Abstract
The recent identification of colon cancer tumor-initiating cells adds further support to the cancer stem cell hypothesis. Ongoing basic and translational research efforts are aimed at gaining an increased understanding of the biology of these cells, as well as methods of targeting them. In addition, the relationship between colon carcinogenesis and inflammatory conditions, such as longstanding colitis and inherited syndromes, might be linked to the effect of the processes on stem cells in the colon. This review summarizes current literature on colon cancer stem cells and proposes strategies aimed at targeting these cells for colon cancer prevention and therapy.
Introduction: cancer stem cells
Traditional models of carcinogenesis posit that cancers might originate in any cell through a series of stochastic genetic events resulting in clonal selection. These genetic events include increases in cellular proliferation and the silencing of genes involved in inhibition of proliferation and apoptosis.
By contrast, the stem cell model proposes that cancers originate in a select cell population from either normal tissue stem cells or progenitor cells and that initiating events disrupt the process that regulates stem cell self-renewal. Self-renewal, one of the defining characteristics of stem cells, is a cell division in which one or both of the resulting daughter cells remains undifferentiated, retaining the ability to give rise to another stem cell with the same capacity to proliferate as the parental cell [1,2]. In addition to self-renewal, stem cells have the capacity to differentiate, generating cells in each organ. Cancer stem cells are defined by similar characteristics, mainly their abilities to self-renew, a characteristic that drives tumorigenesis, and to (aberrantly) differentiate, a property that generates the bulk of cells within a tumor. These self-renewing ‘cancer stem cells’ might constitute only a small fraction of the cells within a tumor, with the bulk of the tumor composed of more differentiated cells that lack self-renewal capacity. The landmark discoveries described in Refs [3,4] substantiated these hypotheses for leukemia. To identify the leukemic stem cell, the authors provided the proof that leukemia was reconstituted even in sublethally irradiated mice with leukemic cells expressing markers that are also found in normal hematopoietic stem cells, including CD34+CD38− and CKIT+. Furthermore, repopulation studies revealed evidence of self-renewal in these leukemic stem cells. With these markers, and further robust enrichment by described by others [5,6], the stage was set to determine whether solid organ malignancies also possessed such tumor-initiating cells, or cancer stem cells.
In addition to the property of self-renewal, cancer stem cells might also be relatively resistant to commonly used cancer therapies, such as radiation and chemotherapy. Indeed, evidence for such a subpopulation of chemotherapy- and radiation-therapy-resistant ‘cancer stem cells’ has been described in brain cancers and breast cancers [7,8]. These studies use both in vitro and animal model systems. In addition, recent evidence for the existence of chemotherapy-resistant cancer stem cells has been directly generated from neoadjuvant studies of human breast cancer [8]. In total, these studies give strong support for the cancer stem cell hypothesis and suggest that improved outcomes of therapies might require targeting of this crucial cancer stem cell population.
Colon cancer stem cells are found in primary colon cancer
Three recent studies provide evidence for the existence of colon cancer tumor-initiating cells, or colon cancer ‘stem cells’ in known colon cancer. Adapting techniques used in the prospective identification of human breast cancer stem cells, flow cytometric analysis was employed to identify cells with surface markers that might correlate with the stem cell tumorigenic phenotype. In the first two of these articles, CD133 was employed to identify the cells that had tumor-initiating potential. In one study, O’Brien et al. used CD133 and isolated cells from seven primary colon cancers and ten extracolonic (metastatic) sites [9]. The tumorigenic cells were placed in the renal capsule of NOD/SCID (non-obese diabetic/severe combined immunodeficiency) mice. The percentages of CD133+ cells in the tumorigenic populations ranged from 3.2–24.5%, whereas in the matching normal tissues, the percentage of CD133+ cells ranged from 0.4–2.1%. The second study also used CD133 as a marker to identify and isolate colon cancer tumor-initiating cells [10]. In this study, these cells were perpetuated in vitro as floating colonies, or ‘tumor spheres’, which were enriched in a tumorigenic population and maintained in serial passages. The CD133+ spheres generated a mix of CD133+ and CD133− populations, of which only the CD133+ population was tumorigenic. This demonstrates the pluripotent potential of this subpopulation. In a third study, Dalerba et al. employed CD44 and epithelial surface antigen (ESA) as stem-cell-specific markers, with further enrichment provided by CD166 [11]. As in the breast cancer model, colon cancer xenografts were dissociated and the putative colon cancer-initiating cells were isolated by flow cytometry and injected into NOD/SCID hosts. In each case, only 200–500 cells were needed to reconstitute a tumor, which phenotypically resembled the original resected colon cancer. By contrast, as many as ten thousand of the ESAlow/CD44neg cells were not capable of forming tumors [11]. Therefore, in colon cancer, as has been reported in brain and breast cancer, cancer stem cells can be prospectively isolated using cell surface markers.
