Abstract
Purpose
Despite tremendous gains in improving prognosis, 10% of patients with Wilms tumor will ultimately experience disease recurrence. The identification of novel prognostic markers and tumor associated targets for patients at risk could enable clinicians to treat recurrences more aggressively and, thus, optimize outcomes. We have previously shown that tumor expression of the T cell coregulatory ligand B7-H1 portends a poor prognosis for adults with renal cell carcinoma and represents a promising target to improve therapy. We hypothesize that this finding may be true for Wilms tumor.
Materials and Methods
We identified 81 patients with Wilms tumor treated at 1 institution between 1968 and 2004. Histopathological features, including Wilms tumor B7-H1 expression, were correlated with clinical observations and outcome.
Results
Tumor recurrences were noted in 22% of patients with Wilms tumor and 14% died. B7-H1 was expressed in 11 tumors (14%) and was more likely to occur in anaplastic Wilms tumor (p = 0.03). Tumor B7-H1 expression was associated with a 2.7-fold increased risk of recurrence, although this difference did not achieve statistical significance (p = 0.06). However, in favorable histology tumors B7-H1 expression was associated with a 3.7-fold increased risk of recurrence (p = 0.03).
Conclusions
B7-H1 is expressed by Wilms tumor, correlates with tumor biology and is associated with an increased risk of recurrence in patients with favorable histology tumors. B7-H1 may prove useful in identifying high risk patients who could benefit from more aggressive initial treatment regimens, and may represent a promising therapeutic target. Multi-institutional studies to elucidate the role of B7-H1 in the treatment of Wilms tumor are warranted.
Keywords: biological markers, Wilms tumor, kidney neoplasms, prognosis, T-lymphocytes
Wilms tumor is the most common solid renal malignancy of childhood, occurring in approximately 500 children annually in the United States.1 Significant advances in multimodality treatment approaches in recent years have resulted in long-term survival rates of greater than 90% in patients with localized disease.2 Coincident with this increase in survivorship has been a heightened interest in minimizing treatment associated morbidity.3–6 One potential avenue for improving the prognosis of children with WT would be to identify prospectively those patients at greatest risk for disease recurrence and cancer specific death. Such patients could then be treated more aggressively with conventional therapies or, potentially, with novel therapies that would ideally target prognostic WT associated proteins. Conversely, low risk patients with WT might be spared unnecessary morbidity by overly aggressive adjunctive treatment.
A cell cytoplasmic membrane glycoprotein, B7-H1, has recently been reported to act as an important coregulator of antigen specific T cell mediated immunity.7,8 B7-H1 is normally expressed by macrophage lineage cells and is aberrantly expressed by multiple human malignancies. Interestingly, tumor B7-H1 has been observed to induce T cell apoptosis or anergy, thereby down-regulating host antitumoral immunity.7 Of particular relevance to patients with WT is the fact that B7-H1 expression has recently been observed to be highly predictive of cancer specific death in adults with ccRCC.9,10 Importantly, tumor B7-H1 expression not only carries prognostic value, but also represents a potential therapeutic target. Preclinical studies indicate that in vivo approaches to block B7-H1 (to abrogate tumor induced impairments of host immunity) may facilitate improvements in the therapy of solid tumors.11–14
If B7-H1 is expressed in WT in a manner similar to its expression in adult ccRCC, B7-H1 could potentially serve as a prognostic marker and a therapeutic target for patients with WT. This application might have significant implications for those children with FH WT who are prone to tumor recurrence. Prospective identification of aggressive FH WT may facilitate earlier and more focused intervention in high risk patients, while simultaneously sparing low risk patients from excessive treatment and morbidity. In this study we examine whether B7-H1 expression in WT predicts disease outcome in a fashion similar to that observed in adult ccRCC.
Materials and Methods
Patient Selection
We identified 191 consecutive patients with WT treated at our institution between 1968 and 2004. We excluded patients whose initial surgery was performed elsewhere (98 patients), those who received neoadjuvant radiation or chemotherapy before biopsy (4) and those whose WT specimen was inadequate for staining (8). All patients included in the study underwent primary surgical intervention followed by therapy as directed by the National Wilms Tumor Study protocols active at the time of patient presentation. Primary surgical therapy was defined as pre-chemotherapy open biopsy, percutaneous biopsy or nephrectomy.
