Abstract
Purpose
Sepsis syndrome represents the leading cause of death in intensive care unit. Patients present features consistent with a decline in immune responsiveness potentially contributing to mortality. We investigated whether CD4+CD25+ regulatory T cells (Treg) participate in the induction of lymphocyte anergy after sepsis.
Method
Observational study in septic shock patients and experimental study in mice.
Results
We took advantage of the recently described flow cytometric gating strategy using the measurement of CD25 and CD127 expressions for monitoring Treg (CD4+CD25+CD127−Foxp3+). In patients the increased circulating Treg percentage significantly correlated with a decreased lymphoproliferative response. In a murine model of sepsis mimicking these observations, the ex vivo downregulation of Foxp3 expression using siRNA was associated with a restoration of this response.
Conclusion
The relative increase in circulating Treg might play a role in lymphocyte anergy described after septic shock and represent a standardizable surrogate marker of declining proliferative capacity after sepsis.
Keywords: Septic shock, Anergy, CD4+CD25+, CD127, Regulatory T cells, Foxp3
Introduction
Sepsis syndrome (i.e. systemic inflammatory response associated with infection) is a common and frequently fatal clinical condition representing a major although largely under-appreciated health care problem worldwide. These are highly lethal conditions, with mortality ranging from 20% in sepsis to over 60% in septic shock [1, 2]. More worrying is a 75% increase in the number of patients diagnosed with severe sepsis observed over the past two decades [1], which is projected to lead to a significant increase in the incidence of sepsis in the forthcoming years; potentially culminating in over a million cases of severe sepsis in 2020 in the USA alone [3].
Pathophysiologically, inflammation was initially thought to play a primal role in the patient’s response to septic challenge [4]. This culminated in the testing of many anti-inflammatory agents, unfortunately resulting in the failure of numerous clinical trials and therefore suggesting that we still do not adequately understand the underlying pathological process of sepsis.
Accordingly, it is now generally understood that, along with the body’s intense hyperinflammatory response designed to eliminate the underlying pathogen, mechanisms are concomitantly initiated to control this initial response. With respect to this latter effect, a number of investigators have suggested that this counter-regulatory process can, if itself not controlled properly, result in dysfunctions of both innate and adaptive immune responses during sepsis [increased levels of anti-inflammatory cytokines such as interleukin-10 (IL-10), reduced capacity to present antigens associated with the decline of cell surface expression of major histo-compatibility complex (MHC) class II molecules such as HLA-DR and increased evidence of immune cell apoptosis], which in turn, may contribute to death [4, 5]. In particular, the occurrence of a state of lymphocyte anergy has been described after sepsis [4, 5]. This has been known for long and is illustrated by the observation of the loss of delayed-type hypersensitivity reaction to recall skin tests antigens in patients [6–9]. Moreover, lymphocyte anergy has been shown to be associated with mortality and with the development of secondary infections after sepsis [6–9]. However, the mechanisms responsible for this phenomenon are still not completely understood.
In this context, the role of a recently described population of naturally occurring regulatory T cells (Treg) has been emphasized [10]. In human, these cells are supposed to play a role not only in cancer, autoimmunity, or allergy, but also in infectious diseases [11, 12]. Treg are characterized by the constitutive expression of CD25 (interleukin-2 receptor α) and of intracellular Foxp3 (a transcription factor known to play a fundamental role in Treg’s regulatory properties) and possess potent immunosuppressive properties affecting both lymphocyte and antigen presenting cell (APC) responses [11]. We have previously demonstrated that human Treg increased Fas/Fas Ligand (FasL)-mediated monocyte apoptosis after stimulation with endotoxin [13]. They can also induce neutrophil apoptosis after LPS challenge [14], suggesting that these cells might play a role in the development of innate immune dysfunctions observed after sepsis. However, whether Treg can affect lymphocyte anergy in sepsis is unknown.
To investigate this, the whole blood percentage of Treg (assessed by using a newly described and reproducible gating strategy based on the CD4, CD25, CD127 and FOXP3 flow cytometric measurement [15, 16]) was correlated with the proliferative response of mononuclear cells purified from septic shock patients after mitogenic stimulation. In a second time and because of the difficulty to perform such experiments in patients, a mouse model of polymicrobial septic challenge was used to test whether the downregulation of Treg’s specific transcription factor Foxp3 was associated with any restoration of lymphocyte proliferation after sepsis.
Results have been partly presented during the 2008 Experimental Biology Meeting, San Diego, CA [17].
Patients and methods
Septic shock patients admitted to the Intensive Care Units at Lyon-Sud University Hospital were prospectively included. The diagnosis of septic shock was based on criteria of the ACCP/SCCM [18]. Detailed methods are available in the Electronic Supplementary Material (ESM).