Controversy exists regarding which markers are the most robust in identifying colon cancer stem cells. Though the first two reports used expression of CD133, the most recent report used expression of CD44 as the dominant selection criterion. In fact, these markers might have significant overlap in their distribution. Furthermore, expression of different stem cell markers in different tumors might reflect the genetic heterogeneity of this disease. In the work of O’Brien et al. [9], many of the primary colon cancers that were engrafted were from the right side of the colon. Though not examined in this article, it is possible that these cancers displayed microsatellite instability that was reflected in the expression pattern of CD133, and this might be helpful as a correlate of ‘stemness’ and microsatellite status, as implied in a recent article by Todaro et al. [12]. In addition, the majority of the cases reported in this series were derived from metastatic sites, which might indicate a more aggressive phenotype. In this study, successful engraftment was possible only in the renal capsule. However, in the studies led by Ricci-Vitiani and Dalerba [10,11], human specimens were retrieved from both sides of the colon, and successful engraftment occurred when these cells were introduced in the heterotopic flank position. In 12 colon cancer cell lines [13], the ability of CD133 to identify cells with stem cell properties has also been examined. Injections of up to 10 000 CD133+ or CD133− cells both gave rise to xenografts in nude mice that could be serially transplanted. Those cells bearing the CD133+ phenotype reportedly led to tumors with decreased latency. However, the finding that in this cell line both CD133+ and CD133− subpopulations were capable of tumorigenic growth suggests that CD133 might not be the most robust marker.
Further studies questioning CD133 as a colon cancer stem cell marker include that of Shmelkov et al. [14]. These investigators used a transgenic mouse model in which the endogenous promoters of CD133 were used to drive the expression of the lacZ reporter gene (which encodes β-galactosidase). This construct was employed to demonstrate that CD133 was expressed by mature and undifferentiated colonic epithelial cells, suggesting that CD133 is not a specific marker of stem cells. Subsequent studies used the interleukin 10 (IL-10)-null mouse, in which chronic intestinal inflammation correlates with neoplastic growth [15–18]. These authors revealed that the entire epithelium of the tumors expressed the reporter construct. Moreover, in their primary human specimens, CD133 was expressed in the majority of the tumor cell population. Furthermore, in those tumors that were metastatic to the liver, isolates from both CD133 high and low expressing components were capable of generating tumorigenic growth in NOD/SCID mice. In fact, compared to CD133+ cells, the CD133− cells gave rise to tumors with decreased latency.
Recent studies in mice suggest that leucine-rich repeat containing G-protein-coupled receptor 5 (Lgr5) might be another marker of both the normal colon stem cell and the colon cancer tumor-initiating cell [19]. This protein was discovered in a screen of proteins downstream of T-cell factor 4 (TCF4), a transcription factor in the WNT pathway. These authors used a series of elegant murine models to provide evidence that Lgr5 expression might provide the most specific markers for normal colon stem cells and colon cancer stem cells in humans. Further studies will be required to determine whether expression of particular stem cell markers reflects cancer stem cell heterogeneity in colon cancer.
Although studies of colon cancer stem cells themselves are intriguing, the role of these cells in understanding the biology of colon carcinogenesis, especially in increasing our understanding of the elusive normal colon stem cell, might be even more informative. The normal human crypt contains roughly 2000 cells and is believed to have ~19 stem cells. Analyses of mitochondrial DNA mutations in these crypt cells have revealed that normal human colon crypts expand by fission, providing further evidence for stem cell expansion [20].Crypt fission occurs with the development of a fissure bisecting the crypt base and ascends longitudinally, resulting in the production of two identical daughter crypts. Other corroborative evidence for the existence of normal colon stem cells has been obtained via labeling index studies, which suggest that the colonic epithelium turns over approximately every five days [21], with more-differentiated daughter cells migrating towards the luminal surface to be shed. Therefore, it is believed that the position of the normal crypt cells remains at the base of the crypt.