Clinical and Pathological Features
Clinical features studied included patient age at surgery, gender and clinical stage. Central pathological review was conducted by a genitourinary pathologist (TJS), who was blinded to patient outcome, to assess tumor histology (FH or AH), the presence of tumor necrosis and the extent of mononuclear inflammatory cell infiltration. The mononuclear cell infiltrate was graded as either absent or present. If present, it was further characterized as focal, moderate or marked. We defined focal as no more than 2 clusters of inflammatory cells appreciated on low magnification. Moderate inflammation was defined as more than 2 clusters of inflammatory cells but not creating confluent growths of inflammation. Marked inflammation was characterized as either sheets of inflammatory cells or clusters greater than roughly 10 in number.
B7-H1 Immunohistochemical Staining and Evaluation
Sections from paraffin embedded primary tumor blocks were stained with anti-B7-H1 antibody as previously described. The specificity of this method in paraffin embedded tissues has been previously demonstrated.10 The percentages of tumor cells that stained positive for B7-H1 were determined by pathologist review, and recorded as absent, or focally, moderately or markedly present. For the purposes of statistical analysis staining was considered to be a dichotomous variable, either present or absent. Focal, moderate and marked B7-H1 staining was defined in a manner similar to that described for the assessment of inflammatory cell infiltrate, and is illustrated in figures 1 to 3.
Fig. 1.
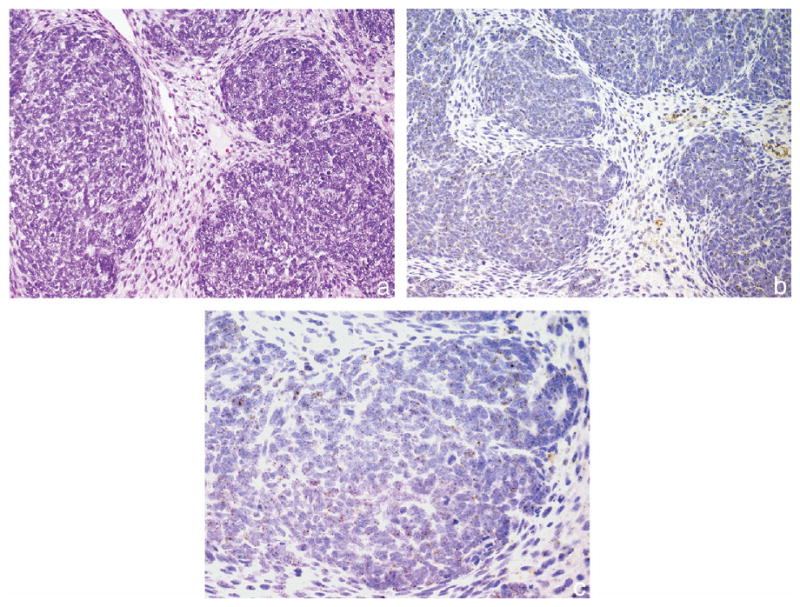
Favorable histology Wilms tumor with absent B7-H1 expression staining. a, H & E, reduced from ×200. b, B7-H1 immunohistochemical staining, reduced from ×200. c, B7-H1 immunohistochemical staining, reduced from ×400.
Fig. 3.