Results
CD4+CD25+CD127− lymphocytes express Foxp3 in healthy individuals and septic shock patients and possess regulatory properties
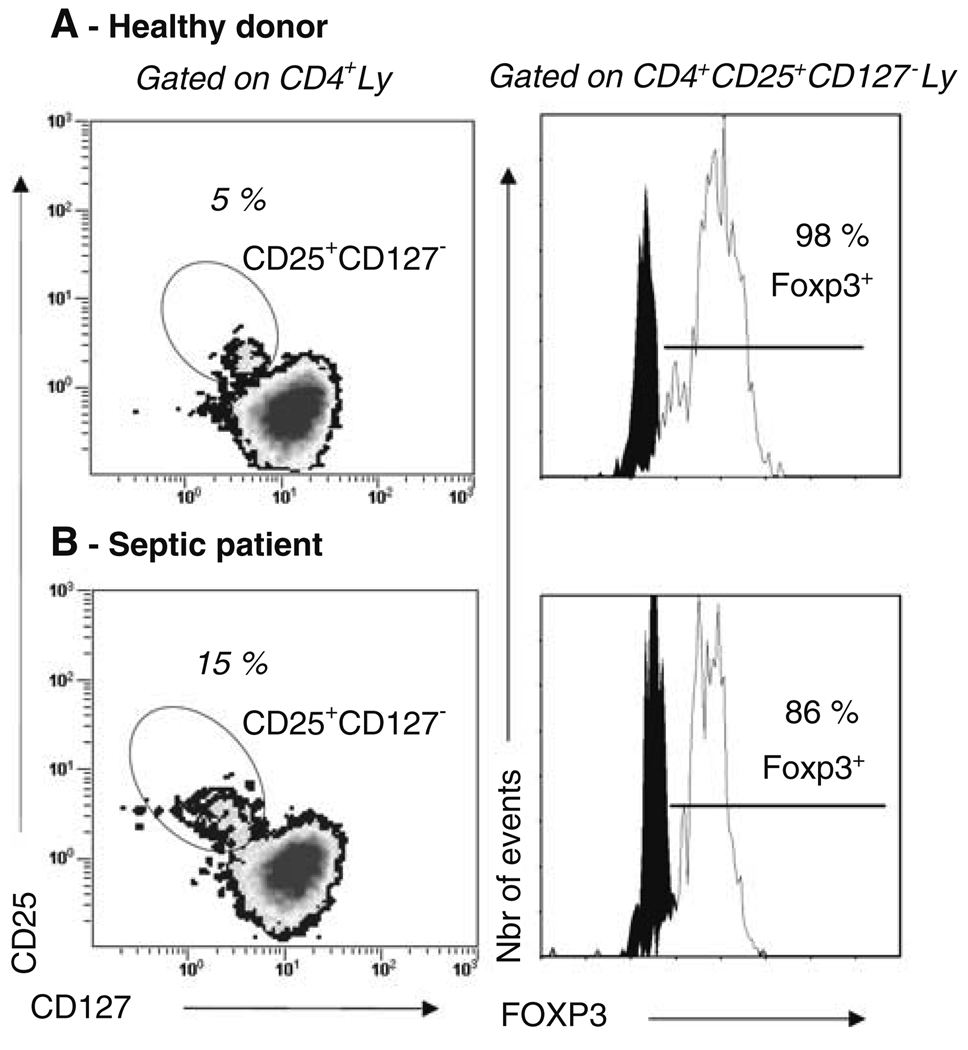
As cell purification is often not feasible in critically ill patients because of the low blood volume available, we investigated Treg phenotype in whole blood from three septic shock patients and five healthy individuals. As observed previously on purified healthy volunteers’ cells (See additional results in the ESM), CD4+CD25+ lymphocytes did not express CD127 either in healthy individuals or in septic shock patients (Fig. 1, left panel). Moreover, using a 4-color flow cytometry staining including intracellular Foxp3 expression measurement, we observed that the large majority of these cells expressed Foxp3 both in healthy volunteers and in septic patients (Fig. 1, right panel). Therefore, the combined expressions of CD4 and CD25 and the lack of expression of CD127 appeared adequate for the characterization of circulating Treg in whole blood of either healthy volunteers or septic shock patients.
Fig. 1.
Flow cytometry phenotyping of whole blood lymphocytes from healthy individuals and septic shock patients. EDTA-anticoagulated blood was harvested from healthy individuals and septic shock patients. Cells were stained using 3-color flow cytometry staining (CD4-ECD–CD25-PC5–CD127-PE) or 4-color flow cytometry staining (CD4-ECD–CD25-PC5–CD127-PE–Foxp3-FITC). Red blood cells were lysed using the automated TQ-Prep lysing system or Versalyse reagent. After gating on CD4+ lymphocytes selected based on a SSC/CD4 dot-plot, CD4+CD25+CD127− cells were selected based on a CD25/CD127 2-color dot-plot (left panel). The percentage of Foxp3 expressing cells among this population was subsequently measured (right panel). One representative experiment for healthy individuals (a) and septic shock patients (b) are presented
The percentage of CD4+CD25+CD127− lymphocytes is significantly increased in septic patients versus healthy individuals
A total of 30 septic shock patients and 17 healthy individuals were prospectively included in the study. The demographic and clinical characteristics for the septic population are presented in Table 1. Patients were monitored once between 3 and 7 days after the onset of shock. The expression of HLA-DR on their monocytes was significantly reduced in comparison with healthy individuals (60 ± 5 vs. >90%, respectively) in accordance with the occurrence of a state of immunoparalysis in these patients [19].
Table 1.
