Abstract
To clarify the relationship between Angiotensin II AT1 and AT2 receptors, we studied AT1 receptor mRNA and binding expression in tissues from AT2 receptor gene-disrupted (AT2 −/−) female mice, where AT2 receptors are not expressed in vivo, using in situ hybridization and quantitative autoradiography. Wild type mice expressed AT1A receptor mRNA and AT1 receptor binding in lung parenchyma, the spleen, predominantly in the red pulp, and in liver parenchyma. In wild type mice, lung AT2 receptors were expressed in lung bronchial epithelium and smooth muscle, and were not present in the lung parenchyma, the spleen or the liver. This indicates that AT1 and AT2 receptors were not expressed in the same cells. In AT2 −/− mice, we found higher AT1A receptor mRNA and AT1 receptor binding in lung parenchyma and in the red pulp of the spleen, but not in the liver, when compared to littermate wild-type controls. Our results suggest that impaired AT2 receptor function upregulates AT1 receptor transcription and expression in a tissue-specific manner and in cells not expressing AT2 receptors. AT1 upregulation explains the increased sensitivity to Angiotensin II characteristic of the AT2 −/− phenotype, consistent with enhanced AT1 receptor activation in a number of tissues.
Keywords: Renin-angiotensin system, Angiotensin receptor types, gene-disruption, autoradiography, in situ hybridization
INTRODUCTION
Angiotensin II (Ang II) is the principal effector peptide of the renin-angiotensin system (RAS) and there are two Ang II receptor types, the AT1 and AT2 receptors [1, 2]. The AT1 receptors are universally recognized as the major physiological Ang II receptors [2, 3] and selective and safe Ang II AT1 receptor blockers are a cornerstone in the treatment of hypertension and other cardiovascular disorders [2].
While humans express a single AT1 receptor type, there are two AT1 receptor subtypes in rodents, the AT1A and AT1B receptors [2, 4]. Their high sequence homology in the translated regions makes them pharmacologically similar and indistinguishable [5, 6]. However, with the use of probes directed to the low homology untranslated domains, it is possible to identify the AT1A and AT1B receptor subtypes by RT-PCR or in situ hybridization techniques [7, 8, 9]. In rodents, the AT1A and AT1B receptor subtypes are distributed and regulated differently [5]. The AT1A receptor is the most abundant AT1 receptor subtype, and most of the Ang II effects are the consequence of AT1A receptor stimulation [7, 10, 11, 12, 13].
On the other hand, the role of the Ang II AT2 receptor is still a matter of controversy [14, 15, 16, 17, 18, 19]. Interest on the role of these receptors emerged from the recognition that therapeutic blockade of AT1 receptors reduces feedback inhibition of renin formation, increasing circulating Ang II levels and possibly over stimulating AT2 receptors [2]. Initially a matter of concern [20] the postulated AT2 receptor activation during therapy with AT1 receptor antagonists was later proposed as a mechanism responsible for some of the therapeutic effects of AT1 receptor blockade [17]. This proposal was based on the cross-talk hypothesis, based on in vitro experiments and initially formulated as a physiological intracellular balance or feedback between AT1 and AT2 receptors exerting opposite effects [21, 22, 23, 24]. A balance shift towards AT2 receptor activation as a consequence of AT1 receptor antagonism could significantly contribute to the beneficial effects of the AT1 receptor blockers [17].
Gene disruption of specific receptors is one useful model to analyze receptor function, and it has been used to study the role of AT2 receptors [25, 26, 27, 28, 29, 30]. The phenotype of AT2 −/− mice largely represents that of enhanced AT1 receptor activation. This includes increased baseline blood pressure [29, 31] enhanced pressor response as a result of peripheral or central administration of Ang II [ 26, 25, 31, 29, 28], enhanced aortic constrictor responses to Ang II [29], decreased urinary sodium excretion after exogenous administration of the peptide and sustained anti-natriuresis [26, 31, 28] and increased renal fibrosis and collagen deposition [32]. In addition, AT2 −/− mice exhibited exaggerated HPA axis activity with increased ACTH and corticosterone release [33], enhanced adrenomedullary activity [33], increased vasopressin secretion [30], enhanced stress-induced hyperthermia [34], increased sensitivity to pain [35], and increased locomotion and anxiety [31, 27]. Thus, the AT2 −/− phenotype appears to resemble overall AT1 receptor activation.
This phenotype was initially explained as a direct result of a deficiency in protective mechanisms, compensatory in nature, in balance with AT1 receptor activation, and mediated by direct AT2 receptor stimulation in the same cells [28]. A number of findings indicated, however, that the original AT1/AT2 receptor cross-talk hypothesis was in need of revision. First, if AT1 and AT2 receptors were normally expressed within the same cells, as initially assumed [13] their reciprocal influence may be the consequence of autocrine regulatory mechanisms. However, there is no in vivo definitive evidence of same-cell localization of AT1 and AT2 receptors [8, 9, 36, 37, 38, 39, 40, 41]. Second, deletion of the AT2 receptor gene is associated with increased expression of AT1 receptors in aorta [29], selected kidney structures [42], adrenal gland [43] and hypothalamic paraventricular nucleus [33]. Third, we were intrigued by our finding that enhanced AT1 receptor expression in AT2 −/− mice occurred in structures such as the hypothalamic paraventricular nucleus and in renal cells were AT2 receptors are may not be present in vivo [33, 42, 44, 45, 46].
These observations indicated that autocrine mechanisms proposed initially may not be sufficient to explain the original hypothesis of an AT1/AT2 interaction. However, the tissues previously studied may contain low levels of AT2 receptors [33, 42, 44, 45, 46] The present study was designed to test to what extent same-cell localization was necessary for the AT2 receptor-induced regulation of AT1 receptor expression. To this effect we chose to analyze, in AT2 −/− mice and in search for AT1 receptor expression, the liver, spleen and lung, organs with established AT1 receptor function and demonstrated lack of AT1 and AT2 receptor cellular coexpression [9, 11, 12, 37, 38, 47, 48, 49, 50, 51, 52].
For our study we combined quantitative autoradiography and in situ hybridization, techniques that have been validated for the study of the distribution and quantification of Ang II receptor types [14].
MATERIALS AND METHODS
Animals
Mice (C57BL/6 and 129 Ola) were initially obtained from Jackson Laboratory (Bar Harbor, MA) and were subsequently housed at the Department of Biochemistry, Nashville University, where they were kept under controlled conditions with free access to water and food. The AT2 −/− mice were produced at the Department of Biochemistry, Nashville University by the injection of AT2 −/− disrupted embryonic stem cells (E14-1) from the 129 Ola mouse line into blastocyts derived from C57BL/6 mice, as described previously [31] . After the genotype of F2 heterozygous females (AT2 −/+) was clearly confirmed by Southern blot analysis of DNA isolated from tail biopsies [31], they were backcrossed with C57BL/6 wild type males for three generations. Female AT2 +/− littermates were crossed with the backcrossed males AT2 +/Y and AT2 -/Y to produce homozygous females AT2 −/− and wild type females AT2 +/+. Female littermates from the third backcross progeny were selected to minimize the effect of differences in genetic background. Adequate measures were taken to minimize pain or discomfort. The experiments were conducted in accordance with international standards on animal welfare as well as being compliant with local and national regulations, as defined by the European Communities Council Directive of 24 November 1986 (86/609/EEC). The University of Nashville and NIMH Animal Care and Use Committees approved all animal protocols. Mice were transported to NIMH when 8 weeks old, kept for one day under controlled conditions as above, and killed by decapitation between 10:00 a.m. and 11:00 a.m.
