Abstract
Perturbation of organellar axonal transport is increasingly recognized as an important contributor in a number of neurodegenerative diseases. Although the specificity of this impairment remains to be elucidated, growing evidence suggests that in certain disease conditions, mitochondria are affected primarily by transport defects. Many hypotheses have been formulated to explain the pathogenic mechanisms involved in amyotrophic lateral sclerosis (ALS). The mutations described so far in genetic forms of ALS (familial ALS, fALS) affect proteins involved in a wide variety of cellular mechanisms, including free radical scavenging, energy metabolism, axonal transport, RNA processing, DNA repair, vesicular transport, and angiogenesis. Here we review the current knowledge on mitochondrial transport and its role in ALS. Antioxid. Redox Signal. 11, 1615–1626.
Introduction
Neurons are polarized cells with extensive processes that connect the soma with the synaptic sites at the cell periphery. Cellular organelles, such as mitochondria and vesicles, are constantly being transported along neurites. Organelles must migrate from their sites of biogenesis in the cell body to the distal portions of axons and dendrites (anterograde transport) to provide their functions to the cell periphery. A similar transport runs in the opposite direction to move organelles out of the peripheral regions of the axon (retrograde transport). This transport is especially relevant in motor neurons, which have long axons that can extend for up to a meter to reach the farthest nerve endings. Thus, any disturbance of axonal transport may have severe consequences on neuronal function and survival. An increasing number of reports link axonal transport impairments with diseases affecting motor neurons, such as amyotrophic lateral sclerosis (ALS) (28), a devastating neurodegenerative disease affecting the upper and lower motor neurons, resulting in paralysis and premature death (3).
Mitochondria are enriched at sites of high ATP utilization and Ca2+-buffering demands, such as cell bodies, nodes of Ranvier, and synaptic terminals. Thus, alterations in mitochondrial transport can cause local energy depletion and Ca2+ imbalance and may trigger synaptic dysfunction and loss.
Both morphologic and functional mitochondrial abnormalities have been described in ALS patients and in laboratory models of the disease (43), whereas disruption of axonal transport has been described in ALS patients (88), and both fast and slow axonal transport are impaired in the ventral roots of mutant Cu,Zn-superoxide dismutase (SOD1) transgenic mice (21, 56, 106, 109, 115).
We review the contribution of mitochondrial damage in ALS, focusing on mitochondrial axonal transport abnormalities in motor neurons. We first discuss the involvement of axonal transport defects in motor neuron diseases and review the evidence supporting mitochondrial transport defects in SOD1-fALS, including unpublished observations from our laboratory. We then summarize the current knowledge of mitochondrial transport mechanisms in relation to potential mechanisms whereby mutant SOD1 could disrupt them and outline the potential consequences of this impairment on motor neuron function and survival.
Axonal Transport Defects in Neurologic Diseases
Evidence for the involvement of axonal transport defects in the pathogenesis of neuropathies comes from the identification of various pathogenic mutations in proteins involved in transport (30). One example is a mutation in the p150glued subunit of dynactin that has been described in a family with distal spinobulbar muscular atrophy, a progressive autosomal dominant lower motor neuron degenerative disease (81). Interestingly, mRNA levels of dynactin 1 are significantly reduced in a distal spinobulbar muscular atrophy mouse model (54), a decrease that also is observed in motor neurons of sporadic ALS patients (50). Another progressive neuropathy is caused by dominant missense mutations in the cytoplasmic dynein heavy chain 1 gene (Loa and Cra1 mutant mice) and associated with impaired axonal transport (42). Although sensory neuropathy rather than motor neuron disease may be responsible for the phenotype of these mutant mice (19, 31), intriguingly, recent studies demonstrated that Loa mice extend the survival of ALS mice (47, 56).
In another example, mutations in kinesin KIF5A cause a dominant inherited spastic paraparesis (hereditary spastic paraplegia; HSP), possibly by disrupting microtubule-dependent axonal transport of neurofilaments (68). Interestingly, mutations in the kinesin motor protein KIF1B isoform β, which transports synaptic vesicle precursors, cause axonal transport defects in one form of Charcot-Marie-Tooth (CMT) neuropathy (119). Among the various mutations that cause peripheral motor and sensory neuropathy in CMT, mutations in mitofusin 2 impair mitochondrial fusion and transport in neurons (4).