Demographic and clinical characteristics of the septic population
| n = 30 (%) | |
| Male | 20 (66) |
| Female | 10 (33) |
| Age (years)* | 65 (48–83) |
| Survivors | 24 (80) |
| Non-survivors | 6 (20) |
| Main diagnosis category | |
| Medical | 13 (43) |
| Surgery | 17 (47) |
| Microbiologically documented diagnosis | |
| Bacilli Gram− | 15 (50) |
| Cocci Gram+ | 20 (67) |
| Fungi | 8 (27) |
| Type of infection | |
| Community acquired | 16 (53) |
| Nosocomial | 14 (47) |
| Site of infection | |
| Pulmonary | 9 (30) |
| Abdominal | 12 (40) |
| Others | 9 (30) |
| Number of co-morbidities | |
| 0 | 17 (57) |
| ≥1 | 13 (43) |
| SOFA score at admission* | 10 (7–16) |
| SAPS II at admission* | 53 (29–77) |
| Secondary nosocomial infections | 13 (43) |
| % mHLA-DR at the time of blood sample* | 60 (10–98) |
Results are presented as numbers of cases (percentage) except for continuous variables (*), which are expressed as mean (95% confidence interval)
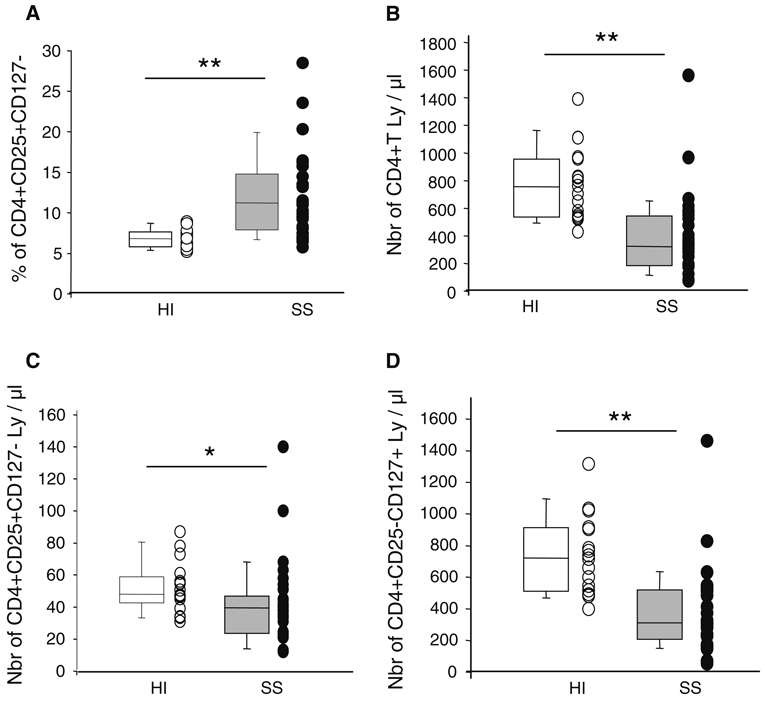
We observed a significantly increased percentage of CD4+CD25+CD127− cells in patients in comparison with healthy volunteers (12.0 ± 1.0 vs. 6.8 ± 0.3%, respectively, P < 0.001, Fig. 2a). This was associated with a decreased number of total CD4+ lymphocytes (410 ± 54 vs. 779 ± 63 cells/µl, respectively, P < 0.001; Fig. 2b); as well as of CD4+CD25+CD127− cells (42 ± 5 vs. 52 ± 4 cells/µl, P < 0.05; Fig. 2c) and CD4+CD25−CD127+ lymphocytes (368 ± 50 vs. 728 ± 60 cells/µl, P < 0.001; Fig. 2d). However, the extent of the decline in CD4+CD25+CD127− cell’s absolute count was significantly less than that of CD4+CD25− lymphocyte population (19 vs. 49%, respectively, P < 0.001).
Fig. 2.
The percentage of CD4+CD25+CD127− cells is increased in whole blood of septic patients in comparison with healthy individuals. EDTA-anticoagulated blood was harvested from 17 healthy individuals (HI, open boxes and circles) and 30 septic shock patients (SS, gray boxes and circles). Cells were stained using 3-color flow cytometry staining (CD4-ECD–CD25-PC5–CD127-PE). Red blood cells were lysed using automated TQ-Prep lysing system. Absolute count measurements were performed using Flow-count fluorospheres™. Results are presented as individual values and box-plots with 5th/95th percentiles. Non-parametric Mann Whitney U test was used for comparison between groups (*P < 0.05, **P < 0.001). a Percentage of CD4+CD25+CD127− lymphocytes measured among CD4+ cells. b Absolute count of peripheral CD4+ T lymphocytes (cells/µl of whole blood). c Absolute count of peripheral CD4+CD25+CD127− T lymphocytes (cells/µl of whole blood). d Absolute count of peripheral CD4+CD25−CD127+ T lymphocytes (cells/µl of whole blood)
