 7 (17.5) | |
| IV | 7 (2.4) | 6 (15.0) | |
| Nuclear grade | <.001 | ||
| 1 | 9 (3.1) | 0 | |
| 2 | 176 (59.9) | 12 (30.0) | |
| 3 | 103 (35.0) | 19 (47.5) | |
| 4 | 6 (2.0) | 9 (22.5) | |
RCC indicates renal cell carcinoma.
There were 17 patients with papillary or chromophobe RCC who had extrarenal disease at nephrectomy, including 9 with regional lymph node involvement, 7 with distant metastases, and 1 with both. (Note that 7 of the 17 patients with extrarenal disease had IMP3-positive tumors.) As such, associations of IMP3 expression with patient outcome were evaluated using the 317 patients (240 papillary and 77 chromophobe) with localized disease at nephrectomy (ie, pNX/pN0; pM0; primary tumor classifications of pT1, pT2, or pT3). In this subset there were 284 (89.6%) papillary or chromophobe RCC tumors with negative IMP3 expression and 33 (10.4%) with positive IMP3 expression.
Twenty-eight of the 317 patients with localized disease progressed to distant metastases at a median of 3.1 years after nephrectomy (range, 0–10). Distant metastasis-free survival rates (SE, number still at risk) at 5 and 10 years after nephrectomy were 94.1% (1.4%, 240) and 88.3% (2.3%, 98), respectively. There was a significant difference in metastasis-free survival between patients with papillary and chromophobe RCC (P =.007; log-rank test). Metastasis-free survival rates (SE, number still at risk) at 5 and 10 years after nephrectomy were 96.4% (1.2%, 192) and 91.6% (2.2%, 79), respectively, for patients with papillary RCC compared with 86.6% (4.2%, 48) and 77.3% (6.3%, 19), respectively, for patients with chromophobe RCC.
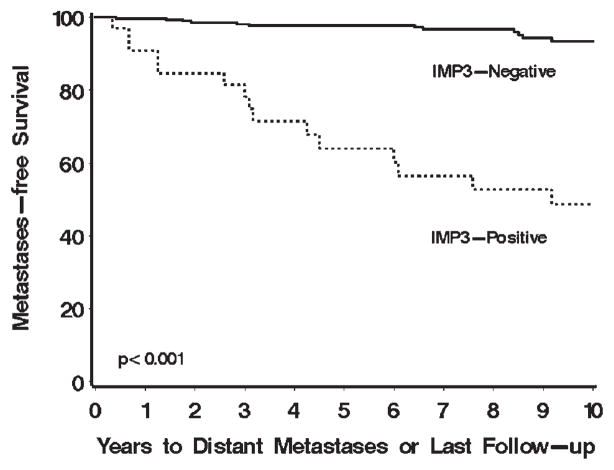
Patients with localized IMP3-positive tumors were over 10 times more likely to progress to distant metastasis compared with patients with localized IMP3-negative tumors (RR, 11.38; 95% CI, 5.40–23.96; P <.001; Table 2). In fact, 15 (45.5%) of the 33 patients with IMP3-positive tumors progressed compared with only 13 (4.6%) of the 284 patients with IMP3-negative tumors. Metastasis-free survival rates (SE, number still at risk) at 5 and 10 years after nephrectomy were 63.9% (8.8%, 17) and 48.6% (9.5%, 12), respectively, for patients with IMP3-positive tumors compared with 97.7% (0.9%, 223) and 93.4% (1.9%, 86), respectively, for patients with IMP3-negative tumors (Fig. 2). In multivariable analysis adjusting for primary tumor classification and nuclear grade, patients with IMP3-positive tumors were still over 10 times more likely to progress compared with patients with IMP3-negative tumors (RR, 13.45; 95% CI, 6.00–30.14; P <.001; Table 2). When stratified by histologic subtype, IMP3 expression was univariately and significantly associated with progression to distant metastasis among patients with papillary RCC (RR, 9.14; 95% CI, 3.39–24.64; P <.001) as well as among patients with chromophobe RCC (RR, 11.91; 95% CI, 3.58–39.61; P <.001), although there were too few patients who progressed in these subsets to evaluate these associations in a multivariable setting.
TABLE 2.
