Abstract
Purpose
Interferon Regulatory Factor 6 (IRF6) encodes a member of the IRF family of transcription factors. Mutations in IRF6 cause Van der Woude (VWS) and popliteal pterygium syndromes (PPS), two related orofacial clefting disorders. Here, we compared and contrasted the frequency and distribution of exonic mutations in IRF6 between two large geographically distinct collections of families with VWS and between one collection of families with PPS.
Methods
We performed direct sequence analysis of IRF6 exons on samples from three collections, two with VWS and one with PPS.
Results
We identified mutations in IRF6 exons in 68% of families in both VWS collections and in 97% of families with PPS. In sum, 106 novel disease-causing variants were found. The distribution of mutations in the IRF6 exons in each collection was not random; exons 3, 4, 7, and 9 accounted for 80%. In the VWS collections, the mutations were evenly divided between protein truncation and missense, whereas most mutations identified in the PPS collection were missense. Further, the missense mutations associated with PPS were localized significantly to exon 4, at residues that are predicted to bind directly to DNA.
Conclusion
The non-random distribution of mutations in the IRF6 exons suggests a two-tier approach for efficient mutation screens for IRF6. The type and distribution of mutations are consistent with the hypothesis that VWS is caused by haploinsufficiency of IRF6. On the other hand, the distribution of PPS-associated mutations suggests a different, though not mutually exclusive, effect on IRF6 function.
Keywords: Cleft lip and palate, mutation, haploinsufficiency, dominant negative, cryptic splice site, CpG
Introduction
The prevalence of orofacial clefting varies from 1 in 500 to 1 in 2500 births, depending on geographic origin, race and socioeconomic background 1-4. About 70% of orofacial clefts occur as isolated cases and the remainder can be attributed to chromosomal abnormalities, maternal exposure to teratogens and syndromes where the phenotype includes other developmental or morphological abnormalities 5.
Van der Woude syndrome (VWS, OMIM 119300) is one of the most common oral cleft syndromes and accounts for ∼2% of all cleft lip and palate cases. VWS is clinically characterized by congenital lower lip pits, cleft lip (CL), cleft lip with or without cleft palate (CLP), cleft palate only (CPO) and hypodontia. Other, less common, features include syndactyly of the fingers, syngnathia and ankyloblepheron 6. VWS is inherited as an autosomal dominant trait with high penetrance (96.7%), but variable expression 7. The phenotype of the lower lip varies from a single barely evident depression to bilateral fistulae of the lower lip, and the orofacial cleft varies from a bifid uvula to a complete cleft lip and palate 6. These facial anomalies are also seen in individuals with popliteal pterygium syndrome (PPS, OMIM 119500), a disorder that includes other physical signs, including bilateral popliteal webs, syndactyly, genital anomalies, ankyloblepharon, oral synechiae and nail abnormalities.
The genetic localization for VWS was assigned by linkage analysis 8 and through chromosome abnormalities involving chromosome 1q32-q41 9-11. Overall, there is little evidence for genetic heterogeneity, although evidence for a second potential VWS locus was reported for chromosome 1p36-p32 12. Sertie et al. 13 suggested that a gene at chromosome 17p11.2-p11.1, together with the VWS gene, enhances the probability of CP in an individual carrying two risk alleles.
Previously, we described a nonsense mutation in the Interferon Regulatory Factor 6 (IRF6) gene in the affected sib of two monozygotic twins discordant for VWS, suggesting IRF6 as a candidate for VWS 14. This hypothesis was confirmed in the same study by the detection of IRF6 mutations in 45 additional unrelated families with VWS. In addition, a unique set of mutations in IRF6 was discovered in 13 families with PPS, demonstrating that VWS and PPS are allelic, as previously suggested 15. Subsequently, mutations in IRF6 were identified in 56 additional families with VWS and three with PPS 16-36.
The objectives of this paper are to determine the prevalence and distribution of mutations in the exons of IRF6 in families with VWS and PPS. We describe the complete sequence analysis of IRF6 exons in two large VWS collections and one PPS collection. Despite geographical diversity between the two VWS collections, the likelihood of finding an exonic mutation in IRF6 was similar as was their distribution. The type and distribution in location of PPS mutations differ significantly from the VWS mutations, but are not mutually exclusive. The results provide the foundation to identify genotype-phenotype correlations in disorders caused by mutations in IRF6 and to determine structure-function relationships in the IRF family of transcription factors.
Materials and Methods
Populations
Each proband was examined by a clinical geneticist or genetic counselor. Two collections of unrelated families affected with VWS were obtained, one from Brazil (N=110) and one of mixed geographic origin (N=197). The collection from Brazil has not been described previously. The geographic origin of the mixed collection is primarily northern Europe, and includes families from the United States (152), Belgium (31), Germany (7), United Kingdom (3), Thailand (2), Phillipines (1) and Brazil (1). Many of these families (175) were described previously 14, 16, 21, 23 and were included in this study to provide a comprehensive analysis of the complete collections of families with VWS and PPS. Diagnostic criteria for individuals to be considered affected with VWS included CLP or CPO, and at least one affected individual in the family with an anomaly in the lower lip, generally bilateral pits.
In addition, a single collection of unrelated families affected with PPS (N=37) was obtained. The geographic origin of the PPS families was mainly northern Europe, but included one family from Brazil. Diagnostic criteria for individuals affected with PPS included the VWS criteria listed above along with the presence of bilateral popliteal webs or a combination of syndactyly, genital anomalies, ankyloblepharon, oral synechiae and nail abnormalities from one or more members in a family. Sample collection and processing was performed as described previously 37. We obtained written informed consent from all subjects and approval for all protocols from the Institutional Review Boards at the University of Iowa, the University of Manchester, the University of São Paulo State and CONEP/Brazil, the Université catholique de Louvain, and Zentrum fur Gynäkologische Endokrinologie, Reproduktionsmedizin und Humangenetik, Regensburg, Germany.
PCR
Exons 1-8 and part of exon 9 of IRF6 were amplified by standard PCR using the primers shown in Table 1. PCR experiments for exons 1-8 were performed in a 10μl total volume mixture containing 20 ng of genomic DNA, 0.5μM each primer, 200μM dNTPs, 0.25% DMSO, 0.2 unit Bio-X-Act Taq polymerase (Bioline, Reno, NV), and 1X PCR buffer supplied by the manufacturer. PCR conditions are as follows: initial denaturation 3 min at 94°C, followed by 35 cycles of denaturation at 94°C for 15 sec, annealing at 57°C for 30 sec, elongation at 68°C for 1min, and final elongation at 68°C for 3 min. Conditions for PCR experiments for exon 9 were performed as above except 0.3μM each primer, Biolase Taq polymerase (Bioline) and initial denaturation 5 min at 94°C, followed by 35 cycles of denaturation at 94°C for 45 sec, annealing at 57°C for 45 sec, elongation at 72°C for 45 sec, and final elongation at 72°C for 3 min.
Table 1. PCR primers used to amplify IRF6 exons.
| Exon | Domaina | Directionb | Primer sequence (5′-3′) | Product size (bp) |
|---|---|---|---|---|
| 1 | 5′UTR | R | atctggaaaagggcgacagg | 537 |
| 1 | F | agaagcggaggagtagggtg | ||
| 2 | 5′UTR | R | aaagttatggaaacagcaac | 382 |
| 2 | F | ttattctagggcttctgagc | ||
| 3 | DBD | R | catgcccccaaaagaggaat | 560 |
| 3 | F | ggctagagcatgaagtgtaa | ||
| 4 | DBD | R | aggctttcttgctttatcca | 512 |
| 4 | F | gctctgggcaatgataggac | ||
| 5 | Proline-rich | R | tgctttcagggcagtggtgg | 425 |
| 5 | F | cagtgaatctagggaggtcc | ||
| 6 | Proline-rich | R | tttacttcttccctggtgac | 432 |
| 6 | F | cagtgtttggttcttgtcta | ||
| 7 | SMIR | R | cttgacctcctccagactaa | 650 |
| 7 | F | agtggccttcctgaatgatg | ||
| 8 | SMIR | R | gtttcagcaagactctaagg | 436 |
| 8 | F | aaagatggtatttgttgagt | ||
| 9 | S/T-rich | R | gtcttcctcagggcctcttt | 446 |
| 9 | F | ggcatatttggatcacaaac |
DNA sequence analysis
The amplified products were sequenced directly using Big Dye sequencing kit (Perkin-Elmer, Foster City, CA) as recommended. Sequence samples were purified with magnetic beads and run on an automated sequencer model ABI Prism 3700 (Perkin-Elmer). DNA sequences were aligned and analyzed using the software PHRED/PHRAP/CONSED 38. Reference sequences for IRF6 cDNA, genomic DNA and protein were NM_006147.2, RP3-434o14 (Genbank AL022398) and NP_006138, respectively. DNA sequence variants were confirmed by sequencing the opposite strand in the proband and, if possible, in at least one other affected family member. To identify non-etiologic polymorphisms, DNA sequence analysis was performed for all IRF6 exons on a minimum of 200 unaffected control samples derived from geographically diverse populations 39.
