Abstract
While it has often been speculated that prior reproductive experience improves subsequent maternal care, few studies have examined specific changes in behavior during a first versus second lactation. During lactation mothers display heightened aggression toward male intruders, purportedly to protect vulnerable young. In the current study, maternal aggression was examined in primiparous and age-matched, multiparous females on postpartum days 5 (PPD5) and PPD15. Expression of oxytocin (OXT), oxytocin receptor (OXT-R), arginine vasopressin (AVP), arginine vasopressin V1a receptors (V1a), and corticotrophin releasing hormone (CRH) mRNA was measured following aggression testing at both time points using real-time quantitative PCR (qPCR) in brain regions previously implicated in the regulation of maternal aggression. Multiparity significantly enhanced maternal aggression on PPD5 but not on PPD15. In addition, this increased aggression was associated with region and gene specific changes in mRNA expression. These findings indicate that reproductive experience enhances maternal aggression, an effect that may be mediated by region specific alterations in neuropeptidergic activity. The adaptations observed in multiparous females provide an innate model for the study of neuroplasticity in the regulation of aggression.
Keywords: Offspring protection, oxytocin, vasopressin, corticotrophin releasing hormone, paraventricular nucleus
Introduction
One important component of maternal care is the protection of vulnerable offspring from potential aggressors. Offspring protection, often referred to as maternal aggression, has been described in numerous species ranging from domesticated cattle to laboratory mice. In rats, numerous factors influence the intensity of maternal aggression including the age of the pups, the age and/or size of the intruder, as well as the testing environment (Erskine, Barfield, & Goldman, 1980; Ferriera & Hansen, 1986; Flannelly & Flannelly, 1985; Flannelly & Flannelly, 1987; Mayer, Reisbick, Siegel, & Rosenblatt, 1987; Stern & Kolunie, 1991; Stern & Kolunie, 1993). In mice, maternal aggression has been shown to increase across the first three litters (Svare & Gandelman, 1976). It is unknown whether this effect is specific to mice or whether a similar effect of parity is present in rats. It has often been suggested that maternal care improves as a function of reproductive experience. Few studies, however, have directly tested this hypothesis. As maternal aggression is a key component of maternal care, enhanced maternal aggression in reproductively experienced rats would certainly provide support for this hypothesis.
Studies conducted over the past decade have demonstrated an important role for neurohormones in the regulation of maternal aggression. Several lines of evidence suggest that oxytocin (OXT), arginine vasopressin (AVP) and corticotrophin releasing hormone (CRH), may all be involved in the control of maternal aggression. For example, lesions of the paraventricular nucleus (PVN), which significantly decrease oxytocin, have been shown to increase maternal aggression and infusion of OXT antisense into the PVN also increases maternal agression (M. Giovenardi, M.J. Padoin, L.P. Cadore, & A.B. Lucion, 1997, 1998). Moreover, OXT injections into the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST) decrease components of maternal aggression without affecting pup retrieval (Consiglio, Borsoi, Pereira, & Lucion, 2005), while OXT antagonist infusion into the CeA increases maternal aggression (Lubin, Elliot, Black, & Johns, 2003). Thus, low levels of OXT in specific brain regions are associated with enhanced maternal aggression. Similarly, studies of AVP indicate that this peptide may also inhibit the display of maternal aggression, as central infusion of V1a antagonist increases maternal aggression (Nephew & Bridges, 2008a, 2008b). Finally, CRH has also been implicated in the control of maternal aggression, as central infusion of CRH impairs maternal aggression in mice (Gammie, Negron, Newman, & Rhodes, 2004). Infusion of a CRH antagonist, however, does not increase maternal aggression. Moreover, CRH receptor 2 knock-out mice display decreased maternal aggression (Gammie, Hasen, Stevenson, Bale, & D’Anna, 2005). Thus, while CRH is involved in the regulation of maternal aggression, the precise role of CRH remains unclear.
The current study was designed to examine the effect of prior reproductive experience on maternal aggression in rats and to examine gene expression related to the neurohormonal regulation of maternal aggression as a function of parity. The intensity of maternal aggression was examined in primiparous females (i.e. first-time mothers) as compared to age-matched, multiparous females (i.e. second-time mothers) during both early (postpartum day 5: PPD5) and mid-late (postpartum day 15: PPD15) lactation. The expression of genes associated with maternal aggression, including OXT, OXT receptor (OXT-R), AVP, AVP V1a receptors, and CRH were determined using real-time quantitative PCR (qPCR) in brain regions relevant to maternal aggression. In addition, plasma corticosterone levels were examined to determine whether any changes in peripheral corticosterone correlate with behavioral effects. Our findings indicate that reproductive experience enhances maternal aggression during early lactation and that these behaviors are associated with differences in gene expression in select brain regions.
METHODS
Animals
Female Sprague-Dawley rats (175–200g) were obtained from Charles River Laboratories (Wilmington, MA) and maintained in temperature (21–25°C) and light (14:10 LD cycle; lights on at 0500h) controlled rooms. Food and water were available ad libidum throughout the studies. All animals were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council and the animal research protocol was approved by Tufts University’s Institutional Animal Care and Use Committee.
Generation of Parity Groups
One week after arriving in the colony, one set of virgin females were mated with colony males. The day of parturition was defined as PPD0. On PPD1, all litters were culled to 5 males and 5 females. Litters were weaned on PPD21. Two weeks after weaning these females were re-mated. At that time, a separate group of age-matched, virgin females were mated for the first time. Thus, two age-matched groups were generated: first-time mothers (primiparous) and second-time mothers (multiparous).
