Abstract
Background
Individuals with diabetes are at two to three-fold increased risk for cardiovascular disease (CVD) relative to those without diabetes. Our objective was to examine CVD risk factor level changes among individuals with and without type 2 diabetes from 1970–2005 in the Framingham Heart Study.
Methods and Results
We included 4,195 participants (3,990 non-diabetes/205 diabetes) aged 50 and 3,495 participants (3,178 non- diabetes/317 diabetes) aged 60. Contemporaneous CVD risk factor levels were measured; linear regression models were used to assess the interaction between diabetes status and calendar year on CVD risk factor levels. Among 50-year olds, for non-diabetes, there was an increase in body mass index (BMI) of 0.39 kg/m2 per 10 years whereas for diabetes there was an increase of 2.52 kg/m2 (p-value for the diabetes by calendar year interaction [p-interaction] <0.0001). For LDL cholesterol, the mean decrease was −7.43 mg/dL per decade [non-diabetes] and −15.5 mg/dL for diabetes (p-interaction=0.002). For systolic blood pressure, the mean decrease was −3.35 mmHg per decade [non-diabetes] and −3.50 mmHg for diabetes (p-interaction=0.97). The direction of the trends for those with diabetes relative to those without diabetes was similar for 60-year olds.
Conclusions
Individuals with diabetes experienced a greater increase in BMI, a greater decrease in LDL-C, and a similar magnitude of decline in systolic blood pressure as compared to those without diabetes. Individuals with diabetes have not experienced the necessary declines in CVD risk factors to overcome their increased risk of CVD. Further efforts are needed to aggressively control CVD risk factors among individuals with diabetes.
Keywords: diabetes mellitus, risk factors, cholesterol, blood pressure, obesity
BACKGROUND
Cardiovascular disease (CVD) risk factor control is critical to reducing the CVD risk of those with diabetes. Recent clinical trials have demonstrated that the use of more aggressive targets for blood pressure and cholesterol control among individuals with diabetes results in reduced incidence of CVD events.1–3 Despite this, data from multiple sources suggests that those with diabetes do not experience optimal risk factor control as compared to those without diabetes.4–6 While individuals with diabetes have experienced substantial declines in dyslipidemia, blood pressure, and cigarette smoking,7 the experience relative to individuals without diabetes has not been well-described. Therefore, individuals with diabetes may not have achieved the level of risk factor control needed to reduce their burden of CVD morbidity and mortality as compared to that of their non-diabetic counterparts.
The primary goal of this analysis is to characterize the trends in body mass index (BMI), blood pressure, cholesterol, and smoking among individuals with and without type 2 diabetes from 1970 through 2005 in the Framingham Heart Study. We also assessed the prevalence of treatment, and control of CVD risk factors among individuals with and without type 2 diabetes.
METHODS
Study Sample
The design of the Framingham Heart Study Original, Offspring, and Third Generation cohorts has been previously described.8–11 Briefly, the Framingham Heart Study Original cohort began in 1948 with the enrollment of 5,209 individuals aged 28–62 years who underwent biennial medical examinations.8, 9 In 1971, 5,124 offspring of the original participants and their spouses were enrolled into the Offspring cohort and underwent examinations approximately every four years.10 In 2002, 4095 children of the offspring participants and their spouses were enrolled in to the Third Generation Cohort.11 As of the present analysis, the Third Generation participants have only attended their first examination.
Our aim was to examine the experience of 50- and 60-year-old individuals with and without diabetes over the time period from 1970 to 2005. We chose this design to describe the experience of a population at a given age over time rather than the aging process of the population, with the goal of examining the changes in risk factor levels cross-sectionally rather than changes within a given person. Individuals aged 50 and 60 were chosen because those ages allowed sufficient sample sizes across each decade to allow for meaningful comparisons. The study has been approved by the Institutional Review Board at Boston Medical Center and written informed consent has been provided by all participants.
For information on diabetes status assessment and risk factor assessment and definition, please see the online supplement.
Statistical Analysis
For all continuous risk factors (BMI, total cholesterol, LDL cholesterol, SBP, DBP), linear regression models were fit to determine the association between calendar year and the risk factor level. The beta coefficients from this model have the interpretation of the change in risk factor level per calendar year. For ease of interpretation, we multiplied the beta coefficients from this model by ten so that they have the interpretation of the change in risk factor level per 10 years. Models were fit separately for those with and without diabetes and by age group (age 50 and 60). The interaction between diabetes status and calendar year was assessed in the total dataset by including a cross-product term in the model and its significance was assessed with a likelihood ratio test. All models were adjusted for sex. For dichotomous characteristics, the prevalence of each risk factor was calculated for each of the 3.5 decades (35 years) of study follow-up (1970–1979, 1980–1989, 1990–1999, and 2000–2005).
In secondary analyses, we further adjusted the linear regression models for BMI and repeated the analysis among those free of prevalent CVD (myocardial infarction, stroke, or coronary heart failure). Since we were also interested in the characteristics of individuals with diabetes over time, we secondarily examined mean fasting blood glucose and prevalence of diabetes treatment over time. Since the original cohort did not have fasting blood glucose measurements available, this analysis was restricted to participants in the Offspring and Third Generation cohorts. All variables were assessed for normality using boxplots and no violations were found. A p-value<0.05 was considered statistically significant. All analyses were performed using SAS version 9.1 (Cary, North Carolina).