Tissue preparation
Spleen, lung and liver were immediately removed, frozen in isopentane at −30°C, and stored at −80°C. For binding studies, tissues were cut in a cryostat at −20°C to obtain 16 μm sections, which were thaw-mounted on gelatin-coated slides, dried overnight in a desiccator at 4°C, and stored at −80°C until use. For in situ hybridization, sections (16 μm) were thaw-mounted onto Superfrost®/Plus microslides (Daigger, Vernon Hills, IL), dried at 50°C for 5 min on a slide warmer and stored at −80°C until hybridization. We used consecutive sections for each set of experiments and for staining with hematoxylin and eosin to localize the structures expressing the binding or the receptor mRNA.
Angiotensin II receptor binding
Receptor binding was performed as described earlier [36]. Tissue sections were pre-incubated for 15 min at 22°C in 10 mM sodium phosphate buffer (pH 7.4), containing 120 mM NaCl, 5 mM MgCl2, 5 mM EDTA, 0.005% bacitracin and 0.2% protease free bovine serum albumin (Sigma Chemical). Pre-incubation was followed by incubation for 2 hours at 22°C in fresh buffer prepared as above with addition of 50 μM Plummer’s inhibitor, 100 μM phenylmethylsulfonyl fluoride and 500 μM phenantrolin. To determine receptor binding, 0.5 nM of Sarcosine1-Angiotensin II ([125I]-Sar1-Ang II) obtained from Peninsula Laboratories (Belmont, CA) and iodinated by New England Nuclear (Boston, MA) to a specific activity of 2200 Ci/mmol were added to the incubation buffer. After the incubation period, sections were washed 4 times for 1 min each in ice-cold 50 mM Tris-HCl buffer (pH 7.4), followed by a 30 sec wash in ice-cold water, and dried under a stream of cold air.
Consecutive sections were incubated with 0.5 nM [125I]Sar1-Ang II to determine total binding. Adjacent sections were incubated as above with addition of 10 μM losartan (DuPont-Merck, Wilmington, DE, USA), to displace binding to AT1 receptors. Binding to AT1 receptors was calculated as the difference between total binding and the binding remaining in adjacent sections incubated in the presence of excess concentration of losartan. Similarly, binding of [125I]Sar1-Ang II to AT2 receptors was determined as the difference between total binding and binding in adjacent sections incubated in the presence of 10 μM of PD 123319 (Parke-Davis, Ann Arbor, MI, USA) or 0.1 μM of CGP42112 (Neosystems Laboratory, Strasbourg, France) to selectively displace binding to AT2 receptors. The concentrations of the AT1 and AT2 receptor selective ligands were selected to give maximum specific displacement [36, 44, 53]. Non-specific or background binding was determined by incubating consecutive sections with 10−6 M Ang II (Peninsula Laboratories, Belmont, CA). The non-specific or background binding was subtracted from all determinations as described above. In the case of areas with only AT1 receptor binding, the values obtained after displacement with excess concentrations of losartan were not significantly different from background values obtained by displacement with excess concentrations of unlabelled Ang II.
In another set of adjacent lung sections, we performed binding to [125I]-CGP42112, a ligand for AT2 receptors that does not recognize AT1 binding sites, to confirm the expression of AT2 receptors [53]. Buffers used in this assay had the same composition as those used for the binding to [125I]-Sar1-Ang II. Tissue sections were pre-incubated for 15 min in incubation buffer followed by incubation for 120 min in fresh buffer containing 0.2 nM [125I]-CGP42112. At the concentrations used, [125I]CGP 42112 labels exclusively AT2, and not AT1, receptors [53]. To determine specific binding to AT2 receptors, consecutive sections were incubated in the presence of 10−6 M of PD 123319 to selectively displace binding to AT2 sites. Non-specific binding was determined by incubating consecutive sections with 5 × 10−6 M of Ang II (Peninsula).
Quantitative receptor autoradiography
We exposed dry sections to Hyperfilm-3H (Amersham Co., Arlington Heights, IL) along with 16 μm sections of autoradiographic [14C] micro scales (Amersham) at 4°C. Films were developed in ice-cold D-19 developer (Eastman Kodak, Rochester, NY) for 4 min, fixed in Kodak rapid fixer for 4 min at 22°C, and rinsed in water for 15 min. We measured optical densities in the autoradiograms by computerized micro densitometry using the NIH Image 1.6 analysis system (NIMH, Bethesda, MD). For quantitative autoradiography, we measured the optical densities separately in lung parenchyma, red pulp of the spleen, and liver parenchyma. The optical densities were related to the concentration of radioactivity present in the sections by comparison with the [14C] micro-scales, and transformed to corresponding values of fmol/mg protein [36, 54].
Emulsion autoradiography
To obtain more precise localization of AT1 and AT2 receptors, we used adjacent sections incubated with [125I]Sar1-Ang II or [125I]CGP 42112 as described above. Adjacent sections were stained with hematoxylin and eosin. After binding experiments sections were fixed for 60 min in paraformaldehyde vapors at 80°C and dipped in photo emulsion (Eastman-Kodak). After exposure for two to three weeks, sections were developed in Kodak D-19 developer (Eastman-Kodak) for three minutes at 15°C, fixed for four minutes in Kodak rapid fixer, counter stained with hematoxylin and eosin, and the silver grains visualized with a Zeiss microscope.
In situ hybridization histochemistry
A full length cDNA clone of the mouse AT1A receptor (I.M.A.G.E clone ID 3497420) was obtained from Invitrogen (Carlsbad, CA). From the sequence of the clone, we selected primers for PCR (Forward -5′TTGAAGAAGTAATTATGCAAAGCTG3′; Reverse -5′GACACGTGGGTCTCCATTG 3′) of a unique 280bp region within the 3′ untranslated region of the cDNA. After amplification, we used a TOPO cloning kit (Invitrogen, Carlsbad, CA) to clone the region into the Eco RI site of the vector pCR®II-TOPO®. The clones containing plasmids were linearized with HindIII for sense or XbaI for antisense template, respectively. Radiolabeled riboprobes were prepared by in vitro transcription in the presence of [35S]-UTP (Amersham, Arlington Heights, IL), 1μg of linearized plasmid and T7 (sense probe) or SP6 (antisense probe) RNA polymerase, using a MAXIScript® T7/SP6 kit (Ambion, Austin, TX) according to the protocol of the manufacturer. Unincorporated nucleotides were removed by centrifugation through ProbeQuant™ G-50 Micro Columns (Amersham Biosciences, Little Chalfont Buckinghamshire, England). The labeling of riboprobes was monitored with liquid scintillation counting and the specificity verified by a preliminary experiment with adrenal gland. In preliminary experiments, we verified the specificity of the riboprobe for the AT1A receptor by performing in situ hybridization in brain sections of wild-type mice. Specific signals for AT1A receptor mRNA were present in hypothalamic paraventricular nucleus, expressing AT1A receptors in the rat, but not in hippocampus, expressing AT1B receptors in the rat [9] (results not shown).
