Abstract
Gambogic acid (GA) has a significant anticancer effect on a wide variety of solid tumors. Recently, many nanoparticles have been introduced as drug-delivery systems to enhance the efficiency of anticancer drug delivery. The aim of this study was to investigate the potential benefit of combination therapy with GA and magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4). The proliferation of K562 cells and their cytotoxicity were evaluated by MTT assay. Cell apoptosis was observed and analyzed by microscope and flow cytometry, respectively. Furthermore, real-time polymerase chain reaction and Western blotting analyses were performed to examine gene transcription and protein expression, respectively. The results showed that MNPs-Fe3O4 dramatically enhanced GA-induced cytotoxicity and apoptosis in K562 cells. The typical morphological features of apoptosis treated with GA and MNPs-Fe3O4 were observed under an optical microscope and a fluorescence microscope, respectively. The transcription of caspase-3 and bax gene in the group treated with GA and MNPs-Fe3O4 was higher than that in the GA-alone group or MNPs-Fe3O4-alone group, but the transcription of bcl-2, nuclear factor-κB, and survivin degraded as did the expression of corresponding proteins in K562 cells. Our data suggests a potential clinical application of a combination of GA and MNPs-Fe3O4 in leukemia therapy.
Keywords: gambogic acid, magnetic nanoparticles of Fe3O4, traditional Chinese medicine, K562 leukemia cells, apoptosis
Introduction
A major problem of cancer therapy is the side effects of chemotherapy. Minimizing side effects and maximizing efficacy is a major goal in the development of tumor treatment.1 Gambogic acid (GA), a naturally occurring brownish orange resin called gamboge,2 possesses diverse biological effects such as anti-inflammatory and antioxidant actions.3 Recent studies showed that GA could inhibit the growth of a wide variety of tumor cells, including hepatoma, pulmonary carcinoma, gastric cancer, and breast cancer cells.4–14 How GA mediates the growth of these tumor cells is not fully understood, but GA has been shown to induce apoptosis, arrest cell cycles, and downregulate bcl-2 and telomerase activity.14,15 Preclinical research revealed that a therapeutic dose of GA did not inhibit the proliferation of bone marrow, peripheral blood leucocyte count, or phagocytotic function of macrophage in tumor-bearing mice.16 Because of its broad spectrum anticancer actions, satisfactory therapeutic effect, and good tolerance, GA is a promising candidate in anticancer drugs.
Another key problem for tumor treatment is the reducing sensitivity of tumor cells to cytotoxic drugs. Thus, many polymer nanospheres and nanoparticles have been introduced as drug-delivery systems to enhance the efficiency of anticancer drug delivery based on the ability to target specific locations in the body.17 The most promising materials are magnetic nanoparticles. Magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4), a biocompatible and superparamagnetic nanomaterial with satisfactory chemical stability and low toxicity, are widely used for targeted-drug carriers with target-orientation and sustained-release properties.18
Our study aims to evaluate the potential benefit of combination therapy with GA and MNPs-Fe3O4 for leukemia and whether MNPs-Fe3O4 could promote the apoptosis induced by GA. To elucidate the mechanisms possibly involved, we also measured the expression of apoptosis-related genes and proteins, including caspase-3, bax, bcl-2, NF-κB, and survivin.
Materials and methods
Main reagents
GA (Kanion Pharmaceutical Co., Ltd, Jiangsu, China) was dissolved in dimethyl sulfoxide (DMSO; Sigma Aldrich, St. Louis, MO), stored at −20 °C, and then diluted as needed in RPMI 1640 medium (Gibco/BRL, Carlsbad, CA). MTT was purchased from Sigma Aldrich. Monoclonal antibodies including caspase-3, bax, bcl-2, NF-κB, survivin, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). MNPs-Fe3O4 (State Key Lab of Bioelectronics, Nanjing, China) were well distributed in RPMI 1640 medium containing 10% (v/v) heat-inactivated new-born calf serum (Sijiqing, Hangzhou, China) by using ultrasound treatment in order to obtain MNPs-Fe3O4 colloidal suspension. GA conjugated with MNPs-Fe3O4 was prepared by mechanical absorption polymerization at 4 °C for 48 hours.
Cell lines and culture conditions
K562 cells, derived from human leukemic cells from a chronic myeloid leukemia patient in blastic crisis and constantly preserved in our laboratory, were cultured in RPMI 1640 medium containing 10% (v/v) heat-inactivated fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified 5% CO2 incubator.