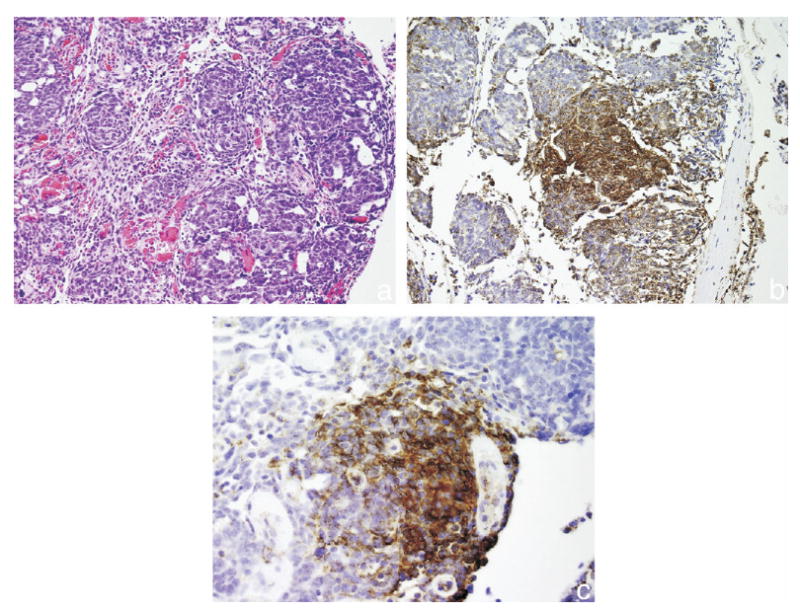
Favorable histology Wilms tumor with moderate B7-H1 expression staining. a, H & E, reduced from ×200. b, B7-H1 immunohistochemical staining, reduced from ×200. c, B7-H1 immunohistochemical staining, reduced from ×400.
Statistical Methods
The associations of tumor B7-H1 expression with the clinical and pathological features of interest were evaluated using chi-square and Fisher's exact tests. Recurrence-free survival and overall survival were estimated using the Kaplan-Meier method. The associations of the clinical and pathological features, as well as tumor B7-H1 expression, with patient outcome were evaluated using Cox proportional hazards regression models, and summarized with RR and 95% CI. Statistical analyses were performed using the SAS® software package. All tests were 2 sided, and p values <0.05 were considered statistically significant.
Results
All Patients With Wilms Tumor
A total of 81 patients met study inclusion criteria and were included in our analyses. Clinicopathological features and B7-H1 expression of all 81 patients are summarized in tables 1 and 2. Average patient age at surgery was 4.8 years (median 3.2, range 0.5 to 38.1). Three patients were older than 18 years at surgery. Of the patient 66 (81%) underwent radical nephrectomy, 14 (17%) underwent open kidney biopsy and 1 (1%) underwent percutaneous kidney biopsy. A total of 11 tumors (14%) expressed B7-H1. Tumors with AH were more likely to express B7-H1 compared to FH tumors (40% vs 9.8%, p = 0.026). B7-H1 expression was predominantly expressed by the blastemal component in FH and AH tumors. Nine of 11 tumors (82%) expressed B7-H1 primarily within the blastema, while 8 (73%) expressed B7-H1 only within the blastemal component. The remaining 3 tumors expressed B7-H1 in the stroma, epithelium, and a mix of primary blastemal and secondary stromal components.
Table 1. Clinical and pathological features.
| No. FH (%) | No. AH (%) | |
|---|---|---|
| Age at primary surgery (yrs): | ||
| Younger than 5 | 56 (79) | 2 (20) |
| 5 or Older | 15 (21) | 8 (80) |
| Sex: | ||
| M | 35 (49) | 7 (70) |
| F | 36 (51) | 3 (30) |
| Clinical stage: | ||
| I | 24 (34) | 2 (20) |
| II | 22 (31) | 1 (10) |
| III | 10 (14) | 1 (10) |
| IV | 8 (11) | 5 (50) |
| V | 7 (10) | 1 (10) |
| Low vs high clinical stage: | ||
| Low (I or II) | 50 (70) | 4 (40) |
| High (III or IV) | 21 (30) | 6 (60) |
| Tumor necrosis: | ||
| Absent | 54 (76) | 6 (60) |
| Present | 17 (24) | 4 (40) |
| Mononuclear cell infiltration: | ||
| Absent | 19 (27) | 1 (10) |
| Focal | 32 (45) | 6 (60) |
| Moderate | 16 (23) | 2 (20) |
| Marked | 4 (6) | 1 (10) |
| Tumor B7-H1 expression: | ||
| Absent | 64 (90) | 6 (60) |
| Focal | 5 (7) | 3 (30) |
| Moderate | 2 (3) | 1 (10) |
Table 2. Clinical and pathological features by tumor B7-H1 expression.