The increase in CD4+CD25+CD127− lymphocytes is correlated with a decreased lymphocyte proliferation in septic shock patients
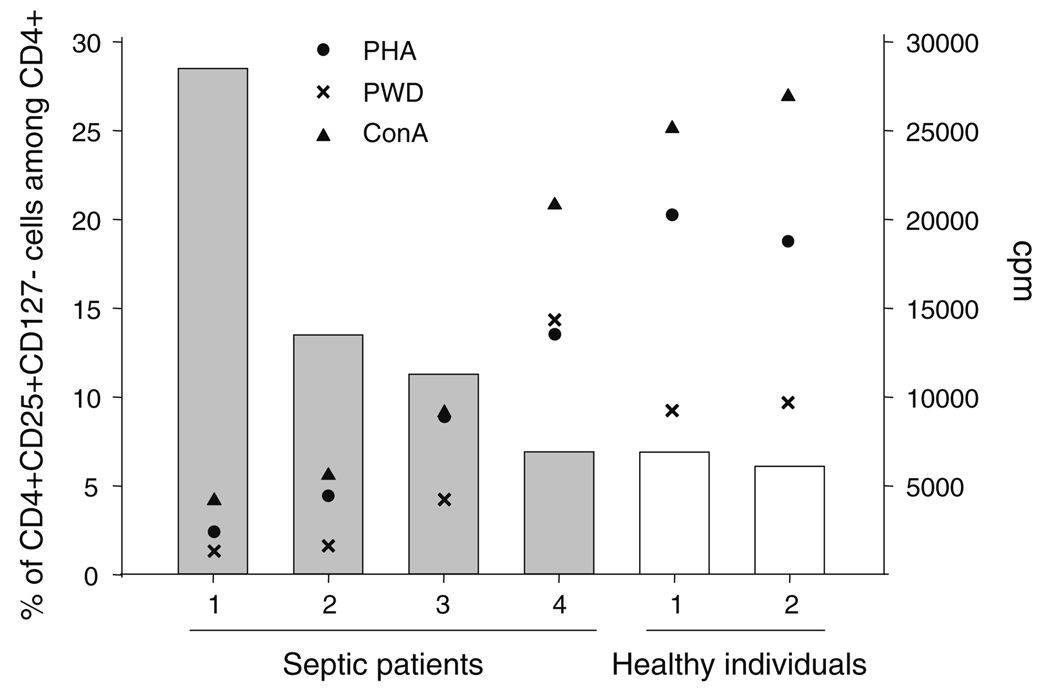
In order to test whether this increased percentage was associated with a functional decline observed in lymphocyte proliferation in patients, the proliferative response of PBMCs purified from a subset of patients and healthy individuals was measured after stimulation with mitogens. This proliferative response was then correlated with the percentage of Treg detected in whole blood using the CD127 gating strategy.
Not surprisingly, we observed that the mitogen-induced proliferative response was severely decreased in patients in comparison with healthy individuals (Fig. 3). Most importantly, we found that the extent of this decrease was closely correlated with the increase in CD4+CD25+CD127− cell’s percentage measured in whole blood (Fig. 3, r = −0.943, P < 0.005 for PHA; r = −1.000, P < 0.005 for ConA and r = −0.829 for PWD). This suggests that the increase in Treg percentage may play a role in the decreased proliferative response observed in patients after septic shock.
Fig. 3.
A decreased cell proliferation is associated with an increased circulating percentage of CD4+CD25+CD127− cells in septic shock patients. EDTA-anticoagulated blood was harvested from healthy individuals (n = 2) and septic shock patients (n = 4). Flow cytometry: Whole blood cells were stained using 3-color flow cytometry staining (CD4-ECD–CD25-PC5–CD127-PE). Red blood cells were lysed using automated TQ-Prep lysing system. Results are expressed as percentages of CD4+CD25+CD127− lymphocytes measured among CD4+ cells (y-axis, gray bars: septic patients, open bars: healthy individuals). Cell proliferation: Peripheral blood mononuclear cells (PBMCs) were harvested using Ficoll-Paque Plus gradient centrifugation and cultured in complete RPMI medium (2 × 105 cells/200 µl) with phytohaemagglutinin (PHA 10 µg/ml, black circles) or concanavalin A (ConA, 0.1 mg/ml, black triangles) or pokeweed (PWD, 20 µg/ml, black crosses). Proliferation was measured by the increased incorporation of [methyl-3H]-Thymidine. The assays were carried out in triplicate. Results are expressed as counts per minute (cpm, z-axis). Normal values from the laboratory: PHA and ConA > 15,000 cpm, PWD > 5,000 cpm
The decreased Foxp3 expression is associated with a restoration of cell proliferation after polymicrobial sepsis in mice
In order to investigate the involvement of Treg in the decreased cell proliferation observed after sepsis, we tested the effect of reducing Foxp3 expression, a transcription factor known to play a fundamental role in Treg’s regulatory properties [20], on lymphocytes’ proliferative response purified from the spleen of mice after polymicrobial sepsis (CLP).