Cell viability assay
Cytotoxicity was determined by the MTT assay. K562 cells (8 × 103/mL) were incubated into 96-well flat-bottomed plates (Costar, Charlotte, NC). Different concentrations of GA were added into these cells and cultured at 37 °C for 24, 48, and 72 hours, respectively. To determine the optimum synergistic effect of MNPs-Fe3O4, different concentrations of MNPs-Fe3O4 were used symphysially with or without GA in graded concentrations. Briefly, 20 μL MTT (5 mg/mL) was added to each well and incubated at 37 °C for 4 hours. The formazan was dissolved with 150 μL dimethyl sulfoxide (Sigma Aldrich) and the reduction of MTT was quantified by absorbance at 570 nm using a plate reader (Model 550; Bio-Rad Laboratories, Tokyo, Japan). The inhibition ratio (IR) of cells was determined as follows: (1-Atreated group/Acontrol group) × 100%. The 50% inhibiting concentration (IC50) was defined as the concentration required for 50% inhibition of cell growth.
Apoptosis assay by flow cytometer
Quantification of apoptotic cells was performed using an Annexin-V-FITC Apoptosis Detection Kit (KenGen, Nanjing, China) according to the manufacturer’s instructions. After incubation in a medium containing different drugs at 37 °C for 48 hours, the cells were collected and suspended in 500 μL of binding buffer, and 5 μL Annexin-V-fluorescein isothiocyanate (FITC) and 5 μL propidium iodide (PI) were added at room temperature in the dark for 15 minutes. Analyses were performed by FACScan flow cytometer (Becton Dickinson, Sunnyvale, CA). The cells in the FITC-positive and PI-negative fraction were regarded as apoptotic cells.
Cell morphological assessment
After being cultured in RPMI-1640 containing 6 mg/L GA, 0.6 mg/L GA conjugated with 10 mg/L MNPs-Fe3O4 or without GA at 37 °C for 48 hours, K562 cells were collected and smeared. Some films were stained with Wright’s stain to observe the morphological changes of apoptosis cells by optical microscope; others were fixed with methanol for 15 minutes, stained with fluorochrome dye DAPI (Santa Cruz Biotechnologies), and then observed under a fluorescence microscope (IX51; Olympus, Tokyo, Japan) with a peak excitation wave length of 340 nm.
Quantitative real-time PCR (QPCR) analysis
As described before, K562 cells (8 × 103/mL) were treated, harvested, and then total RNA was isolated using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. The reverse transcription reactions were performed using SuperScript™ II reverse transcriptase (Invitrogen Life Technologies) and the newly synthetic cDNA was amplified within target and control sequences (primer sequences for caspase-3 (270 bp) forward, 5′-GCTATTGTGAGGCGGTTGT-3′ and reverse, 5′-TGTTTCCCTGAGGTTTGC-3′; Bax (114 bp) forward, 5′-TTTTGCTTCAGGGTTTCATC-3′ and reverse, 5′-GACACTCGCTCAGCTTCTTG-3′; Bcl-2 (452 bp) forward, 5′-GGGAGAACAGGGTACGATAA-3′ and reverse, 5′-CCACCGAACTCAAAGAAGG-3′; NF-κB (227 bp) forward, 5′-TCGTTTCCGTT ATGTATGT-3′ and reverse, 5′-CCTTGGGTCCAGCAGTTA-3′; Survivin (255 bp) forward, 5′-CAAGGACCACCGCATCTC-3′ and reverse, 5′-CCAAGGGTTAATTCTTCAAACT-3′; GAPDH (205 bp) forward, 5′-CGGATTTGGTCGTATTG-3′ and reverse, 5′-GAAGATGGTGATGGGATT-3′). QPCR was performed by monitoring in real-time the increase of fluorescence of SYBR green I dye (Takara, Shiga, Japan) with Rotor-Gene 3000 (Corbett Research, Sydney, Australia). The relative gene copy number was calculated by the concentration-CT standard curve method and normalized using the average expression of GAPDH.
Western blot analysis
In order to examine the expression of caspase-3, bax, bcl-2, NF-κB, and survivin, we next performed Western blot analysis on whole cell protein extracted from cells treated for 48 hours as described previously. In brief, total protein was isolated on ice and subjected to 10% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels using modified radio immunoprecipitation assay buffer, and transferred to a polyvinylidene difluoride membrane (Bio-Rad). Western blotting was performed with a 1:1000–1200 dilutions of monoclonal antibodies against either anti-human caspase-3, bax, bcl-2, NF-κB, surviving, or β-actin anti-body in 5% nonfat dry milk, and then with horseradish peroxidase-conjugated goat anti-rabbit (1:5000) as a secondary antibody. The band was detected by using an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK).