Associations of IMP3 Tumor Expression With Outcome for 317 Patients With Localized Papillary and Chromophobe RCC
| Metastases-Free survival |
Overall survival |
|||
|---|---|---|---|---|
| Univariate | Risk ratio (95% CI) | P | Risk ratio (95% CI) | P |
| IMP3 expression | ||||
| Negative | 1.0 (reference) | 1.0 (reference) | ||
| Positive | 11.38 (5.40–23.96) | <.001 | 1.91 (1.13–3.22) | .016 |
| Multivariable | ||||
| 2002 Primary tumor classification | ||||
| pT1 | 1.0 (reference) | 1.0 (reference) | ||
| pT2 | 4.38 (1.69–11.36) | .002 | 0.97 (0.55–1.68) | .900 |
| pT3 | 10.94 (4.18–28.68) | <.001 | 2.28 (1.29–4.04) | .005 |
| Nuclear grade | ||||
| 1 and 2 | 1.0 (reference) | 1.0 (reference) | ||
| 3 and 4 | 5.30 (1.94–14.49) | .001 | 1.38 (0.93–2.06) | .112 |
| IMP3 expression | ||||
| Negative | 1.0 (reference) | 1.0 (reference) | ||
| Positive | 13.45 (6.00–30.14) | <.001 | 1.95 (1.15–3.31) | .013 |
RCC indicates renal cell carcinoma; CI, confidence interval.
FIGURE 2.
Association of IMP3 tumor expression with progression to distant metastases for 317 patients with localized papillary and chromophobe renal cell carcinoma (RCC). Metastasis-free survival rates (SE, number still at risk) at 5 and 10 years after nephrectomy were 63.9% (8.8%, 17) and 48.6% (9.5%, 12), respectively, for patients with IMP3-positive tumors compared with 97.7% (0.9%, 223) and 93.5% (1.9%, 86), respectively, for patients with IMP3-negative tumors.
At last follow-up 103 patients had died at a median of 4.5 years after nephrectomy (range, 0–16). Among the 214 patients who were still alive at last follow-up the median duration of follow-up was 8.8 years (range, 0–16). Overall survival rates (SE, number still at risk) at 5 and 10 years after nephrectomy were 82.2% (2.2%, 240) and 65.4% (3.1%, 98), respectively. There was not a statistically significant difference in overall survival between patients with localized papillary and chromophobe RCC (P =.997; log-rank test).
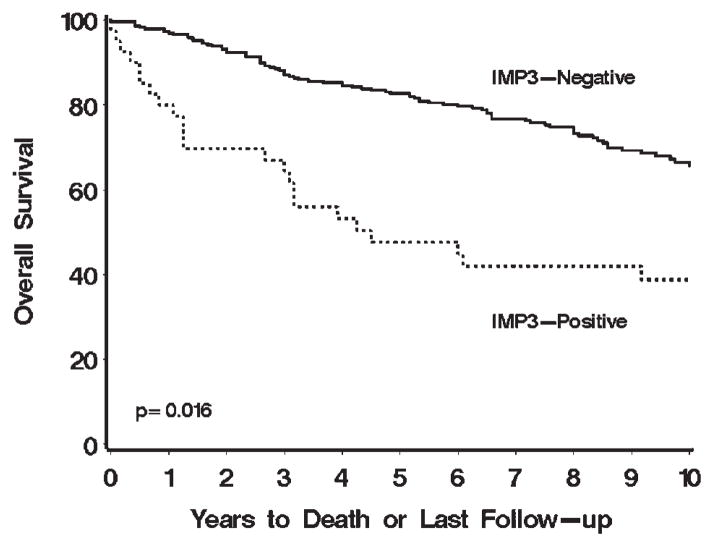
In univariate analyses, patients with localized IMP3-positive tumors were nearly twice as likely to die compared with patients with localized IMP3-negative tumors (RR, 1.91; 95% CI, 1.13–3.22; P =.016; Table 2). Seventeen (51.5%) of the 33 patients with IMP3-positive tumors died compared with 86 (30.3%) of the 284 patients with IMP3-negative tumors. Overall survival rates (SE, number still at risk) at 5 and 10 years after nephrectomy were 57.9% (9.0%, 17) and 47.1% (9.2%, 12), respectively, for patients with IMP3-positive tumors compared with 85.0% (2.2%, 223) and 67.4% (3.3%, 86), respectively, for patients with IMP3-negative tumors (Fig. 3). In multivariable analysis adjusting for primary tumor classification and nuclear grade, patients with IMP3-positive tumors were still nearly twice as likely to die compared with patients with IMP3-negative tumors (RR, 1.95; 95% CI, 1.15–3.31; P =.013; Table 2). In fact, IMP3 status was still significantly associated with death after adjusting for primary tumor classification, nuclear grade, tumor size, and histologic subtype (RR, 1.82; 95% CI, 1.07–3.12; P =.029).
FIGURE 3.
