Abstract
Recent research in breast biology has provided support for the cancer stem-cell hypothesis. Two important components of this hypothesis are that tumors originate in mammary stem or progenitor cells as a result of dysregulation of the normally tightly regulated process of self-renewal. As a result, tumors contain and are driven by a cellular subcomponent that retains key stem-cell properties including self-renewal, which drives tumorigenesis and differentiation that contributes to cellular heterogeneity. Advances in stem-cell technology have led to the identification of stem cells in normal and malignant breast tissue. The study of these stem cells has helped to elucidate the origin of the molecular complexity of human breast cancer. The cancer stem-cell hypothesis has important implications for early detection, prevention, and treatment of breast cancer. Both hereditary and sporadic breast cancers may develop through dysregulation of stem-cell self-renewal pathways. These aberrant stem cells may provide targets for the development of cancer prevention strategies. Furthermore, because breast cancer stem cells may be highly resistant to radiation and chemotherapy, the development of more effective therapies for this disease may require the effective targeting of this cell population.
Introduction
There is both good news and bad news in the fight against breast cancer. The good news is that there has been a steady decline in the death rate from breast cancer in this country and abroad since 1990. A 2% annual decrease in the death rate has resulted in an overall 25% reduction in cancer deaths in 2007 compared with 1990.1 Furthermore, the development of new treatments, such as trastuzumab and the aromatase inhibitors, has offered new hope to women with both early and advanced breast cancer. However, despite these clinical advances, as well as advances in our understanding of the biology of breast cancer, more than 44,000 women still die as a result of breast cancer annually in the United States alone. Recent analysis of the fall in death rates from breast cancer indicates that approximately half of this is the result of improved early detection through mammography screening, and the other half a result of improvements in adjuvant therapies for early-stage disease.2 In contrast, there has been relatively little change in the overall survival for women with metastatic breast cancer during the last several decades.3 Furthermore, even though recurrence rates have been significantly reduced by adjuvant therapies utilizing chemotherapy, hormonal therapy, and most recently, trastuzumab, an inhibitor of human epidermal growth factor receptor 2 (HER-2), recurrence still occurs in a substantial proportion of women after these treatments.
The heterogeneity and molecular complexity of breast cancer poses many challenges for the development of effective strategies to prevent and treat this disease. In addition, there is increasing support for the cancer stem-cell hypothesis, which, if correct, provides an explanation for the limitation of many current breast cancer models and suggests new strategies for breast cancer prevention and therapy. Classical models of carcinogenesis can be described as “stochastic” or “random,” in which any cell in an organ, such as the breast, can be transformed by the right combination of mutations.4 As a result, all or most of the cells in a fully developed cancer are equally malignant. It follows that strategies designed to treat and ultimately cure these cancers require the killing of all these malignant cells. The cancer stem-cell hypothesis is a fundamentally different model composed of two separate, but interrelated, components. The first is that tumors originate in tissue stem and/or progenitor cells through the dysregulation of the normally tightly regulated process of self-renewal.5 As a consequence, tumors contain a cellular component that retains key stem-cell properties including self-renewal, which initiates and drives carcinogenesis and differentiation, albeit aberrant, that contributes to tumor cellular heterogeneity.6 Although the concept that cancers arise from germ cells or stem cells was first proposed more than 150 years ago,7 it is only recently that advances in stem-cell biology have allowed for a more direct testing of the cancer stem-cell hypothesis. We will review recent evidence supporting this hypothesis and discuss its implications for breast carcinogenesis, cancer prevention, and cancer therapy.
Identification of Normal Breast Stem Cells
Stem cells are defined by their ability to undergo self-renewal, as well as multilineage differentiation. Self-renewal may be either symmetric, in which a stem cell produces two daughter stem cells, or asymmetric, in which the stem cell produces a daughter stem cell as well as a cell that leaves the stem-cell niche to differentiate.8 In the mammary gland, these differentiating cells generate three lineages: ductal epithelial cells, which line ducts; alveolar epithelial cells, which are the milk-producing cells; and myoepithelial cells, which are contractile cells lining ducts and alveoli. Until recently, the isolation and characterization of breast stem cells was limited by the lack of identified cell-surface markers for these cells. The existence of stem cells in rodent mammary glands was first demonstrated by Kordon et al,9 who showed the ability to repopulate mouse mammary glands with serial transplantation of retrovirally marked epithelial fragments. Similar mammary fat pad transplantation models have more recently been used to prospectively identify mouse mammary cells with stem-cell properties. Cells expressing CD29 and/or CD49F (β1 and α6 integrin, respectively) as well as CD24 displayed the stem-cell properties of self-renewal and multilineage differentiation.10 A single cell from the CD29high/CD24+ or DC49Fhigh/CD24+ population was able to reconstitute a functional mammary gland when this cell was transplanted into a cleared mouse mammary fat pad.11 The murine mammary stem cell does not express estrogen receptor (ER) or progesterone receptor (ER) but is able to give rise to ER-expressing and PR-expressing cells.12 Recently, our laboratory has provided evidence for the existence of similar stem cell–like populations in the human mammary gland characterized by expression of aldehyde dehydrogenase 1. This enzyme has also been reported to be expressed in hematopoietic and neuronal stem and progenitor cells,13,14 and can be detected utilizing an enzymatic “Aldefluor” assay or by immunohistochemistry utilizing antibodies to ALDH1.