Through colonoscopy, the colon affords an opportunity for non-invasive examination. The rationale for screening colonoscopy was predicated on breakthrough analysis by Fearon and Vogelstein [22] correlating genetic alterations with phenotype in the progression of normal colon mucosa to colorectal cancer. At least one of the genetic alterations – that of adenomatous polyposis coli (APC), which is mutated in at least 50% of colon cancers – occurs early in the pathogenesis of sporadic colorectal cancer [23]. This gene is a member of the WNT pathway, which is implicated as one of the self-renewal pathways responsible for perpetuating stemness. Further implications of these findings include the prospect of delineation of colon carcinogenesis from heritable or preneoplastic conditions in light of recent discoveries in the field of colon cancer-initiating cells. Inherited diseases, such as familial adenomatous polyposis (FAP), that have mutations in APC give insights into the pathogenesis of sporadic colon cancer, and they also provide opportunities for early diagnosis and prevention.
Preneoplastic colon conditions
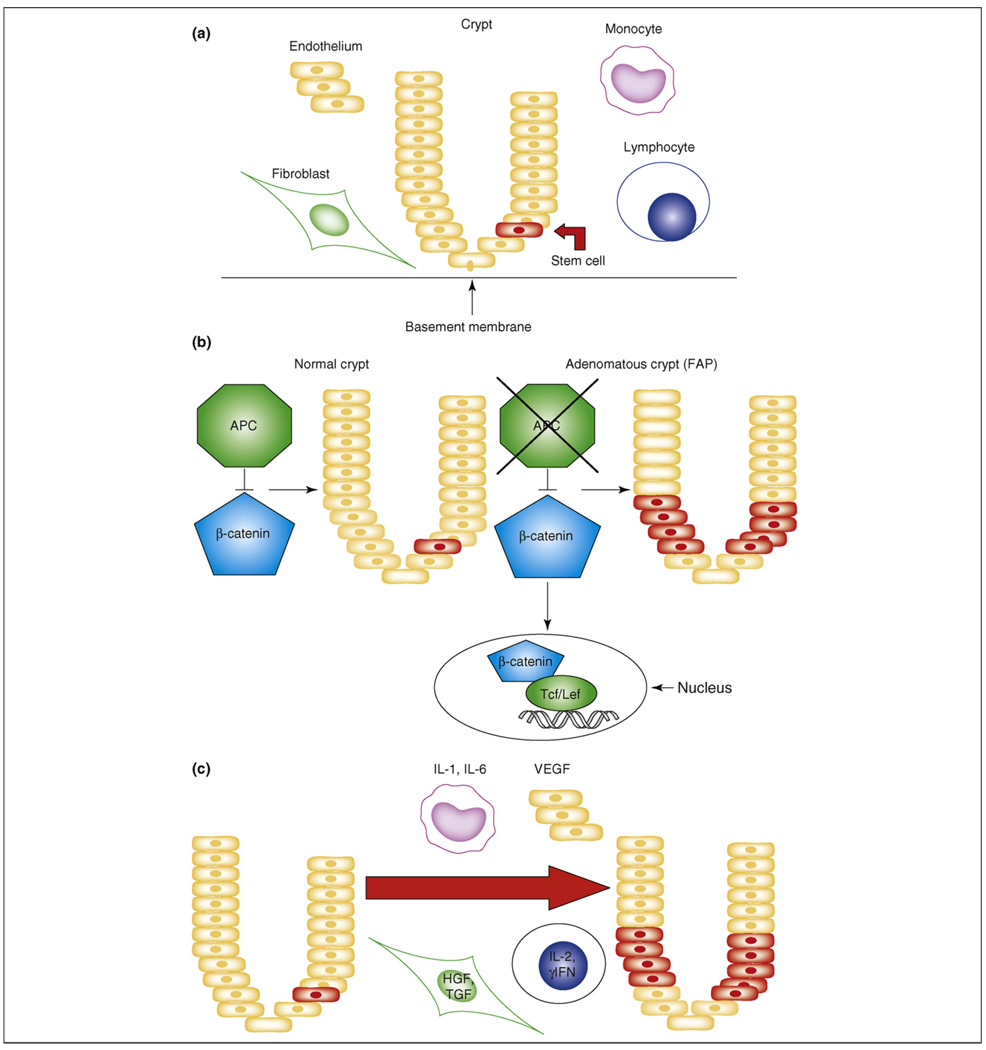
Studies on tumor-initiating cells, or cancer ‘stem cells’, have focused on the phenotype and behavior of these rare cells in established malignancies. In the colon, carcinogenesis occurs through a series of molecular changes in colonic cells. The cancer stem cell hypothesis suggests that normal colonic stem cells might be the targets for these events (Figure 1a). These early events might involve dysregulation of the normally tightly regulated process of stem cell self-renewal, resulting in stem cell expression. These expanded stem cells might then provide targets for additional carcinogenesis events. If this is the case, then strategies centered on targeting these self-renewal pathways might be effective in cancer prevention.