RESULTS
Study Sample Characteristics
The mean cardiovascular disease risk factor levels by decade are presented in Table 1.
Table 1.
Mean cardiovascular disease risk factor levels by time period among participants with and without diabetes in the Framingham Heart Study (1970–2005).
| Time Period | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1970–79 | 1980–89 | 1990–99 | 2000–05 | |||||
| Age 50 | N | % | N | % | N | % | N | % |
| Women | ||||||||
| No Diabetes | 420 | 49.5 | 657 | 50.9 | 653 | 54.3 | 350 | 54.1 |
| Diabetes | 13 | 39.4 | 24 | 40.7 | 33 | 42.3 | 16 | 45.7 |
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Age, years | ||||||||
| No Diabetes | 848 | 50.2 (1.4) | 1292 | 49.9 (1.3) | 1203 | 49.8 (1.1) | 647 | 49.7 (1.4) |
| Diabetes | 33 | 50.5 (1.5) | 59 | 50.1 (1.3) | 78 | 50.1 (1.2) | 35 | 50.2 (1.4) |
| Body mass index, kg/m2 | ||||||||
| No Diabetes | 845 | 26.4 (3.8) | 1288 | 26.6 (4.5) | 1198 | 27.4 (5.0) | 647 | 27.2 (5.2) |
| Diabetes | 33 | 28.3 (4.6) | 59 | 30.6 (5.9) | 78 | 33.3 (6.4) | 34 | 35.7 (8.0) |
| Total cholesterol, mg/dL | ||||||||
| No Diabetes | 785 | 220 (38) | 1268 | 216 (37) | 1198 | 202 (36) | 645 | 200 (36) |
| Diabetes | 30 | 242 (58) | 56 | 224 (54) | 77 | 207 (40) | 35 | 187 (36) |
| LDL cholesterol, mg/dL | ||||||||
| No Diabetes | 781 | 141 (36) | 1218 | 139 (35) | 1177 | 125 (33) | 631 | 119 (33) |
| Diabetes | 30 | 161 (60) | 51 | 140 (46) | 65 | 128 (30) | 35 | 111 (32) |
| Systolic blood pressure, mm Hg | ||||||||
| No Diabetes | 845 | 130 (18) | 1292 | 125 (16) | 1203 | 121 (16) | 647 | 121 (15) |
| Diabetes | 33 | 141 (22) | 59 | 132 (16) | 78 | 134 (19) | 35 | 129 (16) |
| Diastolic blood pressure, mm Hg | ||||||||
| No Diabetes | 845 | 83 (11) | 1292 | 81 (9) | 1203 | 76 (10) | 647 | 77 (9) |
| Diabetes | 33 | 86 (12) | 59 | 84 (10) | 78 | 82 (10) | 35 | 78 (11) |
| Age 60 | N | % | N | % | N | % | N | % |
| Women | ||||||||
| No Diabetes | 530 | 56.6 | 464 | 51.2 | 584 | 52.9 | 131 | 56.7 |
| Diabetes | 33 | 51.6 | 31 | 38.8 | 42 | 33.3 | 23 | 48.9 |
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Age, years | ||||||||
| No Diabetes | 936 | 59.8 (1.4) | 906 | 59.7 (1.2) | 1105 | 59.7 (1.1) | 231 | 59.4 (1.2) |
| Diabetes | 64 | 59.8 (1.4) | 80 | 59.8 (1.3) | 126 | 59.7 (1.1) | 47 | 59.5 (1.2) |
| Body mass index, kg/m2 | ||||||||
| No Diabetes | 932 | 26.4 (4.1) | 899 | 26.6 (4.3) | 1102 | 27.7 (4.7) | 230 | 28.0 (5.0) |
| Diabetes | 63 | 28.1 (4.4) | 79 | 29.7 (5.1) | 124 | 30.7 (5.9) | 43 | 33.8 (7.5) |
| Total cholesterol, mg/dL | ||||||||
| No Diabetes | 487 | 234 (42) | 891 | 224 (40) | 1101 | 209 (39) | 231 | 203 (35) |
| Diabetes | 38 | 233 (61) | 80 | 222 (47) | 123 | 199 (39) | 43 | 189 (37) |
| LDL cholesterol, mg/dL | ||||||||
| No Diabetes | 487 | 152 (40) | 857 | 145 (35) | 1077 | 130 (33) | 229 | 120 (31) |
| Diabetes | 38 | 154 (53) | 73 | 134 (38) | 117 | 122 (36) | 42 | 104 (29) |
| Systolic blood pressure, mm Hg | ||||||||
| No Diabetes | 932 | 138 (20) | 906 | 133 (18) | 1105 | 128 (17) | 231 | 127 (19) |
| Diabetes | 64 | 152 (23) | 80 | 142 (16) | 126 | 137 (21) | 47 | 135 (17) |
| Diastolic blood pressure, mm Hg | ||||||||
| No Diabetes | 932 | 83 (11) | 905 | 81 (9) | 1104 | 76 (9) | 231 | 77 (10) |
| Diabetes | 64 | 85 (14) | 80 | 81 (9) | 126 | 77 (11) | 46 | 79 (9) |
Sample size is 4,195 (3,990 non-DM, 205 DM) for age 50 analysis and 3,495 (3,178 non-DM, 317 DM) for age 60 analysis.
