Abstract
Herein, we report that dihydrolipoic acid (DHLA) and lipoic acid (LA) plus lipoamide dehydrogenase and NADH denitrosate S-nitrosocaspase 3 (CASP-SNO). In HepG2 cells, S-nitrosocysteine ethyl ester (SNCEE) impeded the activity of CASP-SH, while a subsequent incubation of the cells in SNCEE-free medium resulted in endogenous denitrosation and reactivation of CASP-SH. The latter process was inhibited in thioredoxin reductase-deficient HepG2 cells, in which, however, LA markedly reactivated CASP-SH. The data obtained are discussed with focus on low molecular mass dithiols that mimic the activity of thioredoxin in reactions of protein S-denitrosation.
Keywords: lipoic acid, caspase, nitric oxide, dihydrolipoic, denitrosation
Introduction
Caspases are a family of cysteine proteases that play an essential role in the signaling cascade leading to apoptosis. Apoptosis, or programmed cell death, is distinguished from lytic or necrotic cell death by a number of biochemical and structural events. Apogenic signals trigger specific signaling pathways, including activation of proteases, which are followed by the appearance of specific morphologic changes such as condensation of nuclei and cytoplasm, blebbing of cytoplasmic membranes, and finally fragmentation into apoptotic bodies that are phagocytosed by neighboring cells (1). Apoptosis is important to physiologic processes such as cell selection in development and immunologic responses (2), control of organ size in maturation and regeneration (3), and normal cell turnover throughout the organism (4). Dysregulated apoptosis may contribute to pathologic states such as autoimmune disease (5) and malignancy (6). Upon exposure to a proapoptotic signal, zymogen forms of caspases constitutively present in cells are proteolytically cleaved and activated. Initiator caspases such as caspase 8, 9, and 10 can cleave other caspases, while executioner caspases, including caspases 3 (CASP-SH), 6, and 7, cleave death substrates (7).
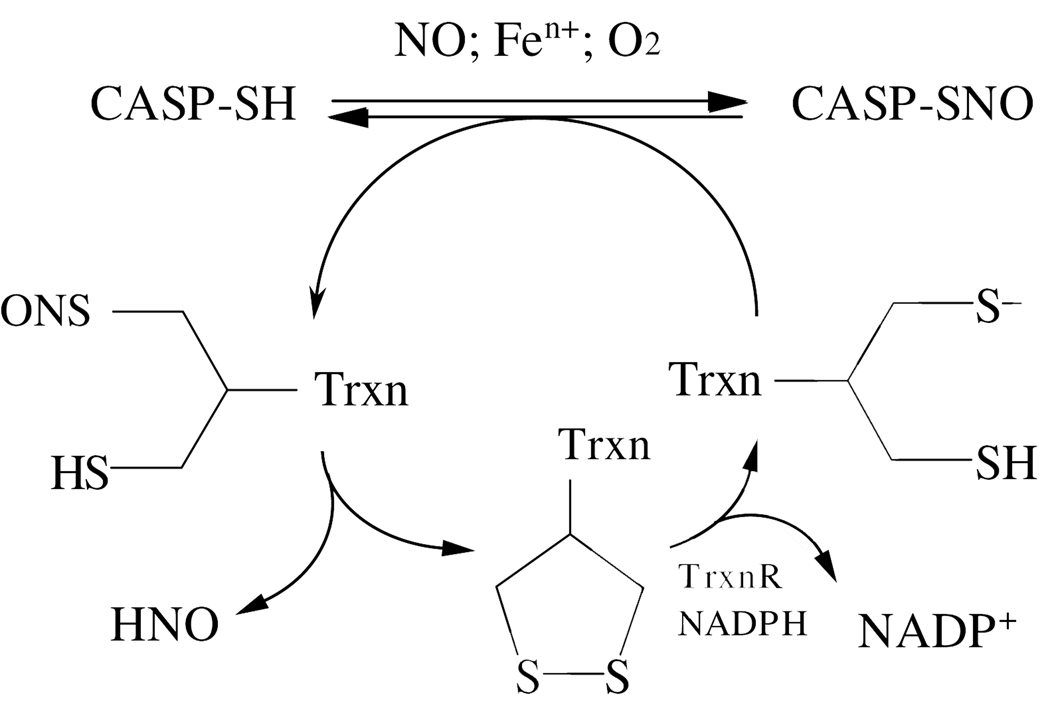
Nitric oxide (NO), produced either extracellularly by low molecular mass (LMM) compounds or intracellularly by nitric oxide synthases, impedes CASP-SH activity via reactions of S-nitrosation (8–10). CASP-SH can undergo poly-S-nitrosation, whereby all SNO functions in its p12 subunit are denitrosated by GSH except for a single SNO group (9). Since the latter was not observed in a mutant form of CASP-SH lacking the active site cysteine, Zach et al. proposed that in cells NO nitrosates this cysteine to form S-nitrosocaspase 3 (CASP-SNO) that is resistant to reduction by GSH (9). However, denitrosation of CASP-SNO back to CASP-SH with reconstitution of its proteolytic activity is catalyzed in cellular cytosol by thioredoxin type 1 (Trxn; (11, 12); scheme 1). In mitochondria, thioredoxin type 2 has been found to mediate denitrosation of mitochondria-associated CASP-SH, a process required for caspase-3 activation (13). Trxn contains a -Cys-Gly-Pro-Cys- motif that is essential for its enzyme activity. Cysteines 32 and 35 in the active site of Trxn(SH)2 can reduce SNO functions in substrate proteins with concomitant disulfide ring closure to Trxn(S)2 and release of nitroxyl (HNO; (12, 14, 15)). In turn, Trxn(S)2 is converted back to Trxn(SH)2 by the NADPH-dependent thioredoxin reductase (TrxnR; (12, 16)).