Splice site prediction
The effect of mutations on splicing activity was modeled using Genscan 40. Wild type and mutant sequences were compared using default settings.
Statistical analysis
Frequency tables showed population specific frequency distribution of mutations across the nine exons. The 2 by 9 tables were analyzed using the Chi-square statistic or Fisher's exact test when appropriate (e.g. when the expected cell count was less than 5 for at least 20% of the cells).
Results and Discussion
Prevalence of Exonic Mutations in IRF6
DNA samples were derived from two distinct VWS collections, one from Brazil (N = 110) and one of mixed origin that was primarily from northern Europe (N = 197). In addition, we screened a PPS collection of mixed geographical origin (N = 37). The mutation screen used in the current study was modified slightly from the screen described previously by Kondo et al14. PCR primers for exon 9 were redesigned (Table 1), and the new primers amplified this region more robustly and generated DNA sequence more reliably. In the VWS collections, we identified IRF6 exonic mutations in 77 of 110 (71%) families from Brazil and identified 132 of 197 (67%) families from the mixed collection (Table 2). The likelihoods for finding exonic mutations in IRF6 between these two diverse VWS collections are not statistically different (p=0.61) and are consistent with common mutation mechanisms.
Table 2.
Likelihood for identifying IRF6 exonic mutation in VWS and PPS populations.
| VWS | PPS | ||||||
|---|---|---|---|---|---|---|---|
| Geographic origin | Families | Families with mutation | fraction with mutation | Families | Families with mutation | fraction with mutation | Reference |
| Mixed* | 197 | 130 | 67 % | 36 | 35 | 97 % | This study, 14, 21, 23,16 |
| Brazil | 110 | 77 | 70 % | 1 | 1 | 100 % | This study. |
| 307 | 207 | 68 % | 37 | 36 | 97 % | ||
In the “Mixed” collection, the previous studies account for 82 VWS and 14 PPS families with mutations.
Exonic mutations in IRF6 in six families are not included in this table because the clinical diagnosis was not specified.
Six previously identified deletions of IRF6 are not included in this table.
Mutations located in the exons of IRF6 have been identified for only 68% of families with VWS analyzed to date. Several possibilities exist to explain the remaining 32%. IRF6 may have gross deletions that are not detected by our DNA sequencing strategy. Etiologic mutations may exist within IRF6, but located outside the exons. Finally, some proportion of the remaining families may be due to mutations located in some other gene. To date, deletions have been found in only six families with VWS 10, 11, 24 29. In general, these have been large deletions and further studies with more sensitive methods are needed to screen for kilobase-sized deletions. Despite the lack of linkage evidence for locus heterogeneity in VWS, it is also possible that VWS-causing mutations may be found in other genes. For example, a polygenic mechanism might contribute to some cases of VWS, but would be difficult to detect in the previous linkage studies. The number and size of families that lack an exonic mutation in IRF6 should be sufficient to test for genetic heterogeneity in the VWS collection.
In the PPS collection, we identified exonic mutations in IRF6 in 36 of 37 unrelated families, demonstrating that IRF6 is the principal gene involved in this disorder. When combined with the VWS mutation studies, IRF6 exonic mutations were identified in 249 unrelated families, representing 170 total and 106 novel alleles (Table 3: Supplemental data online only). None of these mutations were observed in our control samples (see Methods), suggesting that they are etiologic. However, we identified 41 DNA sequence variants from our mutation screen, including four non-synonymous polymorphisms, Asp19Asn, Ala61Pro, Thr224Ser, Val274Ile (Table 4). As these variants were detected in control cases, they are not etiologic for VWS nor PPS. However, Val274Ile is highly associated with isolated cleft lip and palate 41-46, and functional studies must be performed to test Val274Ile and other alleles as potential susceptibility alleles.
Table 3.
IRF6 exonic mutations in unrelated families with VWS and PPS.
| Diagnosisa | Mutation | ntb | nt changec | aa changed | exone | Originf | Citation |
|---|---|---|---|---|---|---|---|
| VWS | truncation | -219 | C>T | 0? | 1 | Brazil | This study |
| VWS | truncation | -151 | G>A | 0? | 1 | Brazil | This study |
| VWS | truncation | -151 | G>A | 0? | 1 | N Eur | This study |
| VWS | truncation | -151 | G>A | 0? | 1 | N Eur | This study |
| NS | truncation | -48 | A>T | 0? | 2 | NS | This study |
| VWS | truncation | -48 | A>T | 0? | 2 | N Eur | 14 |
| VWS | truncation | -19 | C>A | 0? | 2 | NS | This study |
| VWS | truncation | 1 | A>G | 0? | 3 | Brazil | This study |
| VWS | truncation | 3 | G>A | 0? | 3 | N Eur | 14 |
| VWS | missense | 5 | C>T | Ala2Val | 3 | N Eur | 14 |
| VWS | missense | 16 | C>T | Arg6Cys | 3 | Brazil | 14 |
| VWS | missense | 16 | C>T | Arg6Cys | 3 | Brazil | This study |
| VWS | truncation | 16 | del C | Arg6AlafsX3 | 3 | NS | This study |
| VWS | truncation | 16_17 | ins C | Arg6ProfsX13 | 3 | N Eur | This study |
| VWS | missense | 17 | G>T | Arg6Leu | 3 | N Eur | This study |
| VWS | missense | 17 | G>C | Arg6Pro | 3 | N Eur | This study |
| NS | missense | 26 | G>A | Arg9Gln | 3 | NS | This study |
| VWS | missense | 26 | G>A | Arg9Gln | 3 | Brazil | This study |
| VWS | missense | 35 | C>T | Pro12Leu | 3 | N Eur | This study |
| VWS | missense | 39 | G>T | Trp13Cys | 3 | Brazil | This study |
| VWS | truncation | 43_44 | ins ATAG | Val15AspfsX5 | 3 | NS | This study |
| VWS | missense | 47 | C>T | Ala16Val | 3 | Belgium | 21 |
| VWS | truncation | 49_64 | del | Gln17SerfsX23 | 3 | N Eur | 14 |
| VWS | missense | 50 | A>G | Gln17Arg | 3 | Brazil | This study |
| VWS | missense | 52 | G>A | Val18Met | 3 | N Eur | 14 |
| VWS | missense | 53 | T>C | Val18Ala | 3 | N Eur | 14 |
| VWS | missense | 56 | A>G | Asp19Gly | 3 | Germany | This study |
| VWS | missense | 58 | G>A | Ser20Asn | 3 | NS | This study |
| PPS | missense | 65 | T>C | Leu22Pro | 3 | Belgium | 21 |
| VWS | truncation | 69 | C>A | Tyr23X | 3 | N Eur | 14 |
| VWS | missense | 71 | C>T | Pro24Leu | 3 | N Eur | This study |
| VWS | missense | 74 | G>T | Gly25Val | 3 | Belgium | This study |
| VWS | missense | 83 | G>T | Trp28Leu | 3 | Brazil | This study |
| VWS | truncation | 99 | dup T | Lys34X | 3 | N Eur | This study |
| VWS | missense | 101 | A>C | Lys34Thr | 3 | NS | This study |
| VWS | missense | 104 | G>C | Arg35Pro | 3 | Brazil | This study |
| VWS | missense | 107 | T>C | Phe36Ser | 3 | N Eur | This study |
| VWS | missense | 115 | C>G | Pro39Ala | 3 | N Eur | 14 |
| VWS | truncation | 136 | del C | His46IlefsX15 | 3 | N Eur | This study |
| VWS | truncation | 154 | G>T | Glu52X | 3 | N Eur | This study |
| VWS | Truncation | 161 | dup A | Asn54LysfsX3 | 3 | Germany | This study |
| VWS | missense | 167 | T>C | Ile56Thr | 3 | Brazil | This study |
| PPS | splicing | 174 | G>A (80%>8%) | p.? | 3 | NS | This study |
| VWS | missense/splicing | 175 | G>T (58%>8%) | p.? | 4 | Brazil | This study |
| PPS | missense | 178 | T>G | Trp60Gly | 4 | N Eur | 14 |