Figure 1.
Stem cell models in the colon. (a) Normal. During the normal state, each crypt has rare stem cells located near the base of the crypt. Constituents of the surrounding microenvironment include the endothelium, stromal fibroblasts and immune cells, including monocytes and lymphocytes. (b) The colon stem cell in familial adenomatous polyposis (FAP). In this genetic or cell autonomous state, the WNT pathway is activated owing to a functional abnormality in the adenomatous polyposis coli (APC) protein. The defect in this protein permits β-catenin to translocate to the nucleus to activate downstream transcription factors and growth-related targets. This results in the generation of a large number of ‘pre-neoplastic’ colon polyps. (c) The colon stem cell in inflammatory bowel disease. In this state, stem cell self-renewal is driven by extrinsic, or cell non-autonomous, production of cytokines and growth factors in response to injury and subsequent regeneration and repair. The microenvironment, including the monocytes, lymphocytes, fibroblasts and endothelium, all influence the stem cells, which normally participate in tissue repair. However, expanded stem cells also provide targets for further carcinogenic events. Abbreviations: HGF, hepatocyte growth factor; γIFN, gamma interferon; Tcf/Lef, T-cell factor/lymphocyte-enhancing factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Familial adenomatous polyposis (FAP)
Heritable forms of colon cancer constitute 5–10% of all cases of colon cancer. However, these diseases have contributed tremendously to the understanding of the pathogenesis of sporadic colon cancer. FAP is an autosomal dominant colorectal cancer syndrome caused by a mutation in the tumor-suppressor gene APC. The phenotype is characterized by the development of hundreds of colonic polyps, which if untreated leads to the inexorable progression to colorectal cancer by the age of 40. In the pathogenesis of this disease process, a germline mutation of the APC gene, results in a defective protein. This protein is part of a complex that binds β-catenin. In the absence of binding and degradation of β-catenin by the proteasome, β-catenin translocates to the nucleus, thus activating multiple other transcription factors responsible for proliferation, differentiation, migration and apoptosis of cells, including, for example, cyclin D1 and c-myc. Notably, mutations in APC are found in 50% of colorectal cancers, and these mutations are believed to be initiating events in the pathogenesis of colon cancer [22]. Furthermore, APC and β-catenin are central members of the WNT pathway, which is a key regulator of stem cell self-renewal (Figure 1b), and when β-catenin accumulates at high levels, it binds to the transcription factor TCF4, which regulates multiple target proteins.
Patients with FAP have been found to have colonic crypts that display an expansion of cells expressing stem cell markers such as CD44 or ALDH1 (Huang, E.H. et al., unpublished data). Recent literature has substantiated FAP as a stem cell disease characterized by an increased labeling index (a shift of the crypt distribution in S phase upwards towards the luminal surface) [21]. These authors hypothesized that cellular changes would link the genetic mutations with the tissue events in this disease. Mathematical modeling revealed that only an increase in the crypt stem cell number, but not changes in cell-cycle proliferation, differentiation or apoptosis of the non-stem cell populations, could simulate the labeling index shift seen in the crypts of patients with FAP.
As detailed above, crypt bifurcation is noted to be an early event in colon cancer initiation and is likely to be caused by crypt fission [24]. In patients with FAP, and ApcMin mice [25], which bear the same genetic defect, crypt fission was noted to be increased. Because crypt fission is a process that involves division of the crypt stem cell, increased fission implies increased numbers or activity of the stem cells.