Prior to use, the sections were warmed in a desiccator at room temperature and then fixed in 4% formaldehyde in PBS for 10 min. After two washes in PBS, they were acetylated for 10 min in 0.1M triethanolamine HCl, 0.9% NaCl, containing 0.25% acetic anhydride, delipidated in ethanol and chloroform, and air-dried. Each slide-mounted section was covered with 150 μl hybridization buffer containing 50% formamide, 0.3 M NaCl, 1mM EDTA, 20mM Tris, pH 7.5, 1x Denhardt’s solution, 10% dextran sulfate, 100 μg/ml salmon testes DNA, 250 μg/ml yeast RNA, 250 μg/ml yeast tRNA, 150 mM DTT, 0.2% SDS, 0.2% sodium tiosulfate (NTS) and 2×107 cpm/ml sense or antisense probe, and the slide was covered with a glass cover slip. After hybridization for 18 hrs at 54°C, coverslips were removed and sections were rinsed several times in 4X standard saline citrate (SSC). Non-hybridized probes were digested by incubation with 40 μg/ml RNase A (Sigma Chemical, St. Louis, MO) for 30 min at 37°C. After a final high stringency wash in 0.1X SSC at 65°C for 60 min, sections were dehydrated in graded ethanols containing 0.3M ammonium acetate, air dried and exposed to Biomax MR film (Kodak, Rochester, NY) for 9 days. Films were developed as described above. The mRNA expression was analyzed by measuring optical film densities using the Scion Image Program 4.0.2 (Scion Corporation) based on the NIH Image Program. The intensities of hybridization signals were expressed as nCi/g tissue equivalent after calibration with [14C]-microscales [55, 56], and subtraction of the values obtained in the same areas of adjacent sections hybridized with sense (control) probes, which represent nonspecific hybridization. Data were expressed as means ± SEM, for groups of five animals, measured individually.
Statistical analysis
Results were expressed as means ± standard error of the mean (SEM), calculated and analyzed using GraphPad Prism (version 2.00) and Microsoft Excel (version 7.0a), and compared for significance using unpaired Student’s t-test.
RESULTS
Ang II receptors in spleen
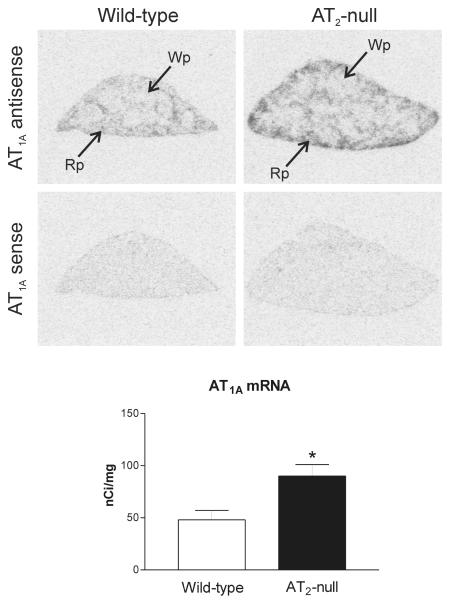
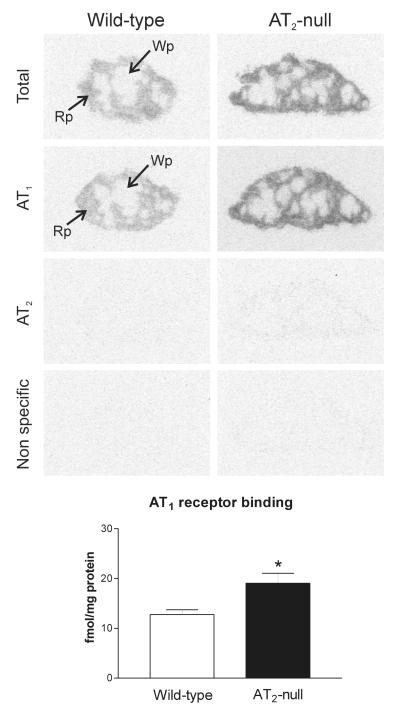
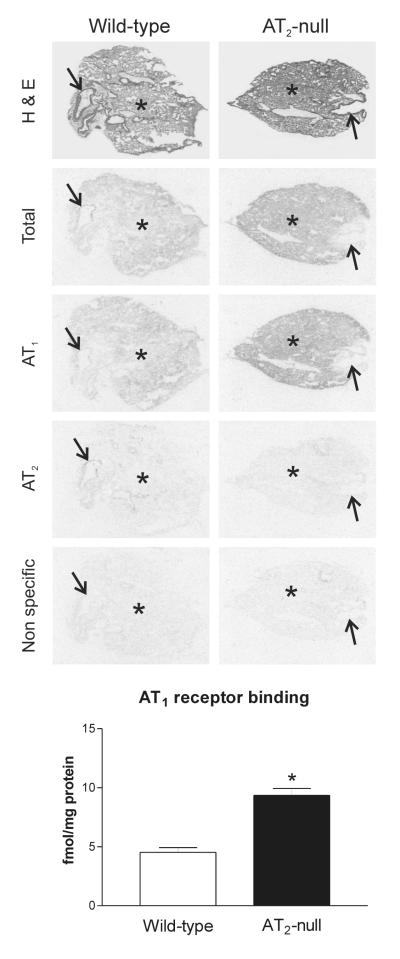
We detected high AT1A mRNA expression in the spleen of wild type mice, primarily localized to the red pulp (Figure 1). This pattern correlated well with high losartan-sensitive [125I]-Sar1-ang II binding, corresponding to AT1 receptors, highly expressed in the red pulp (Figure 2 and 3). Conversely, AT2 receptor binding was not detected in spleen from control wild-type mice (Figure 2).
Figure 1.
Representative film autoradiograms and quantitative analysis of AT1A mRNA in spleen of wild-type (control) and AT2 −/− mice detected by in situ hybridization. Note that the amount of AT1A mRNA is significantly higher (*p<0.05) in AT2 −/− mice as compared with wild-type control. Bars are means ± SEM for group of five animals, measured individually. Wp – white pulp, Rp – red pulp.
Figure 2.
Angiotensin II receptor binding in spleen of wild-type and AT2 −/− mice. Autoradiograms represent [125I]-Sar1-Ang II binding in the absence of selective ligands, in the presence of PD123319, losartan or Angiotensin II to visualize total binding, AT1 receptors, AT2 receptors and nonspecific binding as described in Material and Methods. Quantitative data are means ± SEM for group of five animals, measured individually. Note that AT1 receptor binding is significantly higher (*p<0.05) in AT2 −/− mice and is restricted to the red pulp. Wp – white pulp, Rp – red pulp.
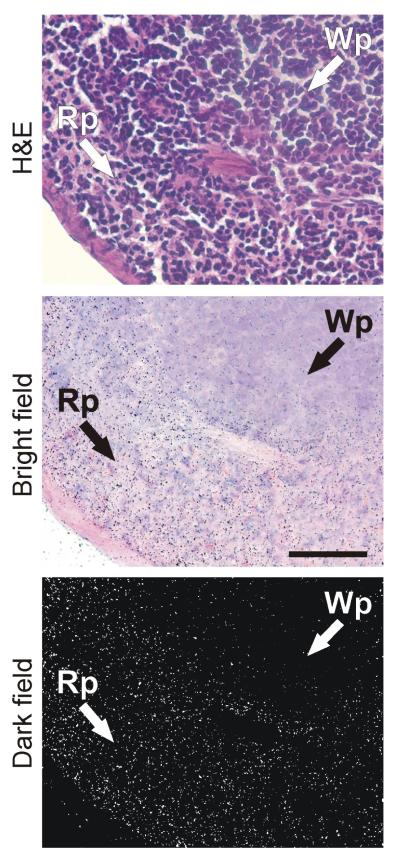
Figure 3.