Maternal Aggression Testing
Treatment groups were tested for maternal aggression between 1330 and 1630 h on either PPD5 or PPD15. Dams and their litters were moved to an empty behavioral observation room one day prior to testing. A digital video camera (Panasonic PV-GS180) was used to record all behavioral observations. Fifteen minute behavioral trials began when an intruder male (50–70 days old) was placed into the female’s clear plastic home cage. Pups remained with the dam throughout the aggression test.
Analysis of Maternal Aggession Data
Digital videotapes were scored by an observer that was blind to the female’s parity status using ODlog video analysis software (Macropod Inc.). The ODlog software records continuous data in 5 seconds bins, and also generates frequency and duration summaries for all behavioral measures over the 15 minute observation period. Attacks consisted of bites, kicking with forelimbs or hind limbs, and pinning the intruder to the floor of the cage. Both frontal and lateral attacks were scored. Latency to initiate the first attack as well as the number, and duration of attacks were recorded. The duration of an attack was defined as the time between the initiation of agonistic contact between the male and female and the moment contact ceased.
Brain and Blood Collection
Five minutes after the conclusion of aggression testing, all females were briefly anesthetized with C02 (< 30 sec) and rapidly decapitated. Brains were removed, snap frozen in methylbutane (−20°C), and stored at −80°C until processed for qPCR. Trunk blood was collected in heparinized tubes and centrifuged. Plasma was stored at −20°C prior to analysis for corticosterone content.
Corticosterone Radioimmunoassay (RIA)
Corticosterone (CORT) was measured by RIA using a kit from Diagnostics Product Corporation (Los Angeles, CA) according to the manufacturer’s instructions. The detection limit of the assay was 0.02 ng/ml with an intraassay variation of 3.9%. All samples were run in duplicate in a single assay.
qPCR with TaqMan®
To collect specific brain regions for subsequent qPCR, bilateral micropunches (either 0.5 or 1.0 mm depending upon brain region) were taken from the following regions: lateral septum (LS), bed nucleus of the stria terminalis (BNST), medial and central amygdala (MeA, CeA), medial preoptic area (MPOA), periaqueductal gray (PAG), paraventricular nucleus (PVN), and supraoptic nucleus (SON). These brain regions were based on previous studies of maternal aggression (Gammie & Nelson, 2001; Hasen & Gammie, 2005). Bilateral tissue punches were homogenized in lysis buffer and total RNA was extracted using the RNAqueous kit from Ambion (Austin, TX) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized using the RETROscript kit (Ambion). PCR was performed using an AB 7500 (Applied Biosystems) under standard amplification conditions: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C and 60 s at 60°C. All PCR primers were TaqMan® Gene Expression Assays purchased from Applied Biosystems. The amplification efficiency of each of these assays has been validated by Applied Biosystems and averages 100% (±10). Assay ID and accession numbers were as follows: OXT - Rn00564446_g1 and NM_012996.2; OXT-R - Rn00563503_m1 and NM_012871.2; AVP - Rn00566449_m1 and NM_016992.1; V1a - Rn00583910_m1 and NM_053019.2; CRH - Rn01462137_m1 and NM_031019.1; GAPDH - Rn99999916_s1 and NM_017008.2.
Relative quantification of gene transcription
Final quantification of mRNA was obtained using the comparative cycle threshold (CT) method (User Bulletin #2, Applied Biosystems). Data are reported as relative transcription or the N-fold difference relative to a calibrator cDNA. In brief, the housekeeping gene for the rat brain tissue, GAPDH, was used as an internal control against which each target signal was normalized, this is referred to as the ΔCT. Validation studies confirmed that the raw CT values of GAPDH did not vary by treatment group, confirming GAPDH as an appropriate housekeeping gene. The ΔCT was then normalized against the calibrator (i.e. the lowest gene transcription for the target gene in each brain region, OXT, OXT-R, AVP, V1a, and CRH), this is referred to as the ΔΔCT. The relative expression of target molecules relative to the calibrator was then calculated using the formula 2−ΔΔCT. Therefore, all gene transcription is expressed as an N-fold difference relative to the calibrator. Individual brain regions were analyzed separately. For each region four samples from each group were run in duplicate which were then averaged. Only samples run on the same plate were compared. The calibrator and target genes were run in separate wells.
Statistical Analyses
All data were analyzed using two-way Analyses of Variance (ANOVA) with parity (primiparous or multiparous) and postpartum day (PPD5 or PPD15) as factors. Significant interactions were followed by post hoc analyses. In addition, any main effect of parity was followed by post hoc analyses of parity effects within each day of lactation. All post hoc analyses were performed using the Tukey’s test. Statistical significance was defined as p ≤ 0.05.
RESULTS
Maternal Aggression
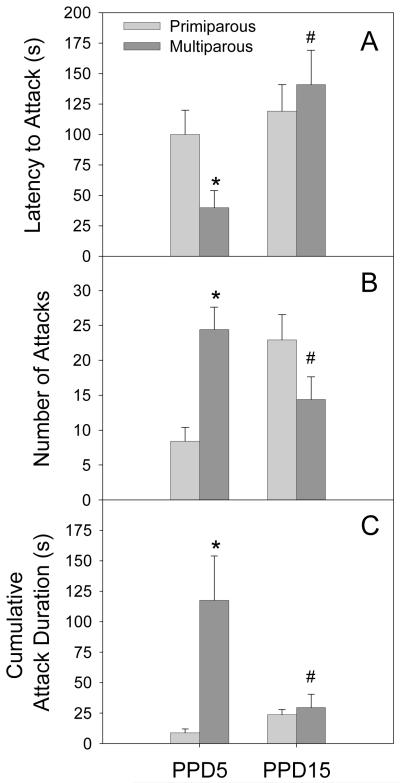
Parity and postpartum day significantly altered the expression of maternal aggression. On the measure of latency to initiate aggression, there was a significant main effect of postpartum day (F[1,36]=9.3 p<0.01,) as well as an interaction between parity and postpartum day (F[1,36]=4.3, p<0.05). As illustrated in Figure 1 (panel A), on PPD5 multiparous dams had significantly shorter attack latencies when compared to primiparous dams on the same day. In addition, multiparous dams had significantly longer attack latencies on PPD15 when compared to PPD5, an effect that was not observed in primiparous dams.