Inflammatory bowel disease
It is estimated that ~15–20% of the world’s cancers are associated with chronic inflammation [26–29]. Patients with longstanding inflammatory bowel disease are at increased risk for the development of colorectal cancer. Inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, affects 4–6/100 000 people in North America [30]. In the colon, the dominant presentation of colitis is ulcerative colitis. In ulcerative colitis, the initial risk of neoplastic transformation is 0.5–1%/year in the first seven years; thereafter, the risk increases 1%/year to 18% by thirty years, representing a relative risk of colon cancer that is 2.6–5.4-fold greater than that found in the general population [31,32]. These colitic patients are often maintained on immunosuppressive agents in an attempt to ameliorate their symptoms. Though the cause of dysplasia is largely believed to precede the advent of invasive adenocarcinoma, the molecular events that control the latency and the progression from dysplasia to cancer are incompletely understood.
Ulcerative colitis is a disease that is characterized by inflammation, which begins distally at the rectum or sigmoid colon and extends proximally. Unlike Crohn’s disease, ulcerative colitis does not have intervening areas or patches of grossly normal tissues. That is, the disease is seemingly continuous but might be somewhat milder at the proximal extent. Clonal expansion of mutant cell populations in the epithelium of these patients might involve crypt fission. Investigators [33,34] have documented genetic changes, such as p53 mutation, in all the crypts in a localized patch of disease. Furthermore, in fissioning crypts, both branches of the crypt contain the same p53 mutation. These findings suggest that crypt fission might involve clonal expansion of mutated stem cells, an event that proceeds phenotypic evidence of neoplasia.
The relationship between chronic inflammation and cancer is provocative, yet the precise mechanisms for this pathogenetic link have not been elucidated. Colitis-associated cancer involves the ‘inflammation–dysplasia–carcinoma’ sequence, whereas the ‘adenoma to carcinoma’ sequence is the prototype for sporadic carcinoma [35]. The molecular events associated with the evolution of these two cancers also seem to proceed in different orders. Sporadic colon cancers are associated with APC mutations early in their pathogenesis. By contrast, colitis-associated cancer is believed to be initiated by p53 mutations or loss of heterozygosity early in the pathogenesis, with APC aberrations occurring at a later stage of carcinogenesis [35–37]. In inflammatory bowel disease, the inflammation might activate Notch or Hedgehog signaling, thus further expanding colonic stem cells. These pathways have been implicated in stem cell self-renewal. These expanded stem cell populations might serve as targets for further carcinogenic events (Figure 1c). The conceptual link between FAP-associated colon carcinogenesis and that associated with chronic inflammation is that both might be initiated by expansion of colon stem cells. In the case of FAP, this process is cell autonomous, whereas in inflammatory bowel disease, stem cell self-renewal might be stimulated by interactions between colonic stem cells and the inflammatory stroma.
This inflammatory stroma includes endothelium, immune cells and fibroblasts. Current therapy for benign colitis includes agents that target immune cells, including one of the cytokines, tumor necrosis factor-α (TNF-α, contained in infliximab), secreted by the local immune cells [38]. Other therapies have included broad spectrum immunosuppressive agents, such as steroids. Whereas older experimental therapies for colorectal cancer have targeted the immune system via dendritic vaccines [39], newer therapies for colorectal cancer have targeted the vascular supply via drugs such as bevacizumab [40,41]. Acute exacerbations of ulcerative colitis are largely mediated by neutrophils with accompanying vascular edema and erythema. If this process is controlled, the infiltrate changes to a chronic healing stroma and the crypts undergo regeneration, probably through crypt fission with augmentation of the stem cell numbers. The environment thus created has other relatively genotoxic influences, including the generation of reactive oxygen species. Numerous studies have demonstrated that the interaction of the stroma and the epithelium might result in a protective or a deleterious outcome to the host. Because local immune responses influence epithelial cell growth, differentiation and crypt homeostasis, these effects might be due to effects on the stem cell population [42].