Change in CVD Risk Factor Levels over Time
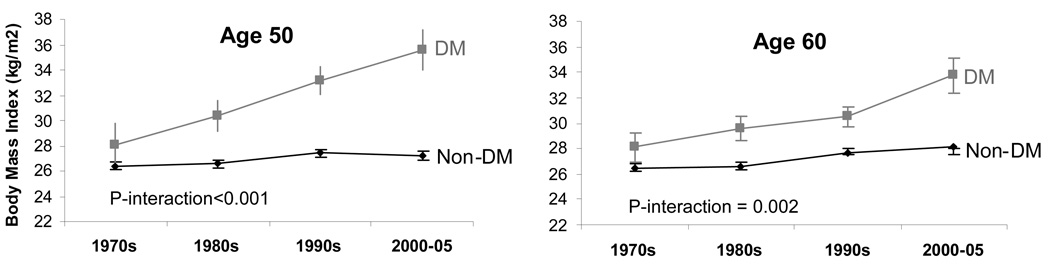
Table 2 shows the difference in CVD risk factor levels per decade in individuals with and without diabetes. Among individuals aged 50, those without diabetes had a BMI of 26.4 kg/m2 in the 1970s, and a mean BMI of 27.4 kg/m2 in the 2000’s, representing a mean adjusted increase in BMI per decade of 0.39 kg/m2. In comparison, those with diabetes had a mean BMI of 28.3 kg/m2 in the 1970s, and a mean BMI of 35.7 kg/m2 in the 2000’s, representing a mean increase of 2.52 kg/m2 per decade. The interaction between calendar year and diabetes status on BMI was statistically significant (age 50: p-interaction<0.001) (Table 2; Figure 1a).
Table 2.
Differences in cardiovascular risk factor levels per decade among participants with and without diabetes in the Framingham Heart Study (1970–2005).
| Risk Factor | No Diabetes | Diabetes | P-value for interaction** |
||
|---|---|---|---|---|---|
| Beta* (95% CI) | P-value | Beta* (95% CI) | P-value | ||
| Age 50 | |||||
| Body mass index, kg/m2 | 0.39 (0.25, 0.53) |
<0.001 | 2.52 (1.68, 3.36) |
<0.001 | <0.001 |
| Total cholesterol, mg/dL | −7.40 (−8.54, −6.26) |
<0.001 | −17.7 (−24.2, −11.2) |
<0.001 | <0.001 |
| LDL cholesterol, mg/dL | −7.43 (−8.47, −6.39) |
<0.001 | −15.5 (−21.4, −9.62) |
<0.001 | 0.002 |
| Systolic blood pressure, mm Hg | −3.35 (−3.82, −2.88) |
<0.001 | −3.51 (−6.04, −0.98) |
0.007 | 0.97 |
| Diastolic blood pressure, mm Hg | −2.28 (−2.75, −1.81) |
<0.001 | −2.51 (−3.90, −1.12) |
<0.001 | 0.78 |
| Age 60 | |||||
| Body mass index, kg/m2 | 0.60 (0.44, 0.76) |
<0.001 | 1.49 (0.86, 2.12) |
<0.001 | 0.002 |
| Total cholesterol, mg/dL | −9.97 (−11.5, −8.44) |
<0.001 | −15.4 (−20.9, −9.89) |
<0.001 | 0.03 |
| LDL cholesterol, mg/dL | −10.0 (−11.4, −8.63) |
<0.001 | −15.0 (−19.8, −10.2) |
<0.001 | 0.03 |
| Systolic blood pressure, mm Hg | −4.17 (−4.78, −3.56) |
<0.001 | −5.93 (−8.05, −3.81) |
<0.001 | 0.05 |
| Diastolic blood pressure, mm Hg | −2.76 (−3.09, −2.43) |
<0.001 | −2.74 (−3.92, −1.56) |
<0.001 | 0.68 |
Abbreviations: SE, standard error; CI, confidence interval
Sample size is 4,195 (3,990 non-DM, 205 DM) for age 50 analysis and 3,495 (3,178 non-DM, 317 DM) for age 60 analysis.
All beta coefficients are adjusted for sex.
Beta represents the change in risk factor level per 10 years
P-value for diabetes by calendar year interaction.