Characterization of stem cells in both human and rodent systems has been facilitated by the development of in vitro culture systems that allow for propagation of mammary stem and progenitor cells in an undifferentiated state. Previously, it had been found that primitive neuronal cells could be propagated as floating spherical colonies termed “neurospheres.”15 On this basis, we hypothesized that normal and malignant stem cells might display anchorage independent growth, and utilized this property to develop a culture system for human mammary epithelial stem and progenitor cells. We demonstrated that such cells isolated from reduction mammoplasties when grown on nonadherent substrata in serum-free conditions in the presence of growth factors generate spherical colonies that we termed “mammospheres.”16 Mammosphere-initiating cells have stem-cell properties and are able to self-renew in vitro as well as differentiating into all three lineages found in the mammary gland. Furthermore, mammosphere-initiating cells express aldehyde dehydrogenase and are capable of generating human mammary structures when transplanted into the humanized fat pad of immunosuppressed nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.17
Breast Cancer Stem Cells
Evidence for existence of cancer stem cells was first reported by Dick et al in acute myelogenous leukemia.18 We utilized a similar approach to prospectively isolate similar populations of cells from human breast cancers. In collaboration with Michael Clarke, we demonstrated that human breast cancers contain a cellular population characterized by the expression of cell-surface markers CD44+/CD24low/lin−, which display stem-cell properties. As few as 200 of these cells, comprising 1% to 10% of the total population, were capable of forming tumors when implanted in NOD/SCID mice.19 In contrast, 20,000 cells that did not express these markers were unable to form tumors. Consistent with the cancer stem-cell model, the stem cells were able to generate tumors that recapitulated the phenotypic heterogeneity of the initial tumor.19 As is the case with normal stem cells, breast tumor stem cells also form mammospheres in vitro.20 Furthermore, as was the case with normal breast stem cells, breast cancer stem cells can be isolated on the basis of their increased expression of aldehyde dehydrogenase. Indeed, there is partial overlap between the CD44+/CD24−/lin− and ALDH-positive populations with cells expressing the phenotype CD44+CD24−/lin−/ALDH-positive able to form tumors from as few as 20 cells.17
Clinical Implications of the Cancer Stem-Cell Hypothesis
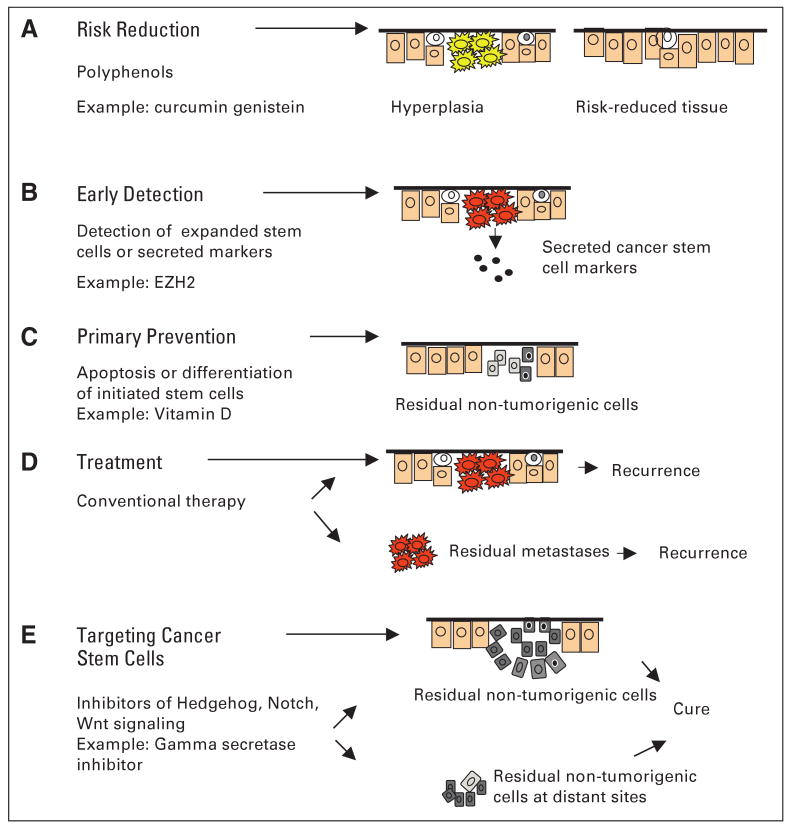
The cancer stem-cell hypothesis has fundamental implications for breast carcinogenesis as well as important clinical implications for prevention and therapy. These are summarized in Figure 1.
Fig 1.
Clinical interventions targeting stem cells (SCs) for cancer prevention and treatment. Cancers arise through dysregulation of SC self-renewal pathways. This produces tumors driven by a cancer SC component. Shown are strategies for cancer risk reduction, early detection, primary prevention, and treatment based on targeting the SC population. ER, estrogen receptor.