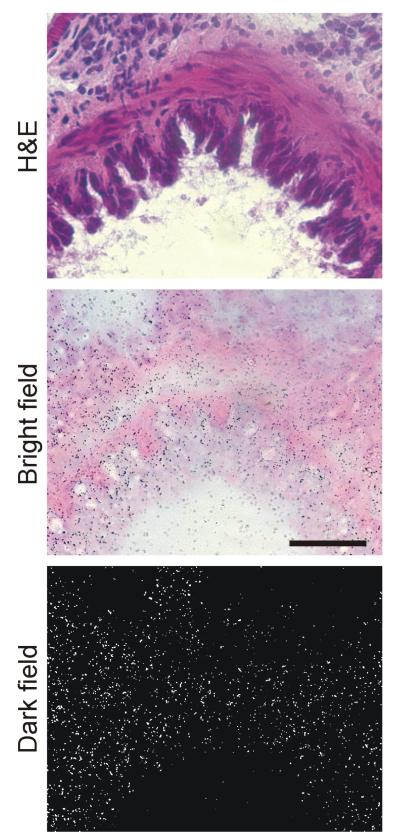
Hematoxylin-eosin staining, bright and dark field emulsion autoradiography of 125I-Sar1-Ang II binding to spleen of wild type mouse. Silver grains over red pulp represent Ang II receptor binding. Magnification 400x. Wp – white pulp, Rp – red pulp.
AT2 −/− mice expressed significantly higher AT1A mRNA and AT1 binding in the spleen when compared to AT2 +/+ mice, and both AT1A mRNA and AT1 binding showed a similar distribution to that present in the wild type mice (Figures 1 and 2). AT2 −/− mice did not express AT2 receptor binding in the spleen (Figure 2).
Ang II receptors in lung
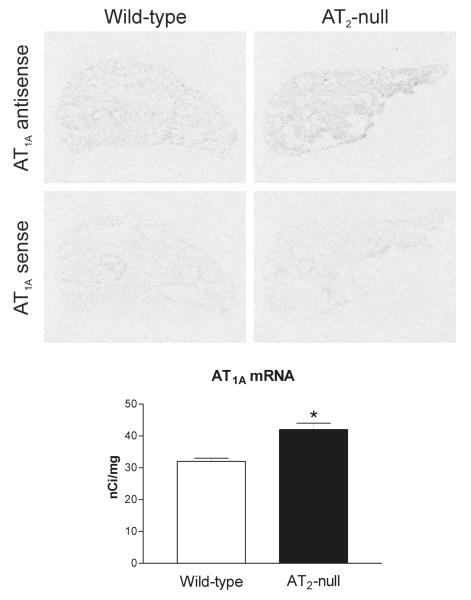
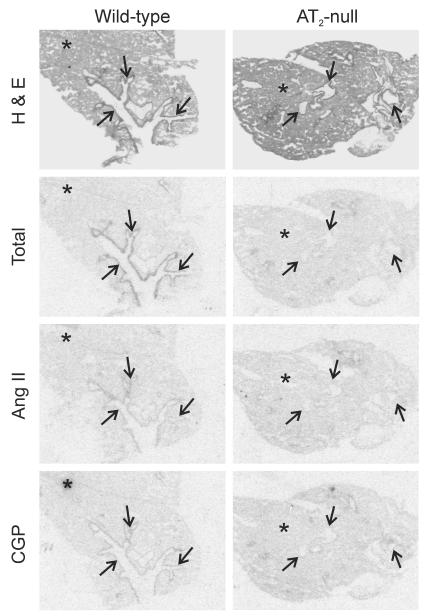
In wild type mice, we found significant AT1A mRNA expression uniformly distributed throughout the lung parenchyma (Figure 4). There was no AT1A mRNA expression associated with lung bronchia (Figure 4). High [125I]Sar1-Ang II binding expression was detected throughout the lung, both in lung parenchyma and bronchia. All binding corresponded to Ang II receptors, because incubation in the presence of Ang II completely prevented binding of [125I]-Sar1-ang II to both parenchyma and bronchia (Figure 5). In wild type mice, high losartan-sensitive binding to [125I]Sar1-Ang II, indicative of AT1 receptor expression was uniformly distributed throughout the lung parenchyma (Figure 5). There were no AT1 receptors in the lung bronchia (Figure 5). Conversely, small and large size bronchia expressed large numbers of [125I]Sar1-Ang II binding sensitive to displacement by PD123319, indicative of the presence of AT2 receptors (Figure 5), with no AT2 receptor present in the lung parenchyma. Ang II-sensitive binding to CGP 24112, indicative of AT2 receptor expression, was also present in lung bronchia, and was undetectable in the lung parenchyma (Figure 6). In bronchia, binding to [125I]-CGP42112 was associated with smooth muscle and epithelial cells as revealed by emulsion autoradiography (Figure 7).
Figure 4.
Representative film autoradiograms and quantitative analysis of AT1A mRNA in lung of wild-type (control) and AT2 −/− mice detected by in situ hybridization. Note that the amount of AT1A mRNA is significantly higher (*p<0.05) in AT2 −/− mice as compared with wild-type control. Bars are means ± SEM for group of five animals, measured individually.
Figure 5.
Hematoxylin-eosin staining, representative film autoradiograms of Angiotensin II receptor binding in lung of wild-type and AT2-null mice. Autoradiograms of [125I]-Sar1-Ang II binding in the presence of selective ligands as described in Material and Methods. Results are means ± SEM for group of five animals, measured individually. AT1 receptor binding is significantly higher (*p<0.05) in lung parenchyma (asterisks) of AT2 −/− mice as compared with wild-type control. AT2 receptor binding in bronchia (arrows) is present in wild-type, but absent in AT2 −/− mice. Bars are means ± SEM for group of five animals, measured individually.
Figure 6.
Hematoxylin-eosin staining, representative autoradiograms of 125I-CGP42112 receptor binding in lung of wild-type and AT2 −/− mice. Incubation of consecutive sections with 125I-CGP42112 and displacement with Angiotensin II confirmed the presence of AT2 receptors in bronchia (arrows) of wild-type animals, and absence in AT2 −/− mice. Asterisk represents parenchyma.
Figure 7.
Hematoxylin-eosin staining, bright and dark field emulsion autoradiography of 125I-CGP42112 binding to lung bronchia of wild type mouse. Silver grains over bronchia represent AT2 receptor binding. Magnification 400x.
In AT2 −/− mice, there was a significant increase in AT1A mRNA and AT1 receptor binding expression restricted to the lung parenchyma, when compared to wild type mice (Figures 4 and 5). As it was the case in wild type mice, no AT1A mRNA or AT1 binding were found in lung bronchia of AT2 −/− mice (Figures 4 and 5). No AT2 receptor binding was detected in the lungs of AT2 −/− mice (Figures 5 and 6).