Scheme 1.
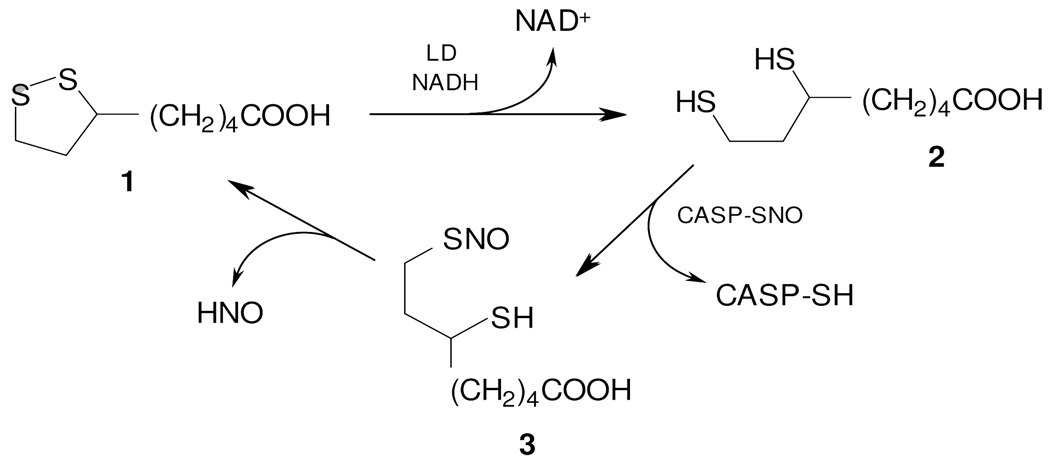
Sustained production of endogenous NO has been shown to decrease both the expression and the activities of Trxn and thioredoxin reductase (TrxnR; (17–19). This suggests that shifts in the rates of S-nitrosation and denitrosation of CASP-SH following changes in either NO production or activity of the Trxn/TrxnR/NADPH system will modulate CASP-SH-dependent apoptotic pathways. Within the scope of this mechanism, we were interested to verify whether LMM dithiols can mimic the activity of Trxn toward CASP-SNO. To this end, we have focused on dihydrolipoic acid (DHLA; 6,8-dimercaptooctanoic acid), which is the reduced form of lipoic acid (LA; 5-[1,2]dithiolan-3-yl-pentanoic acid), a cyclic disulfide that is an essential prosthetic group in the dihydrolipoyl transacetylase component of the •-ketoacid dehydrogenase complex in mitochondria. In cells, LA (Scheme 2, 1) is reduced to DHLA (2) by lipoamide dehydrogenase (LD) with consumption of NADH; in addition, reduction of LA to DHLA is catalyzed by NADPH-dependent reductases (including TrxnR; (20, 21)). Herein, we present experimental evidence that DHLA fully denitrosates CASP-SNO in chemical systems and regenerates the activity of CASP-SH in TrxnR-deficient HepG2 cells exposed to NO.
Scheme 2.
Methods
Reagents
All reagents used were purchased from Sigma Chemical Co. (St. Louis, MO). Human RhoA protein was purchased from Cytoskeleton, Inc. (Denver, CO). The solutions used in the experiments were prepared in deionized and Chelex-100-treated water or potassium phosphate buffer.
Preparation of S-nitrosocysteine ethyl ester (SNCEE)
S-nitrosation of the ethyl ester of cysteine (HS-Cys-OEt) was carried out with nitrosooxy ethane (C2H5ONO) as described previously (22). Briefly, HS-Cys-OEt (0.5 g) was dissolved in methanol (5 mL) containing C2H5ONO (0.5 mL; b.p. 13 °C) and the reaction solutions was incubated for 30 min in ice. Thereafter, the solvent and the remaining C2H5ONO were rotor-evaporated (2 mm Hg) at room temperature and the nitrosothiol formed was recrystallized from methanol. The purity of the nitrosothiols was assessed by UV spectrophotometry (in methanol, •(343)SNCEE = 1019 M−1cm−1 and •(544)SNCEE = 36 M−1cm−1 (22). During the preparation of SNCEE, care must be exercised in handling solutions containing C2H5ONO, as inhalation of its vapor may cause severe headache and heart excitation. The preparation must therefore be conducted in an efficient fume cupboard.
Preparation of CASP-SNO
S-nitrosation of CASP-SH (3 •M) was performed with GSNO (0.3 mM) at 20 °C for 30 min in 0.1 M phosphate buffer containing 0.2 mM EDTA. Thereafter, CASP-SNO was separated from GSH and the excess of GSNO via ultrafiltration (3 kDa Vivaspin cut-off filter; 30 min at 12,000g), which included 4 washing cycles with 0.2 mL of the reaction buffer.
Analysis of CAS-SNO
S-nitrosocaspase 3 was quantified following its Cu+-catalyzed breakdown to CASP-SH and NO with concomitant chemiluminescence measurements of the latter in the gas-phase using a Sievers Nitric Oxide analyzer (NOA ™ 280i; Boulder, CO) (11). The purge vessel of the NO analyzer was filled with 5 mL of 0.1 M phosphate buffer (pH 7.4; 20 °C; gas carrier, He). In the reaction vessel, a steady-state concentration of Cu+ was maintained by a large excess of ascorbic acid over CuCl2 (50 mM vs. 0.2 mM). Thus, multiple injections of aliquots (5 •L) containing CASP-SNO could be made without any significant loss of analytical sensitivity. Under these experimental conditions, NaNO2 (up to 0.1 mM) does not interfere with the analysis of CASP-SNO (11).