Figure 1.
Mean (+SEM) latency to attack (A), number of attacks (B), and cumulative time spent attacking (C) during 15 minute maternal aggression trials on PPD5 and PPD15 in primiparous and age-matched, multiparous females. * p < 0.05 as compared to primiparous females on the same day. # p < 0.05 as compared to PPD5, within parity condition.
On the total number of attacks, there were no main effects, but there was a significant interaction between parity and postpartum day (F[1,36]=14.1, p<0.01). As shown in Figure 1 (Panel B), multiparous dams displayed a higher number of attacks when compared to primiparous dams on PPD5. In addition, while the number of attacks declined between PPD5 and PPD15 in multiparous dams, the number of attacks increased on PPD15 when compared to PPD5 in primiparous dams. No significant effect of parity was evident on PPD15.
Attack duration also differed as a function of reproductive experience with both a main effect of parity (F[1,36]=5.9, p<0.05) as well as a significant parity and postpartum day interaction (F[1,36]=6.9, p<0.05). As shown in Figure 1 (Panel C), multiparous dams had significantly greater attack duration when compared to primiparous dams on PPD5. The duration of multiparous dams averaged more than 5 times that present in primiparous mothers at this time. The duration of attack by multiparous dams significantly declined by PPD15, with the duration similar to that observed in primiparous dams. No significant change in attack duration was observed in primiparous females as a function of postpartum day.
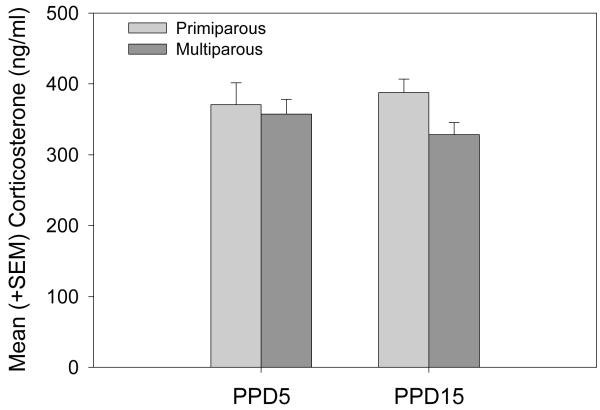
Corticosterone
As shown in figure 2, corticosterone levels 20 minutes after the initiation of aggression testing were comparable between primiparous and multiparous females on PPD5 and PPD15. In addition, no differences in corticosterone were detected as a function of postpartum day.
Figure 2.
Mean (+SEM) plasma concentrations of corticosterone in primiparous and age-matched, multiparous rats immediately after completing a 15 minute maternal aggression trial.
RT-PCR
Differences in the relative expression of the target genes as a function of parity and/or day of lactation were observed in several brain regions as detailed below. As no significant differences were observed in the MPOA, MeA or PAG, those data are not described in detail and instead are included in Table 1.
Table 1.
Mean (±SEM) relative expression of target genes. No statistical differences were observed in these brain regions. N’s = 3–4 per group
| OXT | OXT-R | AVP | V1a | CRH | ||
|---|---|---|---|---|---|---|
| MPOA | ||||||
| Primiparous | PPD5 | 3.8 ± 0.7 | 1.7 ± 0.2 | 26.6 ± 10.9 | 1.3 ± 0.1 | 3.6 ± 0.3 |
| PPD15 | 3.4 ± 1.3 | 2.9 ± 0.3 | 42.5 ± 25.7 | 1.6 ± 0.1 | 1.9 ± 0.2 | |
| Multiparous | PPD5 | 2.3 ± 0.1 | 3.2 ± 0.9 | 23.6 ± 9.9 | 1.7 ± 0.2 | 1.9 ± 0.3 |
| PPD15 | 6.7 ± 1.5 | 2.9 ± 0.3 | 57.9 ± 8.1 | 1.4 ± 0.1 | 2.1 ± 0.6 | |
| MeA | ||||||
| Primiparous | PPD5 | 25.4 ± 8.7 | 1.2 ± 0.1 | 20.5 ± 8.4 | 1.5 ± 0.2 | 2.0 ± 0.3 |
| PPD15 | 18.6 ± 13.8 | 1.2 ± 0.1 | 12.3 ± 6.0 | 1.2 ± 0.2 | 1.4 ± 0.2 | |