Breast Carcinogenesis
The cancer stem-cell hypothesis proposes that cancers arise in breast, stem, and/or progenitor cells through dysregulation of the normally tightly regulated process of self-renewal. It is important to emphasize that this hypothesis does not require that all cancers arise from normal tissue stem cells. Indeed, there is substantial evidence in the hematopoietic system that leukemias may arise from transformed progenitor cells as well as stem cells. Overexpression of the MLL fusion gene in hematopoietic progenitors results in production of leukemias, which are driven by cells that acquire stem-cell properties.21 Interestingly, these progenitors acquire the expression of self-renewal genes normally expressed in hematopoietic stem cells.21 Recent studies have demonstrated a similar phenomenon involving an ETS transcription factor, ETV6, fused to the protein tyrosine kinase domain of MTRK3, a molecular event that is found in human secretory breast carcinoma.22 Li et al, reported that the ETV6/NTRK3 fusion oncogene acts on committed mammary progenitor cells to produce breast cancers in transgenic mice.22 Further evidence that stem/progenitor cells may be targets for transformation have been suggested by mouse mammary tumor virus (MMTV)-driven carcinogenesis models.23 MMTV-Wnt tumors display markers of both epithelial and myoepithelial lineage, whereas MMTV Neu tumors show only luminal differentiation, suggesting that Wnt may effect a primitive bipotent progenitor cells, whereas NEU may target a luminal committed progenitor cell.24 Furthermore, in MMTV-wnt-1 transgenic mice, the number of cells displaying stem-cell markers expanded more than six-fold in the preneoplastic phase, whereas MMTV Neu tumors did not demonstrate stem-cell expansion.10 If similar events occur in human carcinogenesis, it could provide an explanation for aspects of molecular heterogeneity found in human breast cancers. Discrete molecular phenotypes revealed by gene expression analysis may reflect different cells of origin as well as the mutation profile in human breast cancers.25
Mammary Stem-Cell Number As a Determinate of Breast Cancer Risks
If breast tumors can originate in mammary stem cells through dysregulation of the self-renewal process, then breast stem-cell number may be a risk factor for carcinogenesis. Development of the mammary gland in humans and rodents is regulated at three critical periods of development: embryogenesis, puberty, and pregnancy. Changes in the hormonal milieu during these developmental windows may determine the size of the breast stem-cell pool, thereby influencing carcinogenesis.26 Several studies have shown a strong link between birth weight and breast cancer risk in offspring, as well as a strong association of maternal levels of insulin-like growth factor-1 (IGF-1) and birth weight.27 IGF-1 and steroid hormones in utero may modulate subsequent breast cancer risk by regulating the number of mammary stem cells.28,29
The growth hormone/IGF-1 axis may serve as a master regulator of adult stem cells in different organs.30 Growth hormone, an anabolic pulsatile hormone secreted by the pituitary gland, is a major regulator of IFG-1 synthesis and secretion. Not only does growth hormone indirectly regulate cell proliferation mediated by IGF-1 but also acts directly on cells that express growth hormone receptor through stimulation of JAK/STAT (janus kinase/signal transducer and activator of transcription) signaling.28 We have reported that mammary stem/ progenitor cells grown in mammospheres overexpress growth hormone receptors compared with cells induced to differentiate by attachment to a collagen substratum,31 and have found that growth hormone stimulates mammary stem-cell self-renewal (unpublished data). Several clinical lines of evidence support a link between growth hormone levels and breast cancer risk. The rate of increase in height during adolescence, largely regulated by growth hormone, is strongly related to subsequent risk of breast cancer.32 Furthermore, women with acromegaly who have increased growth hormone levels are at increased risk for developing cancers, including breast cancer.30 The link between growth hormone and breast stem cells may also account for the development of aggressive breast cancers associated with pregnancy. Although pregnancy at an early age protects against subsequent breast cancer development,30 breast cancers developing during pregnancy tend to be aggressive and of the basal phenotype, ER/PR/HER-2 negative.33,34 Interestingly, high levels of estrogen and progesterone produced during the third trimester of pregnancy induce local growth hormone production by mammary epithelial cells. This suggests the intriguing possibility that the aggressive basal breast carcinomas associated with pregnancy may be driven by local production of growth hormone, which acts as a paracrine regulator of breast stem cells.35
Hereditary Breast Cancers and BRCA1
Heterozygous germline mutations in the BRCA1 gene predispose women to up to an 80% lifetime risk of developing breast cancer.35 Most of these tumors are of the basal phenotype, characterized by expression of myoepithelial markers, but lack expression of ER, PR, and ERBB2 receptor.36 It is well established that BRCA1 plays an important role in DNA repair, activation of cell-cycle checkpoints, and maintenance of chromosome stability. However, these characteristics do not explain the organ specificity of carcinogenesis. Foulkes et al36 proposed that the clinical, molecular, and pathologic features of breast cancer in BRCA1 mutation carriers suggest the possibility that BRCA1 may function as a stem-cell regulator. The development of in vitro and mouse models for breast stem-cell function has allowed a direct test of this hypothesis. Utilizing these systems, we demonstrated that BRCA1 expression is required for the differentiation of ER-negative stem/progenitor cells into ER-positive luminal cells.37 Knockdown of BRCA1 in primary breast epithelial cells leads to an increase in cells displaying the stem-cell marker ALDH1 and a decrease in cells expressing luminal epithelial markers and ER. Furthermore, in breast tissues from women with germline BRCA1 mutations but not in normal controls, we detected entire lobules that, although histologically normal, were positive for ALDH1 expression but negative for expression of ER. Loss of heterozygosity for BRCA1 was documented in these ALDH1-positive lobules but not in adjacent ALDH1-negative lobules.37 These studies suggest that loss of BRCA1 expression may result in an accumulation of genetically unstable breast stem cells, providing targets for further carcinogenic events.