| VWS | missense | 180 | G>T | Trp60Cys | 4 | Brazil | This study |
| VWS | missense | 181 | G>A | Ala61Thr | 4 | NS | This study |
| VWS | missense | 182 | C>G | Ala61Gly | 4 | N Eur | 14 |
| VWS | missense | 191 | T>C | Thr64Ile | 4 | Belgium | 21 |
| PPS | missense | 197 | A>C | Lys66Thr | 4 | N Eur | 14 |
| VWS | missense | 197_199 | delinsGGG | Lys66_Tyr67delinsArgAsp | 4 | N Eur | This study |
| PPS | missense | 200 | A>G | Tyr67Cys | 4 | Belgium | This study |
| VWS | Truncation | 201 | C>A | Tyr67X | 4 | Germany | 16 |
| VWS | truncation | 202 | C>T | Gln68X | 4 | N Eur | 14 |
| VWS | truncation | 202 | C>T | Gln68X | 4 | N Eur | 14 |
| VWS | missense | 208 | G>C | Gly70Arg | 4 | N Eur | 14 |
| VWS | missense | 208 | G>C | Gly70Arg | 4 | N Eur | 14 |
| VWS | missense | 221 | C>T | Pro74Leu | 4 | Brazil | This study |
| VWS | missense | 221 | C>T | Pro74Leu | 4 | Brazil | This study |
| VWS | missense | 221 | C>G | Pro74Arg | 4 | N Eur | This study |
| VWS | duplication | 223_240 | dup | Asp75_Lys80dup | 4 | N Eur | This study |
| VWS | missense | 226 | C>T | Pro76Ser | 4 | N Eur | 14 |
| VWS | missense | 230 | C>A | Ala77Asp | 4 | Brazil | This study |
| PPS | missense | 244 | C>A | Gln82Lys | 4 | N Eur | 14 |
| VWS | missense | 246 | G>C | Gln82His | 4 | Brazil | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | Belgium | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | Belgium | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | Brazil | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | NS | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | NS | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | Germany | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | 14 |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | 14 |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | 14 |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | This study |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | 14 |
| PPS | missense | 250 | C>T | Arg84Cys | 4 | N Eur | 14 |
| VWS | missense | 250 | C>T | Arg84Cys | 4 | Brazil | This study |
| VWS | missense | 250 | C>T | Arg84Cys | 4 | Brazil | This study |
| VWS | missense | 250 | C>T | Arg84Cys | 4 | NS | This study |
| PPS | missense | 251 | G>A | Arg84His | 4 | NS | This study |
| PPS | missense | 251 | G>A | Arg84His | 4 | NS | This study |
| PPS | missense | 251 | G>A | Arg84His | 4 | N Eur | 14 |
| PPS | missense | 251 | G>A | Arg84His | 4 | N Eur | This study |
| PPS | missense | 251 | G>A | Arg84His | 4 | N Eur | 14 |
| PPS | missense | 251 | G>A | Arg84His | 4 | Belgium | This study |
| VWS | missense | 251 | G>A | Arg84His | 4 | Belgium | This study |
| VWS | missense | 251 | G>A | Arg84His | 4 | N Eur | This study |
| VWS | missense | 251 | G>C | Arg84Pro | 4 | Belgium | This study |
| VWS | missense | 251 | G>C | Arg84Pro | 4 | UK | This study |
| VWS | missense | 259 | C>T | Leu87Phe | 4 | Brazil | This study |
| VWS | missense | 259 | C>T | Leu87Phe | 4 | Brazil | This study |
| NS | missense | 262 | A>T | Asn88Tyr | 4 | NS | This study |
| VWS | missense | 262 | A>C | Asn88His | 4 | Brazil | This study |
| VWS | missense | 262 | A>C | Asn88His | 4 | N Eur | 14 |
| VWS | missense | 263 | A>G | Asn88Ser | 4 | N Eur | This study |
| PPS | missense | 265 | A>G | Lys89Glu | 4 | N Eur | 14 |
| VWS | missense | 265 | A>G | Lys89Glu | 4 | Brazil | This study |
| VWS | missense | 268 | A>G | Ser90Gly | 4 | N Eur | 14 |
| VWS | missense | 269 | G>T | Ser90Ile | 4 | Belgium | This study |
| VWS | missense | 274 | G>A | Glu92Lys | 4 | Brazil | This study |
| VWS | missense | 274 | G>A | Glu92Lys | 4 | Brazil | This study |
| VWS | missense | 274 | G>A | Glu92Lys | 4 | Brazil | This study |
| VWS | truncation | 274 | G>T | Glu92X | 4 | Brazil | This study |
| VWS | truncation | 274 | G>T | Glu92X | 4 | N Eur | 14 |
| VWS | missense | 284 | T>C | Leu95Pro | 4 | N Eur | This study |
| VWS | missense | 292 | G>C | Asp98His | 4 | N Eur | 14 |
| VWS | missense | 293 | A>G | Asp98Gly | 4 | Brazil | This study |
| VWS | missense | 298 | A>G | Thr100Ala | 4 | Belgium | 21 |
| VWS | missense | 299 | C>T | Thr100Ile | 4 | N Eur | This study |
| VWS | missense | 328 | A>T | Ile110Leu | 4 | Belgium | This study |
| VWS | missense | 332 | A>G | Tyr111Cys | 4 | Brazil | This study |
| VWS | missense | 332 | A>G | Tyr111Cys | 4 | Brazil | This study |
| VWS | missense | 332 | A>G | Tyr111Cys | 4 | N Eur | This study |
| VWS | truncation | 352 | C>T | Gln118X | 4 | NS | This study |
| VWS | truncation | 352 | C>T | Gln118X | 4 | N Eur | 14 |
| VWS | missense | 358 | C>A | Gln120Lys | 4 | N Eur | This study |
| VWS | truncation | 358 | C>T | Gln120X | 4 | N Eur | This study |
| VWS | truncation | 358_368 | del | Gln120HisfsX | 4 | Thailand | 23 |
| VWS | truncation | 405_406 | ins TT | Asp135_Glu136LeufsX31 | 5 | Germany | This study |
| VWS | truncation | 427 | G>T | Glu143X | 5 | N Eur | This study |
| VWS | truncation | 466 | dup C | His156ProfsX39 | 5 | N Eur | 14 |
| VWS | missense | 470 | T>G | Val157Gly | 5 | Brazil | This study |
| VWS | truncation | 494 | del T | Phe165SerfsX | 5 | N Eur | This study |
| VWS | truncation | 522_525 | del | Ala174fsX47 | 6 | Belgium | This study |
| VWS | truncation | 558 | C>A | Cys186X | 6 | N Eur | 14 |
| VWS | truncation | 576 | G>A | Try192X | 6 | N Eur | 14 |
| VWS | truncation | 576 | G>A | Try192X | 6 | Germany | This study |
| VWS | truncation | 554_579 | dup | Lys194AsnfsX23 | 6 | Brazil | This study |
| VWS | missense | 589 | C>T | Pro197Ser | 6 | Brazil | This study |
| VWS | truncation | 610 | del C | Gln204ArgfsX18 | 6 | N Eur | This study |
| VWS | truncation | 610 | C>T | Gln204X | 6 | Brazil | This study |
| VWS | truncation | 610 | C>T | Gln204X | 6 | N Eur | This study |
| VWS | truncation | 610 | C>T | Gln204X | 6 | N Eur | This study |
| VWS | truncation | 634_635 | ins CCAC | Ser212ThrfsX14 | 6 | N Eur | 14 |
| PPS | truncation | 651 | G>A | Try217X | 6 | N Eur | This study |
| VWS | truncation | 657_665 | delins TA | Ser219fsX1 | 6 | N Eur | 14 |
| VWS | missense | 665 | C>T | Pro222Leu | 6 | N Eur | This study |
| VWS | truncation | 706 | G>T | Glu236X | 7 | Brazil | This study |
| VWS | truncation | 722_723 | ins C | Met241IlefsX19 | 7 | Brazil | This study |
| VWS | missense | 743 | G>C | Gly248Ala | 7 | N Eur | This study |
| VWS | truncation | 744_748 | del | Gly248fsX10 | 7 | N Eur | 14 |
| VWS | missense | 748 | C>G | Arg250Gly | 7 | Brazil | This study |
| VWS | missense | 748 | C>G | Arg250Gly | 7 | Brazil | This study |
| VWS | missense | 748 | C>G | Arg250Gly | 7 | N Eur | This study |
| VWS | truncation | 748 | C>T | Arg250X | 7 | Belgium | This study |
| VWS | truncation | 748 | C>T | Arg250X | 7 | N Eur | This study |
| VWS | truncation | 748 | C>T | Arg250X | 7 | Belgium | This study |
| VWS | missense | 749 | G>A | Arg250Gln | 7 | Brazil | This study |
| VWS | missense | 749 | G>A | Arg250Gln | 7 | Brazil | This study |
| VWS | missense | 749 | G>A | Arg250Gln | 7 | N Eur | 14 |
| VWS | missense | 749 | G>A | Arg250Gln | 7 | N Eur | This study |
| VWS | missense | 749 | G>A | Arg250Gln | 7 | N Eur | This study |
| VWS | missense | 755 | T>C | Leu251Pro | 7 | Belgium | 21 |
| VWS | truncation | 759 | T>A | Tyr253X | 7 | N Eur | 14 |
| VWS | missense | 775 | C>T | Pro258Ser | 7 | Belgium | 21 |