Clinical implications of the cancer stem cell model
The stem cell model has important implications for cancer prevention and therapy (Figure 2). Because cancers might arise in normal tissue stem cells, elimination or reduction of these ‘initiated’ stem cells might be an effective cancer prevention strategy. It remains a challenge to identify these ‘initiated’ premalignant cells. In preneoplastic conditions, expansion of cells expressing stem cell markers implies that intervention might be directed at the reduction of abnormal stem cell populations. Notably, ESA is a cell surface marker with expression associated with increased proliferation and decreased differentiation. CD44 is a widely expressed protein with a role in cell-to-cell and cell-to-matrix interactions. Though normally expressed at the base of dividing crypts in the proliferative zone, during inflammatory and neoplastic conditions, the distribution of CD44 expression extends to the luminal surface. CD133 expression seems to be restricted to undifferentiated cells, including endothelial progenitor cells, hematopoietic stem cells, prostatic epithelial stem cells and leukemias. The function of CD133 is unknown [43].
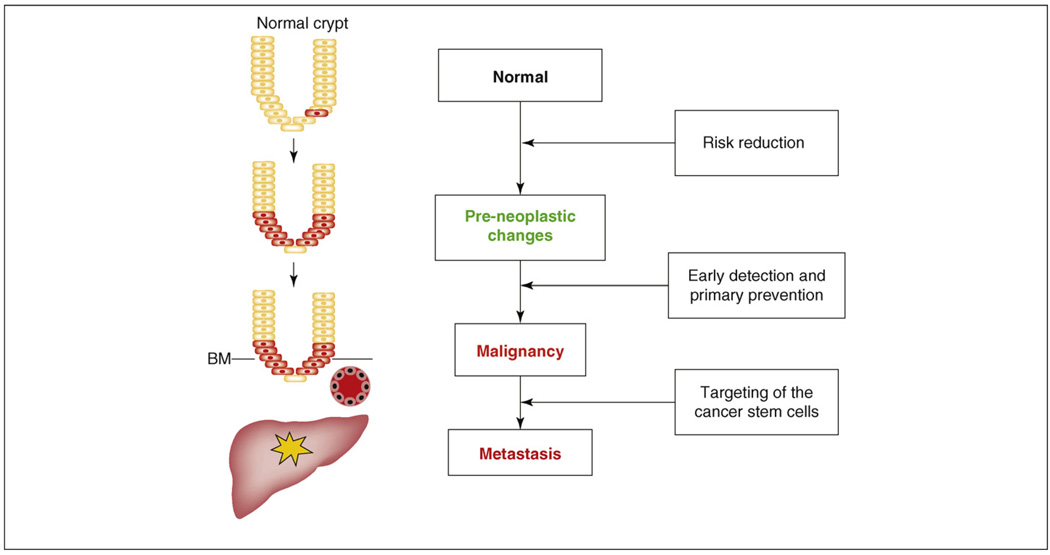
Figure 2.
The adenoma-to-carcinoma sequence: influence on the colon stem cell. In the normal state, there are rare stem cells at the base of the crypt. When either extrinsic or intrinsic influences occur, increased numbers of stem cells become malignant and invasion of the abnormal cells progresses. As disease progresses further, metastasis might be seen in the liver. Abbreviation: BM, basement membrane.
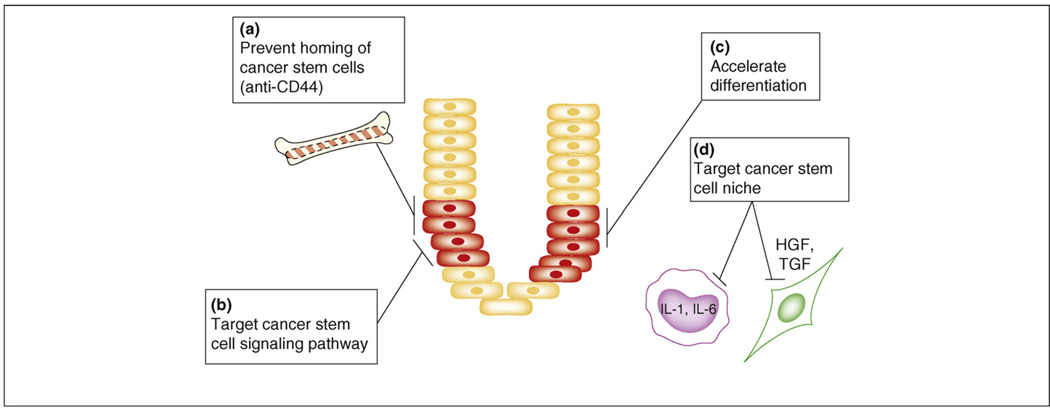
In a mouse model of acute myeloid leukemia, Jin et al. targeted CD44 with a monoclonal antibody [44]. Their strategy used a CD44-activating antibody and resulted in reversal of differentiation blockade. Additionally, the antibody prevented normal homing of these cells to both bone marrow and spleen (Figure 3a). A possible negative consequence of targeting cancer stem cells is that the normal ‘stem cells’ might also be adversely affected. However, recent studies suggest differential sensitivity of normal and malignant stem cells to these agents.