Self-Renewal Pathways in Breast Stem Cells
Elucidation of the pathways that regulate self-renewal of breast stem cells has led to a clearer picture of how dysregulation of these pathways may lead to carcinogenesis. Furthermore, these pathways may provide targets for breast cancer prevention and therapy. Indeed, recent evidence suggests that key oncogenic pathways known to be dysregulated in breast cancer also regulate stem-cell behavior.26
HER-2
An early event in the development of sporadic breast cancer may be the amplification and overexpression of the HER-2 gene, a member of the epidermal growth factor receptor family. Approximately 20% to 25% of human breast cancers display HER-2 amplification. These tumors have a distinct molecular profile and aggressive clinical course associated with the propensity to develop metastasis in areas such as the brain.38 The development of HER-2 inhibitors such as trastuzumab, or more recently, lapatinib, have provided important new agents with demonstrated clinical benefit in both adjuvant and advanced disease. The addition of trastuzumab to adjuvant chemotherapy reduces the recurrence rate by almost 50%.39 Interestingly, in a series of 477 breast carcinomas, we found a significant correlation between expression of the stem-cell marker ALDH1 and HER-2 overexpression.17 Furthermore, we have recently found that HER-2 overexpression in normal human mammary epithelial cells as well as mammary carcinomas increases the proportion of stem cells, as indicated by ALDH expression. Trastuzumab reduces the stem-cell population in trastuzumab-sensitive but not -resistant breast cancer cell lines.40 Together, these results suggest that HER-2 may play a role in mammary carcinogenesis by regulating the stem-cell population. If this is the case, then the remarkable clinical efficacy of HER-2 inhibitors may be a result of the ability of these agents to directly target breast cancer stem cells.
PTEN
Another frequent abnormality in human breast cancers is deletion of the PTEN gene, a defect found in approximately 40% of human breast cancers.41 Furthermore, women with BRCA1 germline mutations develop microdeletions of PTEN.42 PTEN is lipid phosphatase which regulates phosphoinositide-3 kinase Akt signaling. PTEN has previously been shown to regulate self-renewal of hematopoietic and neuronal stem cells.43 We have preliminary evidence that deletion of PTEN has similar effects on normal and malignant breast stem cells. This suggests that development of inhibitors of AKT or mammalian target of rapamycin signaling may be able to target normal and malignant breast stem cells.
Wnt Signaling
Wnt signaling has also been shown to be involved in regulating the self-renewal and differentiation of a variety of stem cells. As indicated previously, MMTV-Wnt mammary tumors display increases in cells expressing stem-cell markers.10 Activation of the canonical Wnt pathway begins with the binding of Wnt proteins to cell-surface receptors in the Frizzled family and the low-density lipoprotein receptor–related proteins LRP5 and -6. This signaling increases cytoplasmic β-catenin, which translocates to the nucleus, where it binds to transcription factors in the LEF1/TCF family.44 Wnt ligands have been shown to be expressed in embryonic mammary development45 Embryos expressing the canonical Wnt inhibitor Dkk1 display a complete block formation of mammary placodes, and mice deficient for LEF1 failed to maintain mammary buds, demonstrating that Wnt signaling is required for normal embryonic mammary development.46
Notch Signaling
Other developmental signaling pathways have been shown to play a role in mammary carcinogenesis in murine models, as well as in human mammary cancer. Notch signaling has been shown to play a role in cell fate determination in neural, hematopoietic, and embryonic stem cells. In mammals, there are four notch receptors (notch 1-4) interacting with surface-bound or secreted ligands (Delta-like 1, Delta-like 3, Delta-like 4, Jagged 1, and Jagged 2).47 Modifier proteins from the Fringe family (Lunatic, Manic, and Radical Fringe) modulate these interactions.48 On ligand binding, Notch receptors are activated by serial cleavage events involving members of the ADAM protease family followed by intramembranous cleavage regulated by gamma secretase (presenilin). After proteolitic cleavage, the intracellular domain of Notch translocates to the nucleus to act on downstream targets such as the Hes and Hay transcription factors.48 Approximately 40% of human breast cancers display reduced expression of the Notch inhibitor NUMB.49 Interestingly, in addition to playing a role in the regulation of Notch signaling, NUMB may also regulate p53.50 Evidence for the role of Notch signaling in mammary development has been provided by transgenic models. Dontu et al51 demonstrated that Notch activation acts as a regulator of asymmetric cell fate decisions in human mammary cells by promoting mammary self renewal. In addition, Notch acts on later stages of mammary development affecting cell-fate commitment. Because the enzyme gamma secretase is necessary for Notch processing, gamma secretase inhibitors are able to inhibit Notch signaling.52 Clinical trials utilizing gamma secretase inhibitors in combination with chemotherapy for women with advanced breast cancer are being initiated. Such trials will directly test the hypothesis that targeting breast cancer stem cells improves the therapeutic outcome in these women.