Statistical analysis
All data were presented as means ± standard deviation in triplicate and analyzed using SPSS software (v. 15.0; SPSS Inc., Chicago, IL). The difference among various groups was analyzed by ANOVA test, and P values of less than 0.05 were considered significant.
Result
Synergistic effect on cytotoxicity of K562 cells
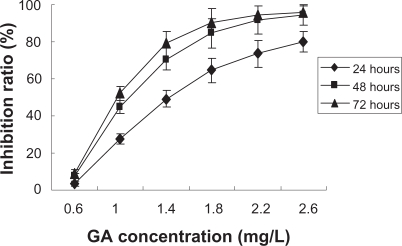
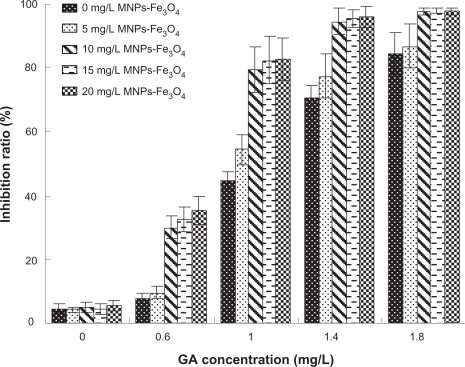
The MTT assay revealed that GA inhibited the survival of K562 cells in a dose- and time-dependent manner and the IC50 was 1.13 mg/L (Figure 1). Furthermore, it was observed that the addition of MNPs-Fe3O4 did enchance the inhibition of GA to K562 cells, and 10 mg/L MNPs-Fe3O4 reduced the IC50 value of GA to 0.72 mg/L (P < 0.05) (Figure 2), suggesting MNPs-Fe3O4 with GA have a synergistic effect on K562 cells.
Figure 1.
Effect of the different concentrations of gambogic acid (GA) on growth inhibition ratio of K562 cells by MTT assay.
Figure 2.
Growth inhibition ratio of GA with or without MNPs-Fe3O4-treated K562 cells for 48 hours.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid.
Synergistic effect on apoptosis of K562 cells
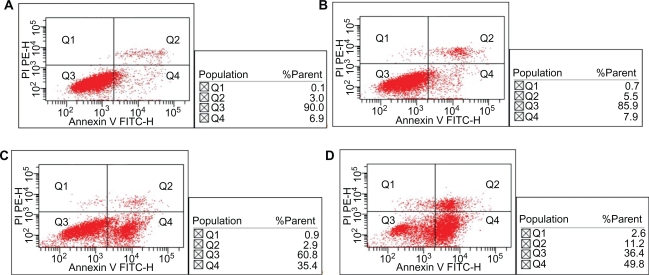
Only (7.1% ± 3.23%) apoptosis of K562 cells were observed under 10 mg/L MNPs-Fe3O4, there was no significant changes compared to the control group (6.1% ± 1.67%) (P > 0.05). The apoptosis of K562 cells induced by 0.6 mg/L GA for 48 hours was (35.2% ± 3.37%) (P < 0.05), while combination of GA with 10 mg/L MNPs-Fe3O4 increased to (48.7% ± 1.47%) (P < 0.05) (Figure 3), which indicated that MNPs-Fe3O4 could enhance GA-induced apoptosis.
Figure 3.
Effect of MNPs-Fe3O4 on GA-induced apoptosis in K562 cells for 48 hours. A) Control; B) Incubated with 10 mg/L MNPs-Fe3O4; C) Incubated with 0.6 mg/L GA; D) Incubated with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid.
Morphological changes of K562 Cells
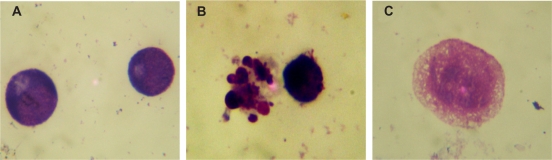
The morphological changes of K562 cells by optical microscope were shown in Figure 4. K562 cells in control group displayed normal, healthy shape demonstrated by the clear skeletons (Figure 4A); After treatment with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4 for 48 hours, typical cytomorphological features of apoptosis in K562 cells were evident, such as cell shrinkage, chromatin condensation, margination, and presence of apoptotic bodies (Figure 4B); While large dose of GA led K562 cells to necrosis (Figure 4C).
Figure 4.
Morphological features of K562 cells after treatment for 48 hours by optical microscope (1000x, Wright staining). A) Control; B) Incubated with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4; C) Incubated with 6 mg/L GA.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid.