| Multiparous | PPD5 | 57.1 ± 37.2 | 1.5 ± 0.1 | 50.1 ± 47.2 | 1.6 ± 0.1 | 1.5 ± 0.4 |
| PPD15 | 33.3 ± 15.7 | 1.3 ± 0.2 | 20.3 ± 8.8 | 1.5 ± 0.1 | 1.9 ± 0.3 | |
| PAG | ||||||
| Primiparous | PPD5 | 10.3 ± 4.9 | 1.5 ± 0.2 | 3.0 ± 0.8 | 1.8 ± 1.2 | 2.1 ± 0.2 |
| PPD15 | 94.1 ± 70.5 | 2.0 ± 0.4 | 4.6 ± 0.9 | 2.2 ± 0.5 | 3.4 ± 0.7 | |
| Multiparous | PPD5 | 23.7 ± 12.9 | 2.5 ± 0.8 | 2.6 ± 0.8 | 1.6 ± 0.2 | 1.8 ± 0.4 |
| PPD15 | 56.7 ± 26.9 | 1.5 ± 0.3 | 7.4 ± 4.4 | 1.4 ± 0.4 | 2.3 ± 0.7 |
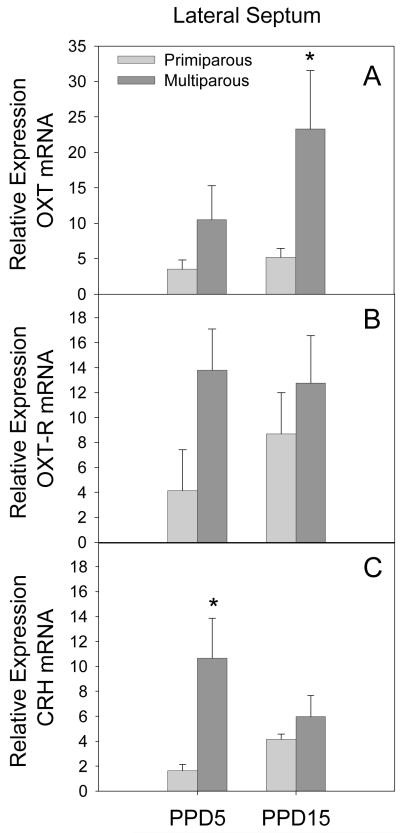
LS
As illustrated in Figure 3 (panel A), there was a significant main effect of parity on OXT mRNA expression (F[1,13] = 7.54, p = 0.02) with multiparous females having higher OXT mRNA expression when compared to primiparous females. This difference achieved significance on PPD15 (p = 0.02). In addition, there was also a trend ((F[1,14] = 3.99, p = 0.07) toward increased OXT receptor mRNA expression in multiparous females across lactation as shown in Figure 3 (panel B). Finally, there was a significant main effect of parity on the expression of CRH mRNA (F[1,15] = 9.78, p = 0.01). As shown in Figure 3 (panel C), this parity effect was due to significantly elevated expression of CRH mRNA in multiparous females on PPD5 (p = 0.005). No significant effects on AVP or V1a were observed in the LS.
Figure 3.
Mean (+SEM) relative expression levels of OXT (Panel A), OXT-R (Panel B) and CRH (Panel C) mRNA within the LS of primiparous and age-matched, multiparous females on PPD5 and PPD15. * p < 0.05 as compared to primiparous females on the same day.
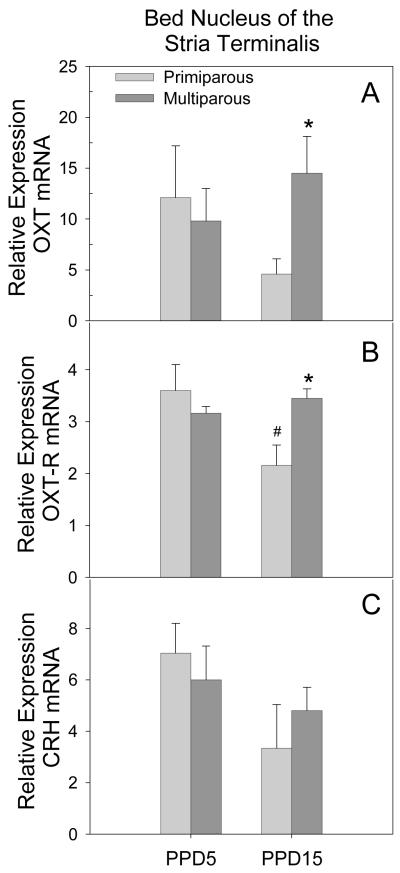
BNST
A trend toward a significant interaction between parity and lactation day was observed for OXT mRNA expression (F[1,14] = 4.42, p = 0.059) in the BNST. As illustrated in Figure 4 (panel A), this trend was due to significantly higher OXT receptor mRNA expression in multiparous females when compared to primiparous females on PPD15 (p = 0.04). A significant parity by lactation day interaction was observed for expression of OXT-R mRNA in the BNST (F[1,14] = 5.62, p = 0.04). As shown in Figure 4 (panel B), this interaction was due to significantly higher expression of OXT-R mRNA in multiparous females when compared to primiparous females on PPD15 (p = 0.04). In addition, there was a significant effect of lactation day in primiparous females with expression reduced on PPD15 when compared to PPD5 (p = 0.01). No effect of lactation day on OXT-R mRNA was observed in multiparous females. Finally, there was a trend toward a main effect of lactation day on the expression of CRH mRNA (F[1,15] = 3.61, p = 0.08) which, as illustrated in Figure 4 (panel C), was due to a tendency toward higher expression of on PPD5 when compared to PPD15. No significant effects on AVP or V1a were observed in the BNST.
Figure 4.
Mean (+SEM) relative expression levels of OXT (Panel A), OXT-R (Panel B) and CRH (Panel C) mRNA within the BNST of primiparous and age-matched, multiparous females on PPD5 and PPD15. * p < 0.05 as compared to primiparous females on the same day. # p < 0.05 as compared to PPD5, within parity condition.