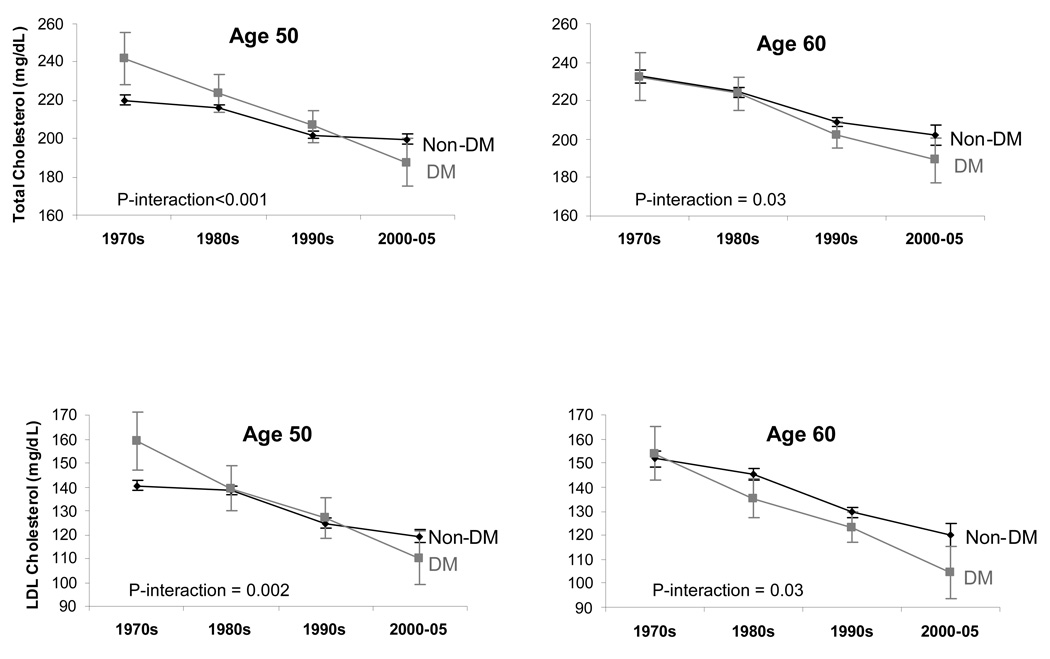
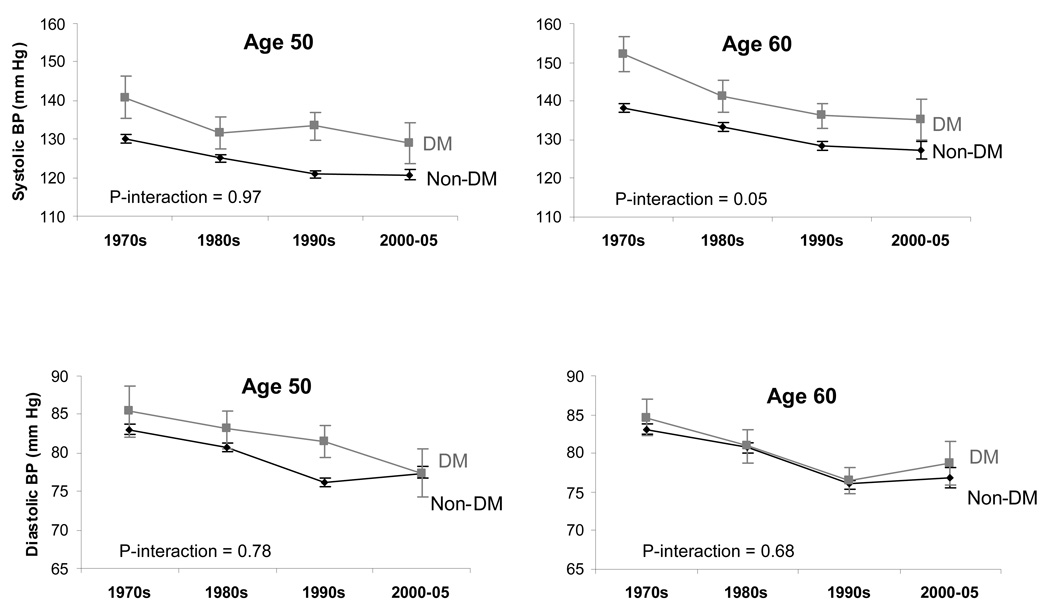
Figure 1.
Figure 1a. Sex-adjusted mean BMI by decade among participants with and without diabetes (DM) in the Framingham Heart Study, 1970–2005.
Figure 1b. Sex-adjusted mean total and LDL cholesterol levels by decade among participants with and without diabetes (DM) in the Framingham Heart Study, 1970–2005.
Figure 1c. Sex-adjusted mean systolic and diastolic blood pressure levels by decade among participants with and without diabetes (DM) in the Framingham Heart Study, 1970–2005.
Those with diabetes had a larger decline in both total and LDL cholesterol than those without diabetes (Table 2; Figure 1b). For example, among those without diabetes, mean LDL cholesterol declined from 141 mg/dL to 119 mg/dL over the 3.5 decades of observation, representing a mean adjusted decrease of 7.43 mg/dL per decade. Among those with diabetes, LDL cholesterol declined from 161 mg/dL to 111 mg/dL, corresponding to a mean adjusted rate of decline of 15.5 mg/dL per decade. The interaction between diabetes status and calendar time on LDL levels was statistically significant (age 50: p-interaction=0.002).