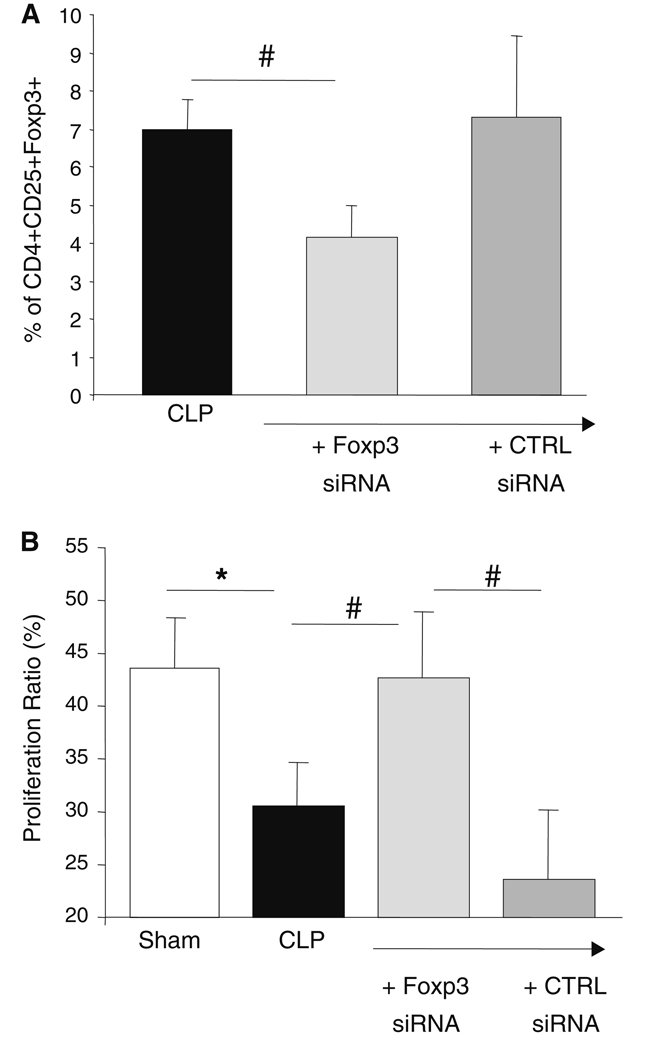
We first confirmed that in our model, as observed in septic patients, the percentages of CD4+CD25+Foxp3+ (10.1 ± 0.5 vs. 8.4 ± 0.5%, P < 0.05), and CD4+CD25+CD127− cells (11.9 ± 0.5 vs. 9.1 ± 0.6%, P < 0.001) were significantly increased 24 h after CLP in comparison with sham animals. Also, as observed in patients, the proliferative response of splenic lymphocytes harvested from mice after CLP was reduced in comparison with sham animals (Fig. 4b).
Fig. 4.
Transfection of CD4+CD25+Foxp3+CD127− regulatory T cells with Foxp3 siRNA is associated with recovery of splenocytes proliferative capacity in septic mice. Murine splenocytes were harvested 24 h after the induction of a polymicrobial septic challenge (cecal ligation and puncture model: CLP, n = 17) or from sham/control animals (Sh, n = 17). Immediately after harvesting, 2 × 106 cells were transfected with 2 µM Foxp3 siRNA or control siRNA or left untouched. a Cells were stained using 3-color flow cytometry staining (CD4-PECy7–CD25-PE–Foxp3-APC) 16 h after transfection or not with siRNA. Results are presented as mean ± SEM [black bar: septic mice, light gray bar: septic mice after transfection with Foxp3 siRNA, dark gray bar: septic mice after transfection with control (CTRL) siRNA]. b After overnight incubation, cells were stained with PKH26 and cultured in 96-well plates in RPMI with or without concanavalin A (0.1 mg/ml, 1 × 106 cells/ml) for 5–6 days. Proliferation was assessed by flow cytometric measurement of the decrease in PKH26 (yellow) fluorescence on viable cells selected based on SSC/FCS characteristics. Results are presented as mean ± SEM of proliferation ratio [i.e. (percentage of cells with decreased fluorescence/percentage of highly fluorescent cells) × 100] measured on splenocytes from sham animals (open bar) or from mice after CLP transfected or not (black bar) with Foxp3 (light-gray bar) or control (CTRL) siRNA (medium-gray bar). The Student t test was used for comparison between groups (*P < 0.05) and the Wilcoxon Signed Ranked test was used for comparison between paired values (#P < 0.05) 682
Most importantly, in those animals, the transfection of splenic lymphocytes with Foxp3 siRNA, which decreased the percentage of Treg (Fig. 4a), was able to restore the proliferative response of murine lymphocytes to a level not different from sham animals, whereas in this model, transfection with control siRNA had no such effect (Fig. 4b).
Discussion
While numerous of clinical trials aiming at treating septic shock have been completed so far, beyond the modest effect of activated protein C, we have still to observe a significant reduction in the number of sepsis-related deaths [3]. Meanwhile, there is now growing recognition that, in addition to an overwhelming pro-inflammatory immune response responsible for the shock phase of sepsis, septic patients appear to also rapidly manifest immune dysfunctions consistent with a state of immune suppression [4, 5]. This state is typically characterized by defects in both innate and adaptive immune responses [4, 5]. However, while the vast majority of clinical and basic science conducted during the last three decades examining the septic process has focused mainly on the roles of myeloid cell populations, the contribution of T lymphocytes, in particular the involvement of sub-populations of regulatory T cells as components of this adaptive immune response, has been somewhat overlooked [10].
Here we used a newly described gating strategy to monitor the change in circulating Treg in septic shock patients as compared to healthy individuals [21]. Moreover, since Treg have been described to be able to decrease T cell’s proliferative response [22] and since lymphocyte anergy has long been known to occur after sepsis, based on the observation of the loss of delayed-type hypersensitivity reaction to recall skin tests antigens in patients [23], we investigated the involvement of Treg in the occurrence of lymphocyte anergy after septic shock.