Hedgehog Signaling and BMI-1
Transgenic models have suggested a role for hedgehog signaling in normal mammary development and carcinogenesis. Furthermore, there is evidence for dysregulation of this pathway in a subset of human breast cancers. Utilizing both in vitro culture systems and NOD/SCID mice, Liu et al53 demonstrated that hedgehog signaling regulates the self-renewal of both normal and malignant human mammary stem cells. Furthermore, this occurs by regulation of a polycomb gene BMI-1. This suggests that hedgehog signaling, acting through BMI-1 is able to regulate the self-renewal of normal and malignant human mammary stem cells. This process is blocked by specific inhibitors such as cyclopamine.53 The development of cyclopamine analogs and other hedgehog inhibitors is currently underway, and clinical trials utilizing these agents are in the planning stages.
Mammary Carcinogenesis: A Conceptual Link Between Hereditary and Sporadic Breast Cancers
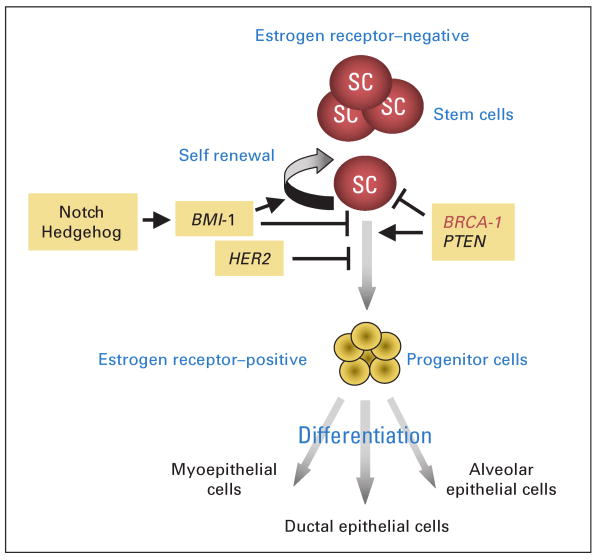
The elucidation of breast stem-cell self-renewal pathways suggests a conceptual link between hereditary and sporadic breast carcinogenesis. Both may be initiated by expansion of mammary stem and/or progenitor cells. In the case of hereditary breast cancer, this may occur via deletion of the normal allele of BRCA1. In sporadic breast cancers, activation of other pathways such as Notch, Hedgehog, or Wnt; amplification of HER-2; or deletion of PTEN may lead to dysregulation of stem-cell self-renewal, resulting in stem-cell expansion. These expanded stem cells provide targets for further carcinogenic events. This conceptual model is depicted in Figure 2. Clonal expansion of breast stem cells might explain the field carcinogenesis observed in human breast cancers. For instance, histologically normal lobules surrounding breast carcinomas share a number of molecular abnormalities with adjacent cancer cells such as PTEN deletion or p53 mutation.54 The detection of expanded stem-cell clusters using markers such as ALDH1 in breast biopsy tissues may identify women with increased risk of subsequent breast cancer development.37
Fig 2.
Self-renewal and differentiation pathways in breast stem cells. Both hereditary and sporadic breast cancers may originate in breast stem/progenitor cells through dysregulation of the normally tightly regulated process of stem-cell self-renewal. This may result from loss of BRCA1 function in hereditary breast cancers. In sporadic cancers this may result from loss of PTEN or activation of the human epidermal growth factor receptor 2, Notch, or Hedgehog pathways. This results in clonal expansion of stem cells providing targets for further carcinogenic events.