The magnitude of decline in SBP and DBP was similar between those with and without diabetes (Table 2; Figure 1c). For example, mean SBP declined from 130 mm Hg to 121 mm Hg (adjusted mean decline of 3.35 mm Hg per decade) among those without diabetes and from 141 mm Hg to 129 mm Hg (adjusted mean decline of 3.50 mm Hg per decade) among those with diabetes. The corresponding decline per decade for DBP was 2.28 mm Hg among individuals without diabetes and 2.51 mm Hg among individuals with diabetes. The interaction between diabetes status and calendar time was not statistically significant for SBP (age 50: p-interaction=0.97) or for DBP (age 50: p-interaction=0.78).
The direction of the trends for those with diabetes relative to those without diabetes was similar for 60-year olds. All analyses presented in Table 2 were repeated further adjusting for BMI and the results were not materially different (Online Supplemental Table 1).
Prevalence, Treatment, and Control of CVD Risk Factors
Overall, among those aged 50, the prevalence of hypertension declined among those without diabetes and did not change among those with diabetes (Table 3). However, the prevalence of hypertension treatment increased from 21.2% to 53.1% among those without diabetes and from 25.9% to 63.0% among those with diabetes over the period from 1970–1979 to 2000–2005. Similarly, the prevalence of hypertension control increased among those without diabetes from 7.8% to 36.0% (p-trend<0.001). Similar trends were observed in those with diabetes as well, although the p-value for trend was not significant (p=0.06). Surprisingly, only about one-third of those without diabetes, and 15% of those with diabetes experienced hypertension control in the 2000s.
Table 3.
Prevalence, treatment, and control of cardiovascular disease risk factors by time among participants with and without diabetes in the Framingham Heart Study (1970–2005).
| n (%) | P-trend | |||||
|---|---|---|---|---|---|---|
| 1970–79 | 1980–89 | 1990–99 | 2000–05 | |||
| Age 50 | (N=881) | (N=1,351) | (N=1,281) | (N=682) | ||
| Hypertension† | ||||||
| Prevalence | No Diabetes | 306 (36.3) | 387 (30.0) | 259 (21.6) | 164 (25.4) | <0.001 |
| Diabetes | 27 (81.8) | 48 (81.4) | 63 (80.8) | 27 (77.1) | 0.64 | |
| Treatment | No Diabetes | 65 (21.2) | 142 (36.7) | 124 (47.9) | 87 (53.1) | <0.001 |
| Diabetes | 7 (25.9) | 20 (41.7) | 30 (47.6) | 17 (63.0) | 0.006 | |
| Controlled | No Diabetes | 24 (7.8) | 70 (18.1) | 93 (35.9) | 59 (36.0) | <0.001 |
| Diabetes | 0 (0.0) | 4 (8.3) | 6 (9.5) | 4 (14.8) | 0.06 | |
| High LDL cholesterol‡ | ||||||
| Prevalence | No Diabetes | 481 (61.8) | 730 (59.9) | 534 (45.2) | 261 (41.2) | <0.001 |
| Diabetes | 26 (86.7) | 41 (80.4) | 59 (88.1) | 26 (74.3) | 0.44 | |
| Treatment | No Diabetes | 12 (2.5) | 11 (1.5) | 65 (12.2) | 61 (23.4) | <0.001 |
| Diabetes | 1 (3.9) | 4 (9.8) | 6 (10.2) | 8 (30.8) | 0.009 | |
| Controlled | No Diabetes | 2 (0.4) | 2 (0.3) | 42 (7.9) | 52 (19.9) | <0.001 |
| Diabetes | 0 (0.0) | 1 (2.4) | 5 (8.5) | 6 (23.1) | 0.001 | |
| Cigarette smoking | No Diabetes | 315 (43.8) | 401 (31.1) | 251 (20.9) | 124 (19.2) | <0.001 |
| Diabetes | 18 (58.1) | 21 (35.6) | 23 (29.5) | 6 (17.1) | <0.001 | |
| Obesity§ | No Diabetes | 141 (16.7) | 242 (18.8) | 288 (24.0) | 152 (23.5) | <0.001 |
| Diabetes | 12 (36.4) | 24 (40.7) | 54 (69.2) | 21 (61.8) | <0.001 | |
| Age 60 | (N=1,000) | (N=986) | (N=1,231) | (N=278) | ||
| Hypertension† | ||||||
| Prevalence | No Diabetes | 474 (50.9) | 421 (46.6) | 463 (42.0) | 99 (42.9) | <0.001 |
| Diabetes | 58 (90.6) | 73 (91.3) | 102 (81.0) | 41 (87.2) | 0.14 | |
| Treatment | No Diabetes | 157 (33.1) | 197 (46.8) | 300 (64.8) | 63 (63.6) | <0.001 |
| Diabetes | (29.3) | 43 (58.9) | 67 (65.7) | 32 (78.1) | <0.001 | |
| Controlled | No Diabetes | 41 (8.7) | 96 (22.8) | 183 (39.5) | 45 (45.5) | <0.001 |
| Diabetes | 1 (1.7) | 5 (6.9) | 19 (18.6) | 11 (26.8) | <0.001 | |
| High LDL cholesterol‡ | ||||||
| Prevalence | No Diabetes | 346 (70.5) | 573 (66.9) | 587 (54.4) | 130 (56.5) | <0.001 |
| Diabetes | 36 (94.7) | 58 (79.5) | 104 (87.4) | 35 (81.4) | 0.40 | |
| Treatment | No Diabetes | 11 (3.2) | 30 (5.2) | 137 (23.3) | 50 (38.5) | <0.001 |
| Diabetes | 3 (8.3) | 0 (0.0) | 37 (35.6) | 17 (48.6) | <0.001 | |
| Controlled | No Diabetes | 6 (1.7) | 5 (0.9) | 81 (13.8) | 42 (32.3) | <0.001 |