In agreement with our previous reports [24, 25], we observed a significant increase in the percentage of circulating CD4+CD25+CD127− Treg in septic shock patients in comparison with healthy individuals. This was associated with a decrease in the number of CD4+ lymphocytes. Consequently, as previously hypothesized [25], we observed that this increase was mainly due to a decrease in CD4+CD25−CD127+ T cells number and not so much a rise in the Treg cell count after sepsis (Fig. 2). Once again, this suggested that Treg may be less sensitive to the potentiated apoptotic process that has been reported in patients after septic shock [4, 5]. Although their resistance to Fas/FasL and dexamethasone-induced apoptosis (both known to play a role in injury-induced apoptosis) has been described in vitro [26, 27], this remains to be demonstrated in vivo in the context of sepsis.
A similar increase in Treg percentage has been observed in trauma patients [28] and in mice after polymicrobial septic challenge and stroke [29–32]. However, despite several observations of an increased regulatory capacity of Treg after trauma/sepsis in patients and in mice (i.e., increased Foxp3 expression and increased suppressive potency after trauma/sepsis in comparison with controls) [28, 29, 32] suggesting that injury primes Treg for enhanced regulatory activity, the actual role of these cells in lymphocyte anergy let alone in mortality/morbidity after severe injury remains unclear [10].
Anergy is defined as a state of non-responsiveness to antigen. T cells are anergic when they fail to proliferate or secrete cytokines in response to their specific antigens. Lymphocyte anergy after sepsis is illustrated by the observation of the loss of delayed-type hypersensitivity reaction to recall skin tests antigens in patients. This has long been described and is known to be associated with mortality and with the development of secondary infections [6–9]. In accordance, we observed that the proliferative response of patients’ PBMCs after mitogenic stimulation was decreased in comparison with healthy individuals. A similar state of anergy has been described in patients after major surgery [33], severe burn and trauma [34–36]. Anergy is associated with a diminished production of cytokines such as IL-2 and IFN-γ by peripheral blood mononuclear cells [37] as well as with a decreased expression of activating co-receptors such as monocyte HLA-DR and CD86 or lymphocyte CD28 and CD3 and an increased expression of inhibitory co-receptors (CTLA-4 or PD-1) [36, 38]. Therefore, the development of lymphocyte anergy in sepsis is likely to be a multifactorial process involving soluble factors (IL-10), cell–cell interactions (increased expression of inhibitory receptors associated with a decreased expression activating co-receptors on lymphocytes, decreased antigen-presentation by APCs) and increased apoptosis [5]. Also, intrinsic defects in intracellular lymphocyte activation pathways are likely to occur after sepsis as shown by Bandyopadhyay et al. in trauma patients [increased levels of inhibitory signal transduction proteins (c-Cbl, and SHP-1) concomitant with depressed phosphorylation of activating signal kinases (Erk, Zap70, and CD3ε)] [36]. T-cell receptor complex phosphorylation and activation of the interleukin-2 pivotal transcriptional complex protein CREB (cAMP response element-binding) were also simultaneously depressed [36]. Most importantly and from a clinical perspective, the severity of this state of anergy has been demonstrated to correlate with poor outcome and the increased occurrence of infectious complications and subsequent multiorgan failure in injured patients [39].
In this context, we observed a close negative correlation between the increase in the percentage of Treg measured in patients’ whole blood and the decline in their proliferative response to mitogens. This suggests that Treg may play a role in the development of lymphocyte anergy after septic shock. In order to investigate this hypothesis, we used the murine model of cecal ligation and puncture (CLP) to induce polymicrobial sepsis in mice. This model is supposed to be the most clinically relevant murine model of sepsis [40]. We first recapitulated in septic mice the characteristics observed in patients (i.e. increased percentage of Treg and decreased splenic lymphocytes’ proliferative response to mitogens). We then observed that the downregulation of Foxp3 expression in those cells using ex vivo transfection with Foxp3 targeting siRNA was associated with the restoration of their proliferative response to mitogens, therefore confirming the role of Treg in the decreased proliferative response observed after sepsis. These results are concordant with the deleterious role of Treg in the regulation of immune responses described in various animal models of infection (i.e. excessive control of the effector immune response, development of a bystander immunosuppression) [12]. Along with our previous study and others demonstrating that Treg increase monocyte and neutrophil apoptosis after LPS stimulation [13, 14], this indicates that Treg may produce deleterious effects on both arms (innate and adaptive) of the immune response after septic shock and thus may represent central players in the regulation of the developing immune dysfunctions encountered in patients.