CASP-SH assay
In cell lysates, CASP-SH activity was measured fluorometrically on a LS50B Perkin Elmer spectrofluorimeter (•ex = 380 nm; (•em = 420 nm; excitation/emission slit, 5 nm) using 0.15 mM Asp-Glu-Val-Asp-7-amido-4-methyl-coumarin as a substrate (Sigma, Inc. St. Louis, MO).
Cell culture and treatments
Human hepatoma (HepG2) cells were cultured in the Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM l-glutamine in a humidified atmosphere in 5% CO2 at 37°C.
CASP-SH activation in HepG2 cells - Cells (2 × 106 cells/flask) were grown in T75 flasks for 24 hours and then incubated for 4.5 hours with medium (control) or with medium containing TNF-• (15 nM) and cycloheximide (40 µM; Sigma; St. Louis, MO; (23)).Thereafter, the cells were washed with PBS (3 × 10 mL) and incubated for 15 min at 37 °C with standard incubation medium containing SNCEE. Whole-cell lysates for analysis of CASP-SH were harvested by repeated freeze and thaw cycles followed by centrifugation at 15,000g for 15 min at 4°C.
siRNA transfection
The siRNA directed against TrxnR was 5'-AGACCACGUUACUUGGGCAdTdT-3' and the control was a scrambled sequence (5'-AGGCAAAUCACGGUGUCCUdT dT-3') that does not match any sequence in the GenBank human database for >16 nt ((24); Dharmacon RNA Technologies; Chicago, IL). Approximately 2 × 105 HepG2 cells were plated per well in a six-well plate. The following day, siRNA were transfected with lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), with 30 pmoles siRNA per well (25), according to the manufacturer’s recommendations. Transfection with the same amount of nonspecific siRNA was performed as control. The cells were harvested and analyzed at 24, 48 and 72 hours after transfection for cell viability, activity, and level of TrxnR protein.
Western Blots
Cells were disrupted by three consecutive freeze and thaw cycles and then centrifuged at 15,000 g for 15 min at 4 °C to remove membrane fractions. Equal amounts of protein (20–30 µg) were resolved by SDS PAGE (10%) and transferred on to a nitrocellulose membrane. The membrane was blocked in 5% (w/v) dried milk powder in TBS (Tris-buffered saline) with (v/v) 0.01% Tween-20 at room temperature and was incubated with anti-TrxR1 (Rabbit IgG, Upstate) (1:500 dilution) overnight in 5% (w/v) BSA in TBS with 0.01% (v/v) Tween-20. Secondary antibody (HRP-conjugated anti-rabbit IgG; 1:1,000 dilution; Pierce; Rockforf, IL) was then added to the membrane for 1 hour at room temperature, washed, and thereafter the membrane was exposed to HRP substrate (Pierce SuperSignal West Pico Chemiluminescent Substrate) and visualized by chemiluminescence on autoradiography film.
TrxnR assay
TrxnR activity was determined in a coupled assay with E. coli Trxn (10 •M) and 5,5•-Dithiobis(2-nitrobenzoic acid) (DTNB) as described in ref . (11). One unit of TrxnR activity was defined as the formation of 74 •moles of 5-mercapto-2-nitro-benzoic acid (1 absorbance unit at 412 nm; •412 = 13500 M−1.cm−1) per min per mL at pH 7.0 at 25 °C.
Data Analysis
Results are given as mean ± S. E. (n = 3 – 6).
Results and discussion
S-denitrosation of CASP-SNO by DHLA
LMM compounds containing vicinal dithiols such as threo-1,4-dimercapto-2,3-butanediol (Cleland’s reagent; DTT) and DHLA react with RSNO to form RSH and disulfides with generation of HNO, NH3, NH2OH and NO. In this reaction, the ratio between end products depends on the presence of nitrate anion and the degree of S-nitrosation of the parent dithiols. Mono-S-nitroso thiols tend to release HNO (12, 14, 26), whereas di-S-nitrosothiols undergo cyclization to disulfides with release of NO (14, 27).
At mM concentrations, DTT and both LA and DHLA have been shown to cause toxicity via induction of reductive stress (28) and activation of CASP-SH, respectively ((29) and the references therein). However, pharmacological experiments are often carried out with 10 mg of LA per kg of body weight (30, 31), whereas therapeutic results in humans are achieved at plasma concentrations of 10–20 •g/mL (~50–100 µM) LA (31, 32). Hence, we were interested to verify whether CASP-SNO can be denitrosated by pharmacological concentrations DHLA (Scheme 2).