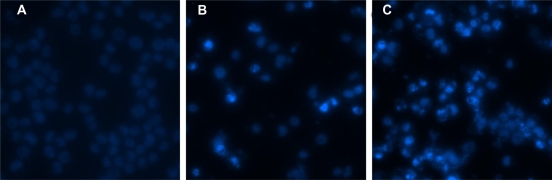
Under the fluorescence microscope, the nucleolus changes of K562 cells were observed (Figure 5). K562 cells in control group were stained equably blue fluorescence, indicating that the chromatin equably distributed in nucleolus (Figure 5A), but 0.6 mg/L GA led a few K562 cells to display chromatin condensation and nucleolus pyknosis (Figure 5B). After incubated with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4 for 48 hours, the cells emitting bright fluorescence increased and displayed the typical phenomena of apoptosis including chromatin condensation, nucleolus pyknosis, and nuclear fragmentation (Figure 5C).
Figure 5.
Nucleolus morphological changes of K562 cells after different treatment for 48 hours under fluorescence microscope (400x, DAPI staining). A) Control; B) Incubated with 0.6 mg/L GA; C) Incubated with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid.
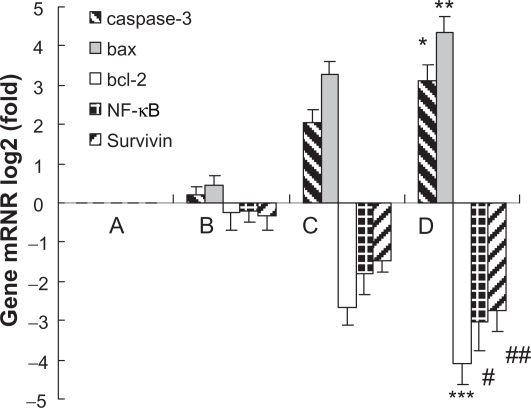
Transcription of caspase-3, bax, bcl-2, NF-κB and survivin by QPCR
10 mg/L MNPs-Fe3O4 could not influence the expression of caspase-3, bax, bcl-2, NF-κB, and survivin mRNA, but the synergia of 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4 for 48 hours could dramatically upregulate the transcription of caspase-3 and bax mRNA in K562 cells (P < 0.05), surpassing the effects of 0.6 mg/L GA alone (Figure 6C) (P < 0.05). Meanwhile, the co-treatment of agents mentioned above for 48 hours seemed to induce degradation of bcl-2, NF-κB, and survivin mRNA on K562 cells (P < 0.05), also surpassing the effects of GA (0.6 mg/L) alone (P < 0.05).
Figure 6.
Transcription of caspase-3, bax, bcl-2, NF-κB, and survivin in K562 cells after treatment of GA with or without MNPs-Fe3O4 for 48 hours. A) Control; B) Incubated with 10 mg/L MNPs-Fe3O4; C) Incubated with 0.6 mg/L GA; D) Incubated with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4.
Notes: *,**P < 0.05, synergia of GA with MNPs-Fe3O4 compared to GA alone; #,##,***P < 0.05, synergia of GA with MNPs-Fe3O4 compared to GA alone.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid; NF-κB, nuclear factor-kappaB.
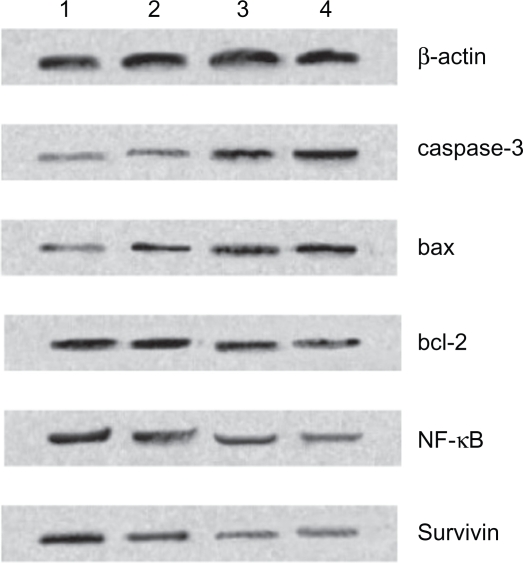
Expression of caspase-3, bax, bcl-2, NF-κB, and survivin protein by Western blot
Based on computer-assisted image analysis, it seems that caspase-3, bax, bcl-2, NF-κB, and survivin proteins in K562 cells treated with 10 mg/L MNPs-Fe3O4 had no significant changes when compared to control group (P > 0.05). However, the level of caspase-3 and bax proteins in K562 cells treated with 0.6 mg/L GA dramatically elevated, compared to control group (P < 0.05) (Figure 7). Furthermore, these two kinds of proteins, whose genes were upregulated in 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4 group in K562 cells (Figure 6), were more than those of 0.6 mg/L GA alone (P < 0.05). Reversely, compared with the control group, the level of other three proteins in cells after co-treatment as described previously was lower than those of 0.6 mg/L GA alone (P < 0.05) (Figure 7).