| No. Tumor B7-H1 Expression (%) | |||
|---|---|---|---|
| Neg | Pos | p Value | |
| Age at primary surgery (yrs): | |||
| Younger than 5 | 50 (71) | 8 (73) | 1.00 |
| 5 or Older | 20 (29) | 3 (27) | |
| Sex: | |||
| M | 36 (51) | 6 (55) | 0.848 |
| F | 34 (49) | 5 (45) | |
| Clinical stage: | |||
| I | 24 (34) | 2 (18) | |
| II | 20 (29) | 3 (27) | |
| III | 10 (14) | 1 (9) | 0.329 |
| IV | 11 (16) | 2 (18) | |
| V | 5 (7) | 3 (27) | |
| Histology: | |||
| FH | 64 (91) | 7 (64) | 0.026 |
| AH | 6 (9) | 4 (36) | |
| Tumor necrosis: | |||
| Absent | 54 (77) | 6 (55) | 0.142 |
| Present | 16 (23) | 5 (45) | |
| Mononuclear cell infiltration: | |||
| Absent | 19 (27) | 1 (9) | 0.277 |
| Present | 51 (73) | 10 (91) | |
At last followup 18 patients had recurrence at a mean of 2.6 years postoperatively (median 1.1, range 0.1 to 15.1). Estimated recurrence-free survival rates (SE, number still at risk) for all patients at 1, 3, 5 and 10 years postoperatively were 88.8% (3.5%, 70), 81.1% (4.4%, 62), 81.1% (4.4%, 59) and 79.7% (4.5%, 46), respectively. The associations of clinical and pathological features and B7-H1 expression with recurrence are summarized in table 3. As expected, patients with high stage tumors were significantly more likely to have recurrence compared to those with low stage tumors (RR 3.94; 95% CI 1.52 to 10.16, p = 0.005). Five of the 18 tumors that would ultimately recur expressed B7-H1. Tumor B7-H1 expression was associated with a nearly 3-fold increased risk of recurrence among all patients with WT, although this increase did not attain statistical significance (p = 0.057).
Table 3. Association of clinical and pathological features and B7-H1 expression with recurrence.
| RR (95% CI) | p Value | |
|---|---|---|
| Age at primary surgery (yrs): | ||
| Younger than 5 | 1.0 (referent) | 0.178 |
| 5 or Older | 1.92 (0.74–4.96) | |
| Sex: | ||
| M | 1.0 (referent) | 0.916 |
| F | 1.05 (0.42–2.65) | |
| Clinical stage: | ||
| Low (I or II) | 1.0 (referent) | 0.005 |
| High (III or IV) | 3.94 (1.52–10.16) | |
| Histology: | ||
| FH | 1.0 (referent) | 0.294 |
| AH | 1.95 (0.56–6.80) | |
| Tumor necrosis: | ||
| Absent | 1.0 (referent) | 0.283 |
| Present | 0.51 (0.15–1.75) | |
| Mononuclear cell infiltration: | ||
| Absent | 1.0 (referent) | 0.186 |
| Present | 2.70 (0.62–11.73) | |
| Tumor B7-H1 expression: | ||
| Absent | 1.0 (referent) | 0.057 |
| Present | 2.73 (0.97–7.70) |
A total of 11 patients died at a mean of 3.8 years postoperatively (median 2.9, range 0.3 to 15.7). All deaths were attributable to the diagnosis of WT. Among the 70 patients who were still alive at last followup the mean duration of followup was 16.1 years (median 13.4, range 0.9 to 34.8). Estimated overall survival rates (SE, number still at risk) of all patients at 1, 3, 5 and 10 years postoperatively were 96.3% (2.1%, 77), 92.4% (3.0%, 71), 88.5% (3.6%, 65) and 87.0% (3.8%, 50), respectively. Patients 5 years or older at surgery were more than 5 times as likely to die as younger patients (RR 5.34, 95% CI 1.56 to 18.29, p = 0.008). Each 1-year increase in age at surgery was associated with an 8% increase in the risk of death (RR 1.08, 95% CI 1.03 to 1.14, p = 0.001). As with recurrence, patients with high stage tumors were significantly more likely to die compared to those with low stage tumors (RR 6.31, 95% CI 1.67 to 23.88, p = 0.007). Additionally, AH was associated with a significantly increased risk of death compared to FH (RR 5.36, 95% CI 1.51 to 19.01, p = 0.009). Tumor B7-H1 expression was not significantly associated with death among the 81 patients with WT.