| Diabetes | 0 (0.0) | 0 (0.0) | 14 (13.5) | 14 (40.0) | <0.001 | |
| Cigarette smoking | No Diabetes | 255 (35.1) | 227 (25.1) | 169 (15.3) | 39 (16.9) | <0.001 |
| Diabetes | 20 (41.7) | 22 (27.5) | 23 (18.3) | 6 (12.8) | <0.001 | |
| Obesity§ | No Diabetes | 161 (17.3) | 166 (18.5) | 289 (26.2) | 76 (33.0) | <0.001 |
| Diabetes | 18 (28.6) | 34 (43.0) | 57 (46.0) | 29 (67.4) | <0.001 | |
Hypertension is defined as >140/90 mm Hg or treatment for individuals without diabetes and >130/80 mm Hg or treatment for individuals with diabetes. Hypertension treatment was calculated by dividing the number of participants receiving antihypertensive medication by the total number of individuals with hypertension. Hypertension control was calculated by dividing the number of participants with SBP <140/90 mm Hg (<130/80 mm Hg for individuals with diabetes12) by the total number of individuals with hypertension.
High LDL cholesterol is defined as ≥130 mg/dL or treatment for individuals without diabetes and ≥100 mg/dL or treatment for individuals with diabetes. LDL treatment was calculated by dividing the number of participants receiving lipid lowering medication by the total number of individuals with high LDL. LDL control was calculated by dividing the number of participants with LDL <130 mg/dL (<100 mg/dL for individuals with diabetes12) by the total number of individuals with high LDL cholesterol.
Obesity is defined as a body mass index of ≥30 kg/m2.
The prevalence of high LDL cholesterol declined among those without diabetes and remained stable among those with diabetes. The proportion of individuals treated and controlled for high LDL cholesterol increased among both those with and without diabetes, although only about 20% of either those with or without diabetes experienced LDL cholesterol control in 2000–2005.
Among those aged 50, the prevalence of obesity increased markedly, from 16.7% to 23.5% among those without diabetes, and 36.4% to 61.8% among those with diabetes (Table 3). The prevalence of cigarette smoking decreased from 43.8% to 19.2% among those without diabetes and from 58.1% to 17.1% among those with diabetes.
Overall, the results were similar for the age 60 group compared to the age 50 group. From the 1970–1979 to 2000–2005, hypertension prevalence declined among those without diabetes and did not change among those with diabetes. Treatment and control of hypertension increased among both those with and without diabetes. The prevalence of high LDL cholesterol decreased among those without diabetes and did not change among those with diabetes. The proportion of individuals treated and achieving LDL control increased in both groups. Cigarette smoking declined among those with and without diabetes and obesity increased in both groups.
Secondary Analyses
Table 4 shows the glycemia-related characteristics of individuals with diabetes from the Offspring and Third Generation cohorts. Among individuals aged 50, mean fasting blood glucose did not appreciably decline and diabetes treatment increased from 40.9% to 71.4% (p-trend=0.004) from 1970–1979 to 2000–2005. Among those aged 60, mean fasting blood glucose decreased (p-trend<0.001). The prevalence of treatment increased, although the difference was not statistically significant.
Table 4.
Glycemia-related characteristics of participants with diabetes, by decade, in the Framingham Heart Study, 1970–2005.
| 1970–79 | 1980–89 | 1990–99 | 2000–05 | P-trend | |
|---|---|---|---|---|---|
| Age 50 | |||||
| Fasting blood glucose, mg/dL, mean (SD) | 154 (31) | 178 (73) | 158 (54) | 158 (48) | 0.43 |
| Fasting blood glucose <126 mg/dL, n (%) | 0 (0.0) | 7 (13.0) | 15 (20.0) | 7 (20.0) | 0.11 |
| Diabetes treatment*, n (%) | 9 (40.9) | 21 (35.6) | 30 (38.5) | 25 (71.4) | 0.004 |
| Age 60 | |||||
| Fasting blood glucose, mg/dL, mean (SD) | 240 (106) | 173 (65) | 160 (54) | 150 (40) | <0.001 |
| Fasting blood glucose <126 mg/dL, n (%) | 0 (0.0) | 13 (17.1) | 29 (24.0) | 10 (23.8) | 0.30 |
| Diabetes treatment*, n (%) | 3 (37.5) | 42 (52.5) | 60 (48.0) | 28 (59.6) | 0.48 |
Note: This analysis contains only diabetic participants in the Offspring (age 50: n=165; age 60: n=249) and Third Generation (age 50: n=29; age 60: n=12) cohorts since fasting blood glucose measurements were not available for the Original cohort.
Treatment with insulin or a hypoglycemic agent.