Ang II receptors in liver
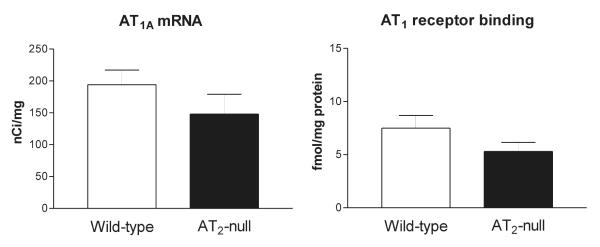
In wild type mice, we detected significant AT1A receptor mRNA and AT1 receptor binding (Figure 8), and no AT2 receptor binding (not shown). In the liver, there were no significant differences in expression of AT1A receptor mRNA and AT1 receptor binding between AT2 +/+ and AT2 −/− mice (Figure 8).
Figure 8.
Quantitative autoradiography of the AT1A mRNA and receptor binding in liver of wild-type and AT2 −/− mice. Bars are means ± SEM for group of five animals, measured individually. Non significant differences were found between wild-type and AT2 −/− mice.
DISCUSSION
The novel finding in our study is the demonstration of a significant increase, in adult AT2 −/− mice, in the expression of AT1A receptor mRNA and AT1 receptor binding in tissues and cells where AT2 receptors are never expressed, the red pulp of the spleen and the lung parenchyma [9, 11, 12, 37, 38, 47, 48, 49, 50, 51, 52].
Although upregulation of AT1 receptor transcription and translation was reported earlier in other tissues such as the aorta, kidney, adrenal gland and hypothalamic paraventricular nucleus, the possibility remained that these tissues may express low levels of AT2 receptors [33, 42, 44, 45, 46]. In the present study, we focused on structures where AT2 receptors were unequivocally demonstrated to be absent [9, 11, 12, 37, 38, 47, 48, 49, 50, 51, 52]. Thus, the main implication of our findings is that life-long gene-disruption of one Ang II receptor type, the AT2 receptor, coincides, in different cell populations, with life-long enhanced transcription and overexpression of the main physiological Ang II receptor, the AT1 receptor.
Analysis of Ang II receptor binding in lung, spleen and liver of AT2 +/+ type mice confirmed expression of AT1 receptors in lung parenchyma, red pulp of the spleen and liver. Conversely, AT2 receptor binding was only present in lung bronchia, and was absent in lung parenchyma, spleen or liver. Expression of AT1 and AT2 receptors in the mouse is identical to that of the rat, confirming previous reports [9, 11, 12, 38, 41, 48, 49, 50, 51, 52]. Our results reveal that in the mouse, like in the rat, AT1 and AT2 receptors were expressed in different cells not anatomically associated.
Our results are an extension of previous work reporting increased AT1 receptor mRNA and binding in the hypothalamic paraventricular nucleus, kidney glomeruli, adrenal gland and aorta [29, 33, 42, 43]. These studies, however, utilized a riboprobe directed to the translated region of the rat AT1 receptor, with very high homology to the translated region of the mouse AT1 receptor [33, 42, 43]. Although this probe successfully demonstrated mouse AT1 receptor transcription, its use did not clarify the AT1 receptor subtype involved. In the mouse and rat liver and lung, and in the rat spleen, AT1 receptors have been characterized as of the AT1A subtype [7, 8, 57, 58]. For this reason we constructed a riboprobe selective for the mouse AT1A receptor. We took advantage of the low homology in the untranslated regions of the mouse AT1A and AT1B receptors, and we designed a subtype-specific riboprobe complementary to the 3′ noncoding region of the AT1A receptor mRNA [5]. With the use of this riboprobe, we now demonstrate mRNA expression for AT1A receptors in lung parenchyma, the red pulp of the spleen and the liver, what is in agreement with previous reports [7, 8, 57, 58, 59]. Although we did not study AT1B mRNA, this AT1 receptor subtype has a very restricted expression in adult male rodents, it is not present in the lung or the liver [7, 8, 51, 57, 58, 59] and its presence has not been reported in spleen.
Our combined autoradiography and in situ hybridization results reveal that AT1A mRNA expression and AT1 receptor binding are very significantly increased in spleen and lung parenchyma of AT2 −/− mice. These results and our previous observations [29, 33, 42, 43] suggest a revised mechanism for the AT1/AT2 cross-talk hypothesis. We postulate that, instead of intracellular, autocrine interactions, AT2 receptor expression physiologically limits and controls AT1 receptor transcription and expression, and therefore AT1 receptor activity in cells devoid of AT2 receptor expression. As a consequence, disruption of the AT2 receptor should be expected to promote enhanced AT1 receptor activity. Increased activity of AT1 receptors, as a result of their transcription and translation upregulation, explains the AT2 −/− receptor phenotype of increased anxiety, response to stress, vasoconstriction and hypertension [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 42, 43].
Accordingly, AT2 −/− mice would be expected to present a phenotype consistent with AT1 receptor overactivity in the lung and the spleen. Basal lung functions were not altered in AT1A or AT2 gene-deleted mice [11]. However, indirect evidence strongly suggest a vulnerability to inflammatory responses when AT2 receptor activity is decreased, and, reciprocally, diminished inflammatory responses when AT1 receptor activity is reduced. For example, the inflammatory response of the lung to bacterial endotoxin was decreased in AT1A−/− mice [12]. Inactivation of the AT2 receptor but not AT2 receptor blockade aggravates acute lung inflammatory injury, indicating that the role of the AT2 receptor was indirect, possibly through AT1 receptor activation [11]. In addition, both gene deletion of the AT1A receptor and AT1 receptor blockade protected against lung injury [11] and AT1 receptor blockade reduces inflammatory responses in the lung [60]. These findings demonstrate that AT1 receptor activity contributes to pulmonary vulnerability to injury and inflammation, and may be best explained by our present finding of enhanced AT1A receptor transcription and expression in AT2−/− mice. Increased AT1 receptor expression in the spleen, in turn, may contribute to explain the enhanced inflammatory responses in AT2 −/− mice, because spleen AT1 receptor blockade decreases inflammatory responses to bacterial endotoxin [61].
Spontaneous, life-long, complete absence of AT2 receptor activity has not been reported and probably represents a condition not existing in nature. This questions the physiological significance of findings in this model. For this reason it is of interest that pharmacological inhibition of AT2 receptors in adult rats enhances AT1 receptor expression in the brain [62]. Moreover, cardiac-specific over-expression of AT2 receptors attenuated AT1 receptor-mediated pressor and chronotropic effects [23, 63] a finding indicative of decreased AT1 receptor function. This indicates that AT2-dependent modulation of AT1 receptor activity does not only occur after complete AT2 gene disruption, but also after partial AT2 receptor blockade or organ-selective over-expression. Moreover, it appears that the expression of AT1 and AT2 receptors is reciprocally regulated, since AT1 receptor blockade enhances AT2 receptor expression in selected brain areas [64, 65, 66] and in peripheral tissues such as the muscular gastric layer and adipose tissue [67, 68] .The conclusion is that while AT2 receptor activity may limit and control AT1 receptor expression, AT1 receptor activity, in turn, limits the expression of AT2 receptors.