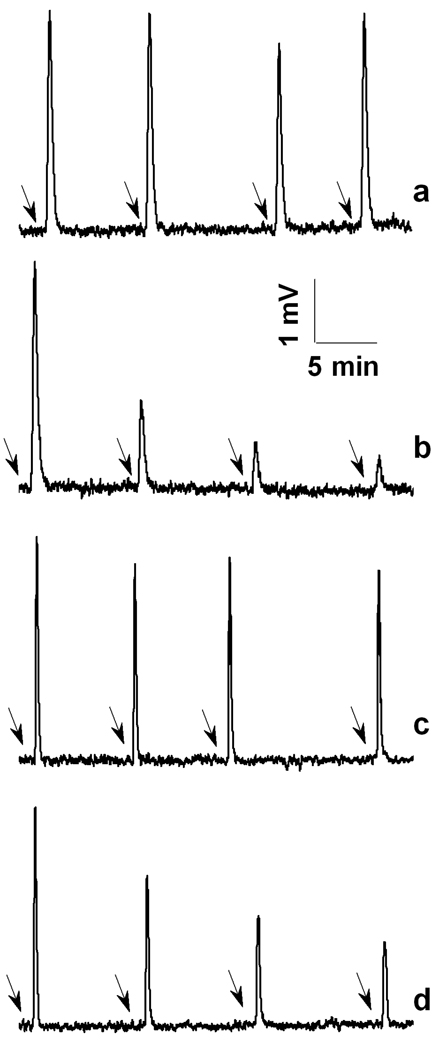
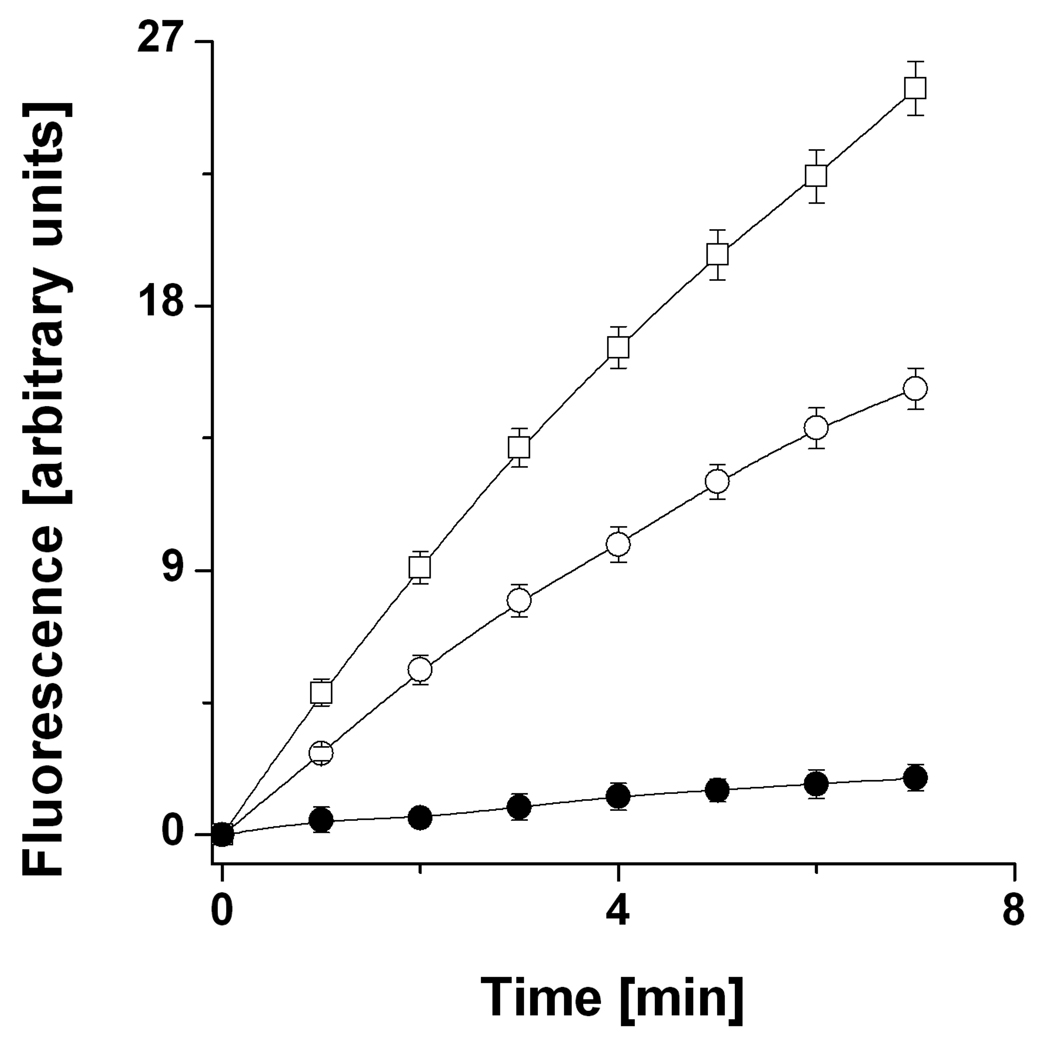
Purified CASP-SNO, which contains 5 SNO functions per mol of protein (12), exhibited insignificant proteolytic activity and did not decompose to any significant extent in the presence of LA or LD plus NADH (Figure 1a,c). However, both DHLA and the complete LA/LD/NADH system denitrosated CASP-SNO (Fig. 1b,d) with concomitant reconstitution of the enzyme activity of CASP-SH (Fig. 2). Notably, CASP-SNO was fully denitrosated by DHLA. In contrast to LA, a series of LMM disulfides have been shown to inhibit CASP-SH via formation of protein mixed disulfides (33, 34).
Figure 1.
DHLA denitrosates CASP-SNO. Reactions were carried out at 25 °C in 0.1 M phosphate buffer (pH 7.4) containing 0.1 mM EDTA. CASP-SNO, DHLA, NADH, and LA were used at concentrations of 0.8 µM, 50 µM, 400 µM and 50 µM, respectively; LD, 1 unit/mL. At given time points (arrows), aliquots from the reaction solutions were assessed for CASP-SNO content as described in Methods. Trace a- CASP-SNO and LA; b- CASP-SNO and DHLA; c- CASP-SNO, LD and NADH; d- CASP-SNO, LA, LD and NADH. Results are representative of three independent experiments.
Figure 2.
S-denitrosation of CASP-SNO by DHLA leads to reconstitution of the proteolytic activity of CASP-SH. Reactions were carried out as indicated in Fig. 1. At given time points (0 min (•), 10 min (•), and 20 min (•)), aliquots from the reaction solution consisting of CASP-SNO and DHLA were assessed for CASP-SH activity. Data are presented as mean values of three independent experiments ± SE.