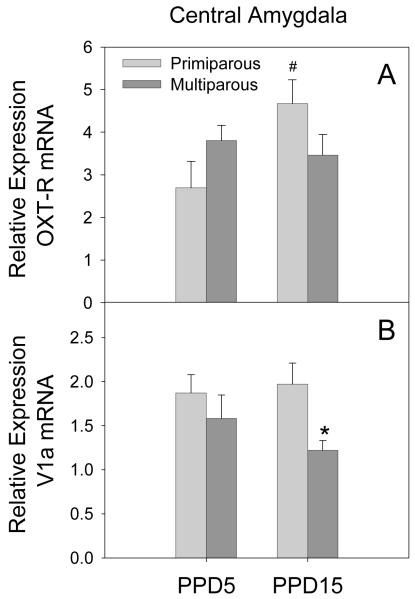
CeA
There was a significant parity by lactation day interaction on the expression of OXT-R mRNA in the CeA (F[1,15] = 4.91, p = 0.05). As shown in Figure 5 (panel A), this interaction effect was due to an effect of lactation day in primiparous females, with OXT-R mRNA expression higher on PPD15 when compared to PPD5 (p = 0.025). No effect of lactation day was observed in multiparous females. A significant main effect of parity on V1a receptor mRNA expression was also observed in the CeA (F[1,15] = 5.54, p = 0.04). As illustrated in Figure 5 (panel B), this effect was due to significantly lower V1a mRNA expression in multiparous females on PPD15 when compared to primiparous females on PPD15 (p = 0.03). No significant effect on OXT, AVP or CRH mRNA expression was observed in the CeA.
Figure 5.
Mean (+SEM) relative expression levels of OXT-R (Panel A) and V1a (Panel B) mRNA within the CeA of primiparous and age-matched, multiparous females on PPD5 and PPD15. * p < 0.05 as compared to primiparous females on the same day. # p < 0.05 as compared to PPD5, within parity condition.
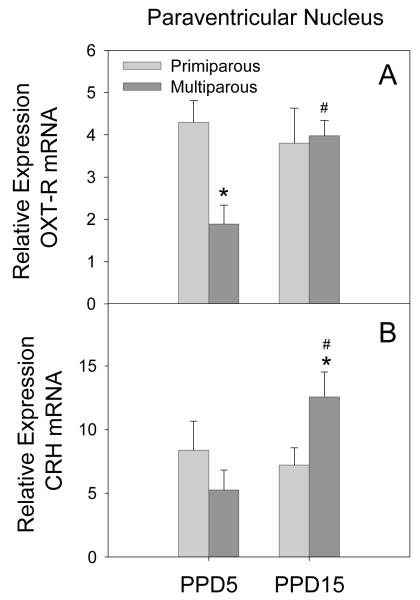
PVN
There was a significant parity by lactation day interaction on the expression of OXT-R mRNA in the PVN (F[1,15] = 4.65, p = 0.05). As illustrated in Figure 6 (panel A), this effect was due to significantly lower expression of OXT-R mRNA in multiparous females on PPD5 when compared to both primiparous females on PPD5 (p = 0.01) and multiparous females on PPD15 (p = 0.01). In addition, there was a significant parity by lactation day interaction on the expression of CRH mRNA in the PVN (F[1,14] = 5.59, p = 0.04). As shown in Figure 6 (panel B), this effect was due to a significantly higher expression in multiparous females on PPD15 when compared both to primiparous females on PPD15 (p = 0.05) and multiparous females on PPD5 (p = 0.01). No significant effects on V1a receptors mRNA expression was observed in the PVN.
Figure 6.
Mean (+SEM) relative expression levels of OXT-R (Panel A) and CRH (Panel B) mRNA within the PVN of primiparous and age-matched, multiparous females on PPD5 and PPD15. * p < 0.05 as compared to primiparous females on the same day. # p < 0.05 as compared to PPD5, within parity condition.
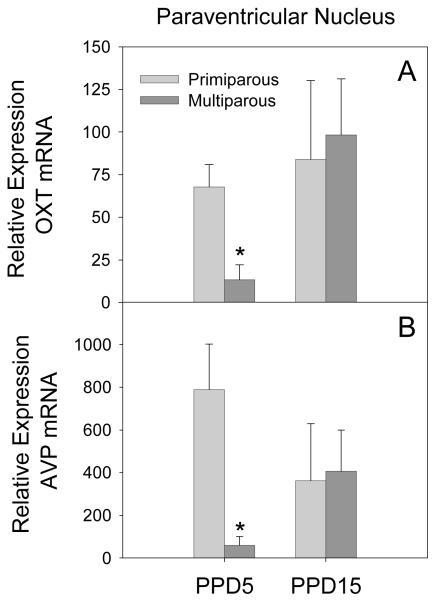
The data for the expression of OXT and AVP mRNA were highly variable in this brain region, especially on PPD15. Thus, no statistically significant effects were found using a two-way ANOVA. As shown in Figure 7, however, there were clear parity-related differences on PPD5. Given that our behavioral effects were only observed on PPD5, we conducted post-hoc t-tests examining the effects of parity on OXT and AVP mRNA within the PVN on PPD5. These analyses yielded significant effects, with multiparous females having reduced OXT (t[5] = 3.59, p = 0.02) and AVP (t[5] = 3.94, p = 0.01) mRNA when compared to primiparous females.
Figure 7.
Mean (+SEM) relative expression levels of OXT (Panel A) and AVP (Panel B) within the PVN of primiparous and age-matched, multiparous females on PPD5 and PPD15. * p < 0.05 as compared to primiparous females on the same day. # p < 0.05 as compared to PPD5, within parity condition.