| VWS | truncation | 784 | C>T | Gln262X | 7 | Brazil | This study |
| VWS | truncation | 795 | del C | Leu265fsX36 | 7 | N Eur | 14 |
| VWS | missense | 803 | C>T | Pro268Leu | 7 | Brazil | This study |
| VWS | missense | 818 | A>G | Gln273Arg | 7 | N Eur | 14 |
| VWS | truncation | 842 | del A | His281LeufsX20 | 7 | N Eur | 14 |
| VWS | deletion | 870_897 | del/ins A | Phe290_Asp296delinsLeu | 7 | N Eur | 14 |
| VWS | missense | 881 | T>C | Leu294Pro | 7 | N Eur | 14 |
| VWS | missense | 889 | G>A | Val297Ile | 7 | N Eur | 14 |
| VWS | missense | 905 | T>C | Leu302Pro | 7 | Brazil | This study |
| VWS | missense | 953 | A>C | Gln318Pro | 7 | N Eur | This study |
| VWS | missense | 954 | G>C | Gln318His | 7 | NS | This study |
| VWS | missense | 955 | T>C | Cys319Arg | 7 | N Eur | This study |
| VWS | truncation | 957 | C>A | Cys319X | 7 | Brazil | This study |
| VWS | truncation | 957 | C>A | Cys319X | 7 | Brazil | This study |
| VWS | missense | 958 | A>G | Lys320Glu | 7 | N Eur | 14 |
| VWS | missense | 958 | A>G | Lys320Glu | 7 | N Eur | 14 |
| VWS | missense | 961 | G>A | Val321Met | 7 | Belgium | This study |
| VWS | missense | 961 | G>A | Val321Met | 7 | N Eur | 14 |
| VWS | missense | 965 | A>G | Tyr322Cys | 7 | Brazil | This study |
| VWS | missense | 974 | G>A | Gly325Glu | 7 | Belgium | This study |
| VWS | missense | 974 | G>A | Gly325Glu | 7 | N Eur | 14 |
| VWS | Truncation | 1011_1020 | del | Ile337fsX22 | 7 | Germany | This study |
| VWS | missense | 1034 | T>C | Leu345Pro | 7 | N Eur | 14 |
| VWS | missense | 1040 | G>T | Cys347Phe | 7 | N Eur | 14 |
| VWS | truncation | 1087 | del A | Ile363X | 8 | N Eur | This study |
| VWS | missense | 1106 | T>C | Phe369Ser | 8 | N Eur | This study |
| VWS | truncation | 1107 | del T | Phe369LeufsX27 | 8 | N Eur | 14 |
| VWS | truncation | 1116 | C>G | Tyr372X | 8 | N Eur | This study |
| VWS | missense | 1118 | T>C | Leu373Ser | 8 | Brazil | This study |
| VWS | missense | 1120 | T>C | Cys374Arg | 8 | Brazil | This study |
| VWS | missense | 1122 | C>G | Cys374Try | 8 | N Eur | 14 |
| VWS | missense | 1123 | T>C | Phe375Leu | 8 | Brazil | This study |
| VWS | missense | 1126 | G>C | Gly376Arg | 8 | Brazil | This study |
| VWS | missense | 1126 | G>C | Gly376Arg | 8 | N Eur | This study |
| VWS | truncation | 1136 | G>A | Try379X | 8 | Brazil | This study |
| VWS | truncation | 1136 | G>A | Try379X | 8 | Brazil | This study |
| VWS | missense | 1162 | A>G | Lys388Glu | 8 | N Eur | 14 |
| PPS | truncation | 1177 | C>T | Gln393X | 8 | N Eur | 14 |
| VWS | missense | 1190 | T>C | Val397Ala | 9 | NS | This study |
| VWS | missense | 1198 | C>T | Arg400Try | 9 | Belgium | This study |
| VWS | missense | 1198 | C>T | Arg400Try | 9 | Brazil | This study |
| VWS | missense | 1198 | C>T | Arg400Try | 9 | Brazil | This study |
| VWS | missense | 1198 | C>T | Arg400Try | 9 | Brazil | This study |
| VWS | missense | 1198 | C>T | Arg400Try | 9 | Brazil | This study |
| VWS | missense | 1198 | C>T | Arg400Try | 9 | Brazil | This study |
| VWS | missense | 1199 | G>A | Arg400Gln | 9 | Brazil | This study |
| VWS | missense | 1210 | G>A | Glu404Lys | 9 | Brazil | This study |
| VWS | missense | 1212_1226 | del | Glu404_Gly408delAsp | 9 | Brazil | This study |
| VWS | truncation | 1219 | del T | Ser407LeufsX28 | 9 | Brazil | This study |
| NS | truncation | 1234 | C>T | Arg412X | 9 | N Eur | This study |
| NS | truncation | 1234 | C>T | Arg412X | 9 | N Eur | This study |
| PPS | truncation | 1234 | C>T | Arg412X | 9 | N Eur | This study |
| PPS | truncation | 1234 | C>T | Arg412X | 9 | N Eur | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | Brazil | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | Brazil | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | Brazil | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | Brazil | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | Brazil | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | NS | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | NS | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | N Eur | This study |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | N Eur | 14 |
| VWS | truncation | 1234 | C>T | Arg412X | 9 | N Eur | This study |
| VWS | missense | 1262 | T>G | Leu421Arg | 9 | N Eur | This study |
| VWS | truncation | 1264 | C>T | Gln422X | 9 | Brazil | This study |
| VWS | missense | 1268 | T>C | Ile423Thr | 9 | NS | This study |
| PPS | missense | 1288 | G>A | Asp430Asn | 9 | N Eur | 14 |
| PPS | truncation | 1291_1311 | del/ins GTAGAGGCTAAACTCCCTGGAA | Asn431ValfsX70 | 9 | N Eur | This study |
| VWS | missense | 1296_97 | delins TT | Val433Phe | 9 | Germany | This study |
| VWS | truncation | 1303_1307 | del | Gln435GlufsX64 | 9 | Brazil | This study |
| VWS | truncation | 1327 | del C | Leu443PhefsX46 | 9 | N Eur | This study |
| VWS | truncation | 1357_1368 | del | QPTP Gln453fsX43 | 9 | N Eur | This study |
| VWS | truncation | 1368 | dup C | Ser457GlnfsX43 | 9 | N Eur | This study |
| VWS | truncation | 1369_1370 | ins TGCAGCCCAT | Ser457MetfsX27 | 9 | N Eur | This study |
| VWS | truncation | 1372 | dup A | Met458AsnfsX43 | 9 | Brazil | This study |
| VWS | truncation | 1385 | del C | Pro462LeufsX27 | 9 | Brazil | This study |
| VWS | truncation | 1385 | dup C | Pro462fsX39 | 9 | N Eur | 14 |
| VWS | truncation | 1386_1387 | ins C | Pro462_Ala463ArgfsX38 | 9 | N Eur | This study |
| NS | splicing | -2 | A>G (100%>0%) | p.? | IVS2 | NS | This study |
| VWS | splicing | -2 | A>G (100%>0%) | p.? | IVS2 | Brazil | This study |
| VWS | splicing | -2 | A>G (100%>0%) | p.? | IVS2 | Brazil | This study |
| VWS | splicing | -1 | G>A (100%>0%) | p.? | IVS3 | Brazil | This study |
| VWS | splicing | -1 | G>T (100%>0%) | p.? | IVS3 | N Eur | This study |
| PPS | splicing | 1 | G>T (100%>0%) | Val18_Lys58del | IVS3 | Belgium | This study |
| VWS | splicing | 1 | G>A (100%>0%) | Val18_Lys58del | IVS3 | Belgium | This study |
| PPS | splicing | 3 | A>C (71%>2%) | Val18_Lys58del | IVS3 | N Eur | This study |
| VWS | splicing | -5 | T>G (22%>5%) | p.? | IVS6 | Brazil | This study |
| VWS | splicing | -1 | G>A (100%>0%) | p.? | IVS6 | Brazil | This study |
| VWS | splicing | -3 | C>G (55%>1%) | p.? | IVS7 | Brazil | This study |
| VWS | splicing | -2 | A>G (100%>0%) | p.? | IVS7 | Brazil | This study |
In six cases, the phenotype was not specified (NS). Table does not include six cases with microdeletions of IRF6 (see text).
Nucleotide (nt) counted from start codon if mutation is located in an exon or counted from nearest exon if located in an intron. Reference sequences for cDNA and genomic DNA were NM_006147.2 and RP3-434o14 (Genbank AL022398), respectively.
All protein and exon splicing changes are deduced and not experimentally verified. Reference protein sequence is NP_006138. Frameshift mutations (fs) are predicted to add the indicated number of amino acids beyond the mutation (fsXnumber). Residues located in the DNA binding domain (yellow) or protein binding domain (green) are indicated. For splicing mutations, the values represent the change in probability of finding the indicated nucleotide at a splice junction (Zhang et al., 1998). Predicted protein for splicing mutation is based on computational analysis shown in Figure 3.
Exon and intron (IVS) numbering are based on ENSG00000117595 (www.ensembl.org).