Expression of Stem-Cell Markers and Prognosis
A number of studies have demonstrated that expression of stem-cell markers in mammary tumors has prognostic significance. For instance, in a series of 477 breast carcinoma patients, expression of ALDH1 was associated with poor clinical outcome.17 Expression of this marker also identified a subset of patients with inflammatory breast carcinoma with an increased risk of recurrence. In addition, a 186-gene signature identified from CD44+/CD24low tumorigenic cells isolated from human tumors correlated significantly both with overall and metastasis-free survival in patients with breast cancer, as well as other malignancies.55
Implications of the Cancer Stem-Cell Hypothesis for Breast Cancer Prevention
The cancer stem-cell hypothesis suggests that strategies targeting breast stem-cell populations may prove effective for cancer prevention and therapy. The effectiveness of prevention strategies aimed at modulating stem-cell number during key developmental windows including in utero and adolescence have been suggested by animal models. Hilakivi-Clark et al56 demonstrated that phytoestrogens administered to pregnant mice decreased breast cancer development in their offspring. Similarly, phytoestrogens have protective effects when administered during adolescent, but not adult, stages of rodent development.56 These studies have implications for dietary modifications in women. In addition, chemopreventive agents such as curcumin from turmeric may function by modulating stem-cell self-renewal pathways including Wnt and Notch.57,58 In addition, several dietary polyphenoids including apple-derived quercetin, and epigallocetechin-galleate have been shown to regulate molecules in the Wnt-β catenin and Notch pathways.59 Vitamin D3 has also been shown to be involved in stem-cell differentiation, and therefore may have applications for cancer prevention strategies aimed at the stem cell.60
Metastasis and the Stem-Cell Phenotype
The most important prognostic factor influencing the outcome of patients with invasive breast cancer is whether the tumor has spread regionally or systemically.61 There is increasing evidence that cancer stem cells play an important role in mediating tumor metastasis. As described previously, breast cancer stem cells have been characterized as having the cell-surface phenotype CD44+/CD24−. CD44 is a cell-adhesion molecule involved in binding of cells to hyaluronic acid, whereas CD24 is a negative regulator of the chemokine receptor CXCR4, a molecule involved in breast cancer metastasis.62 To determine the relationship of the stem-cell phenotype to metastasis, Balic et al63 examined the expression of stem-cell markers in metastatic bone marrow sites in patients with breast carcinoma and found an increased in CD44+-/CD24−-expressing cells. Although the presence of micrometastasis in the bone marrow is associated with poor prognosis, approximately 50% of patients with such micrometastasis do not develop clinically apparent macrometastasis with a 10-year follow-up. Studies are currently in progress to determine whether expression of stem-cell markers in bone marrow and lymph node micrometastasis predict relapse.
We have recently demonstrated the invasive and metastatic characteristics of cancer stem cells. Aldefluor-positive populations of mammary carcinoma cell lines display increased invasive characteristics as well as increased ability to metastasize when injected into the left ventricle of NOD/SCID mice.64
There is increasing evidence that the tumor microenvironment plays an important role in tumor growth and metastasis. Indeed, breast density is an important risk factor for breast cancer development.65,66 Breast density appears to be related to characteristics of breast stromal fibroblasts.67 Growth factors produced by these stromal elements may play a role in regulating mammary stem-cell behavior. In addition to mammary fibroblasts, the role of endothelial cells and adipocytes in mammary stem cell behavior is currently being investigated.
Cancer Treatment
The cancer stem-cell hypothesis has important implications for the development of cancer therapeutics. Recent evidence indicates that breast cancer stem cells,68 as well as cancer stem cells from other tumor types, are relatively resistant to both radiation and chemotherapy.69 There are several postulated mechanisms for this resistance. Stem cell are slowly proliferating largely in the G0 phase of the cell cycle for extended periods of time, making them resistant to cell-cycle active chemotherapeutic agents. In addition, these cancer stem cells express increased adenosine triphosphate–binding cassette proteins known to efflux chemotherapeutic drugs. Indeed, ABCG2, or breast cancer–resistance protein, was initially identified in breast cancers. This molecule is overexpressed in stem cells and has been utilized to purify breast and other stem cells by exclusion of Hoechst dye, generating the so-called side population detected by flow cytometry.70 In addition, enzymes such as ALDH that are highly expressed in stem cells are able to metabolize chemotherapeutic agents such as cyclophosphamide.71 Cancer stem cells may also express increased levels of antiapoptotic molecules such as survivin and BCL2-family proteins.72
Current clinical trial designs have largely been based on strategies aimed at producing tumor regression. Indeed, the Response Evaluation Criteria in Solid Tumors (RECIST) criteria measuring tumor response have been used to assess efficacy of new therapeutic agents. However, in breast cancer, as is the case with other malignancies, tumor regression does not correlate well with patient survival.61 In the neoadjuvant setting, only a complete pathologic response correlates with recurrence and survival, whereas partial response does not.73 Together with studies demonstrating resistance of breast cancer stem cells to chemotherapy and radiation therapy, these studies suggest that limitations of present therapies may relate to their inability to target the cancer stem cell component. Recent neoadjuvant studies demonstrating an increase in the proportion of CD44+/CD24− breast cancer stem cells after chemotherapy suggest that this is the case.74,75 Most importantly, recent reports by Chang et al75 suggest that targeting HER-2 with lapatinib in a neoadjuvant clinical trial was able to reduce the cancer stem-cell population, and that this resulted in a significantly increased pathologic complete response rate. This provides strong clinical support for the cancer stem-cell hypothesis because HER-2 drives the cancer stem-cell population. The effectiveness of HER-2 inhibitors such as trastuzumab and lapatinib may relate directly to the ability of these agents to target the cancer stem-cell population. The elucidation of pathways that regulate breast cancer stem cells, such as Notch, Hedgehog, and Wnt, provide new targets for therapeutic development. Furthermore, the ability to directly measure the effect of these interventions on breast cancer stem-cell populations utilizing a neoadjuvant trial design should permit a direct test of the cancer stem-cell hypothesis. The ultimate test of this hypothesis, however, will be the demonstration that the successful targeting of cancer stem cells results in improved clinical outcomes for patients with breast cancer.