Association of IMP3 tumor expression with death for 317 patients with localized papillary and chromophobe renal cell carcinoma (RCC). Overall survival rates (SE, number still at risk) at 5 and 10 years after nephrectomy were 57.9% (9.0%, 17) and 47.1% (9.2%, 12), respectively, for patients with IMP3-positive tumors compared with 85.0% (2.2%, 223) and 67.4% (3.3%, 86), respectively, for patients with IMP3-negative tumors.
DISCUSSION
Papillary and chromophobe subtypes of RCC encompass relatively understudied forms of malignancy in comparison to clear-cell RCC. In this study we demonstrate that expression of IMP3 in papillary and chromophobe RCCs can predict tumor metastasis and provide important prognostic information in patients with localized disease who undergo nephrectomy.
Currently, surgical resection of a tumor (nephrectomy) is the standard of care for almost all patients with RCC.1,2,25 After nephrectomy, patients with metastatic disease typically receive systemic treatment (eg, immunotherapy), which can be associated with potentially significant toxicity.1,2,25 As such, to avoid toxicities associated with systemic treatment, watchful waiting is the standard of care for patients lacking overt metastases after radical nephrectomy.1,2,25 Recently, 3 new drugs have been used for treatment of patients with metastatic RCC. Sorafenib and sunitinib, multikinase receptor inhibitors, can block the signaling cascade of the vascular endothelial growth factor (VEGF) 2, 3, and R1, as well as platelet-derived growth factor (PDGF) that are critical to angiogen-esis.26–29 Temsirolimus inhibits the mammalian target of rapamycin complex 1 kinase that regulates protein translation and appears to have very promising activity in nonclear-cell RCCs.30 However, these new drugs have only been evaluated in patients with clinically metastatic RCC. For localized RCC, patients at high risk for metastasis should be selected for immediate adjuvant therapy trials, yet the metastatic potential of localized tumors is often unpredictable.
The literature clearly supports that histologic subtype influences RCC outcomes, and that conventional clear-cell RCC and nonclear-cell RCC (chromophobe and papillary) exhibit different propensities for metastatic dissemination.7–14 Specifically, patients with localized papillary and chromophobe RCCs tend to experience less aggressive disease spread and, thus, have a markedly better prognosis compared with patients with clear-cell RCC.7–14 However, as we report in this study, patients with papillary or chromophobe RCCs also develop metastasis, albeit rarely. Furthermore, patients with IMP3-positive tumors show significantly higher risk of developing metastasis compared with patients with IMP3-negative tumors. In the current study, 45% of patients with IMP3-positive tumors developed metastasis compared with only 4.6% of patients with IMP3-negative tumors. In addition, IMP3 status independently predicted metastatic spread in patients with localized papillary and chromophobe RCC; in multivariable analysis, patients with IMP3-positive papillary or chromophobe RCCs were 13 times more likely to develop subsequent metastases than those with IMP3-negative tumors, even after adjustment for other well-known clinical variables such as tumor stage and grade. Thus, our results strongly suggest that patients with IMP3-positive papillary or chromophobe RCC should not be regarded as having indolent disease and should be followed up aggressively relative to patients with IMP3-negative tumors.
In vitro studies have demonstrated that IMP3, an oncofetal protein, plays a crucial role for tumor proliferation and invasion21,22 and, therefore, may play a direct role in facilitating RCC metastasis. Interestingly, Yaniv et al31 reported that IMP3 in Xenopus laevis is required for the migration of cells forming the roof plate of the neural tube and, subsequently, for neural crest migration, which suggested that IMP3 is important for promoting cell migration. These findings could explain why IMP3 expression is associated with tumor progression and metastasis. However, further study is required to investigate whether IMP3 plays a direct role in the biological behavior of RCC.
Our previous and current studies demonstrate that IMP3 encompasses an important biomarker for the 3 most common subtypes of RCC. However, there are some limitations to our current study that warrant discussion. First, to our knowledge, we are the first group to describe IMP3 as a prognostic marker for papillary and chromophobe RCC; as such, our results need to be externally validated by other groups using independent cohorts of patients. Second, papillary RCC can be divided into 2 further subtypes (types 1 and 2) based on a combination of histologic as well as cytogenetic features. Specifically, type 1 papillary RCC tumors tend to be of lower grade and less aggressive, whereas type 2 papillary RCC tumors are often associated with chromosomal aberrations (ie, 9p loss) as well as higher tumor grade, positive N stage and M stage, and poorer patient survival.32–34 Because cytogenetic analysis to distinguish type 1 from type 2 papillary RCC is not routinely conducted at our institutions, in the current study we were unable to address whether IMP3 expression differs based on papillary RCC subtype. Thus, future studies will be required to test whether type 2 papillary RCCs express higher levels of IMP3 than type 1 papillary RCCs, a finding that would be consistent with the general observation that more aggressive forms of RCC tend to express increased levels of IMP3. Third, the relationship between IMP3 and other prognostic markers (both among clear-cell RCC and among papillary and chromophobe RCC) remains unclear and is currently the focus of future research by our group.