Geographic origins of pedigrees include the indicated countries, or Northern Europe (N Eur) if proband is white, but country is not known or origins not specified (NS).
Table 4.
DNA variants in IRF6 that do not cause VWS or PPS.
| Locationa | SNP | amino acid | MAFb (%) | Sequence |
|---|---|---|---|---|
| promoter | -156 G>A | 35 | AGGGTGGGACRCTGGACGGAC | |
| promoter | -134 G>C | 36 | CCGCTGGGCCSGGCAGCCCAG | |
| promoter | -50 T>A | 18 | CTGGGAGGCGWGGCCGGGCGG | |
| promoter | -39 A>T | 18 | GGCCGGGCGGWTGCGAAGGCT | |
| 1i | -4 A>G | 2 | TTTTCTCCATRCAGAATCTTT | |
| 2e | -73 T>C | 46 | CCATACAGAAYCTTTGAGCGG | |
| 2i | +102 T>C | 1 | CCTTTAGTTGYCTTGTTTAAA | |
| 2i | -73 G>C | 2 | AGATGGGAAASGTGGCTGGGA | |
| 3e | 9 C>T | L3L | 3 | TCATGGCCCTYCACCCCCGCA |
| 3e | 55 G>A | D19N | 1 | GGCCCAGGTGRATAGTGGCCT |
| 3i | +36 TT>T | 24 | CCTTTCTGGATTTTTTTTTTT | |
| 3i | -138 G>C | 12 | TGATGGGGCASTCATGCAAAA | |
| 3i | -84 GTGT>GT | 12 | GTGTGTGTGTGTTTGTGTCTA | |
| 3i | -5 C>G | 49 | GTTTCTTGTTSTCAGGCCTGG | |
| 4e | 181 C>G | A61P | 5 | TCAGGCCTGGSCTGTAGAGAC |
| 4e | 339 G>T | V113V | 2 | TATATCAAGTKTGTGACATCC |
| 4i | -174 A>G | 1 | AGGTCCTTCCRTGAGAGAAGT | |
| 4i | -155 C>T | 9 | GTGTTCATTCYCTTGATTCTC | |
| 4i | -106 C>T | 1 | TGTACTGAACYTGAGGAGCCT | |
| 4i | -102 G>A | 1 | CTGAACCTGARGAGCCTCTGG | |
| 5e | 459 G>T | S153S | 25 | TGGATCAGTCKCAGCACCATG |
| 5i | +55 A>C | 32 | AGGAGTTTTGMCCTTGGGACT | |
| 6i | +27 C>G | 40 | CTTTCTTGCTSGGTCTTCTGC | |
| 7e | 671 C>G | T224S | 2 | CTTGCAGTGASTGACCTGGAC |
| 7e | 711 C>T | Y237Y | 1 | GGAAGGAGTAYGGGCAGACCA |
| 7e | 726 C>T | T242T | 2 | AGACCATGACYGTGAGCAACC |
| 7e | 820 G>A | V274I | 13 | CCTGGAGCAGRTCAAATTCCC |
| 7i | +37 C>T | 21 | GTGGGAATCAYTCTCTGGAAG | |
| 7i | -75 A>T | 47 | TGTAATGGACWGCATAAAAGA | |
| 8e | 1153 T>C | L385L | 5 | TGGGAAACCAYTGGAAAGGAA |
| 8i | +34 T>C | 2 | CAACTCTTCAYCTTTTTGCCA | |
| 8i | +42 A>C | 1 | CATCTTTTTGMCAATGCTTAA | |
| 8i | +93 G>T | 3 | GCATCCATCAKCCCATGTAGG | |
| 9e | 1608 C>T | 2 | TTCAAATCTCYTAATGGTAGT | |
| 9e | 1692 A>T | 4 | CTTTGCTTCCAATGTGACCTT | |
| 9e | 1703 G>A | 2 | ATGTGACCTTRAACAAGTCCT | |
| 9e | 1751 A>T | 5 | TTATAAAGTGWAGAGATTGGA | |
| 9e | 1757 T>C | 1 | AGTGAAGAGAYTGGAGTAGTG | |
| 9e | 1855 A>G | 11 | ATCCTTCTGCRTTGTTCTTGT | |
| 9e | 1922 C>T | 1 | TGTCCAGGATYGAGCTCTGTT | |
| 9e | 1962 C>T | 4 | AGTAAGCTGGYTCCCTGATGG |
Mutations are located upstream of exon 1 (promoter) or in the indicated intron (i) and exon (e).
Minor Allele Frequency (MAF) is based on CEPH diversity panel.
![]()
Non-random Distribution of IRF6 Exonic Mutations in VWS Collections
The distribution of all exonic mutations in IRF6 in the VWS collections is not random (p<0.0001; Table 5, row A). More mutations were located in exons 3, 4, 7, and 9 than expected, suggesting a multi-tier approach for mutation screening of IRF6 in VWS cases. This pattern was observed in both the Brazilian (Figure 1A) and mixed origin (Figure 1B) VWS collections, suggesting that the mutation mechanisms for IRF6 are independent of origin of the population.
Table 5.
Distribution of mutations in IRF6 exons in VWS and PPS collections.
| ************* EXONS ********* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Row Totala | Row Averageb | p-valuec | ||
| VWS Total | A | 4 | 2 | 36 | 54 | 5 | 13 | 44 | 15 | 34 | 207 | 23 | <0.0001 |
| VWS Trunc | B | 4 | 2 | 11 | 9 | 4 | 11 | 13 | 5 | 21 | 80 | 9 | 0.05 |
| VWS Miss | C | 0 | 0 | 22 | 42 | 1 | 2 | 29 | 8 | 13 | 117 | 13 | <0.0001 |
| VWS Splice | D | 0 | 0 | 3 | 3 | 0 | 0 | 2 | 2 | 0 | 10 | 1 | 0.45 |
| PPS-Total | E | 0 | 0 | 4 | 26 | 0 | 1 | 0 | 1 | 4 | 36 | 4 | <0.0001 |
| PPS-Trunc | F | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 5 | ≤1 | 0.73 |
| PPS-Miss | G | 0 | 0 | 1 | 26 | 0 | 0 | 0 | 0 | 1 | 28 | 4 | <0.0001 |
| PPS-Splice | H | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | ≤1 | 0.79 |
Total number of mutations in each row.
Number of mutations expected in each exon if distributed randomly.
p-value comparing observed to expected distribution of mutations in each exon.
Figure 1.
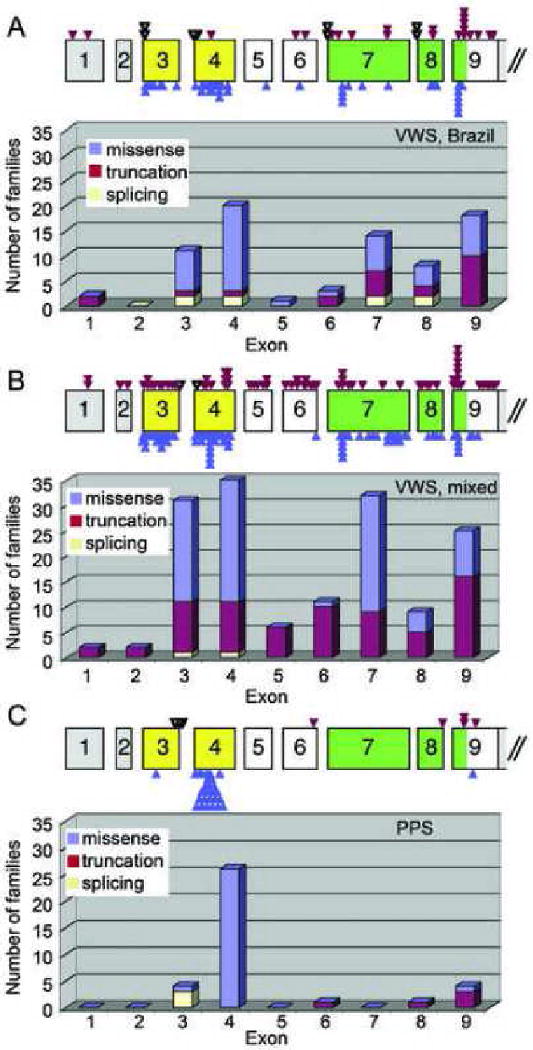
Distribution of exonic mutations in IRF6. Each panel shows the genomic structure for IRF6. Exons (rectangles) are color coded as untranslated (gray), encode DNA binding domain (yellow), or encode the protein binding domain (green). The introns (space between exons) are not drawn to scale. The relative position of protein truncation mutations (red triangle), missense mutations (blue triangle) and splicing mutations (black triangle) is shown. Below each genomic structure is the distribution of missense (blue; includes in-frame deletions and insertions), protein trunctation (red; includes nonsense, frameshift and large deletions), and splicing (white) mutations in each exon for each population. A) Mutations found in VWS collection from Brazil. B) Mutations found in VWS collection from mixed geographic origin. C) Mutations found in PPS collection.