Patients With Favorable Histology Wilms Tumor
Clinical and pathological features and B7-H1 expression for the 71 patients with FH WT are summarized in table 1. Average patient age at surgery was 4.5 years (median 3.0, range 0.5 to 38.1). All 3 patients who were older than 18 years at surgery had FH. Seven patients (10%) had tumor B7-H1 expression. However, none of the features studied in this subset was significantly associated with tumor B7-H1 expression.
At last followup 15 patients in this subset had recurrence at a mean of 3.0 years postoperatively (median 1.6, range 0.1 to 15.1). Estimated recurrence-free survival rates (SE, number still at risk) for patients with FH at 1, 3, 5 and 10 years postoperatively were 90.1% (3.5%, 63), 82.9% (4.5%, 56), 82.9% (4.5%, 53) and 81.3% (4.7%, 42), respectively. The associations of clinicopathological features and B7-H1 expression with recurrence are summarized in table 4. Patients with high stage tumors were significantly more likely to have recurrence compared to those with low stage tumors (RR 3.12, 95% CI 1.13 to 8.60, p = 0.028). Four of the 15 patients with FH WT recurrence had expression of B7-H1. Patients with FH and tumor B7-H1 expression were more than 3 times as likely to have recurrence compared to those without tumor B7-H1 expression (RR 3.65, 95% CI 1.16 to 11.48, p = 0.027).
Table 4. Association of clinical and pathological features and B7-H1 expression with recurrence in patients with FH WT.
| RR (95% CI) | p Value | |
|---|---|---|
| Age at primary surgery (yrs): | ||
| Younger than 5 | 1.0 (referent) | 0.505 |
| 5 or Older | 1.48 (0.47–4.65) | |
| Sex: | ||
| M | 1.0 (referent) | 0.916 |
| F | 1.06 (0.38–2.92) | |
| Clinical stage: | ||
| Low (I or II) | 1.0 (referent) | 0.028 |
| High (III or IV) | 3.12 (1.13–8.60) | |
| Tumor necrosis: | ||
| Absent | 1.0 (referent) | 0.632 |
| Present | 0.73 (0.21–2.60) | |
| Mononuclear cell infiltration: | ||
| Absent | 1.0 (referent) | 0.113 |
| Present | 5.15 (0.68–39.17) | |
| Tumor B7-H1 expression: | ||
| Absent | 1.0 (referent) | 0.027 |
| Present | 3.65 (1.16–11.48) |
Seven patients with FH tumors died at a mean of 4.8 years postoperatively (median 2.9, range 0.8 to 15.7). Among the 64 patients who were still alive at last followup the mean duration of followup was 16.6 years (median 14.8, range 0.9 to 34.8). Estimated overall survival rates (SE, number still at risk) for patients with FH at 1, 3, 5 and 10 years postoperatively were 98.6% (1.4%, 69), 94.2% (2.8%, 63), 92.7% (3.1%, 59) and 91.0% (3.5%, 46), respectively. Patients 5 years or older at surgery were more than 3 times as likely to die compared to younger patients. Each 1-year increase in age at surgery was associated with an 8% increase in the risk of death (RR 1.08, 95% CI 1.02 to 1.15, p = 0.012). High tumor stage (RR 3.60, 95% CI 0.80 to 16.15, p = 0.094) and tumor necrosis (RR 0.73, 95% CI 0.21 to 2.60, p = 0.067) were associated with an increased risk of death, although there were too few deaths observed to identify these associations as statistically significant.