To assess the potential of DHLA to denitrosate CASP-SNO in intact cells, we have conducted experiments with control and TrxnR-deficient HepG2 cells. In cells, TrxnR was silenced using siRNA, while release of CASP-SH in the cellular cytosol was attained with TNF- and cycloheximide (11). In contrast to hepatocytes, HepG2 cells are deficient in some phase I enzymes (35–37), including alcohol dehydrogenase class III (ADH; also referenced as GSNO reductase), which catalyses the denitrosation of GSNO (38). Hence, control and TrxnR-deficient HepG2 cells offered the advantage to study Trxn-catalyzed reactions of S-denitrosation without interference of ADH.
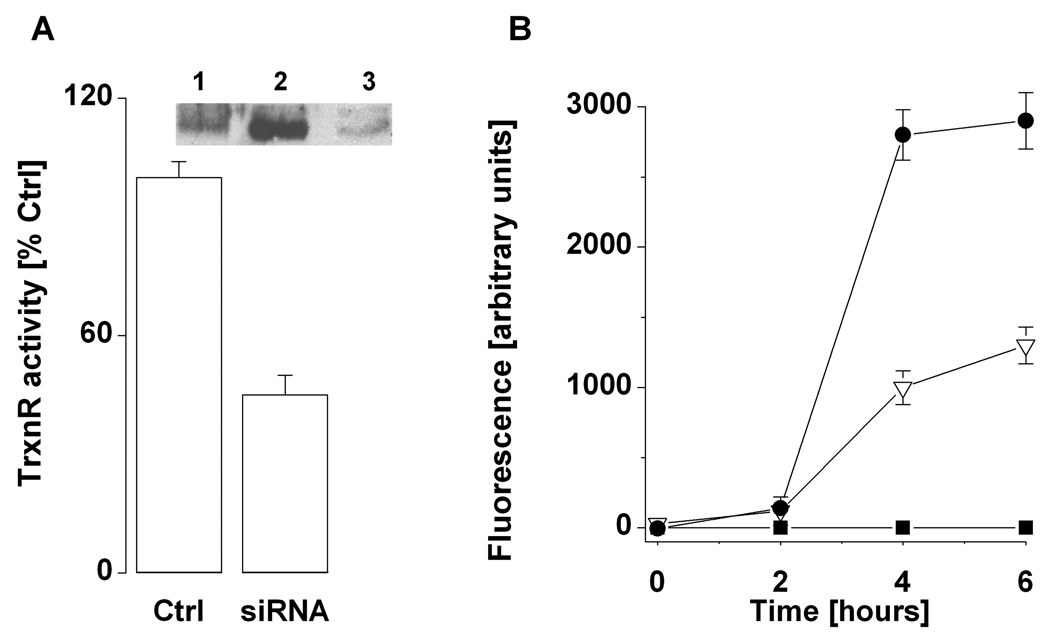
Incubation of HepG2 cells for 72 hours with siRNA (30 nM) caused a ~ 50% decrease in the activity of TrxnR (Figure 3A) without affecting the levels of intracellular GSH to any significant extent (data not shown). Western blot analysis established that, in siRNA-treated cells, the protein levels of TrxnR had markedly decreased (Figure 3A, inset). For S-nitrosation of thiols, cells were incubated with SNCEE, which readily crosses cell membranes and trans-S-nitrosates intracellular proteins (11, 12, 22). While at concentrations of 0.2 – 0.8 mM SNCEE impedes the activity of TrxnR and causes toxicity in HepG2 cells (11), experiments aimed to assess the nitrosative inactivation of CASP-SH were carried out with 50 µM SNCEE.
Figure 3.
Inhibition of TrxnR and activation of caspase 3 in HepG2 cells. Experiments aimed to silence TrxnR in HepG2 cells with siRNA (A) and to activate caspase 3 (B) were carried out as described in Methods. A, Inset- Western analysis of cells transfected with scrambled siRNA (lane 1), untransfected cells (lane 2), and siRNA-transfected cells (lane 3). B- Activity of caspase 3 in HepG2 cells exposed to TNF-•(5 nM, triangles; 15 nM, circles) and cycloheximide (40 •M); no addition, rectangles. The results represent the mean ± S.E (n = 3).
Exposure of HepG2 cells to cycloheximide and TNF- resulted in a dose- and time-dependent activation of CASP-SH, whereby maximal activity of the protease was attained after and incubation for 4.5 hours with 15 nM TNF- (Fig. 3B). Thereafter, control and TrxnR-deficient HepG2 cells were incubated for 15 min with 50 µM SNCEE. The cells were then washed with PBS and the activity of CASP-SH was assessed either immediately (time for preparation of reaction solutions for spectral analysis, 5 min) or after an additional incubation for 60 min at 37 °C in SNCEE-free incubation medium.