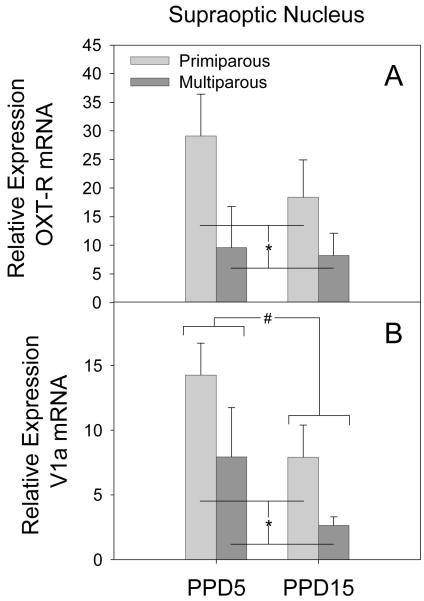
SON
A significant main effect of parity was observed for expression of the OXT-R mRNA expression in the SON (F[1,13] = 4.83, p = 0.05). As illustrated in Figure 8 (panel A), overall multiparous females had lower expression of OXT-R mRNA across lactation. Significant main effects of both parity (F[1,14] = 5.68, p = 0.04) and lactation day (F[1,14] = 5.73, p = 0.04) were also observed for V1a receptor mRNA expression in the SON. As shown in Figure 8 (panel B) multiparous females had lower expression of V1a mRNA across lactation. In addition, expression of V1a mRNA was reduced on PPD15 when compared to PPD5 regardless of parity. No significant effects on the expression of OXT, AVP, or CRH mRNA were observed in the SON. These data, however, were not normally distributed due to a high degree of variability. Therefore negative findings should be interpreted with caution.
Figure 8.
Mean (+SEM) relative expression levels of OXT-R (Panel A) and V1a (Panel B) within the SON of primiparous and age-matched, multiparous females on PPD5 and PPD15. * p < 0.05 primiparous versus multiparous collapsed across lactation day. # p < 0.05 PPD5 versus PPD15 collapsed across parity condition.
DISCUSSION
The current findings indicate that one functional consequence of prior reproductive experience is enhanced maternal aggression during a subsequent lactation. Multiparous females initiated aggression more rapidly, attacked more frequently, and had more prolonged attacks than primiparous females on PPD5. This heightened aggression, however, was not observed on PPD15 with multiparous females displaying an increased latency to attack as well as a decrease in the number and duration of attacks when compared to PPD5. A different pattern of behavior was observed in primiparous females, with some aspects of aggression (i.e. attack latency and duration) unchanged between PPD5 to PPD15 and other components (i.e. number of attacks) increased. Previous studies examining aggression across lactation in primiparous females have observed similar results. For example, Mayer et al (Mayer et al., 1987) reported consistent high levels of aggression on PPD1, PPD4, PPD9, and PPD14 in Sprague-Dawley rats. Erskine et al (Erskine et al., 1980) reported elevated maternal aggression in Long-Evans rats during the first two weeks of lactation with a peak in the frequency of aggression on PPD9, while other studies have observed a more stable level of heightened maternal aggression across the first two weeks of lactation in this strain (Flannelly & Flannelly, 1987). Only one prior study has systematically examined maternal aggression in multiparous females. In that study, which was conducted in mice, maternal aggression was highest during early lactation in multiparous females with levels of aggression declining by PPD15 (Svare & Gandelman, 1976). Thus, while primiparous females typically demonstrate a relatively constant level of aggression during the first two weeks of lactation, multiparous females appear to have significantly higher levels of aggression during early lactation which then decline by mid-lactation to levels similar to those observed in first-time mothers.
In the current study only two time points during lactation were examined. Thus, it is not possible to know when the high level of aggression observed in multiparous females emerges or when it wanes. It has been suggested that heightened maternal aggression during early lactation may be mediated by hormonal priming. Indeed, maternal aggression can be induced in virgin females administered a hormone regimen designed to mimic the hormones of pregnancy, even in the absence of pup exposure (Mayer & Rosenblatt, 1987). Yet, aggression is also regulated by tactile and olfactory cues emanating from the pups (Ferriera & Hansen, 1986; Stern & Kolunie, 1993). Overall, the preponderance of evidence suggests that it is the interaction of both hormones and pup stimuli that produce the most robust expression of maternal aggression (Lonstein & Gammie, 2002). It is possible that prior reproductive experience renders the female more responsive to the hormones of pregnancy and therefore stimulates heightened aggression. It is also possible, however, that experienced females are more responsive to the tactile and/or olfactory cues provided by pups. Furthermore, multiparous mothers may spend more time caring for their pups, thereby increasing their exposure to pup stimuli. Based on the present findings, however, it is evident that multiparous mothers are far more aggressive than their primiparous counterparts during a period of lactation when their pups would be particularly vulnerable to predation.
The present findings also demonstrate changes in gene expression that correlate with enhanced maternal aggression in multiparous females as well as more general changes as a function of both parity and lactation. Perhaps the most relevant changes with regard to maternal aggression were observed in the PVN. On PPD5, multiparous females had decreased expression of both OXT and OXT-R in the PVN when compared to primiparous females. When examined on PPD15, however, no differences were detected. Numerous studies have implicated OXT in the modulation of aggression in females. For example, female OXT gene knock-out mice demonstrate significantly increased offensive aggression during a resident intruder test. These same knock-outs also display extremely high levels of infanticide and cannibalism when presented with foster pups (Ragnauth et al., 2005), indicating that the loss of OXT augments aggression while disrupting maternal responding. These knockout findings suggest that a fine balance must exist in the postpartum female which allows for high levels of offensive aggression in conjunction with appropriate pup-directed behaviors. With regard to maternal aggression, several previous studies have demonstrated enhanced aggression when OXT activity in the PVN is reduced (M. Giovenardi, M. J. Padoin, L. P. Cadore, & A. B. Lucion, 1997, 1998), however other studies have demonstrated that the precise role of OXT in both the PVN and CeA may be dependent upon the innate anxiety of the female (Bosch, Meddle, Beiderbeck, Douglas, & Neumann, 2005). It is certainly possible that multiparous females are less anxious than their primiparous counterparts and may therefore be similar to rats demonstrating a low anxiety phenotype. Nonetheless, the present findings support the hypothesis that reduced OXT activity in the PVN is associated with enhanced maternal aggression.