Protein truncation mutations (nonsense and frameshifts) were observed in all exons prior to the endogenous stop codon in exon 9. Interestingly, we identified point mutations in six families in exons 1 and 2 that create new start codons in the 5′ untranslated region. These new start sites should not make IRF6 protein as they are in the wrong reading frame, but may not prevent initiation at the native site. The protein truncation mutations are evenly distributed across the gene, except for exon 9 (Table 5, row B). The spike in protein truncation mutations in exon 9 appears to be due to one of five mutational hotspots in IRF6 (see below). Overall, the high prevalence of protein truncation mutations in families with VWS (80 of 207), in addition to the six known IRF6 deletions10, 11, 14, 24, 29, provides further support that VWS can be caused by haploinsufficiency of IRF6.
Nearly all of the 117 mutations that do not truncate the protein (missense and in-frame insertions and deletions) are localized to regions encoding the DNA binding domain (64 families) and the protein binding domain (45 families). The significant over-representation of missense mutations in the DNA binding (exons 3 and 4) and protein binding (exons 7-9) domains (Table 5, row C) reinforces the importance of these domains for IRF6 function.
Non-random Distribution of IRF6 Exonic Mutations in the PPS Collection
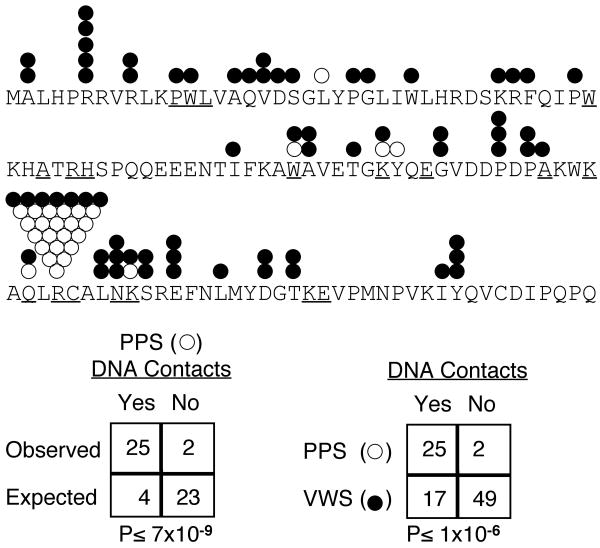
The location of mutations identified in families with PPS is non-random (Table 5, row E). In 34 of 36 families with PPS, the mutation is located in exons 3, 4 or 9 (Figure 1C). Like VWS, these observations suggest a multi-tier approach for efficient mutation screens for PPS. However, the distribution of mutations among the exons for the PPS collection differs significantly from the VWS collections (p < 0.0001; Table 5, row A versus E). Another difference is the low frequency of protein truncation mutations in the PPS versus VWS collections (5/36 vs 80/207; p = 0.036), and the high frequency of missense mutations in exon 4 in the PPS versus VWS collections (26/36 vs 42/207; p < 0.0001). In addition, the distribution of missense mutations within the DNA binding domain (exons 3 and 4) is non-random for the PPS collection (Figure 2). Specifically, the missense mutations in the PPS collection are more likely to be located at residues that are predicted to contact DNA, when compared with random chance (P≤ 7×10-9) and when compared with missense mutations in the VWS collection (P≤ 1×10-6). Based on the significant differences in the frequency of the type of mutation and distribution in location of mutations found in the PPS versus the VWS collections, we conclude that the PPS-associated mutations affect IRF6 function differently than VWS-associated mutations.
Figure 2.
Distribution of missense mutations in the DNA binding domain of IRF6. Mutations were identified in families with VWS (closed circles) and families with PPS (open circles). Amino acids predicted to directly contact DNA (underline) are based on crystal structure of IRF1 (see text). The expected number of mutations that contact DNA is based on the ratio of 17 amino acids that are predicted to contact the DNA (underlined, see text) out of 120 total amino acids in the DNA binding domain.
How might VWS and PPS-associated mutations affect IRF6 function differently? The identification of six large deletions of IRF6 10, 11, 24, 29, along with the high frequency of protein truncation mutations, demonstrates that VWS can be caused by loss of function of IRF6. For families with PPS, we hypothesized previously that mutations have a dominant negative effect on IRF6 14. The rationale for this hypothesis is that the Arg84Cys and Arg84His mutations abrogate DNA binding 47, but are not predicted to affect protein binding. Consequently, protein dimers are predicted to form between a wild type isoform and the Arg84Cys and Arg84His isoform, but such a dimer will not be able to bind DNA. This model is supported by two main observations. First, in a previous study, mice heterozygous for a PPS-associated Irf6 allele (Arg84Cys) had a more severe and more penetrant phenotype than mice that were heterozygous for a loss of function allele 47, 48. Second, in the current study, we observed that mutations identified in families with PPS are much more likely to be missense mutations than in families with VWS, and that mutations are more likely to be located at residues that are predicted to directly contact the DNA. Such mutations are more likely to affect DNA binding without affecting protein stability or protein interaction. The most common examples of this class of mutations are Arg84Cys and Arg84His (Table 3: Supplemental data online only).
However, current data do not fully support a simple model whereby VWS is caused by IRF6 loss-of-function mutations and PPS is caused by IRF6 dominant negative mutations. Foremost, the same mutations were identified in patients with VWS and with PPS. For example, we identified missense mutations at Arg84 in seven families diagnosed with VWS and 21 with PPS (Table 3: Supplemental data online only). The mutations Arg84Cys and Arg84His were found in five families diagnosed with VWS. Moreover, individuals with VWS and PPS have been diagnosed in the same family 21. These data suggest that while the association between the Arg84Cys and Arg84His mutations and PPS is strong, it is not absolute. In sum, the data is most consistent with the model that VWS is most likely caused by loss (or partial loss) of function mutations, but can also be caused by dominant negative mutations and that PPS is most likely caused by dominant negative mutations but can also be caused by loss (or partial loss) of function mutations. The range of phenotypes for VWS and PPS, including their overlap, suggests the likely contributions of stochastic events and genetic modifiers 13 for IRF6-related disorders.
Three other observations are relevant to the effect of VWS and PPS mutations on IRF6 function. First, we identified a novel missense change at Arg84, Arg84Pro, in two families where affected individuals were diagnosed with VWS. In addition, Item et al., 22 identified an Arg84Gly mutation in a family where both affected individuals were diagnosed with VWS. The Arg84Pro and Arg84Gly mutations challenge the dominant negative hypothesis, since this residue is predicted to contact the DNA but these mutations are only found in individuals with VWS. However, the residue Arg84 is located in the middle of helix 3 in IRF6. The amino acids proline and glycine are known to disrupt alpha helices 49. Consequently, the Arg84Pro and Arg84Gly mutations are predicted to disrupt the secondary and/or tertiary structure of IRF6, whereas Arg84Cys and Arg84His would not. Thus, we hypothesize that the Arg84Pro and Arg84Gly alleles cause complete loss of IRF6 function and result in VWS through haploinsufficiency of IRF6. Further biochemical and molecular studies are needed to test this hypothesis.
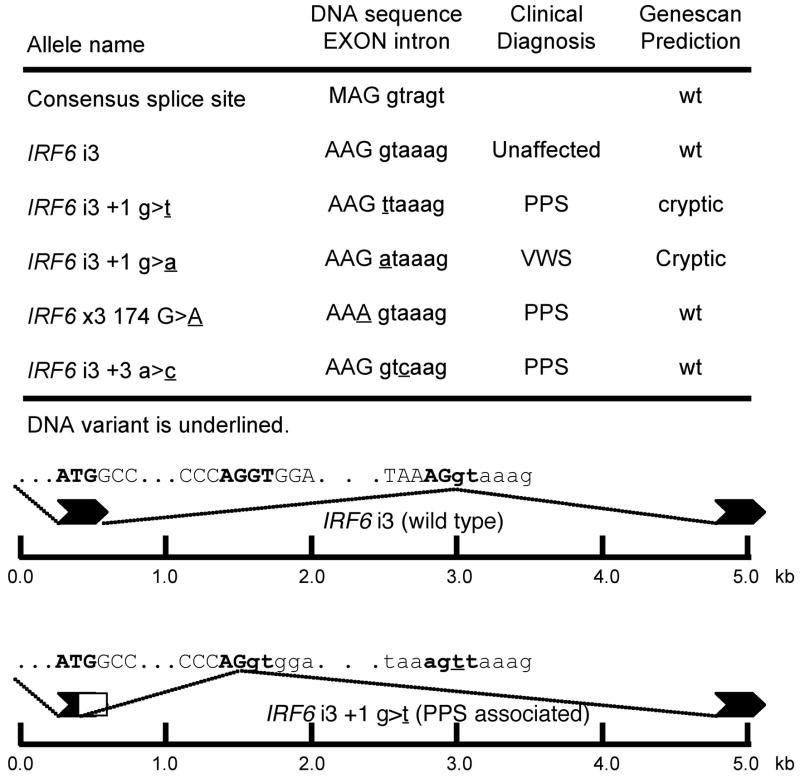
Secondly, the splicing mutations at the 5′ splice site of intron 3 and the protein truncation mutations in exon 9 also challenge the dominant negative hypothesis for mutations that cause PPS. To produce a dominant negative allele, a defective, but stable protein must be produced. We hypothesize that the splicing mutations at the 5′ splice site of intron 3 activate a cryptic splice site that produces a mutant IRF6 allele that is stable, but unable to bind DNA. To test this hypothesis, we used Genscan 40, a program that predicts splice sites, to model the effect of the four splicing mutations at intron 3. For the two mutations at the highly conserved position +1 of intron 3, Genscan analysis predicts the loss of the endogenous splice site and the use of a cryptic splice site in the middle of exon 3 (Figure 3). Moreover, the cryptic splice site rejoins exon 4 in frame, but deletes 41 amino acids from the DNA binding region encoded in exon 3. Thus, these splicing mutations create a potentially stable protein with a mutation in the DNA binding domain and are consistent with the dominant negative model for PPS mutations. However, like the Arg84Cys and Arg84His mutations, these mutations do not always cause PPS, as one of these mutations was identified in a family with VWS. Also, for the other two splice mutations in intron 3 found in families with PPS, Genscan did not predict loss of the endogenous splice site (Figure 2).