Figure 7.
Expression of caspase-3, bax, bcl-2, NF-κB, and survivin protein in K562 cells by western blot after treatment of GA and/or MNPs-Fe3O4 for 48 hours. Line 1: Control; Line 2: Incubated with 10 mg/L MNPs-Fe3O4; Line 3: Incubated with 0.6 mg/L GA; Line 4: Incubated with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid; NF-κB, nuclear factor-kappaB.
Discussion
Although many chemotherapy drugs are used clinically, the overall survival of leukemia patients is still unsatisfactory. The majority of chemotherapy medicines have serious adverse effects in addition to their clinical effects. Patients find these side effects hard to tolerate, which often causes chemotherapy failure. GA differs from other anticancer drugs as it is an apoptotic inducer from traditional Chinese medicine. It can induce tumor cell death selectively without toxicity to normal tissue, which offers a unique prospect in the development of new antitumor medicine.4,19 Apoptosis is an important metabolic step in regulating the number of cells and their growth. If apoptosis is blocked, the metabolism will become disordered and tumors will develop and grow.20 Most anticancer agents exert their anticancer effects by inducing apoptosis. 21 Recently, MNPs-Fe3O4 are widely used for targeteddrug carriers to enhance the efficiency of anticancer drug delivery based on the ability of target-orientation and sustained-release properties.18 Our previous studies have demonstrated the synergistic effect of MNPs-Fe3O4 with anticancer drug on the intracellular accumulation in leukemia cells.22–28 Thus, in the present study, we wanted to demonstrate the potential synergistic effects of MNPs-Fe3O4 and GA on apoptosis in leukemia cells.
Data from our cytotoxicity assay showed that MNPs-Fe3O4 enhanced the toxicity of GA in K562 cells and the addition of MNPs-Fe3O4 decreased the IC50 of GA in K562 cells. This phenomenon is consistent with our previous studies that reported that less than 20 mg/L of MNPs-Fe3O4 did not influence the multiplication of K562 cells.26 We also investigated the synergistic effects of GA with MNPs-Fe3O4 on the apoptosis of K562 cells. The addition of 10 mg/L MNPs-Fe3O4 caused the apoptotic percentage of K562 cells induced by 0.6 mg/L GA for 48 hours to increase by 14.4%. Our outcomes clearly indicate that a MNPs-Fe3O4-drug delivery system can decrease the IC50 of GA and enhance apoptosis in leukemia cells.
In order to check whether the effects of MNPs-Fe3O4 combining with a small dose of chemotherapeutic agent was different from the effects of a large dose of chemotherapeutic agent on K562 cells,28 we demonstrated that the K562 cells in 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4 group for 48 hours showed a typical morphological features of apoptosis under the optical microscope, while 6 mg/L GA led cells to necrosis. This effect supports our previous assumption. Meanwhile, the cells incubated with 0.6 mg/L GA and 10 mg/L MNPs-Fe3O4 displayed the typical apoptosis under the fluorescence microscope, compared with that of 0.6 mg/L alone. These results suggest that a combination of MNPs-Fe3O4 and GA could be a feasible candidate in the development of anticancer drugs.
Apoptosis is the consequence of a series of precisely regulated events that are frequently altered in tumor cells. In general, the sequence of events has been broadly categorized into two pathways: the extrinsic pathway, which involves the activation of the tumor necrosis factor (TNF)/Fas death receptor family and the intrinsic pathway, which involves the mitochondria. In both pathways, an apoptotic death stimulus results in the activation of caspases, the major executioners of this process, either directly or via activation of the mitochondrial death program.10,29–31 It is well known that caspase-3 is the most characterized effector caspase, and its activation leads to the final stages of cellular death by proteolytic dismantling of a large variety of cellular components on one hand, and activation of proapoptotic factors on the other hand.29–31 Our study showed that GA combined with MNPs-Fe3O4 dramatically upregulated the transcription and expression of caspase-3 in K562 cells. This result supports the promotion of GA-induced apoptosis by MNPs-Fe3O4 was related to the level of genes and proteins expression.