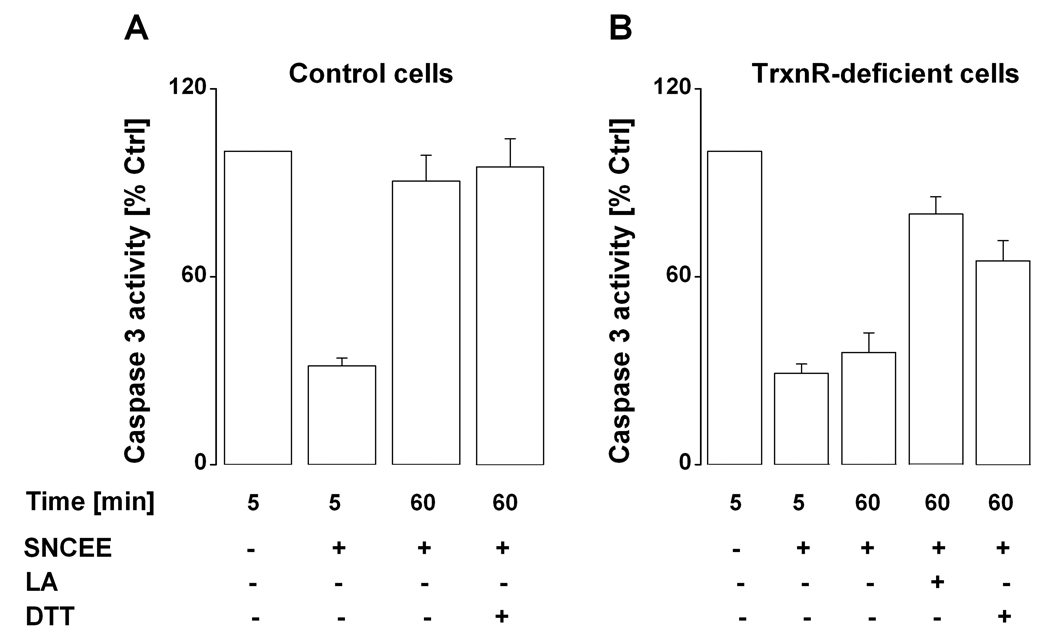
The rationale for this experimental design was based on previously established kinetics of denitrosation of CASP-SNO by the Trxn/TrxnR/NADPH system (11), with the hypothesis that time-dependent increases in CASP-SH activity would reflect the rates of endogenous reactions of S-denitrosation. Treatment of control HepG2 cells with SNCEE led to ~ 80 % (5 min) and 5 % (60 min in SNCEE-free incubation medium) inhibition of CASP-SH (Figure 4A). The regeneration of CASP-SH activity was insignificant in TrxnR-deficient cells (Figure 4B), which suggests the requirement for Trxn catalysis in this process. However, substitution of SNCEE with LA followed by incubation for 60 min led to a marked reactivation of CASP-SH activity in TrxnR-deficient HepG2 cells (Fig. 4B), presumably via intracellular reduction of LA to DHLA and reaction of the latter with CASP-SNO. Notably, CASP-SH activity was reconstituted more efficiently by 50 •M LA than by 5 mM DTT (Fig. 4B). In this set of experiments, qualitatively the same effects were observed when SNCEE was substituted for S-nitroso-N-acetyl-DL-penicillamine (SNAP; incubation time, 1 hour; data not shown).
Figure 4.
SNCEE and LA modulate CASP-SH activity in HepG2 cells. CASP-SH activity was stimulated with TNF-alpha (15 nM) plus cycloheximide (40 •M) as described in Methods; then, the cells were incubated for 15 min at 37 °C with SNCEE (50 •M), washed with PBS, and assayed for CASP-SH activity either immediately or after an additional incubation for 60 min with SNCEE-free incubation medium in the absence and in the presence of LA (50 •M) or DTT (5 mM). Control activity of TrxnR, 0.12 units/mg protein (Panel A); in lysates of (TNF-• plus cycloheximide)-treated HepG2 cells, 7-amino-4-methylcoumarin was generated at a rate of 2.8 nmol/min/mg protein/mL. The results represent the mean ± S.E (n = 3).
The data presented herein provide a proof of concept that LMM dithiols can mimic the activity of Trxn in reactions of protein S-denitrosation. Several disease states have been associated with both increased S-nitrosation of proteins and modulation of the homeostasis of Trxn. Examples of such diseases are liver steatosis and cirrhosis (39–41), rheumatoid arthritis (42–45), bronchopulmonary dysplasia (46–48), and asthma (49, 50). Hence, DHLA and structurally similar LMM disulfides/dithiols could be instrumental in assessments of the involvement and specific roles of reactions of S-nitrosation. It could be further hypothesized that denitrosation of S-nitrosothiols by LMM dithiols may be used to counteract the toxicity of NO and drugs that act as S-nitrosating agents, such as glyceryl trinitrate, nitrosooxyethane and nitrosoaspirine (22, 51–53). This is an experimentally verifiable hypothesis for which support has not previously been generated. Hence, further studies are needed to assess the structure/activity relationship of the reduction of LMM disulfides to dithiols by NADH-and NADPH-dependent reductases, as well as to define the factors that affect the rates of protein S-denitrosation by LMM dithiols.
ACKNOWLEDGMENT
This work is supported by NIH grant GM044100 and Walter Reed Army Institute of Research grants W81XWH-09-P-0631 and W81XWH-06-1-0247.