Acknowledgments
Supported by National Institutes of Health Grants No. CA101860, CA66233, and P 30 C CA46592, and by the Taubman Institute.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Authors' Disclosures of Potential Conflicts of Interest: Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Max S. Wicha, OncoMed Pharmaceuticals (C) Stock Ownership: Max S. Wicha, OncoMed Pharmaceuticals Honoraria: None Research Funding: Max S. Wicha, Merck Expert Testimony: None Other Remuneration: None
Author Contributions: Conception and design: Max S. Wicha
Financial support: Max S. Wicha
Provision of study materials or patients: Madhuri Kakarala, Max S. Wicha
Collection and assembly of data: Madhuri Kakarala
Data analysis and interpretation: Madhuri Kakarala, Max S. Wicha
Manuscript writing: Madhuri Kakarala, Max S. Wicha
Final approval of manuscript: Max S. Wicha
References
- 1.Heron M. Deaths: Leading causes for 2004. Natl Vital Stat Rep. 2007;56:1–95. [PubMed] [Google Scholar]
- 2.Calvocoressi L, Sun A, Kasl SV, et al. Mammography screening of women in their 40s: Impact of changes in screening guidelines. Cancer. 2008;112:473–480. doi: 10.1002/cncr.23210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minino AM, Heron MP, Smith BL. Deaths: Preliminary data for 2004. Natl Vital Stat Rep. 2006;54:1–49. [PubMed] [Google Scholar]
- 4.Martínez-Climent JA, Andreu EJ, Prosper F. Somatic stem cells and the origin of cancer. Clin Transl Oncol. 2006;8:647–663. doi: 10.1007/s12094-006-0035-7. [DOI] [PubMed] [Google Scholar]
- 5.Graziano A, d'Aquino R, Tirino V, et al. The stem cell hypothesis in head and neck cancer. J Cell Biochem. 2008;103:408–412. doi: 10.1002/jcb.21436. [DOI] [PubMed] [Google Scholar]
- 6.Wicha MS, Liu S, Dontu G. Cancer stem cells: An old idea: A paradigm shift. Cancer Res. 2006;66:1883–1890. 1895–1896. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 7.Julius Cohnheim. (1839-1884) experimental pathologist. JAMA. 1968;206:1561–1562. [PubMed] [Google Scholar]
- 8.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 9.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 10.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 11.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 12.Vaillant F, Asselin-Labat ML, Shackleton M, et al. The emerging picture of the mouse mammary stem cell. Stem Cell Rev. 2007;3:114–123. doi: 10.1007/s12015-007-0018-2. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong L, Stojkovic M, Dimmick I, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 14.Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolis SK. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol Dis. 2007;25:217–229. doi: 10.1016/j.nbd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: Implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:75–86. doi: 10.1007/s10911-005-2542-5. [DOI] [PubMed] [Google Scholar]
- 17.Ginestier C, Hur M, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant breast stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 21.Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Tognon CE, Godinho FJ, et al. ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex. Cancer Cell. 2007;12:542–558. doi: 10.1016/j.ccr.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GH. Stem cells and mammary cancer in mice. Stem Cell Rev. 2005;1:215–223. doi: 10.1385/SCR:1:3:215. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Welm B, Podsypanina K, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Dontu G, Liu S, Wicha MS. Stem cells in mammary development and carcinogenesis: Implications for prevention and treatment. Stem Cell Rev. 2005;1:207–213. doi: 10.1385/SCR:1:3:207. [DOI] [PubMed] [Google Scholar]
- 27.Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119:2007–2025. doi: 10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- 28.Savarese TM, Strohsnitter WC, Low HP, et al. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: Implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. doi: 10.1186/bcr1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginestier C, Wicha MS. Mammary stem cell number as a determinate of breast cancer risk. Breast Cancer Res. 2007;9:109. doi: 10.1186/bcr1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins PJ. Acromegaly and cancer. Horm Res. 2004;62(suppl):108–115. doi: 10.1159/000080768. [DOI] [PubMed] [Google Scholar]
- 31.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 33.Russo J, Balogh GA, Heulings R, et al. Molecular basis of pregnancy-induced breast cancer protection. Eur J Cancer Prev. 2006;15:306–342. doi: 10.1097/00008469-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr Relat Cancer. 2007;14:907–933. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 35.van Garderen E, Schalken JA. Morphogenic and tumorigenic potentials of the mammary growth hormone/growth hormone receptor system. Mol Cell Endocrinol. 2002;197:153–165. doi: 10.1016/s0303-7207(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 36.Foulkes WD. BRCA1 functions as a breast stem cell regulator. J Med Genet. 2004;41:1–5. doi: 10.1136/jmg.2003.013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Ginestier C, Charafe-Jauffret E, et al. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci U S A. 2008;105:1680–1685. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 39.Slamon D, Pegram M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol. 2001;28:13–19. doi: 10.1016/s0093-7754(01)90188-5. [DOI] [PubMed] [Google Scholar]