Recessive mutations in the gigaxonin gene cause giant axonal neuropathy, a progressive neurodegenerative disorder associated with abnormal accumulations of intermediate filaments in a variety of cell types. Gigaxonin promotes the proteasome-dependent degradation of microtubule-associated proteins, including MAP1B, MAP8, and tubulin cofactor B (111), and therefore influences microtubule stability. Mutations in the small heat-shock proteins Hsp22 and Hsp27 lead to the formation of aggregates that can interfere with axonal transport and cause lower motor neuron degeneration in hereditary motor neuropathy type 2 and distal hereditary neuropathy/CMT2F, respectively (2, 32, 48).
Taken together, these observations strongly support the view that perturbation and impairment of axonal transport is a common pathogenic cause of neuronal degeneration in a wide variety of motor neuron diseases, including ALS.
Mitochondrial Morphologic Abnormalities in ALS
Mitochondria with abnormal morphology, which includes fragmented network, swelling, and increased cristae, have been observed in the soma and proximal axons of motor neurons in the anterior horns of patients with sporadic ALS (sALS) (89). Among the pathologic features observed in the neuronal processes and soma of motor neurons of mice carrying the G93A (23) and the G37R SOD1 mutations (110) are membrane vacuoles derived from degenerating mitochondria. In G93A mice, the onset of the disease is immediately preceded by a rapid increase in degenerating mitochondria, with little motor neuron death (7, 52, 58). Interestingly, these abnormal mitochondria first appear distally, at the neuromuscular junction (NMJ) (39). Thus, mitochondrial alterations may represent a triggering factor for distal axonal degeneration and denervation, in both ALS patients and animal models (34).
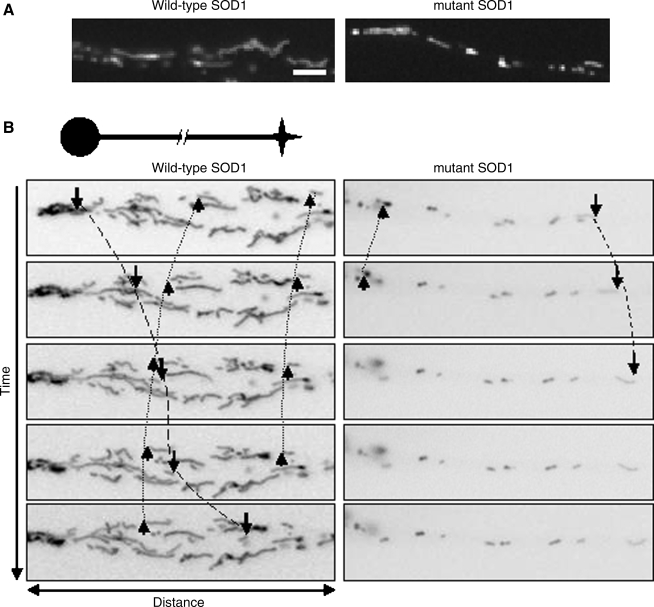
Morphologic alterations of mitochondria were observed in a well-characterized motor neuron–like cell model (NSC34 cells) (13) expressing G93A SOD1 (71, 84). In undifferentiated NSC34 cells, these abnormalities were studied in the cell soma. They affected mitochondria, but not other organelles, and were present in NSC34 cells expressing only mutant but not wild-type (WT) SOD1 (84). To analyze mitochondrial morphology, NSC34 cells stably expressing WT or SOD1 mutants can also be induced to differentiate, as shown in the example in Fig. 1A. Preliminary unpublished data from our laboratory suggest that mutant, but not WT, SOD1s induce mitochondrial fragmentation along neurites. These changes, which must be further characterized, could be the consequence of altered mitochondrial dynamics, such as transport, fusion, and fission mechanisms, which regulate number, distribution, size, and shape of mitochondria (76).
FIG. 1.