Abbreviations
- ADH
alcohol dehydrogenase
- SNCEE
S-nitroso-L-cysteine ethyl ester
- GSH
reduced glutathione
- GSNO
S-nitrosoglutathione
- Trxn
human thioredoxin type 1
- LA
Lipoic acid
- DHLA
dihydrolipoic acid
- LMM
low molecular mass
- CASP-SH
caspase 3
- CASP-SNO
S-nitrosocaspase 3
- TrxnR
human thioredoxin reductase
- LD
lipoamide dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Pospisil R, Young-Cooper GO, Mage RG. Preferential expansion and survival of B lymphocytes based on VH framework 1 and framework 3 expression: "positive" selection in appendix of normal and VH-mutant rabbits. Proc Natl Acad Sci U S A. 1995;92:6961–6965. doi: 10.1073/pnas.92.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raff MC. Size control: the regulation of cell numbers in animal development. Cell. 1996;86:173–175. doi: 10.1016/s0092-8674(00)80087-2. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 5.Reap EA, Leslie D, Abrahams M, Eisenberg RA, Cohen PL. Apoptosis abnormalities of splenic lymphocytes in autoimmune lpr and gld mice. J Immunol. 1995;154:936–943. [PubMed] [Google Scholar]
- 6.Liebermann DA, Hoffman B, Steinman RA. Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene. 1995;11:199–210. [PubMed] [Google Scholar]
- 7.Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 9.Zech B, Wilm M, van Eldik R, Brune B. Mass spectrometric analysis of nitric oxide-modified caspase-3. J Biol Chem. 1999;274:20931–20936. doi: 10.1074/jbc.274.30.20931. [DOI] [PubMed] [Google Scholar]
- 10.Melino G, Bernassola F, Knight RA, Corasaniti MT, Nistico G, Finazzi-Agro A. S-nitrosylation regulates apoptosis. Nature. 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR, Stoyanovsky DA. Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry. 2007;46:8472–8483. doi: 10.1021/bi700449x. [DOI] [PubMed] [Google Scholar]
- 12.Stoyanovsky DA, Tyurina YY, Tyurin VA, Anand D, Mandavia DN, Gius D, Ivanova J, Pitt B, Billiar TR, Kagan VE. Thioredoxin and lipoic acid catalyze the denitrosation of low molecular weight and protein S-nitrosothiols. J Am Chem Soc. 2005;127:15815–15823. doi: 10.1021/ja0529135. [DOI] [PubMed] [Google Scholar]
- 13.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liebeskind S, Korth HG, de Groot H, Kirsch M. Dependence of product formation from decomposition of nitroso-dithiols on the degree of nitrosation. Evidence that dinitroso-dithiothreitol acts solely as an nitric oxide releasing compound. Org Biomol Chem. 2008;6:2560–2573. doi: 10.1039/b801583j. [DOI] [PubMed] [Google Scholar]
- 15.Hashemy SI, Holmgren A. Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J Biol Chem. 2008;283:21890–21898. doi: 10.1074/jbc.M801047200. [DOI] [PubMed] [Google Scholar]
- 16.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 17.Patel JM, Block ER. Sulfhydryl-disulfide modulation and the role of disulfide oxidoreductases in regulation of the catalytic activity of nitric oxide synthase in pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1995;13:352–359. doi: 10.1165/ajrcmb.13.3.7544597. [DOI] [PubMed] [Google Scholar]
- 18.Patel JM, Zhang J, Block ER. Nitric oxide-induced inhibition of lung endothelial cell nitric oxide synthase via interaction with allosteric thiols: role of thioredoxin in regulation of catalytic activity. Am J Respir Cell Mol Biol. 1996;15:410–419. doi: 10.1165/ajrcmb.15.3.8810647. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Li YD, Patel JM, Block ER. Thioredoxin overexpression prevents NO-induced reduction of NO synthase activity in lung endothelial cells. Am J Physiol. 1998;275:L288–L293. doi: 10.1152/ajplung.1998.275.2.L288. [DOI] [PubMed] [Google Scholar]
- 20.Handelman GJ, Han D, Tritschler H, Packer L. Alpha-lipoic acid reduction by mammalian cells to the dithiol form, and release into the culture medium. Biochem Pharmacol. 1994;47:1725–1730. doi: 10.1016/0006-2952(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 21.Arner ES, Nordberg J, Holmgren A. Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase. Biochem Biophys Res Commun. 1996;225:268–274. doi: 10.1006/bbrc.1996.1165. [DOI] [PubMed] [Google Scholar]
- 22.Clancy R, Cederbaum AI, Stoyanovsky DA. Preparation and properties of Snitroso-L-cysteine ethyl ester, an intracellular nitrosating agent. J Med Chem. 2001;44:2035–2038. doi: 10.1021/jm000463f. [DOI] [PubMed] [Google Scholar]
- 23.Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 24.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy PB, Edes K, Nelson CC, Parsawar K, Fitzpatrick FA, Moos PJ. Thioredoxin reductase is required for the inactivation of tumor suppressor p53 and for apoptosis induced by endogenous electrophiles. Carcinogenesis. 2006;27:2538–2549. doi: 10.1093/carcin/bgl111. [DOI] [PubMed] [Google Scholar]
- 26.Arnelle DR, Stamler JS. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 27.Petit C, Hoffmann P, Souchard JP, Nepveu F, Labidalle S. Thionitrites as potent donors of nitric oxide: example of S-nitroso- and S,S'-dinitroso-dihydrolipoic acids. C R Seances Soc Biol Fil. 1996;190:641–650. [PubMed] [Google Scholar]
- 28.Halleck MM, Holbrook NJ, Skinner J, Liu H, Stevens JL. The molecular response to reductive stress in LLC-PK1 renal epithelial cells: coordinate transcriptional regulation of gadd153 and grp78 genes by thiols. Cell Stress Chaperones. 1997;2:31–40. doi: 10.1379/1466-1268(1997)002<0031:tmrtrs>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamasaki M, Kawabe A, Nishimoto K, Madhyastha H, Sakakibara Y, Suiko M, Okamoto T, Suda T, Uehira K, Nishiyama K. Dihydro-alpha-lipoic acid has more potent cytotoxicity than alpha-lipoic acid. In Vitro Cell Dev Biol Anim. 2009;45:275–280. doi: 10.1007/s11626-008-9164-3. [DOI] [PubMed] [Google Scholar]