We repeated our main analysis excluding individuals with prevalent CVD (Online Supplemental Table 2). Overall, the results were very similar to those from the main analysis presented in Table 2. Additionally, we repeated our main analysis using the generalized estimating equations procedure to account for familial correlations, and found that the results were essentially unchanged (data not shown).
DISCUSSION
Principal Findings
Mean BMI levels have increased, whereas smoking prevalence and levels of cholesterol and blood pressure have decreased among individuals with and without diabetes. Individuals with diabetes had a greater increase in BMI and a greater decrease in cholesterol than those without diabetes. However, individuals with diabetes had a similar magnitude of decline for blood pressure compared to those without diabetes. The prevalence of treatment and control of hypertension and high LDL cholesterol increased from 1970–2005 among both those with and without diabetes; glucose lowering treatment among individuals with diabetes has markedly increased with concomitant declines in mean glucose. Finally, prevalence of control of both hypertension and LDL cholesterol remain sub-optimal for both individuals with and without diabetes.
In the Context of the Current Literature
In order to further reduce the differences in CVD rates between those with and without diabetes, efforts must be intensified to achieve guideline targets for SBP and cholesterol levels among those with diabetes. In this context, our results reveal parallel declines of blood pressure among those with and without diabetes. A study using NHANES data showed 27% decline in hypertension prevalence among those with diabetes over the period 1971 to 2000.7 In our study, the proportion of individuals with diabetes achieving hypertension control is low, even in more recent years, and individuals with diabetes are less likely to achieve hypertension control than those without diabetes. This may be partially explained by the fact that we used a lower threshold (<130/80 mm Hg) for hypertension control among individuals with diabetes, which is harder to achieve. In addition, a prior study indicated that only one-third of individuals with diabetes and hypertension were able to meet their blood pressure goals, even after behavioral management and pharmacotherapy.13 Further, a recent study of hypertension trends in the US population from 1988 to 2000 showed that among individuals with diabetes, the proportion of individuals who achieved hypertension control did not change significantly between 1988 to 2000.14
In contrast to trends in hypertension, we observed steeper declines for cholesterol levels among those with as compared to without diabetes. This is likely due to the increased use of lipid lowering drugs, as evidenced by the increase in treatment and control of LDL cholesterol. It is important to note that before the existence of statins, there were few effective lipid lowering therapies available. However, the results from the current study highlight that the proportion of individuals achieving control, particularly among those with diabetes, is still relatively low. Our results are similar to data from NHANES which showed a prevalence of hypercholesterolemia control in 1999–2000 of 22% for individuals with diabetes and 6% for individuals without diabetes.15 A study of HMO patients showed that 61% of individuals with diabetes did not reach their LDL cholesterol goal of <100 mg/dL after 6 months of lipid treatment.16
Our study demonstrated a dramatic increase in the prevalence of obesity among individuals with diabetes relative to those without diabetes which has been observed in a prior study of Spanish individuals.17 We also observed a decline in the prevalence of smoking among both individuals with and without diabetes, which has been documented in a prior studies.17
Implications
In the present study, individuals with diabetes had greater declines in total and LDL cholesterol compared to those without diabetes and similar declines in blood pressure levels. The improvement in risk factor levels was paralleled by an increase in drug treatment, which likely contributes to the observed trends. Given that diabetes is still associated with approximately an overall two to three-fold increased risk of CVD mortality18–20, these findings highlight the need for further improvements in blood pressure and cholesterol control among individuals with diabetes. Previous work in the Framingham Heart Study has demonstrated the increasing burden of CVD due to type 2 diabetes.21 Lifestyle modifications which result in weight loss have also been shown to result in an improved cardiometabolic profile among individuals with diabetes, however, long-term weight maintenance is often not successful.22, 23
Recent clinical trials have focused on intensive blood glucose control as a way of reducing the morbidity and mortality associated with type 2 diabetes.24–27 However, the benefits of intensive control of glucose levels on major CVD endpoints were not observed in three recently published major trials.24, 25, 27 In addition, in the ACCORD trial, it has been shown that intensive blood glucose control is associated with increased mortality compared to more standard blood glucose control regimens.24, 25
While improved blood glucose control may have benefits with respect to microvascular complications, intensified efforts to control other major CVD risk factors are currently the most effective way to reduce the risk of CVD among patients with diabetes.28, 29 In this context, it is notable that we observed a steep increase in the use of glucose-lowering medications that outweighed the pharmacologic management for elevated LDL cholesterol. Trials have repeatedly shown that reduction of cholesterol and blood pressure in individuals with diabetes results in reduced rates of CVD.1, 28, 30–32 Several trials of statin use among individuals with diabetes have shown that lipid lowering, particularly intensive regimens, is associated with a reduction in CVD events.1, 30, 31 Trials of intensive blood pressure lowering among individuals with diabetes have demonstrated reductions in both microvascular and macrovascular events.2,28
Strengths and Limitations
One of the major strengths of the study is that we were able to assess temporal trends over a 35-year period from 1970 to 2005. Additionally, diabetes status and CVD risk factor levels were rigorously collected at periodic examinations. One of the limitations of our study is that our study sample was predominantly white, thus our results may not be completely generalizable to other ethnic or racial groups. For some of the categorical characteristics, we had a relatively small sample size. We had relatively few data points in the 2000–2005 time period relative to the other periods; this may have affected the precision of the results. We did not have a sufficient sample size to examine the data separately by sex. Additionally, since the participants in our study underwent routine screening for diabetes and other CVD risk factors, the improvements in CVD risk factors observed this study may represent an optimistic picture relative to the general U.S. population.