In tumor cells, apoptosis can be induced either by activation of molecules upstream of apoptosis signaling or by inhibition of antiapoptotic factors. Survivin, a member of the inhibitor of apoptosis protein (IAP) family, is overexpressed in virtually every human cancer. In several tumor cell lines, the presence of survivin correlates with resistance to apoptosis and is associated with increased malignancy.14,32,33 Previous in vitro studies showed that inhibition of survivin restored or enhanced the cytotoxicity of chemoreagents,34,35 and animal studies showed a superb efficacy against xenografts using an adenoviral strategy targeted to survivin.36,37 At present, survivin is validated as a cancer therapeutic target.38 Our data showed that the expression of antiapoptotic genes such as bcl-2, survivin of K562 cells were significantly downregulated after co-treatment of GA with MNPs-Fe3O4, whereas the expression of bax was upregulated. Bax and bcl-2 both belong to the bcl-2 family.39 Overexpression of Bax has been shown to accelerate cell death,40,41 while overexpression of antiapoptotic proteins such as bcl-2 represses the death function of bax.42 Thus, the ratio of bcl-2/bax might be a critical factor of a cell’s threshold for undergoing apoptosis.43 Although bcl-2 and survivin are both apoptosis inhibitors, they work through different pathways in the regulation of cell apoptosis. The antiapoptotic protein bcl-2 mainly inhibits the mitochondrial pathways,44,45 while survivin directly blocks the processing and activation of effector caspase-3 and caspase-7, which commonly acts downstream of both apoptosis signaling pathways,46 which suggests that MNPs-Fe3O4 loaded with GA induced cell apoptosis through various pathways.
NF-κB is a transcription factor,47 which regulates the expression of several genes whose products are involved in tumorigenesis. Apoptosis and tumorigenesis are known to be regulated by NF-κB-regulated gene products.48 The transcription factor NF-κB involves the extrinsic death receptor signaling pathway of apoptosis. Suppression of NF-κB activation promotes TNF-induced apoptosis.49 As our results showed, GA combined with MNPs-Fe3O4 induced the degradation of NF-κB genes and proteins in K562 cells. We presumed that they inhibited the activation of NF-κB and worked through downregulated NF-κB-regulated gene products involved in antiapoptosis such as IAP1, IAP2, bcl-2, Bcl-xL, and TRAF1.2
Kasibhatla and colleagues10 reported an undiscovered link between transferrin receptor (TfR) and the rapid activation of apoptosis. GA binding to TfR induced a unique signal leading to rapid apoptosis of tumor cells. TfR, the molecular target for GA, was significantly overexpressed in different types of cancers. However, GA bound to the TfR independent of the transferring-binding site. Binding of GA to TfR activated the apoptosis cascade rapidly by using caspase-8 and the mitochondrial pathway. They also demonstrated that GA and transferrin bound to independent sites on the receptor and it appeared that GA was not competed by transferring. Yong Yang and colleagues19 proved that GA not only banded to transmembrane protein TfR, but also permeated the cell membrane and distributed in the cell matrix. Apart from the “extrinsic” pathway, they hypothesized that the intrinsic mitochondrial pathway for the activation of caspases might also be involved in GA-induced apoptosis. In our research, GA combined with MNPs-Fe3O4 induced apoptosis not only through influencing the regulatory factors in the intrinsic mitochondrial pathway such as bax and bcl-2, but also through regulating the transcription factor NF-κB involved in the extrinsic death receptor signaling pathway in apoptosis. We hypothesized that both pathways might be involved in apoptosis induced by the combination of MNPs-Fe3O4 with GA. Besides, in our study, a significant change was observed in apoptosis of K562 cells after GA combined with MNPs-Fe3O4, which obviously surpassed the effects of GA alone. We supposed that in addition to binding to TfR and stimulating a unique signal of rapid apoptosis, GA loaded on MNPs-Fe3O4 also permeated the cell membrane through binding to the transferring-binding site of TfR and the endocytosis, which were potential routes into cells for MNPs-Fe3O4. Our assumption should be proven by future research.
In conclusion, our study demonstrates for the first time that MNPs-Fe3O4 can promote apoptosis induction of GA in vitro in leukemic cells, and the synergistic effect of that composite on apoptosis induction may owe to the regulation of various proliferative and antiapoptotic gene products, including caspase-3, bax, bcl-2, NF-κB, and survivin. Thus, it may be possible that a combination of MNPs-Fe3O4 and GA may be a sufficient and less toxic method in leukemia therapy.