In addition to altered OXT activity, AVP mRNA in the PVN was also lower in multiparous females on PPD5. Previous studies demonstrated that antagonism of the AVP V1a receptor augments maternal aggression (Nephew & Bridges, 2008b), suggesting that the reduction in AVP observed in the present study may be related to the heightened aggression displayed by multiparous dams. Moreover, recent findings demonstrate a significant reduction in maternal aggression in multiparous females during early lactation following ICV infusions of AVP (Nephew & Bridges, 2009). Thus, a reduction in AVP during early lactation appears to play a significant role in the enhanced aggression observed in multiparous females.
Finally, a significant increase in CRH mRNA was observed in the LS of multiparous females on PPD5. The precise role of CRH in the regulation of maternal aggression is unclear. Studies using ICV infusions have demonstrated an inhibitory effect of CRH and CRH-related peptides, urocortin 1 and urocortin 3, on maternal aggression in lactating mice (Gammie et al., 2004). However, no significant effects of CRH antagonists have been observed (Gammie et al., 2004)and CRH receptor 2 knock-out mice actually demonstrate decreased aggression (Gammie et al., 2005). These discrepancies may be due to brain region specificity with regard to CRH-mediated regulation of maternal aggression. A recent paper by D’Anna and Gammie (2009) observed decreased maternal aggression in mice following direct infusion of CRH, urocortin 1 or urocortin 3 into the LS. These findings strongly suggest that the increased CRH mRNA expression in multiparous females on PPD5 is not associated with increased CRH release within the LS. LS neurons project to a number of brain regions relevant to maternal aggression. For example, LS neurons project to the PVN. Thus, one possibility is that alterations in CRH release within the PVN may play a role in the enhanced aggressive behavior of multiparous females. Interestingly, infusion of CRH into the LS of mice was associated with both decreased maternal aggression and increased c-Fos expression in the ventromedial hypothalamus and lateral hypothalamus (D’Anna & Gammie, 2009). These two regions, which are both associated with aggressive behavior, were not examined in the current study but would be interesting targets in future studies on the role of CRH in the regulation of maternal aggression in reproductively experienced females. In addition to alteration in CRH mRNA expression on PPD5, multiparous females had higher CRH mRNA in the PVN on PPD15 when compared to PPD5, suggesting that increased CRH mRNA expression in the PVN may be associated with decreased levels of maternal aggression. Once again, the extent to which differences in mRNA expression are related to the release of CRH in specific brain regions remains unknown. Finally, while no significant differences in circulating corticosterone secretion as a function of parity were observed, this does not suggest that alterations in stress responsiveness do not play a role in the enhanced aggression. Indeed, significant differences in CRH mRNA expression within brain regions known to regulate stress responsiveness certainly suggest that alterations in stress and/or fear may underlie the effects of reproductive experience on aggression.
A number of other parity-related alterations in gene expression were observed in the current data set. For example, both OXT-R and V1a receptor mRNA expression was reduced in the SON of multiparous mothers, while these same females tended toward higher OXT and OXT-R mRNA expression in the LS and BNST. These alterations may be related to other aspects of maternal behavior, including lactation and/or grooming. Indeed, in the rat, more pup-responsive females displaying elevated levels of grooming possess higher oxytocin levels in the MPOA, LS, CeA, PVN, and BNST (Champagne, Diorio, Sharma, & Meaney, 2001). The current study did not examine the quality of maternal care. It is certainly possible that differences in maternal behavior may underlie or be affected by parity-induced alterations in the OXT system. Indeed, previous studies in sheep have demonstrated significant parity-mediated alterations in OXT levels which are associated with differences in maternal care (Dwyer, 2008)
In addition to parity-mediated alterations, changes in gene expression as a function of lactation were also observed. Certainly several studies have documented the critical role of OXT in lactation not only within the SON but in the LS and BNST as well (Ingram & Moos, 1992; Lambert et al., 1993; Moos et al., 1989). These lactation effects, however, were observed more frequently in primiparous females. For example, primiparous females had higher OXT-R mRNA expression in the CeA on PPD15 when compared to PPD5, while in multiparous females there was no effect of lactation day. These changes in primiparous females may be a consequence of their increasing experience with pups as lactation proceeds(Lalmansingh & Uht, 2008). It is possible, that in more experienced mothers (i.e. multiparous dams) changes occurring during their first lactation are maintained at a steady state during subsequent lactations.
Overall, the results suggest significant differences in neural gene expression in second-time versus first-time mothers. Of course these are correlational studies and do not indicate causative effects on behavior. It is also important to consider the time course involved in changes in mRNA expression. For example, differences in CRH mRNA expression following aggression testing likely reflect dynamic changes as a function of the test session. CRH is an immediate early gene (Lalmansingh & Uht, 2008) and as such responds rapidly to changing environmental conditions. It is likely that other differences in gene expression, however, represent the basal state of the gene expression on that particular day of lactation rather than a response to the test condition. Moreover, while increases and decreases in gene expression often lead to differences in protein expression, divergence in translation could mitigate differences in gene expression. Finally, it is impossible to discern whether these changes in gene expression occur within the cell body or in distal processes. Thus, while informative, these studies represent a first step in identifying potential neural alterations associated with differences in maternal aggression following reproductive experience.