The reciprocal influence of AT1 and AT2 Ang II receptors is not universal, since there were no alterations in liver AT1A receptor transcription and expression in AT2 −/− mice. This is not surprising, because it is well known that AT1 receptor transcription is regulated independently in a cell and tissue-specific manner [69, 70]. In the liver, increased AT1 receptor stimulation is associated with fibrosis [71], and fibrosis as a consequence of bile duct ligation is reduced in AT1A −/− mice [51].
The question remains as to the mechanism of the reciprocal regulation of AT1/AT2 receptor expression. Our present results appear to rule out intracellular (intracrine) and close-range intercellular (paracrine) mechanisms to explain AT1/AT2 receptor cross-talk. Instead, two possible mechanisms of interregulation should be considered. AT2/AT1 regulation may occur through factors acting at a distance of their site of production, a hormonal mechanism. For example, the increased sensitivity to Ang II in AT2 −/− mice has been explained by a deficiency of AT2 receptor-mediated local generation of bradykinin and nitric oxide [28, 72]. Expression of endothelial nitric oxide synthase was lower and nitric oxide synthesis was decreased in AT2 −/− mice [28, 73, 74], and the endothelial nitric oxide synthase −/− phenotype is consistent with AT1 receptor upregulation [72].
Alternatively, we must consider the possibility of genomic regulatory mechanisms. Genomic regulations are complex and poorly understood. However, it is not unreasonable to suggest that expression of genes belonging to a given system may influence each other. Multiple epistatic interactions between genes regulating blood pressure, and a major influence of the genetic background, are being demonstrated with the use of genetically hypertensive congenic rat strains [75]. The genetic background may be of fundamental importance for AT2 receptor function and its effects on AT1 receptor expression. For example, AT2 −/− mice were generated in strains with different genetic background by two independent groups [26, 31] While one of the strains, studied here, expressed a phenotype consistent with AT1 receptor stimulation [31] the other strain did not [26].
In conclusion, the results presented here indicate that AT2 receptor activity regulates AT1 receptor transcription and expression at a distance, in cells not expressing AT2 receptors. These observations clearly demonstrate that the interaction between Ang II receptor types is of an indirect nature, possibly involving still undetermined hormonal and/or genomic mechanisms.
Acknowledgements
This study was supported by the Division of Intramural Research Programs, National Institute of Mental Health, Department of Health and Human Services, USA (J.P., J.A.T, J.B., A.F-N, J.M.S.) and NIH grant HL58205 (A.R. and T.I.).
J.A.T. was on leave from the Department of Pharmacology, CINVESTAV-IPN, Mexico City, Mexico. J.P. is currently an employee of the Institute of Neurobiology, the Slovak Academy of Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsusaka T, Ichikawa I. Biological functions of angiotensin and its receptors. Annu. Rev. Physiol. 1997;59:395–412. doi: 10.1146/annurev.physiol.59.1.395. [DOI] [PubMed] [Google Scholar]
- 2.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharm. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 3.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology. XXIII. The Angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 4.Sasamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, Dzau VJ. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem. Biophys. Res. Commun. 1992;185:253–259. doi: 10.1016/s0006-291x(05)80983-0. [DOI] [PubMed] [Google Scholar]
- 5.Iwai N, Inagami T. Identification of two subtypes in the rat type I angiotensin II receptor. FEBS Lett. 1992;298:257–260. doi: 10.1016/0014-5793(92)80071-n. [DOI] [PubMed] [Google Scholar]
- 6.Iwai N, Inagami T, Ohmichi N, Nakamura Y, Saeki Y, Kinoshita M. Differential regulation of rat AT1a and AT1b receptor mRNA. Biochem. Biophys. Res. Commun. 1992;188:298–303. doi: 10.1016/0006-291x(92)92384-a. [DOI] [PubMed] [Google Scholar]
- 7.Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in the mouse. Am. J. Physiol. 1994;267:E260–E267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
- 8.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase polymerase chain reaction analysis. Hypertension. 1994;24:538–548. doi: 10.1161/01.hyp.24.5.538. [DOI] [PubMed] [Google Scholar]
- 9.Jöhren O, Inagami T, Saavedra JM. AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. Neuroreport. 1995;15:2549–2552. doi: 10.1097/00001756-199512150-00024. [DOI] [PubMed] [Google Scholar]
- 10.Kato H, Ishida J, Nagano K, Honjo K, Sugaya T, Takeda N, Sugiyama F, Yagami K, Fujita T, Nangaku M, Fukamizu A. Deterioration of atherosclerosis in mice lacking angiotensin II type 1A receptor in bone marrow-derived cells. Lab. Invest. 2008;88:731–739. doi: 10.1038/labinvest.2008.42. [DOI] [PubMed] [Google Scholar]
- 11.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Poi H Leong, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohwada K, Watanabe K, Okuyama K, Ohkawara Y, Sugaya T, Takayanagi M, Ohno I. The involvement of type 1a angiotensin II receptors in the regulation of airway inflammation in a murine model of allergic asthma. Clin. Exp. Allergy. 2007;37:1720–1727. doi: 10.1111/j.1365-2222.2007.02815.x. [DOI] [PubMed] [Google Scholar]
- 13.Gembardt F, Heringer-Walther S, van Esch JH, Sterner-Kock A, van Veghel R, Le TH, Garrelds IM, Coffman TM, Danser AH, Schultheiss HP, Walther T. Cardiovascular phenotype of mice lacking all three subtypes of angiotensin II receptors. FASEB J. 2008;22:3068–3077. doi: 10.1096/fj.08-108316. [DOI] [PubMed] [Google Scholar]
- 14.Saavedra JM. Emerging features of brain angiotensin receptors. Regul. Pept. 1999;30(85):31–45. doi: 10.1016/s0167-0115(99)00081-6. [DOI] [PubMed] [Google Scholar]
- 15.Saavedra JM. Brain Angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell. Mol. Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu YC, Zhu YZ, Lu N, Wang MJ, Wang YX, Yao T. Role of angiotensin AT1 and AT2 receptors in cardiac hypertrophy and cardiac remodelling. Clin. Exp. Pharmacol. Physiol. 2003;30:911–918. doi: 10.1111/j.1440-1681.2003.03942.x. [DOI] [PubMed] [Google Scholar]
- 17.Volpe M, Musumeci B, De Paolis P, Savoia C, Morganti A. Angiotensin II AT2 receptor subtype: an uprising frontier in cardiovascular disease? J. Hypertens. 2003;21:1429–1443. doi: 10.1097/00004872-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 18.de Gasparo M, Siragy HM. The AT2 receptor: fact, fancy and fantasy. Regul. Pept. 1999;81:11–24. doi: 10.1016/s0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 19.Porrello ER, Delbridge LMD, Thomas WG. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front. Biosc. 2009;14:958–972. doi: 10.2741/3289. [DOI] [PubMed] [Google Scholar]