Figure 3.
Therapeutic strategies targeting the colon cancer stem cell. Depicted in this figure are multiple approaches to destruction of the colon cancer stem cell. (a) Prevent homing of the cancer stem cells. There are some proponents of the possibility that cells external to the local environment migrate to sites of malignancy, or indeed are the genesis of malignancy. This strategy would prevent cells external to this microenvironment from establishing residence in the local milieu. (b) Target the cancer stem cell signaling pathway. Pathways unique to the colon cancer stem cell would be targets for novel agents. Combination therapy would then facilitate obliteration of these rare cells. (c) Accelerate differentiation. In this modality, medical therapies for enhancing differentiation would promote the destruction of colon cancer stem cells by conventional chemotherapy. (d) Target the cancer stem cell niche. New strategies invoke approaches aimed at the environment that supports the cancer stem cell, thus preventing further proliferation and spread of the malignancy.
Todaro et al. [12] recently described the autocrine production of IL-4 by CD133+ colon cancer stem-like cells. Though their cell isolates were more resistant to the chemotherapeutic agents 5-fluorouracil or oxaliplatin, the ability of these agents to decrease tumorigenic growth was significantly increased when the cells were first treated with antibodies to IL-4. In xenografts, the addition of anti-IL-4 antibodies significantly reduced tumor growth after chemotherapy. In vivo, this enhancement was expressed as a substantial slowing of growth, and normal growth resumed once the chemotherapeutic agent was withdrawn. These authors concluded that IL-4 exerts a protective effect on the CD133+ stem-like colon cancer isolates.
Another possible interventional approach for cancer prevention and therapy involves targeting self-renewal pathways, such as WNT, Hedgehog or Notch (Figure 3b). As above, such an approach would require the identification of distinctive molecular pathways used by cancer stem cells and the further identification of agents that can either block the pathway(s) or propel the tumor-initiating cells to differentiate (Figure 3c), thus enhancing sensitivity to chemotherapeutic agents.
The microenvironment, or niche, of the stem cell includes fibroblasts, endothelia and inflammatory cells, all contained within the lamina propria. If the stem cells are removed, the niche survives but the stem cells lose their pluripotency. The microenvironment of a stem cell influences the capacity of these cells to proliferate, migrate or invade. Genetic and epigenetic changes in the stroma have been identified before the advent of gross neoplasia [45]. The niche is an anchoring site for stem cells, and adhesion molecules and cytokines facilitate communication between the stromal elements and the stem cells. The niche generates growth factors, including fibroblast growth factor, and factors that are members of major developmental pathways, including the WNT, bone morphogenic and Notch pathways [46]. This suggests that strategies aimed at the stem cell microenvironment might prove effective (Figure 3d). Furthermore, because current strategies for follow-up surveillance currently view only the epithelial growth capacity, alternatives for surveillance might also address the potential of the niche as fertile ground for implantation of the putative cancer stem cell. Moreover, interpretation of the stem cell niche might induce dormancy of cancer stem cells. This might result in cancers becoming more indolent chronic diseases.
Summary
The identification of colon cancer tumor-initiating cells provides further support for the cancer stem cell hypothesis. Future therapy will be directed not only at these rare cells themselves but also at the microenvironment that regulates stem cell behavior. Furthermore, because cancers might originate from disregulation of normal stem cell homeostasis, strategies for cancer prevention might also target this cell population.