- 40.Korkaya H, Paulson A, Iovino F, et al. HER-2 Signaling regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Cancer Cell. doi: 10.1038/onc.2008.207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panigrahi AR, Pinder SE, Chan SY, et al. The role of PTEN and its signalling pathways, including AKT, in breast cancer; an assessment of relationships with other prognostic factors and with outcome. J Pathol. 2004;204:93–100. doi: 10.1002/path.1611. [DOI] [PubMed] [Google Scholar]
- 42.Saal LH, Gruvberger-Saal SK, Persson C, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi DJ, Weissman IL. Pten, tumorigenesis, and stem cell self-renewal. Cell. 2006;125:229–231. doi: 10.1016/j.cell.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Bu G. LRP5/6 in Wnt signaling and tumorigenesis. Future Oncol. 2005;1:673–681. doi: 10.2217/14796694.1.5.673. [DOI] [PubMed] [Google Scholar]
- 45.Turashvili G, Bouchal J, Burkadze G, et al. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73:213–223. doi: 10.1159/000098207. [DOI] [PubMed] [Google Scholar]
- 46.Uyttendaele H, Soriano JV, Montesano R, et al. Notch4 and Wnt-1 proteins function to regulate branching morphogenesis of mammary epithelial cells in an opposing fashion. Dev Biol. 1998;196:204–217. doi: 10.1006/dbio.1998.8863. [DOI] [PubMed] [Google Scholar]
- 47.Brennan K, Brown AM. Is there a role for Notch signalling in human breast cancer? Breast Cancer Res. 2003;5:69–75. doi: 10.1186/bcr559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 2006;6:905–918. doi: 10.2174/156652406779010830. [DOI] [PubMed] [Google Scholar]
- 49.Pece S, Serresi M, Santolini E, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167:215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Echeverri CJ, Beachy PA, Baum B, et al. Minimizing the risk of reporting false positives in large-scale RNAi screens. Nat Methods. 2006;3:777–779. doi: 10.1038/nmeth1006-777. [DOI] [PubMed] [Google Scholar]
- 51.Dontu G, Jackson KW, McNicholas E, et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: A new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- 53.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patocs A, Zhang L, Xu Y, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 55.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 56.Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–348. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Zhang Y, Banerjee S, et al. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–2513. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 58.Jaiswal AS, Marlow BP, Gupta N, et al. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 59.Pahlke G, Ngiewih Y, Kern M, et al. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006;54:7075–7082. doi: 10.1021/jf0612530. [DOI] [PubMed] [Google Scholar]
- 60.Nagler A, Riklis I, Kletter Y, et al. Effect of 1,25 dihydroxyvitamin D3 and retinoic acid on normal human pluripotent (CFU-mix), erythroid (BFU-E), and myeloid (CFU-C) progenitor cell growth and differentiation patterns. Exp Hematol. 1986;14:60–65. [PubMed] [Google Scholar]
- 61.Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007;43:867–876. doi: 10.1016/j.ejca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Abraham BK, Fritz P, McClellan M, et al. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- 63.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 64.Charafe-Jauffret E, Ginestier C, Iovino F, et al. The metastatic phenotype of inflammatory breast cancer is associated with ALDH1-positive stem cells. Proc Natl Acad Sci U S A. in press. [Google Scholar]
- 65.Filip S, Mokry J, English D. Stem cell plasticity and carcinogenesis. Neoplasma. 2006;53:87–91. [PubMed] [Google Scholar]
- 66.Reinier KS, Vacek PM, Geller BM. Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and postmenopausal women. Breast Cancer Res Treat. 2007;103:343–348. doi: 10.1007/s10549-006-9375-9. [DOI] [PubMed] [Google Scholar]
- 67.Savarese TM, Low HP, Baik I, et al. Normal breast stem cells, malignant breast stem cells, and the perinatal origin of breast cancer. Stem Cell Rev. 2006;2:103–110. doi: 10.1007/s12015-006-0016-9. [DOI] [PubMed] [Google Scholar]
- 68.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: Status and controversies. Neoplasia. 2007;9:882–892. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang C, Chua CL, Ang BT. Insights into the cancer stem cell model of glioma tumorigenesis. Ann Acad Med Singapore. 2007;36:352–357. [PubMed] [Google Scholar]
- 70.Kim M, Turnquist H, Jackson J, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 71.Smalley MJ, Clarke RB. The mammary gland “side population”: A putative stem/progenitor cell marker? J Mammary Gland Biol Neoplasia. 2005;10:37–47. doi: 10.1007/s10911-005-2539-0. [DOI] [PubMed] [Google Scholar]
- 72.Litingtung Y, Lawler AM, Sebald SM, et al. Growth retardation and neonatal lethality in mice with a homozygous deletion in the C-terminal domain of RNA polymerase II. Mol Gen Genet. 1999;261:100–105. doi: 10.1007/s004380050946. [DOI] [PubMed] [Google Scholar]
- 73.Arnould L, Arveux P, Couturier J, et al. Pathologic complete response to trastuzumab-based neoadjuvant therapy is related to the level of HER-2 amplification. Clin Cancer Res. 2007;13:6404–6409. doi: 10.1158/1078-0432.CCR-06-3022. [DOI] [PubMed] [Google Scholar]
- 74.Yu F, Yao H, Zhu P, et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]