Figure 3.
Cryptic splice site in exon 3 revealed by computer modeling. The wild type (wt) and mutant sequences for the 5′ splice site for intron 3 are shown below the consensus sequence. In the consensus, M represents A or C and r represents G or A. The panel below contains the output from GENESCAN and shows the cryptic splice site in exon 3 revealed by the mutation at the endogenous site.
Thirdly, protein truncation mutations in exon 9 were identified in families with either VWS or PPS. While the effect of these mutations on IRF6 function is not known, previous studies with the other members of the IRF family showed that the C terminus contains an auto-inhibitory domain 50. Recently, we discovered that IRF6 binds to maspin, a tumor suppressor gene, and that the C terminus blocks this interaction 51. Additional molecular and biochemical studies are needed to understand the effects of the PPS-causing mutations in exon 9.
Source of exonic mutations in IRF6
To date, we identified IRF6 exonic mutations in 249 unrelated families and represent 170 different disease-causing alleles in IRF6. Thus, 68% of exonic mutations in IRF6 are private and represent a wide array of potential mutational mechanisms. However, we identified five apparent hotspots. Mutations in the codons for Arg6, Arg84, Arg250, Arg400 and Arg412 were identified in 6, 26, 11, 7 and 14 unrelated families, respectively. The codon sequence for each of these residues contains a CpG dinucleotide. In humans, approximately one third of germline mutations result from loss of the CpG dinucleotide, and 90% of those are consistent with a mutation mechanism of cytosine methylation and deamination 52. Similarly, in this report, 55 of 64 (86%) of the mutations in these CpG codons were consistent with the cytosine methylation/deamination mechanism.
This study shows that exonic mutations in IRF6 are found in 68% of families with Van der Woude syndrome and nearly all families with popliteal pterygium syndrome. A few percent of families with VWS are caused by microdeletions of IRF6. Although the majority of the mutations are private, the distributions of exonic mutations suggest that future mutation searches should focus on exons 3, 4, 7 and 9 for families with VWS and on exons 3, 4 and 9 for families with PPS. In addition, since the distribution of mutations is consistent between geographically distinct populations, this multi-tier approach for mutation discovery should be widely applicable. Further, the distributions of mutations in the VWS and PPS collections suggest some limited guides for risk assessment and suggest a molecular rationale for clinical heterogeneity caused by genetic variation in IRF6.
Acknowledgments
The authors thank the families that participated in this study, Amy Mach and Jamie L'Heureux for administrative assistance with samples, Kristin Orr, Sally Santiago, Marla Johnson and Theresa Zucchero for technical assistance, and the following clinicians for participating in this study, Melissa Lees, Koen Devriendt, Geert Mortier, Lionel van Maldergem, Mieke Bouma, David Genevieve, Aurora Sanchez. This work was supported in part by NIH grants R01-DE013513 (BCS, MJD), R01-DE08559 (JCM) and P50-DE016215 (BCS, JCM, MLM, MJD) and by the Fonds Spéciaux de Recherche - Université catholique de Louvain, the Fonds national de la recherche scientifique (F.N.R.S.) (to M.V., a “Maître de recherches du F.N.R.S.”). M. Ghassibé was supported by a fellowship from F.R.I.A. (Fonds pour la formation à la recherche dans l'industrie et dans l'agriculture).
Funding for this work was received from NIH, the Fonds Spéciaux de Recherche - Université catholique de Louvain, the Fonds national de la recherche scientifique and Fonds pour la formation à la recherche dans l'industrie et dans l'agriculture.
Footnotes
Conflict of Interest
The authors have no conflict of interest with the information presented in any submitted manuscript
References
- 1.Cooper ME, Stone RA, Liu Y, Hu DN, Melnick M, Marazita ML. Descriptive epidemiology of nonsyndromic cleft lip with or without cleft palate in Shanghai, China, from 1980 to 1989. Cleft Palate Craniofac J. 2000 May;37(3):274–280. doi: 10.1597/1545-1569_2000_037_0274_deoncl_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 2.Murray JC, Daack-Hirsch S, Buetow KH, et al. Clinical and epidemiologic studies of cleft lip and palate in the Philippines. Cleft Palate-Craniofacial Journal. 1997;34(1):7–10. doi: 10.1597/1545-1569_1997_034_0007_caesoc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 3.Tolarova MM, Cervenka J. Classification and birth prevalence of orofacial clefts. Am J Med Genet. 1998 Jan 13;75(2):126–137. [PubMed] [Google Scholar]
- 4.Vanderas AP. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J. 1987;24(3):216–225. [PubMed] [Google Scholar]
- 5.Jugessur A, Murray JC. Orofacial clefting: recent insights into a complex trait. Curr Opin Genet Dev. 2005 Jun;15(3):270–278. doi: 10.1016/j.gde.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorlin RJ, Cohen AA, Hennekam RCM. Syndromes of the head and neck. 4th. Oxford: Oxford University Press; 2001. [Google Scholar]
- 7.Cheney ML, Cheney WR, LeJeune FE., Jr Familial incidence of labial pits. Am J Otolaryngol. 1986;7(4):311–313. doi: 10.1016/s0196-0709(86)80055-2. [DOI] [PubMed] [Google Scholar]
- 8.Murray JC, Nishimura DY, Buetow KH, et al. Linkage of an autosomal dominant clefting syndrome (Van der Woude) to loci on chromosome 1q. Am J Hum Genet. 1990;46(3):486–491. [PMC free article] [PubMed] [Google Scholar]
- 9.Bocian M, Walker AP. Lip pits and deletion 1q32-q41. Am J Med Genet. 1987;26(2):437–443. doi: 10.1002/ajmg.1320260223. [DOI] [PubMed] [Google Scholar]
- 10.Sander A, Schmelzle R, Murray J. Evidence for a microdeletion in 1q32-41 involving the gene responsible for Van der Woude syndrome. Hum Mol Genet. 1994;3(4):575–578. doi: 10.1093/hmg/3.4.575. [DOI] [PubMed] [Google Scholar]
- 11.Schutte BC, Basart AM, Watanabe Y, et al. Microdeletions at chromosome bands 1q32-q41 as a cause of Van der Woude syndrome. Am J Med Genet. 1999;84(2):145–150. doi: 10.1002/(sici)1096-8628(19990521)84:2<145::aid-ajmg11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Koillinen H, Wong FK, Rautio J, et al. Mapping of the second locus for the Van der Woude syndrome to chromosome 1p34. Eur J Hum Genet. 2001 Oct;9(10):747–752. doi: 10.1038/sj.ejhg.5200713. [DOI] [PubMed] [Google Scholar]
- 13.Sertie AL, Sousa AV, Steman S, Pavanello RC, Passos-Bueno MR. Linkage analysis in a large Brazilian family with van der Woude syndrome suggests the existence of a susceptibility locus for cleft palate at 17p11.2-11.1. Am J Hum Genet. 1999;65(2):433–440. doi: 10.1086/302491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo S, Schutte BC, Richardson RJ, et al. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002 Sep 3; doi: 10.1038/ng985. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lees MM, Winter RM, Malcolm S, Saal HM, Chitty L. Popliteal pterygium syndrome: a clinical study of three families and report of linkage to the Van der Woude syndrome locus on 1q32. J Med Genet. 1999;36(12):888–892. [PMC free article] [PubMed] [Google Scholar]
- 16.Brosch S, Baur M, Blin N, Reinert S, Pfister M. A novel IRF6 nonsense mutation (Y67X) in a German family with Van der Woude syndrome. Int J Mol Med. 2007 Jul;20(1):85–89. [PubMed] [Google Scholar]
- 17.de Medeiros F, Hansen L, Mawlad E, et al. A novel mutation in IRF6 resulting in VWS-PPS spectrum disorder with renal aplasia. Am J Med Genet A. 2008 Jun 15;146A(12):1605–1608. doi: 10.1002/ajmg.a.32257. [DOI] [PubMed] [Google Scholar]