- 20.Wharton J, Morgan K, Rutherford RA, Catravas JD, Chester A, Whitehead BF, De Leval MR, Yacoub MH, Polak JM. Differential distribution of angiotensin AT2 receptors in the normal and failing human heart. J. Pharmacol. Exp. Ther. 1998;284:323–336. [PubMed] [Google Scholar]
- 21.Gelband CH, Zhu M, Lu D, Reagan LP, Fluharty SJ, Posner P, Raizada MK, Sumners C. Functional interactions between neuronal AT1 and AT2 receptors. Endocrinology. 1997;138:2195–2198. doi: 10.1210/endo.138.5.5236. [DOI] [PubMed] [Google Scholar]
- 22.Jin XH, Wang ZQ, Siragy HM, Guerrant RL, Carey RM. Regulation of jejunal sodium and water absorption by angiotensin subtype receptors. Amer. J. Physiol. 1998;275:R515–R523. doi: 10.1152/ajpregu.1998.275.2.R515. [DOI] [PubMed] [Google Scholar]
- 23.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J. Clin. Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagami T, Eguchi S, Numaguchi K, Motley ED, Tang H, Matsumoto T, Yamakawa T. Cross-talk between angiotensin II receptors and the tyrosine kinases and phosphatases. J. Am. Soc. Nephrol. 1999;(Suppl 11):S57–S61. [PubMed] [Google Scholar]
- 25.Akishita M, Yamada H, Dzau VJ, Horiuchi M. Increased vasoconstrictor response of the mouse lacking angiotensin II type 2 receptor. Biochem. Biophys. Res. Commun. 1999;261:345–349. doi: 10.1006/bbrc.1999.1027. [DOI] [PubMed] [Google Scholar]
- 26.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor gene in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 27.Okuyama S, Sakagawa T, Chaki S, Imagawa Y, Ichiki T, Inagami T. Anxiety-like behavior in mice lacking the angiotensin II type-2 receptor. Brain Res. 1999;821:150–159. doi: 10.1016/s0006-8993(99)01098-7. [DOI] [PubMed] [Google Scholar]
- 28.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc. Natl. Acad. Sci. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Tsuchida S, Imai T, Fujii N, Miyazaki H, Ichiki T, Naruse M, Inagami T. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem. Biophys. Res.Commun. 1999;258:194–198. doi: 10.1006/bbrc.1999.0500. [DOI] [PubMed] [Google Scholar]
- 30.Gross V, Obst M, Luft FC. Insights into angiotensin II receptor function through AT2 receptor knockout mice. Acta Physiol. Scand. 2004;181:487–494. doi: 10.1111/j.1365-201X.2004.01322.x. [DOI] [PubMed] [Google Scholar]
- 31.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BLM, Inagami T. Effects on blood pressure and explorative behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Nishimura H, Fogo A, Kon V, Inagami T, Ichikawa I. Accelerated fibrosis and collagen deposition develop in the renal interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstruction. Kidney Int. 1998;53:937–944. doi: 10.1111/j.1523-1755.1998.00893.x. [DOI] [PubMed] [Google Scholar]
- 33.Armando I, Terrón JA, Falcón-Neri A, Takeshi I, Häuser W, Inagami T, Saavedra JM. Increased angiotensin II AT(1) receptor expression in paraventricular nucleus and hypothalamic-pituitary-adrenal axis stimulation in AT(2) receptor gene disrupted mice. Neuroendocrinology. 2002;76:137–147. doi: 10.1159/000064525. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Hashimoto M, Okuyama S, Inagami T, Nakamura S. Effects of targeted disruption of the mouse angiotensin II type 2 receptor gene on stress-induced hyperthermia. J. Physiol. 1999;515:881–885. doi: 10.1111/j.1469-7793.1999.881ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakagawa T, Okuyama S, Kawashima N, Hozumi S, Nakagawasai O, Tadano T, Kisara K, Ichiki T, Inagami T. Pain threshold, learning and formation of brain edema in mice lacking the angiotensin II type 2 receptor. Life Sci. 2000;67:2577–2585. doi: 10.1016/s0024-3205(00)00841-9. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptors subtypes (AT1 and AT2) in rat brain. Amer. J. Physiol. 1991a;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 37.Tsutsumi K, Saavedra JM. Angiotensin-II receptor subtypes in median eminence and basal forebrain areas involved in regulation of pituitary function. Endocrinology. 1991b;129:3001–3008. doi: 10.1210/endo-129-6-3001. [DOI] [PubMed] [Google Scholar]
- 38.Bullock GR, Steyaert I, Bilbe G, Carey RM, Kips J, De Paepe B, Pauwels R, Praet M, Siragy HM, de Gasparo M. Distribution of type-1 and type-2 angiotensin receptors in the normal human lung and in lungs from patients with chronic obstructive pulmonary disease. Histochem. Cell. Biol. 2001;115:117–124. doi: 10.1007/s004180000235. [DOI] [PubMed] [Google Scholar]
- 39.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin II type-2 mRNA expression in the adult rat brain. J. Comp. Neurol. 1996;373:322–339. doi: 10.1002/(SICI)1096-9861(19960923)373:3<322::AID-CNE2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience. 1998;82:827–841. doi: 10.1016/s0306-4522(97)00328-x. [DOI] [PubMed] [Google Scholar]
- 41.Tsutsumi K, Strömberg C, Saavedra JM. Characterization of angiotensin II receptor subtypes in the rat spleen. Peptides. 1992;13:291–296. doi: 10.1016/0196-9781(92)90111-f. [DOI] [PubMed] [Google Scholar]
- 42.Saavedra JM, Häuser W, Ciuffo G, Egidy G, Hoe KL, Jöhren O, Sembonmatsu T, Inagami T, Armando I. Increased AT(1) receptor expression and mRNA in kidney glomeruli of AT(2) receptor gene-disrupted mice. Am. J. Physiol. Renal Physiol. 2001a;280:F71–F78. doi: 10.1152/ajprenal.2001.280.1.F71. [DOI] [PubMed] [Google Scholar]
- 43.Saavedra JM, Armando I, Terrón JA, Falcón-Neri A, Jöhren O, Häuser W, Inagami T. Increased AT(1) receptors in adrenal gland of AT(2) receptor gene-disrupted mice. Regul. Pept. 2001b;102:41–47. doi: 10.1016/s0167-0115(01)00303-2. [DOI] [PubMed] [Google Scholar]
- 44.Häuser W, Jöhren O, Saavedra JM. Characterization and distribution of angiotensin II receptor subtypes in the mouse brain. Eur. J. Pharm. 1998;348:101–114. doi: 10.1016/s0014-2999(98)00134-4. [DOI] [PubMed] [Google Scholar]
- 45.Jain P, Armando I, Juorio AV, Barden N, Benicky J, Saavedra JM. Decreased hypothalamic and adrenal angiotensin II receptor expression and adrenomedullary catecholamines in transgenic mice with impaired glucocorticoid receptor function. Neuroendocrinology. 2004;80:171–180. doi: 10.1159/000082358. [DOI] [PubMed] [Google Scholar]
- 46.Jöhren O, Imboden H, Häuser W, Maye I, Sanvitto GL, Saavedra JM. Localization of angiotensin-converting enzyme, angiotensin II, angiotensin II receptor subtypes, and vasopressin in the mouse hypothalamus. Brain Res. 1997;757:218–227. doi: 10.1016/s0006-8993(97)00220-5. [DOI] [PubMed] [Google Scholar]