From a methodological point of view, the present results demonstrate that the measurement of Treg frequency may represent a simple and valuable surrogate marker of lymphocyte anergy usable on a routine basis for the monitoring of sepsis-induced immune dysfunctions. Indeed, this study shows the validity of the 3-color flow cytometry staining (CD4/CD25/CD127) for the standardized routine monitoring of Treg in patients. While Foxp3 has been described as a specific marker for T cells with regulatory properties [41], the intracellular staining procedure required for its determination (4 h for completion) renders this marker unsuitable for a routine analysis of these cells in patients. Thus, until recently, Treg were solely characterized in human whole blood based on the combined expression of CD4 and CD25. Among CD4+CD25+ cells, only the CD25high cells are thought to possess regulatory properties in humans [42]. However, since CD25 is not a specific marker for Treg, as it is also over-expressed on activated CD4+ T cells and since no method has been agreed on as a reproducible way to select this CD25high Treg population, this leads to a high variability in the published percentage of Treg measured in healthy individuals (ranging, for example, from less than 1% to more than 10% of CD4+ T lymphocytes) [43]. This becomes even more problematic in a pathological context, e.g., type 1 diabetes, where it has led to contradictory/-reproducible measured variations of Treg frequency in patients as opposed to controls, thus rendering the determination of their putative involvement in the pathophysiology of the disease hard to reconcile [44]. In this context, the use of the combined expression of CD25 and CD127 (a chain of the IL-7 receptor) has been recently proposed for accurately identify Treg in human whole blood and as a biomarker for human Treg cells in diseases (e.g. type 1 diabetes, organ transplant rejection…) [15, 16, 45]. CD127 expression is suggested to allow an unambiguous flow-cytometry based distinction of Treg and activated CD4+ T cells [21]. Here we have confirmed these results since we observed that CD4+CD25+CD127− cells (either purified or in whole blood) expressed a substantially higher percentage of Foxp3+ lymphocytes and possessed regulatory properties. The accuracy of this 3-color staining for the monitoring of Treg in human blood is also further illustrated by the small variation in the percentage of Treg measured in healthy individuals in different studies (6.35% of CD4+ T cells for Seddiki et al. [15], 7–8% for Liu et al. [16], 6% for Ndhlovu et al. [46], 8.34% for Hoffmann et al. [47] and 6.8% in the present study). Therefore the measurement of CD127 in addition with CD4 and CD25 allows for a more rapid (below 30 min) and standardizable estimation of Treg cell numbers, usable on in multi-centered clinical studies, and should help clarify our understanding of the contribution of these cells to sepsis pathophysiology.
Conclusion
We observed in the current study that the increased percentage of circulating CD4+CD25+CD127− Treg was strongly and negatively correlated with the decrease in septic patients’ proliferative response to mitogens, suggesting that Treg measurement may represent a simple and valuable surrogate biological marker for lymphocyte anergy. In addition, the downregulation of Treg-specific transcription factor Foxp3 in murine splenocytes after sepsis was associated with the restoration of their proliferative response, thus demonstrating for the first time that the relative increase in Treg count might play a role in the state of anergy described after septic shock. Therefore, Treg appear to participate in the defects in both innate and adaptive immune responses observed in septic patients (increased monocyte apoptosis [13] and increased lymphocyte anergy), thus suggesting a major role for these cells in sepsis-induced immune dysfunctions.
Supplementary Material
Acknowledgments
Aspects of the work presented here were supported by funds from the Hospices Civils de Lyon (to G.M.) and a grant from the NIH R01 GM46354 (to A.A.). The study was conducted thanks to the logistic support of Centre d’Investigation Clinique (CIC 201) of INSERM and Hospices Civils de Lyon.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-008-1337-8) contains supplementary material, which is available to authorized users.
None of the authors has any potential financial conflict of interest related to this manuscript.
Contributor Information
Fabienne Venet, Division of Surgical Research, Rhode Island Hospital, Brown University, Providence, RI, USA.
Chun-Shiang Chung, Division of Surgical Research, Rhode Island Hospital, Brown University, Providence, RI, USA.
Hakim Kherouf, Flow Cytometry Unit, Immunology Laboratory, Hôpital E. Herriot, Hospices Civils de Lyon, 5, Place d’Arsonval, 69437 Lyon Cedex 03, France.
Anne Geeraert, Flow Cytometry Unit, Immunology Laboratory, Hôpital E. Herriot, Hospices Civils de Lyon, 5, Place d’Arsonval, 69437 Lyon Cedex 03, France.
Chistophe Malcus, Flow Cytometry Unit, Immunology Laboratory, Hôpital E. Herriot, Hospices Civils de Lyon, 5, Place d’Arsonval, 69437 Lyon Cedex 03, France.
Françoise Poitevin, Flow Cytometry Unit, Immunology Laboratory, Hôpital E. Herriot, Hospices Civils de Lyon, 5, Place d’Arsonval, 69437 Lyon Cedex 03, France.
Julien Bohé, Intensive Care Units, Centre Hospitalier Lyon-Sud, Hospices Civils de Lyon, Lyon, France.
Alain Lepape, Intensive Care Units, Centre Hospitalier Lyon-Sud, Hospices Civils de Lyon, Lyon, France.
Alfred Ayala, Division of Surgical Research, Rhode Island Hospital, Brown University, Providence, RI, USA.
Guillaume Monneret, Flow Cytometry Unit, Immunology Laboratory, Hôpital E. Herriot, Hospices Civils de Lyon, 5, Place d’Arsonval, 69437 Lyon Cedex 03, France, guillaume.monneret@chu-lyon.fr.