- 30.Baillie JK, Thompson AA, Irving JB, Bates MG, Sutherland AI, Macnee W, Maxwell SR, Webb DJ. Oral antioxidant supplementation does not prevent acute mountain sickness: double blind, randomized placebo-controlled trial. QJM. 2009;102:341–348. doi: 10.1093/qjmed/hcp026. [DOI] [PubMed] [Google Scholar]
- 31.Teichert J, Hermann R, Ruus P, Preiss R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J Clin Pharmacol. 2003;43:1257–1267. doi: 10.1177/0091270003258654. [DOI] [PubMed] [Google Scholar]
- 32.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12:343–351. [PubMed] [Google Scholar]
- 33.Nobel CS, Burgess DH, Zhivotovsky B, Burkitt MJ, Orrenius S, Slater AF. Mechanism of dithiocarbamate inhibition of apoptosis: thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem Res Toxicol. 1997;10:636–643. doi: 10.1021/tx970006a. [DOI] [PubMed] [Google Scholar]
- 34.Nobel CS, Kimland M, Nicholson DW, Orrenius S, Slater AF. Disulfiram is a potent inhibitor of proteases of the caspase family. Chem Res Toxicol. 1997;10:1319–1324. doi: 10.1021/tx970131m. [DOI] [PubMed] [Google Scholar]
- 35.Kabakibi A, Morse CR, Laposata M. Fatty acid ethyl esters and HepG2 cells: intracellular synthesis and release from the cells. J Lipid Res. 1998;39:1568–1582. [PubMed] [Google Scholar]
- 36.Wolfla CE, Ross RA, Crabb DW. Induction of alcohol dehydrogenase activity and mRNA in hepatoma cells by dexamethasone. Arch Biochem Biophys. 1988;263:69–76. doi: 10.1016/0003-9861(88)90614-5. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Lopez JM, Carrasco MP, Segovia JL, Marco C. Resistance of HepG2 cells against the adverse effects of ethanol related to neutral lipid and phospholipid metabolism. Biochem Pharmacol. 2002;63:1485–1490. doi: 10.1016/s0006-2952(02)00896-1. [DOI] [PubMed] [Google Scholar]
- 38.Jensen DE, Belka GK, Du Bois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J. 1998;331(Pt 2):659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ottesen LH, Harry D, Frost M, Davies S, Khan K, Halliwell B, Moore K. Increased formation of S-nitrothiols and nitrotyrosine in cirrhotic rats during endotoxemia. Free Radic Biol Med. 2001;31:790–798. doi: 10.1016/s0891-5849(01)00647-5. [DOI] [PubMed] [Google Scholar]
- 40.Grattagliano I, Caraceni P, Calamita G, Ferri D, Gargano I, Palasciano G, Portincasa P. Severe liver steatosis correlates with nitrosative and oxidative stress in rats. Eur J Clin Invest. 2008;38:523–530. doi: 10.1111/j.1365-2362.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 41.Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Mutual changes of thioredoxin and nitrosothiols during biliary cirrhosis: results from humans and cholestatic rats. Hepatology. 2007;45:331–339. doi: 10.1002/hep.21519. [DOI] [PubMed] [Google Scholar]
- 42.Kabuyama Y, Kitamura T, Yamaki J, Homma MK, Kikuchi S, Homma Y. Involvement of thioredoxin reductase 1 in the regulation of redox balance and viability of rheumatoid synovial cells. Biochem Biophys Res Commun. 2008;367:491–496. doi: 10.1016/j.bbrc.2007.12.178. [DOI] [PubMed] [Google Scholar]
- 43.Tsuji G, Koshiba M, Nakamura H, Kosaka H, Hatachi S, Kurimoto C, Kurosaka M, Hayashi Y, Yodoi J, Kumagai S. Thioredoxin protects against joint destruction in a murine arthritis model. Free Radic Biol Med. 2006;40:1721–1731. doi: 10.1016/j.freeradbiomed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Hilliquin P, Borderie D, Hernvann A, Menkes CJ, Ekindjian OG. Nitric oxide as S-nitrosoproteins in rheumatoid arthritis. Arthritis Rheum. 1997;40:1512–1517. doi: 10.1002/art.1780400820. [DOI] [PubMed] [Google Scholar]
- 45.Rocks SA, Davies CA, Hicks SL, Webb AJ, Klocke R, Timmins GS, Johnston A, Jawad AS, Blake DR, Benjamin N, Winyard PG. Measurement of S-nitrosothiols in extracellular fluids from healthy human volunteers and rheumatoid arthritis patients, using electron paramagnetic resonance spectrometry. Free Radic Biol Med. 2005;39:937–948. doi: 10.1016/j.freeradbiomed.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Das KC, Guo XL, White CW. Hyperoxia induces thioredoxin and thioredoxin reductase gene expression in lungs of premature baboons with respiratory distress and bronchopulmonary dysplasia. Chest. 1999;116:101S. [PubMed] [Google Scholar]
- 47.Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med. 2007;176:291–299. doi: 10.1164/rccm.200605-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorch SA, Foust R, 3rd, Gow A, Arkovitz M, Salzman AL, Szabo C, Vayert B, Geffard M, Ischiropoulos H. Immunohistochemical localization of protein 3-nitrotyrosine and S-nitrosocysteine in a murine model of inhaled nitric oxide therapy. Pediatr Res. 2000;47:798–805. doi: 10.1203/00006450-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 49.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153–160. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 51.Janero DR, Bryan NS, Saijo F, Dhawan V, Schwalb DJ, Warren MC, Feelisch M. Differential nitros(yl)ation of blood and tissue constituents during glyceryl trinitrate biotransformation in vivo. Proc Natl Acad Sci U S A. 2004;101:16958–16963. doi: 10.1073/pnas.0406075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moya MP, Gow AJ, Califf RM, Goldberg RN, Stamler JS. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet. 2002;360:141–143. doi: 10.1016/S0140-6736(02)09385-6. [DOI] [PubMed] [Google Scholar]
- 53.Carini M, Aldini G, Orioli M, Piccoli A, Tocchetti P, Facino RM. Chemiluminescence and LC-MS/MS analyses for the study of nitric oxide release and distribution following oral administration of nitroaspirin (NCX 4016) in healthy volunteers. J Pharm Biomed Anal. 2004;35:277–287. doi: 10.1016/S0731-7085(03)00531-4. [DOI] [PubMed] [Google Scholar]