Acknowledgments
This work was supported by 863 Project of People’s Republic of China (No. 2007AA0222007) National Nature Science Foundation of People’s Republic of China (No. 30740062, 30872970) and Special-purpose Science Research Foundation for High School (No. 20070286042). The authors report no conflicts of interest in this work.
References
- 1.Min LW. New therapeutic aspects of flavones: The nticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Pandey MK, Sung B, Ahn KS, et al. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-κB signaling pathway. Blood. 2007;110(10):3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panthong A, Norkaew P, Kanjanapothi D, Taesotikul T, Anantachoke N, Reutrakul V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook. J Ethnopharmacol. 2007;111(2):335–340. doi: 10.1016/j.jep.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull. 2004;27(7):998–1003. doi: 10.1248/bpb.27.998. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Guo QL, You QD, Zhao L, Hong Y, Yuan ST. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J Gastroenterol. 2005;11(24):3655–3659. doi: 10.3748/wjg.v11.i24.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacol Sin. 2004;25(6):769–774. [PubMed] [Google Scholar]
- 7.Wu ZQ, Guo QL, You QD, et al. Growth inhibitory effect of GGAs on experimental tumor in mice and human tumor cell cultured in vitro. Chin J Nat Med. 2003;1(2):99–102. [Google Scholar]
- 8.Yu J, Guo QL, You QD, et al. Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis. 2007;28(3):632–638. doi: 10.1093/carcin/bgl168. [DOI] [PubMed] [Google Scholar]
- 9.Tisdale EJ, Slobodov I, Theodorakis EA. Unif ied synthesis of caged Garcinia natural products based on a siteselective Claisen/Diels-Alder/Claisen rearrangement. Proc Natl Acad Sci U S A. 2004;101(33):12030–12035. doi: 10.1073/pnas.0401932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasibhatla S, Jessen KA, Maliartchouk S, et al. A role for transferrin receptor intriggering apoptosis when targeted with gambogic acid. Proc Natl Acad Sci U S A. 2005;102(34):12095–12100. doi: 10.1073/pnas.0406731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo QL, Lin SS, You QD, et al. Inhibition of human telomerase reverse transcriptase gene expression by gambogic acid in human hepatoma SMMC-7721 cells. Life Sci. 2006;78(11):1238–1245. doi: 10.1016/j.lfs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Wu ZQ, Guo QL, You QD, Zhao L, Gu HY. Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cells. Biol Pharm Bull. 2004;27(11):1769–1774. doi: 10.1248/bpb.27.1769. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HZ, Kasibhatla S, Wang Y, et al. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg Med Chem. 2004;12(2):309–317. doi: 10.1016/j.bmc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Wang TT, Wei J, Qian XP, Ding YT, Yu LX, Liu BR. Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Lett. 2008;262(2):214–222. doi: 10.1016/j.canlet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Guo QL, You QD, et al. Repression of telomerase reverse transcriptase mRNA and hTERT promoter by gambogic acid in human gastric carcinoma cells. Cancer Chemother Pharmacol. 2006;58(4):434–443. doi: 10.1007/s00280-005-0177-2. [DOI] [PubMed] [Google Scholar]
- 16.Guo QL, Wu ZQ, You QD, et al. Effect of gambogic acid on the hemopoietic and immune functions in experimental animals. Chin J Nat Med. 2003;1(4):229–232. [Google Scholar]
- 17.Gilles KK, Joseph I. A nanoparticle-based immobilization assay for prion-kinetics study. J Nanobiotechnology. 2006;4:8. doi: 10.1186/1477-3155-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin BL, Shen XD, Cui S. Application of nanosized Fe3O4 in anticancer drug carriers with target-orientation and sustained-release properties. Biomed Mater. 2007;2(2):132–134. doi: 10.1088/1748-6041/2/2/011. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Yang L, You QD, et al. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007;256(2):259–266. doi: 10.1016/j.canlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Kerr JF, Winterford CM, Harmon BV. Apoptosis:its significance in cancer and cancer therapy. Cancer. 1994;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7(6):541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Chen BA, Sun Q, Wang XM, et al. Reveral in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/AO2 leukemic cells. Int J Nanomedicine. 2008;3(2):277–286. doi: 10.2147/ijn.s2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang RY, Wang XM, Wu CH, et al. Synergistic enhancement effect of magnetic nanoparticles on anticancer drug accumulation in cancer cells. Nanotechnology. 2006;17(14):3622–3626. doi: 10.1088/0957-4484/17/14/043. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q, Chen BA, Wang XM, et al. Preparation of Fe3O4-magnetic nanoparticles loaded with adriamycin and its reversal of multidrug resistance in vitro. J Exper Hematol. 2007;15(4):748–751. [PubMed] [Google Scholar]