In summary, experienced mothers demonstrate significantly more robust maternal aggression during early lactation when compared to first-time mothers. As maternal aggression is an important component of maternal behavior, these findings suggest an improvement in maternal care with prior experience. Overall these findings support the hypothesis that prolonged experience caring for offspring leads to greater facility with regard to certain aspects of maternal care and that these behavioral adaptations are mediated by changes in neural gene expression. Understanding the precise neural changes underlying enhanced maternal aggression in multiparous females provides greater insight into the long-term effects of reproductive experience on both brain and behavior. In addition, comparing maternal aggression in first- and second-time mothers provides a natural model for the study of heightened aggression.
Acknowledgements
This work was supported by National Institutes of Health grants R37 HD19789 (RSB) and F32 HD048103 (BCN) from the NIH.
References
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behaivor in the rat are associated with differences in estrogen-inducible central oxytocin receptors. PNAS. 2001;98(22) doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GAM, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiology & Behavior. 2005;85(3):354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- D’Anna KL, Gammie SC. Activation of corticotropin-releasing factor receptor 2 in lateral septum negatively regulates maternal defense. Behav Neurosci. 2009;123(2):356–368. doi: 10.1037/a0014987. [DOI] [PubMed] [Google Scholar]
- Dwyer CM. Individual variation in the expression of maternal behaviour: a review of the neuroendocrine mechanisms in the sheep. J Neuroendocrinol. 2008;20(4):526–534. doi: 10.1111/j.1365-2826.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Postpartum aggression in rats: II. Dependence on maternal sensitivity to young and effects of experience with pregnancy and parturition. J. Comp. and Physiol. Psych. 1980;94(3):495–505. doi: 10.1037/h0077677. [DOI] [PubMed] [Google Scholar]
- Ferriera A, Hansen S. Sensory control of maternal aggression in Rattus norvegicus. J. Comp. Psych. 1986;100(2):173–177. [PubMed] [Google Scholar]
- Flannelly KJ, Flannelly L. Opponents size influences maternal aggression. Psych. Reports. 1985;57:883–886. doi: 10.2466/pr0.1985.57.3.883. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Flannelly L. Time Course of Postpartum Aggression in Rats (Rattus norvegicus) Journal of Comparative Psychology. 1987;101(1):101–103. [Google Scholar]
- Gammie SC, Hasen NS, Stevenson SA, Bale TL, D’Anna KL. Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behavioural Brain Research. 2005;160(1):169–177. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav. Neurosci. 2004;118(4):105–114. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898(2):232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus, oxytocin, and maternal aggression in rats. Annals of the New York Academy of Sciences. 1997;807:606–609. doi: 10.1111/j.1749-6632.1997.tb51981.x. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus, oxytocin, and maternal aggression in rats. Ann N Y Acad Sci. 1997;807:606–609. doi: 10.1111/j.1749-6632.1997.tb51981.x. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: Effects of Ibotenic acid lesion and oxytocin antisense. Physiol. and Behav. 1998;63(3):351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav. 1998;63(3):351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav. 2005;84(5):681–695. doi: 10.1016/j.physbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Ingram CD, Moos F. Oxytocin-containing pathway to the bed nuclei of the stria terminalis of the lactating rat brain: immunocytochemical and in vitro electrophysiological evidence. Neuroscience. 1992;47(2):439–452. doi: 10.1016/0306-4522(92)90258-4. [DOI] [PubMed] [Google Scholar]
- Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3′,5′-cyclic adenosine 5′-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;149(1):346–357. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert RC, Moos FC, Ingram CD, Wakerley JB, Kremarik P, Guerne Y, Richard P. Electrical activity of neurons in the ventrolateral septum and bed nuclei of the stria terminalis in suckled rats: statistical analysis gives evidence for sensitivity to oxytocin and for relation to the milk-ejection reflex. Neuroscience. 1993;54(2):361–376. doi: 10.1016/0306-4522(93)90258-h. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural controls of maternal aggression in laboratory rodents. Neuroscience and Biobehav. Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliot JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav. Neurosci. 2003;117(2):195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AD, Reisbick S, Siegel HI, Rosenblatt JS. Maternal aggression in rats: Changes over pregnancy and lactation in a Sprague-Dawley strain. Aggress. Behav. 1987;13:29–43. [Google Scholar]
- Mayer AD, Rosenblatt JS. Hormonal factors influence the onset of maternal aggression in laboratory rats. Horm Behav. 1987;21(2):253–267. doi: 10.1016/0018-506x(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76(3):593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Nephew B, Bridges R. Vasopressin Mediates Enhanced Offspring Protection in Multiparous Rats. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiology & Behavior. 2008a;95(1–2):182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology Biochemistry and Behavior. 2008b;91(1):77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Muglia LJ, Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4(4):229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kolunie JM. Trigeminal lesions and maternal behavior in Norway rats: Effects of cutaneous rostral snout denervation on maintenance of nurturance and maternal aggression. Behavioral Neuroscience. 1991;105(6):984–997. doi: 10.1037//0735-7044.105.6.984. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiol Behav. 1993;54(5):861–868. doi: 10.1016/0031-9384(93)90293-o. [DOI] [PubMed] [Google Scholar]
- Svare B, Gandelman R. A longitudinal analysis of maternal aggression in Rockland-Swiss albino mice. Dev. Psychobiol. 1976;9(5):437–446. doi: 10.1002/dev.420090506. [DOI] [PubMed] [Google Scholar]