- 18.Del Frari B, Amort M, Janecke AR, Schutte BC, Piza-Katzer H. Van-der-Woude Syndrome. Klin Padiatr. 2008 Jan-Feb;220(1):26–28. doi: 10.1055/s-2007-971049. [DOI] [PubMed] [Google Scholar]
- 19.Du X, Tang W, Tian W, et al. Novel IRF6 mutations in Chinese patients with Van der Woude syndrome. J Dent Res. 2006 Oct;85(10):937–940. doi: 10.1177/154405910608501013. [DOI] [PubMed] [Google Scholar]
- 20.Gatta V, Scarciolla O, Cupaioli M, Palka C, Chiesa PL, Stuppia L. A novel mutation of the IRF6 gene in an Italian family with Van der Woude syndrome. Mutat Res. 2004 Mar 22;547(12):49–53. doi: 10.1016/j.mrfmmm.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Ghassibe M, Revencu N, Bayet B, et al. Six families with van der Woude and/or popliteal pterygium syndrome: all with a mutation in the IRF6 gene. J Med Genet. 2004 Feb;41(2):e15. doi: 10.1136/jmg.2003.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Item CB, Turhani D, Thurnher D, et al. Van Der Woude syndrome: variable penetrance of a novel mutation (p.Arg 84Gly) of the IRF6 gene in a Turkish family. Int J Mol Med. 2005 Feb;15(2):247–251. [PubMed] [Google Scholar]
- 23.Kantaputra PN, Limwongse C, Assawamakin A, et al. A novel mutation in IRF6 underlies hearing loss, pulp stones, large craniofacial sinuses, and limb anomalies in Van der Woude Syndrome patients. Oral Biosciences & Medicine. 2004;4:277–282. [Google Scholar]
- 24.Kayano S, Kure S, Suzuki Y, et al. Novel IRF6 mutations in Japanese patients with Van der Woude syndrome: two missense mutations (R45Q and P396S) and a 17-kb deletion. J Hum Genet. 2003;48(12):622–628. doi: 10.1007/s10038-003-0089-0. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Park JY, Lee TJ, Yoo HW. Identification of two novel mutations of IRF6 in Korean families affected with Van der Woude syndrome. Int J Mol Med. 2003 Oct;12(4):465–468. [PubMed] [Google Scholar]
- 26.Matsuzawa N, Shimozato K, Natsume N, Niikawa N, Yoshiura K. A novel missense mutation in Van der Woude syndrome: usefulness of fingernail DNA for genetic analysis. J Dent Res. 2006 Dec;85(12):1143–1146. doi: 10.1177/154405910608501215. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzawa N, Yoshiura K, Machida J, et al. Two missense mutations in the IRF6 gene in two Japanese families with Van der Woude syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Oct;98(4):414–417. doi: 10.1016/j.tripleo.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Mostowska A, Wojcicki P, Kobus K, Trzeciak WH. Gene symbol: IRF6. Disease: Van der Woude syndrome. Hum Genet. 2005 May;116(6):534. [PubMed] [Google Scholar]
- 29.Osoegawa K, Vessere GM, Utami KH, et al. Identification of novel candidate genes associated with cleft lip and palate using array comparative genomic hybridisation. J Med Genet. 2008 Feb;45(2):81–86. doi: 10.1136/jmg.2007.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peyrard-Janvid M, Pegelow M, Koillinen H, et al. Novel and de novo mutations of the IRF6 gene detected in patients with Van der Woude or popliteal pterygium syndrome. Eur J Hum Genet. 2005 Sep 14; doi: 10.1038/sj.ejhg.5201493. [DOI] [PubMed] [Google Scholar]
- 31.Shotelersuk V, Srichomthong C, Yoshiura K, Niikawa N. A novel mutation, 1234del(C), of the IRF6 in a Thai family with Van der Woude syndrome. Int J Mol Med. 2003 Apr;11(4):505–507. [PubMed] [Google Scholar]
- 32.Tan EC, Lim EC, Yap SH, et al. Identification of IRF6 gene variants in three families with Van der Woude syndrome. Int J Mol Med. 2008 Jun;21(6):747–751. [PubMed] [Google Scholar]
- 33.Wang X, Liu J, Zhang H, et al. Novel mutations in the IRF6 gene for Van der Woude syndrome. Hum Genet. 2003 Oct;113(5):382–386. doi: 10.1007/s00439-003-0989-2. [DOI] [PubMed] [Google Scholar]
- 34.Ye XQ, Jin HX, Shi LS, et al. Identification of novel mutations of IRF6 gene in Chinese families with Van der Woude syndrome. Int J Mol Med. 2005 Nov;16(5):851–856. [PubMed] [Google Scholar]
- 35.Zechi-Ceide RM, Guion-Almeida ML, de Oliveira Rodini ES, Jesus Oliveira NA, Passos-Bueno MR. Hydrocephalus and moderate mental retardation in a boy with Van der Woude phenotype and IRF6 gene mutation. Clin Dysmorphol. 2007 Jul;16(3):163–166. doi: 10.1097/MCD.0b013e3280739753. [DOI] [PubMed] [Google Scholar]
- 36.Bertele G, Mercanti M, Gangini GN, Carletti V. A familiar case of popliteal pterygium syndrome. Minerva Stomatol. 2008 Jun;57(6):309–322. [PubMed] [Google Scholar]
- 37.Schutte BC, Bjork BC, Coppage KB, et al. A Preliminary Gene Map for the Van der Woude Syndrome Critical Region Derived from 900 kb of Genomic Sequence at 1q32-q41. Genome Res. 2000;10(1):81–94. [PMC free article] [PubMed] [Google Scholar]
- 38.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998 Mar;8(3):175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 39.Cann HM, de Toma C, Cazes L, et al. A human genome diversity cell line panel. Science. 2002 Apr 12;296(5566):261–262. doi: 10.1126/science.296.5566.261b. [DOI] [PubMed] [Google Scholar]
- 40.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268(1):78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 41.Blanton SH, Cortez A, Stal S, Mulliken JB, Finnell RH, Hecht JT. Variation in IRF6 contributes to nonsyndromic cleft lip and palate. Am J Med Genet A. 2005 Sep 1;137(3):259–262. doi: 10.1002/ajmg.a.30887. [DOI] [PubMed] [Google Scholar]
- 42.Ghassibe M, Bayet B, Revencu N, et al. Interferon regulatory factor-6: a gene predisposing to isolated cleft lip with or without cleft palate in the Belgian population. Eur J Hum Genet. 2005 Nov;13(11):1239–1242. doi: 10.1038/sj.ejhg.5201486. [DOI] [PubMed] [Google Scholar]
- 43.Park JW, McIntosh I, Hetmanski JB, et al. Association between IRF6 and nonsyndromic cleft lip with or without cleft palate in four populations. Genet Med. 2007 Apr;9(4):219–227. doi: 10.1097/GIM.0b013e3180423cca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scapoli L, Palmieri A, Martinelli M, et al. Strong evidence of linkage disequilibrium between polymorphisms at the IRF6 locus and nonsyndromic cleft lip with or without cleft palate, in an Italian population. Am J Hum Genet. 2005 Jan;76(1):180–183. doi: 10.1086/427344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zucchero TM, Cooper ME, Maher BS, et al. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004 Aug 19;351(8):769–780. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]
- 46.Jugessur A, Rahimov F, Lie RT, et al. Genetic variants in IRF6 and the risk of facial clefts: single-marker and haplotype-based analyses in a population-based case-control study of facial clefts in Norway. Genet Epidemiol. 2008 Jul;32(5):413–424. doi: 10.1002/gepi.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson RJ, Dixon J, Malhotra S, et al. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006 Nov;38(11):1329–1334. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- 48.Ingraham CR, Kinoshita A, Kondo S, et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006 Nov;38(11):1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunasekaran K, Nagarajaram HA, Ramakrishnan C, Balaram P. Stereochemical punctuation marks in protein structures: glycine and proline containing helix stop signals. J Mol Biol. 1998 Feb 6;275(5):917–932. doi: 10.1006/jmbi.1997.1505. [DOI] [PubMed] [Google Scholar]
- 50.Qin BY, Liu C, Lam SS, et al. Crystal structure of IRF-3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nat Struct Biol. 2003 Nov;10(11):913–921. doi: 10.1038/nsb1002. [DOI] [PubMed] [Google Scholar]
- 51.Bailey CM, Khalkhali-Ellis Z, Kondo S, et al. Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership. J Biol Chem. 2005 Oct 7;280(40):34210–34217. doi: 10.1074/jbc.M503523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper DN, Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]