In summary, IMP3 is an independent prognostic biomarker for patients with localized papillary or chromophobe RCC. Although relatively rare, patients with localized papillary and chromophobe RCC can develop metastasis after nephrectomy, and IMP3 expression can be used to help identify subgroups of patients with high potential for developing metastasis after surgery. IMP3 expression may provide important prognostic information for patients with papillary and chromophobe RCC, and will assist physicians in selecting high-risk patients who might benefit from early and tailored systematic therapy after nephrectomy.
Acknowledgments
The authors thank Karen Dresser (University of Massachusetts Medical Center) for technical support.
References
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma [review] N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma [see comment] [review] N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States [see comment] JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:987–989. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours [review] J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases [see comment] Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS. Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases [see comment] Am J Surg Pathol. 1997;21:621–635. doi: 10.1097/00000478-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Lau WK, Cheville JC, Blute ML, Weaver AL, Zincke H. Prognostic features of pathologic stage T1 renal cell carcinoma after radical nephrectomy. Urology. 2002;59:532–537. doi: 10.1016/s0090-4295(01)01589-8. [DOI] [PubMed] [Google Scholar]
- 11.Ljungberg B, Alamdari FI, Stenling R, Roos G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol. 1999;36:565–569. doi: 10.1159/000020049. [DOI] [PubMed] [Google Scholar]
- 12.Lohse CM, Cheville JC. A review of prognostic pathologic features and algorithms for patients treated surgically for renal cell carcinoma [review] Clin Lab Med. 2005;25:433–464. doi: 10.1016/j.cll.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. 2000;89:604–614. [PubMed] [Google Scholar]
- 14.Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71–77. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–1270. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller-Pillasch F, Pohl B, Wilda M, et al. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev. 1999;88:95–99. doi: 10.1016/s0925-4773(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 17.Monk D, Bentley L, Beechey C, et al. Characterisation of the growth regulating gene IMP3, a candidate for Silver-Russell syndrome. J Med Genet. 2002;39:575–581. doi: 10.1136/jmg.39.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller-Pillasch F, Lacher U, Wallrapp C, et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14:2729–2733. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Fan L, Watanabe Y, et al. L523S, an RNA-binding protein as a potential therapeutic target for lung cancer. Br J Cancer. 2003;88:887–894. doi: 10.1038/sj.bjc.6600806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yantiss RK, Woda BA, Fanger GR, et al. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol. 2005;29:188–195. doi: 10.1097/01.pas.0000149688.98333.54. [DOI] [PubMed] [Google Scholar]
- 21.Liao B, Hu Y, Herrick DJ, Brewer G. The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem. 2005;280:18517–18524. doi: 10.1074/jbc.M500270200. [DOI] [PubMed] [Google Scholar]
- 22.Vikesaa J, Hansen TV, Jonson L, et al. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J. 2006;25:1456–1468. doi: 10.1038/sj.emboj.7601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Z, Chu PG, Woda BA, et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006;7:556–564. doi: 10.1016/S1470-2045(06)70732-X. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann NE, Sheinin Y, Lohse CM, et al. External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer. 2008;112:1471–1479. doi: 10.1002/cncr.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma [review] J Urol. 2000;163:408–417. [PubMed] [Google Scholar]
- 26.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma [see comment] N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma [review] J Clin Oncol. 2006;24:5601–5608. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 28.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma [see comment] N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 29.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma [see comment] JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 30.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 31.Yaniv K, Fainsod A, Kalcheim C, Yisraeli JK. The RNA-binding protein Vg1 RBP is required for cell migration during early neural development. Development. 2003;130:5649–5661. doi: 10.1242/dev.00810. [DOI] [PubMed] [Google Scholar]
- 32.Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10:537–544. [PubMed] [Google Scholar]
- 33.Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol. 2001;32:590–595. doi: 10.1053/hupa.2001.24984. [DOI] [PubMed] [Google Scholar]
- 34.Gunawan B, von Heydebreck A, Fritsch T, et al. Cytogenetic and morphologic typing of 58 papillary renal cell carcinomas: evidence for a cytogenetic evolution of type 2 from type 1 tumors. Cancer Res. 2003;63:6200–6205. [PubMed] [Google Scholar]