We evaluated treatment and control relative to current guidelines that were not in place when much of the clinical data was collected. It is possible that the results may be explained by the emergence of new diabetes treatments and the development of new treatment guidelines over time. Lastly, since we wanted to apply a uniform definition of diabetes across all of the time periods, we used the fasting blood glucose cutoff of 126 mg/dL which was not adopted until 1998. Thus, it is likely that low proportion of individuals in the earlier decades who were untreated for diabetes may be explained by the fact that they were simply not diagnosed at the time because of the different criteria that were in place. It is also possible that they received their diabetes diagnosis at their examination visit and thus may not have had the opportunity to be treated.
Conclusions
Individuals aged 50 and 60 with diabetes have had declines in major CVD risk factors over the past several decades. However, these improvements have not been sufficient to overcome their CVD risk factor burden as compared to those without diabetes. Further efforts are needed to aggressively control CVD risk factors among individuals with diabetes.
Clinical Summary.
Cardiovascular disease (CVD) risk factor control is critical to reducing the CVD risk of those with diabetes. Recent clinical trials have demonstrated that the use of more aggressive targets for blood pressure and cholesterol control among individuals with diabetes results in reduced incidence of CVD events. Despite this, data from multiple sources suggests that those with diabetes do not experience optimal risk factor control as compared to those without diabetes. While individuals with diabetes have experienced substantial declines in CVD risk factors, the experience relative to individuals without diabetes has not been well-described. The primary goal of this analysis is to characterize the trends in body mass index (BMI), blood pressure, cholesterol, and smoking among individuals with and without type 2 diabetes from 1970 through 2005 in the Framingham Heart Study. Our results showed that individuals with diabetes experienced a greater increase in BMI, a greater decrease in LDL-C, and a similar magnitude of decline in systolic blood pressure as compared to those without diabetes. Smoking prevalence has declined among both those with and without diabetes. Prevalence of control of both hypertension and LDL cholesterol remain sub-optimal for both individuals with and without diabetes. Overall, individuals with diabetes have not experienced the necessary declines in CVD risk factors to overcome their increased risk of CVD. Further efforts are needed to aggressively control CVD risk factors among individuals with diabetes.
Supplementary Material
ACKNOWLEDGEMENTS
From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. Sarah R. Preis and Caroline S. Fox had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING SOURCES
This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195).
Footnotes
DISCLOSURES
Sarah Rosner Preis – none
Michael J. Pencina – none
Shih-Jen Hwang – none
Ralph B. D’Agostino – none
Peter J. Savage – none
Daniel Levy - none
Caroline S. Fox - none
REFERENCES
- 1.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S, Waters D. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 2.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 4.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–574. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 5.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 6.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 7.Imperatore G, Cadwell BL, Geiss L, Saadinne JB, Williams DE, Ford ES, Thompson TJ, Narayan KM, Gregg EW. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971–2000. Am J Epidemiol. 2004;160:531–539. doi: 10.1093/aje/kwh232. [DOI] [PubMed] [Google Scholar]
- 8.Dawber TR, Meadors GF, Moore FEJ. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 10.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 11.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 12.Standards of medical care in diabetes--2008. Diabetes Care. 2008;31 Suppl 1:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 13.Aparasu RR, Aparasu A. Hypertension management in outpatient visits by diabetic patients. Res Social Adm Pharm. 2008;4:284–291. doi: 10.1016/j.sapharm.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Nag SS, Daniel GW, Bullano MF, Kamal-Bahl S, Sajjan SG, Hu H, Alexander C. LDL-C goal attainment among patients newly diagnosed with coronary heart disease or diabetes in a commercial HMO. J Manag Care Pharm. 2007;13:652–663. doi: 10.18553/jmcp.2007.13.8.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez-Garcia R, Hernandez-Barrera V, Jimenez-Trujillo I, Garrido PC, Lopez de AA, Gil de MA. Trends in cardiovascular risk factors and lifestyle behaviors among Spanish adults with diabetes (1993–2003) J Diabetes Complications. 2008 doi: 10.1016/j.jdiacomp.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D'Agostino RB, Sr, Wilson PW, Savage PJ. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 19.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 20.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281:1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Coady S, Sorlie PD, D'Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 22.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van DB, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto AD, Garcia DO, Jakicic JM. Lifestyle intervention strategies to prevent and control type 2 diabetes. Curr Diab Rep. 2008;8:407–412. doi: 10.1007/s11892-008-0070-6. [DOI] [PubMed] [Google Scholar]
- 24.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 26.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 27.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2008 doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 28.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 29.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EAM, Howard BV, Kirkman S, Kosiborod M, Reaven P, Sherwin RS. Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials. A Position Statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 30.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 31.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 32.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.