- 25.Wang JQ, Chen BA, Cheng J, et al. Comparison of reversal effects of 5-bromotetrandrine and tetrandrine on P-glycoprotein-dependent resistance to adriamycin in human leukemia cell line K562/A02. Ai Zheng. 2008;27(5):491–495. [PubMed] [Google Scholar]
- 26.Chen BA, Dai YY, Wang XM, et al. Synergistic effect of the combination of nanoparticulate Fe3O4 and Au with daunomycin on K562/A02 cells. Int J Nanomedicine. 2008;3(3):343–350. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen BA, Cheng J, Wu YN, et al. Reversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5-bromotetrandrine in xenograft nude-mice. Int J Nanomedicine. 2009;4(1):73–78. doi: 10.2147/ijn.s5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen BA, Cheng J, Shen MF, et al. Magnetic nanoparticle of Fe3O4 and 5-bromotetrandrin interact synergistically to induce apoptosis by daunorubicin in leukemia cells. Int J Nanomedicine. 2009;4(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6(11):1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 30.Yacobi K, Wojtowicz A, Tsafriri A, Gross A. Gonadotropins enhance caspase-3 and -7 activity and apoptosis in the theca-interstitial cells of rat preovulatory follicles in culture. Endocrinology. 2004;145(4):1943–1951. doi: 10.1210/en.2003-1395. [DOI] [PubMed] [Google Scholar]
- 31.Yin XL, Zhou JB, Jie CF, Xing DM, Zhang Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004;75(18):2233–2244. doi: 10.1016/j.lfs.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Takai N, Ueda T, Nishida M, Nasu K, Miyakawa The relationship between oncogene expression and clinical outcome in endometrial carcinoma. Curr Cancer Drug Targets. 2004;4(6):511–520. doi: 10.2174/1568009043332871. [DOI] [PubMed] [Google Scholar]
- 33.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22(53):8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Zhang GM, Feng ZH. Down-regulation of survivin expression reversed multidrug resistance in adriamycin- resistant HL-60/ADR cell line. Acta Pharmacol Sin. 2003;24(12):1235–1240. [PubMed] [Google Scholar]
- 35.Ling X, Bernacki RJ, Brattain MG, Li FZ. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol mediated G2/M arrest. J Biol Chem. 2004;279(15):15196–15203. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- 36.Uchida H, Tanaka T, Sasaki K, et al. Adenovirus-mediated transfer of siRNA against survivin induced apoptosis and attenuated tumor cell growth in vitro and in vivo. Mol Ther. 2004;10(1):162–171. doi: 10.1016/j.ymthe.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Tu SP, Cui JT, Liston P, et al. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of survivin Cys84Ala mutant. Gastroenterology. 2005;128(2):361–375. doi: 10.1053/j.gastro.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 38.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3(1):46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 39.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 40.Mehta U, Kang BP, Bansal G, Bansal MP. Studies of apoptosis and bcl-2 in experimental atherosclerosis in rabbit and influence of selenium supplementation. Gen Physiol Biophys. 2002;21(1):15–29. [PubMed] [Google Scholar]
- 41.Mertens HJMM, Heineman MJ, Evers JLH. The expression of apoptosis-related proteins bcl-2 and ki67 in endometrium of ovulatory men strual cycles. Gynecol Obstet Invest. 2002;53(4):224–230. doi: 10.1159/000064569. [DOI] [PubMed] [Google Scholar]
- 42.Tilli CMLJ, Stavast-Koey AJW, Ramaekers FCS, Neumann HAM. Bax expression and growth behavior of basal cell carcinomas. J Cutan Pathol. 2002;29(2):79–87. doi: 10.1034/j.1600-0560.2002.290203.x. [DOI] [PubMed] [Google Scholar]
- 43.Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87(5):555–561. doi: 10.1038/sj.bjc.6600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:2–3. 229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Manion MK, Hockenbery DM. Targeting BCL-2-related proteins in cancer therapy. Cancer Biol Ther. 2003;2(4):S105–S114. [PubMed] [Google Scholar]
- 46.Suzuki A, Ito T, Hayashida M, et al. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene. 2000;19(10):1346–1353. doi: 10.1038/sj.onc.1203429. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal BB. Nuclear factor-kappa B: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Pandey MK, Sung B, Ahn KS. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-κB signaling pathway. Blood. 2007;110(10):3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]