- 47.Castrén E, Kurihara M, Saavedra JM. Autoradiographic localization and characterization of angiotensin II binding sites in the spleen of rats and mice. Peptides. 1987;8:737–742. doi: 10.1016/0196-9781(87)90050-7. [DOI] [PubMed] [Google Scholar]
- 48.Chassagne C, Eddahibi S, Adamy C, Rideau D, Marotte F, Dubois-Randé JL, Adnot S, Samuel JL, Teiger E. Modulation of angiotensin II receptor expression during development and regression of hypoxic pulmonary hypertension. Am. J. Respir. Cell. Mol. Biol. 2000;22:323–332. doi: 10.1165/ajrcmb.22.3.3701. [DOI] [PubMed] [Google Scholar]
- 49.Cox SL, Trendelenburg AU, Starke K. Prejunctional angiotensin receptors involved in the facilitation of noradrenaline release in mouse tissues. Br. J. Pharmacol. 1999;127:1256–1262. doi: 10.1038/sj.bjp.0702652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–1676. doi: 10.1053/gast.2002.36561. [DOI] [PubMed] [Google Scholar]
- 51.Yang L, Bataller R, Dulyx J, Coffman TM, Ginés P, Rippe RA, Brenner DA. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J. Hepatol. 2005;43:317–323. doi: 10.1016/j.jhep.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 52.Tsutsumi K, Strömberg C, Viswanathan M, Saavedra JM. Angiotensin-II receptor subtypes in fetal tissue of the rat: autoradiography, guanine nucleotide sensitivity, and association with phosphoinositide hydrolysis. Endocrinology. 1991;129:1075–1082. doi: 10.1210/endo-129-2-1075. [DOI] [PubMed] [Google Scholar]
- 53.Heemskerk FM, Saavedra JM. Quantitative autoradiography of angiotensin II AT2 receptors with [125I]CGP 42112. Brain Res. 1995;677:29–38. doi: 10.1016/0006-8993(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 54.Nazarali AJ, Gutkind JS, Saavedra JM. Calibration of [125I]-polymer standards with [125I]-brain paste standards for use in quantitative receptor autoradiography. J. Neurosci. Meth. 1989;30:247–253. doi: 10.1016/0165-0270(89)90135-0. [DOI] [PubMed] [Google Scholar]
- 55.Miller JA. The calibration of 35S or 32P with 14C-labeled brain paste or 14C-plastic standards for quantitative autoradiography using LKB Ultrofilm or Amersham Hyperfilm. Neurosci Lett. 1991;121:211–214. doi: 10.1016/0304-3940(91)90687-o. [DOI] [PubMed] [Google Scholar]
- 56.Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. Situ Hybridization Protocols for the Brain. Academic; San Diego: 1994. pp. 9–34. [Google Scholar]
- 57.Gasc JM, Shanmugam S, Sibony M, Corvol P. Tissue-specific expression of type 1 angiotensin II receptor subtypes. An in situ hybridization study. Hypertension. 1994;24:531–537. doi: 10.1161/01.hyp.24.5.531. [DOI] [PubMed] [Google Scholar]
- 58.Shanmugam S, Monnot C, Corvol P, Gasc JM. Distribution of type 1 angiotensin II receptor subtype messenger RNAs in the rat fetus. Hypertension. 1994;23:137–141. doi: 10.1161/01.hyp.23.1.137. [DOI] [PubMed] [Google Scholar]
- 59.Shanmugam S, Corvol P, Gasc JM. Angiotensin II type 2 receptor mRNA expression developing cardiopulmonary system of the rat. Hypertension. 1996;28:91–97. doi: 10.1161/01.hyp.28.1.91. [DOI] [PubMed] [Google Scholar]
- 60.Tanabe Y, Morikawa Y, Kato T, Kanai S, Watakabe T, Nishijima A, Iwata H, Isobe K, Ishizaki M, Nakayama K. Effects of olmesartan, an AT1 receptor antagonist, on hypoxia-induced activation of ERK1/2 and pro-inflammatory signals in the mouse lung. Naunyn Schmiedebergs Arch. Pharmacol. 2006;374:235–248. doi: 10.1007/s00210-006-0110-1. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Lemus E, Benicky J, Pavel J, Larrayoz IM, Zhou J, Baliova M, Nishioku T, Saavedra JM. Angiotensin II AT1 blockade reduces the lipopolysaccharide-induced innate immune response in rat spleen. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1376–R1384. doi: 10.1152/ajpregu.90962.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macova M, Pavel J, Saavedra JM. A peripherally administered, centrally acting angiotensin II AT2 antagonist selectively increases brain AT1 receptors and decreases brain tyrosine hydroxylase transcription, pituitary vasopressin and ACTH. Brain Res. 2009;1250:130–140. doi: 10.1016/j.brainres.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K, Miwa T, Kawada N, Mori Y, Shibasaki Y, Tanaka Y, Fujiyama S, Koyama Y, Fujiyama A, Takahashi H, Iwasaka T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seltzer A, Bregonzio C, Armando I, Baiardi G, Saavedra JM. Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res. 2004;1028:9–18. doi: 10.1016/j.brainres.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 65.Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, Nishioku T, Dou J, Delgiacco E, Saavedra JM. AT1 receptor blockade regulates the local Angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- 66.Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM. Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold-restraint stress-induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress. 2008;11:457–466. doi: 10.1080/10253890801892040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G414–G423. doi: 10.1152/ajpgi.00058.2003. [DOI] [PubMed] [Google Scholar]
- 68.Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARγ. Eur. J. Pharm. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitami Y, Okura T, Wakamiya R, Marumoto K, Iwata T, Hiwada K. Regulation of the gene expression of type-1 angiotensin II receptor in spontaneously hypertensive rats. Blood Press. 1992;(Suppl 3):12–16. [PubMed] [Google Scholar]
- 70.Amiri F, Garcia R. Differential regulation of renal glomerular and preglomerular vascular angiotensin II receptors. Am. J. Physiol. 1996;270:E810–E815. doi: 10.1152/ajpendo.1996.270.5.E810. [DOI] [PubMed] [Google Scholar]
- 71.Yoshiji H, Noguchi R, Ikenaka Y, Kitade M, Kaji K, Tsujimoto T, Uemura M, Fukui H. Renin-angiotensin system inhibitors as therapeutic alternatives in the treatment of chronic liver diseases. Curr. Med. Chem. 2007;14:2749–2754. doi: 10.2174/092986707782360169. [DOI] [PubMed] [Google Scholar]
- 72.Brede M, Roell W, Ritter O, Wiesmann F, Jahns R, Haase A, Fleischmann BK, Hein L. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension. 2003;42:1177–1182. doi: 10.1161/01.HYP.0000100445.80029.8E. [DOI] [PubMed] [Google Scholar]
- 73.Toda N, Ayajiki K, Okamura T. Interaction of endotelial nitric oxide and angiotensin in the circulation. Pharm. Rev. 2007;9:54–87. doi: 10.1124/pr.59.1.2. [DOI] [PubMed] [Google Scholar]
- 74.Obst M, Gross V, Bonartsev A, Janke J, Müller DN, Park JK, Kärgel E, Luft FC. Nitric oxide synthase expression in AT2 receptor-deficient mice after DOCA-salt. Kidney Int. 2004;65:2268–2278. doi: 10.1111/j.1523-1755.2004.00646.x. [DOI] [PubMed] [Google Scholar]
- 75.Saavedra JM. Opportunities and limitations of genetic analysis of hypertensive rat strains. J. Hypertension. Editorial. 2009 doi: 10.1097/HJH.0b013e32832bb832. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]