Discussion
B7-H1, also known as PD-L1, was initially described in 1999 by Dong et al,8 and is a cell surface glycoprotein and a member of the B7 family of T cell coregulatory molecules. In the context of aberrant tumor expression B7-H1 exerts an inhibitory effect on host antitumoral immunity by inducing either antigen specific T cell apoptosis or anergy. Tumor B7-H1 expression has been observed to inhibit significantly activated T cell responses by abrogating cell cycle progression and proliferation.15
Although B7-H1 is typically expressed only by macrophage lineage cells, multiple human malignancies, including melanoma, lymphoma and carcinomas of the breast, ovary, lung, colon, head and neck, and kidney, can express B7-H1 aberrantly.7,8,12 Studies by our group have revealed associations of aberrant B7-H1 tumor expression with increased cancer recurrence and cancer specific death in adults with renal cell carcinoma.9,10 These findings become particularly important because of data showing that blockade of B7-H1 or one of its T cell cognate receptors, PD-1, robustly enhances antitumoral T cell responses.11–14 This result suggests that blockade of B7-H1 may represent an avenue to improve immunotherapeutic cancer treatment. As such, it appears that B7-H1 may be not only a useful prognostic feature for WT, but also a potential therapeutic target for patients at high risk for disease recurrence or cancer specific death.
Our study suggests that tumor expression of B7-H1 may be useful as a prognostic feature to facilitate the evaluation of patients with WT. B7-H1 expression was observed in 14% of all WT specimens surveyed, and demonstrated correlations with other previously described markers of WT aggressiveness, including AH and tumor necrosis. Moreover, tumor B7-H1 expression was significantly predictive of cancer recurrence. However, due to the relatively limited size of our study cohort (and, thus, the low number of cancer specific deaths observed), the associations seen between tumor B7-H1 expression and cancer specific death did not achieve statistical significance. Clearly, a larger multi-institutional study will be required to establish the usefulness of tumor B7-H1 as a prognostic indicator for WT progression and patient outcomes.
Dome et al recently reviewed the results from the fifth National Wilms Tumor Study, and concluded that there is room for improvement in the current treatment of WT.16 We noted that for the subset of patients with FH WT no traditional prognostic features correlate with B7-H1 expression. This finding implies that B7-H1 expression may represent an independent prognostic marker that portends aggressive behavior for FH WT. As such, B7-H1 may prove useful to distinguish patients with FH WT who require aggressive treatment from those who are at relatively low risk for disease recurrence and death, and who might be spared unnecessary overtreatment. Our finding that tumor B7-H1 expression is associated with a nearly 4-fold increased risk of recurrence in FH tumors may have significant implications for advancing the management of WT. B7-H1 expression by AH tumors may also find usefulness as a prognostic indicator for the assessment of patients with WT. However, the small number of patients with AH limits our ability to draw firm conclusions regarding B7-H1 and AH WT.
Several limitations of our study warrant comment. Most notable is the fact that our study represents the experience of a single institution with multiple patients undergoing multiple therapeutic regimens during different eras, although all patients were treated according to then current NWTSG guidelines. Given the dramatic advances in WT chemotherapy, radiotherapy and surgical technique during the last several decades, this variation could conceivably have influenced our outcomes analyses. Future investigation of B7-H1 as it pertains to WT will clearly benefit from the study of a more uniform cohort of patients.
In addition, as mentioned previously, our small cohort size prohibited in-depth analyses of factors that may have confounded our results. Specifically, the small number of recurrences in our series precluded multivariate analyses. Also, we acknowledge that the relative merits of treating patients at increased risk for recurrence of WT are controversial, given the high salvage rates with modern WT therapy. However, it is noteworthy that B7-H1 has potential as a therapeutic as well as a prognostic adjunct to current WT treatment modalities. Clearly, our preliminary observations display potential and merit further study.
In summary, WT exhibits the ability to express B7-H1. Consistent with what we have previously reported for adult ccRCC, B7-H1 expression within WT correlates with known features of biological aggressiveness, including stage and histology. B7-H1 expressed by WT is also associated with an increased risk of cancer recurrence in patients with FH tumors, and may portend an increased risk of cancer specific death, although larger multi-institutional studies will be required to establish better the potential of this T cell coregulatory molecule as a prognostic marker and therapeutic target to advance the treatment of WT.
Conclusions
B7-H1 is expressed by WT, correlates with tumor biology and is associated with an increased risk of recurrence in patients with FH tumors. B7-H1 may prove useful in identifying high risk patients who could benefit from more aggressive initial treatment regimens, and may represent a promising therapeutic target. Multi-institutional studies to elucidate the role of B7-H1 in the treatment of WT are warranted.
Fig. 2.