Acknowledgements
Sources of financial support: National Institute of Health National Cancer Institute (NCI), K08 CA91915–01A1 (E.H.H.), Comprehensive Cancer Center, Will and Jeanne Caldwell Fund for Cancer Research (E.H.H.), Comprehensive Cancer Center, Weatherwax Foundation (E.H.H.), NCI RO1 CA101860 and RO1 CA129765 (M.S.W.). The authors are grateful to Jain R. Dunckel, Jason Cline and Denise Poirier for preparation of the manuscript.
References
- 1.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 2.Wicha MS, et al. Cancer stem cells: an old idea – a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M, et al. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 5.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz OH, et al. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 8.Yu F, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien CA, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 11.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todaro M, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ieta K, et al. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann. Surg. Oncol. 2008;15:638–648. doi: 10.1245/s10434-007-9605-3. [DOI] [PubMed] [Google Scholar]
- 14.Shmelkov SV, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J. Clin. Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeilstra J, et al. Deletion of the WNT target and cancer stem cell marker CD44 in ApcMin/+ mice attenuates intestinal tumorigenesis. Cancer Res. 2008;68:3655–3661. doi: 10.1158/0008-5472.CAN-07-2940. [DOI] [PubMed] [Google Scholar]
- 16.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg DJ, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang EH, et al. Induction of inflammatory bowel disease accelerates adenoma formation in Min+/− mice. Surgery. 2006;139:782–788. doi: 10.1016/j.surg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 20.Greaves LC, et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc. Natl. Acad. Sci. U. S. A. 2006;103:714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boman BM, et al. How dysregulated colonic crypt dynamics cause stem cell overpopulation and initiate colon cancer. Cancer Res. 2008;68:3304–3313. doi: 10.1158/0008-5472.CAN-07-2061. [DOI] [PubMed] [Google Scholar]
- 22.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 23.Powell SM, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 24.Bjerknes M. Expansion of mutant stem cell populations in the human colon. J. Theor. Biol. 1996;178:381–385. doi: 10.1006/jtbi.1996.0034. [DOI] [PubMed] [Google Scholar]
- 25.Moser AR, et al. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 26.Blaser MJ, et al. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995;55:562–565. [PubMed] [Google Scholar]
- 27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuper H, et al. Infections as a major preventable cause of human cancer. J. Intern. Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 29.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–226. 229. [PubMed] [Google Scholar]
- 30.Loftus EV, Jr, et al. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gut. 2000;46:336–343. doi: 10.1136/gut.46.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein CN, et al. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71–74. doi: 10.1016/s0140-6736(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 32.Eaden JA, et al. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R, et al. The initiation of colon cancer in a chronic inflammatory setting. Carcinogenesis. 2005;26:1513–1519. doi: 10.1093/carcin/bgi106. [DOI] [PubMed] [Google Scholar]
- 34.Chen R, et al. DNA fingerprinting abnormalities can distinguish ulcerative colitis patients with dysplasia and cancer from those who are dysplasia/cancer-free. Am. J. Pathol. 2003;162:665–672. doi: 10.1016/S0002-9440(10)63860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 36.Colliver DW, et al. Molecular profiling of ulcerative colitis-associated neoplastic progression. Exp. Mol. Pathol. 2006;80:1–10. doi: 10.1016/j.yexmp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Krok KL, Lichtenstein GR. Colorectal cancer in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2004;20:43–48. doi: 10.1097/00001574-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Jarnerot G, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–1811. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Saha A, et al. Therapy of established tumors in a novel murine model transgenic for human carcinoembryonic antigen and HLA-A2 with a combination of anti-idiotype vaccine and CTL peptides of carcinoembryonic antigen. Cancer Res. 2007;67:2881–2892. doi: 10.1158/0008-5472.CAN-06-3045. [DOI] [PubMed] [Google Scholar]
- 40.de Gramont A, et al. Targeted agents for adjuvant therapy of colon cancer. Semin. Oncol. 2006;33:S42–S45. doi: 10.1053/j.seminoncol.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Rubio E. Vascular endothelial growth factor inhibitors in colon cancer. Adv. Exp. Med. Biol. 2006;587:251–275. doi: 10.1007/978-1-4020-5133-3_20. [DOI] [PubMed] [Google Scholar]
- 42.Dahan S, et al. Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology. 2008;134:192–203. doi: 10.1053/j.gastro.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shmelkov SV, et al. AC133/CD133/Prominin-1. Int. J. Biochem. Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Jin L, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 45.Deng G, et al. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]