References
- 1.Brun-Buisson C, Meshaka P, Pinton P, Valley P EPISEPSIS Study group. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30:580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 2.Brun-Buisson C, Roudot-Thoraval F, Girou E, Grenier-Sennelier C, Durand-Zaleski I. The costs of septic syndromes in the intensive care unit and influence of hospital-acquired sepsis. Intensive Care Med. 2003;29:1464–1471. doi: 10.1007/s00134-003-1877-x. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christou NV, Meakins JL, Gordon J, Yee J, Hassan-Zahraee M, Nohr CW, Shizgal HM, MacLean LD. The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg. 1995;222:534–546. doi: 10.1097/00000658-199522240-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rode HN, Christou NV, Bubenik O, Superina R, Gordon J, Meakins JL, MacLean LD. Lymphocyte function in anergic patients. Clin Exp Immunol. 1982;47:155–161. [PMC free article] [PubMed] [Google Scholar]
- 8.Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, Holzmann B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–292. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 9.Roth G, Moser B, Krenn C, Brunner M, Haisjackl M, Almer G, Gerlitz S, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. Susceptibility to programmed cell death in T-lymphocytes of septic patients: a mechanism of lymphopenia and Th2 predominance. Biochem Biophys Res Comm. 2003;308:840–846. doi: 10.1016/s0006-291x(03)01482-7. [DOI] [PubMed] [Google Scholar]
- 10.Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2007;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 11.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 12.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 13.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Powell WS, Monneret G. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 14.Lewkowicz P, Lewkowicz N, Sasiak A, Tchórzewski H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol. 2006;177:7155–7163. doi: 10.4049/jimmunol.177.10.7155. [DOI] [PubMed] [Google Scholar]
- 15.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venet F, Chung CS, Lepape A, Ayala A, Monneret G. Anergy in septic patients: correlating the increased percentage of circulating CD4+CD25+CD127− regulatory T cells with a decline in lymphocyte proliferation. FASEB J. 2008;22:848–849. [Google Scholar]
- 18.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 19.Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 20.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 21.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–544. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 23.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186:241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 25.Venet F, Pachot A, Debard AL, Bohé J, Bienvenu J, Lepape A, Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25− lymphocytes. Crit Care Med. 2004;32:2329–2331. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- 26.Banz A, Pontoux C, Papiernik M. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of regulatory T cells and effector T cells. J Immunol. 2002;169:750–757. doi: 10.4049/jimmunol.169.2.750. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Murakami T, Oppenheim JJ, Howard OM. Differential response of murine CD4+CD25+ and CD4+CD25− T cells to dexamethasone-induced cell death. Eur J Immunol. 2004;34:859–869. doi: 10.1002/eji.200324506. [DOI] [PubMed] [Google Scholar]
- 28.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. Increased CD4+CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scumpia PO, Delano MJ, Kelly KM, O’Malley KA, Efron PA, McAuliffe PF, Brusko T, Ungaro R, Barker T, Wynn JL, Atkinson MA, Reeves WH, Salzler MJ, Moldawer LL. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–7949. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- 30.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Bäumel M, Männel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 32.Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2006;27:251–257. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W, Wagner H, Siewert JR, Holzmann B. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–2291. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puyana JC, Pellegrini JD, De AK, Kodys K, Silva WE, Miller CL. Both T-helper-1- and T-helper-2-type lymphokines are depressed in osttrauma anergy. J Trauma. 1998;44:1037–1045. doi: 10.1097/00005373-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrini JD, De AK, Kodys K, Puyana JC, Furse RK, Miller-Graziano C. Relationships between T lymphocyte apoptosis and anergy following trauma. J Surg Res. 2000;88:200–206. doi: 10.1006/jsre.1999.5797. [DOI] [PubMed] [Google Scholar]
- 36.Bandyopadhyay G, De A, Laudanski K, Li F, Lentz C, Bankey P, Miller-Graziano C. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35:794–801. doi: 10.1097/01.CCM.0000256847.61085.A5. [DOI] [PubMed] [Google Scholar]
- 37.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Manjuck J, Saha DC, Astiz M, Eales LJ, Rackow EC. Decreased response to recall antigens is associated with depressed costimulatory receptor in septic critically ill patients. J Lab Clin Med. 2000;135:153–160. doi: 10.1067/mlc.2000.104306. [DOI] [PubMed] [Google Scholar]
- 39.De AK, Kodys KM, Pellegrini J, Yeh B, Furse RK, Bankey P, Miller-Graziano CL. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96:52–66. doi: 10.1006/clim.2000.4879. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24S1:52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 42.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 43.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Tree TI, Roep BO, Peakman M. A mini meta-analysis of studies on CD4+CD25+ T cells in human type 1 diabetes: report of the Immunology of Diabetes Society T Cell Workshop. Ann N Y Acad Sci. 2006;1079:9–18. doi: 10.1196/annals.1375.002. [DOI] [PubMed] [Google Scholar]
- 45.Codarri L, Vallotton L, Ciuffreda D, Venetz JP, Garcia M, Hadaya K, Buhler L, Rotman S, Pascual M, Pantaleo G. Expansion and tissue infiltration of an allospecific CD4+CD25+CD45RO+IL-7Ralphahigh cell population in solid organ transplant recipients. J Exp Med. 2007;207:1533–1541. doi: 10.1084/jem.20062120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. FOXP3 expressing CD127lo CD4+ T cells inversely correlates with CD38+CD8+ cell activation levels in primary HIV-1 infection. J Leukoc Biol. 2008;83:254–262. doi: 10.1189/jlb.0507281. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann HJ, Malling TM, Topcu A, Ryder LP, Nielsen KR, Varming K, Dahl R, Omland O, Sigsgaard T. CD4dimCD25bright Treg cell frequencies above a standardized gating threshold are similar in asthmatics and controls. Cytometry A. 2007;71:371–378. doi: 10.1002/cyto.a.20389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.