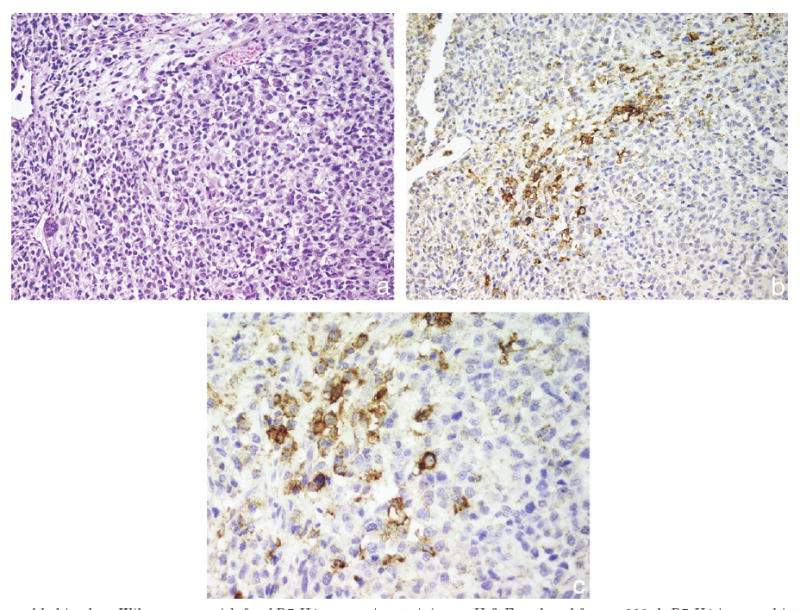
Favorable histology Wilms tumor with focal B7-H1 expression staining. a, H & E, reduced from ×200. b, B7-H1 immunohistochemical staining, reduced from ×200. c, B7-H1 immunohistochemical staining, reduced from ×400.
Acknowledgments
Supported partly by the Richard M. Schulze Family Foundation, and the Helen and Martin Kimmel Foundation.
Abbreviations and Acronyms
- AH
anaplastic histology
- ccRCC
clear cell renal cell carcinoma
- CI
confidence intervals
- FH
favorable histology
- NWTSG
National Wilms Tumor Study Group
- RR
relative risk
- SE
standard error
- WT
Wilms tumor
References
- 1.Green DM, D'Angio GJ, Beckwith JB, Breslow NE, Grundy PE, Ritchey ML, et al. Wilms tumor. CA Cancer J Clin. 1996;46:46. doi: 10.3322/canjclin.46.1.46. [DOI] [PubMed] [Google Scholar]
- 2.Pritchard-Jones K. Controversies and advances in the management of Wilms' tumour. Arch Dis Child. 2002;87:241. doi: 10.1136/adc.87.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green DM, Grigoriev YA, Nan B, Takashima JR, Norkool PA, D'Angio GJ, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study group. J Clin Oncol. 2001;19:1926. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 4.Green DM, Breslow NE, Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, et al. Treatment with nephrectomy only for small, stage I/favorable histology Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol. 2001;19:3719. doi: 10.1200/JCO.2001.19.17.3719. [DOI] [PubMed] [Google Scholar]
- 5.Breslow NE, Takashima JR, Whitton JA, Moksness J, D'Angio GJ, Green DM. Second malignant neoplasms following treatment for Wilm's tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol. 1995;13:1851. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 6.Breslow NE, Collins AJ, Ritchey ML, Grigoriev YA, Peterson SM, Green DM. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol. 2005;174:1972. doi: 10.1097/01.ju.0000176800.00994.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 8.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 11.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 12.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501. [PubMed] [Google Scholar]
- 13.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089. [PubMed] [Google Scholar]
- 14.Thompson RH, Webster WS, Cheville JC, Lohse CM, Dong H, Leibovich BC, et al. B7-H1 glycoprotein blockade: a novel strategy to enhance immunotherapy in patients with renal cell carcinoma. Urology. 2005;66(suppl):10. doi: 10.1016/j.urology.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 16.Dome JS, Cotton CA, Perlman EJ, Breslow NE, Kalapurakal JA, Ritchey ML, et al. Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol. 2006;24:2352. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]