Morphology and transport abnormalities in SOD1-fALS. A well-characterized motor neuron–like cell model (NSC34 cells) is used to study the effects of mutant SOD1 on mitochondrial dynamics. (A) In this example, neurites in wild-type neurons contain mostly tubular mitochondria, whereas in mutant SOD1, mitochondria appear fragmented. Scale bar, 2.5 μm. (B) With live-imaging microscopy, movements of mitochondria can be followed. Mitochondria move anterogradely (arrows), retrogradely (arrowheads), or remain stationary. In this example, note the reduction in mobile mitochondria along mutant SOD1 neurites.
Mitochondrial Function and Axonal Transport in ALS
Early deficits in the metabolic activity of mitochondria in fALS-SOD1 mice have been described and therefore support a direct role of mitochondria dysfunction in motor neuron degeneration and ALS pathogenesis (69). Mitochondrial dysfunction, such as altered Ca2+ homeostasis (12), decrease in mitochondrial respiration and ATP synthesis (9, 10, 37, 52, 57, 70, 71), alteration of mitochondria-related gene expression (114), and increase in reactive oxygen species (40, 86), have been reported both in in vitro and in vivo models of ALS. We have found that, in G93A SOD1 transgenic mice, a significant decrease in mitochondrial Ca2+ capacity in brain and spinal cord is observed early on in the course of the disease (24). Because one of the main functions of mitochondria is buffering Ca2+ released into the cytosol of excitable cells (8) and motor neurons have a particularly low Ca2+ buffering capacity (100), altered mitochondrial Ca2+ capacity may play a pathogenic role in lower motor neurons. Indeed, Ca2+-mediated glutamate excitotoxicity has been proposed as one of the potential mechanisms for the motor neuron–selective death in ALS (87). Moreover, mutant SOD1 has been shown to increase the formation of damaging hydroxyl radicals (108, 113) and peroxynitrite derivatives (5), which caused inhibition of the mitochondrial electron-transport chain (82, 117). Intracellular free radical species are known to affect mitochondrial proteins and DNA and to inhibit the activities of specific mitochondria enzymes (83).
It has been shown that mitochondria and lysosomes accumulate in proximal axons of the anterior horn neurons in ALS patients (88), suggesting s block of axonal trafficking into proximal neurons. Moreover, the presence of metabolically dysfunctional mitochondria could also be a consequence of poor recycling or degradation of abnormal mitochondria due to impaired axonal transport. In support of this view, decreased retrograde transport was described in G93A SOD1 mice at an early stage of disease, coincident with NMJ degeneration and muscle weakness (62).
To study mitochondrial transport in SOD1 mutant motor neurons, we induced differentiation in NSC34 cells stably expressing WT or mutant SOD1, and analyzed mitochondrial axonal transport by live imaging. The results indicate that both anterograde and retrograde mitochondrial transport are affected in mutant SOD1, but not in WT-expressing cells (Fig. 1B). Recently, De Vos and colleagues (27) reported impaired organelle transport in mutant SOD1-expressing neurons. Both anterograde and retrograde transport were altered for membrane-bound organelles, which are vesicles transporting a variety of proteins along the axons to the synapses, whereas only anterograde transport was affected for mitochondria. These data would suggest that mutant SOD1 targets mitochondrial anterograde transport machinery or its regulatory signaling mechanisms. Alternatively, mutant SOD1 may decrease mitochondrial membrane potential (12), which has been shown to determine direction of movement (72); thus, mitochondria with low membrane potential are sent mainly to the soma for recycling or degradation.
These findings strongly support the rationale for a more in-depth investigation of the pathogenic and functional significance of mitochondrial axonal transport impairment, not only in the context of isolated motor neurons, but also at a more complex organism level.
Mitochondrial Dynamics in Neurons
Mitochondria must be positioned strategically at neuronal sites where the metabolic demand is high, such as active growth cones, nodes of Ranvier, and synapses (17, 61). In this section, we briefly review the current knowledge of the mechanisms of mitochondrial dynamics, because each step of its complex regulation could be a pathogenic target in ALS, as outlined later.
In isolated hippocampal neurons, at any given time, ∼10–30% of mitochondria are subject to fast axonal transport, whereas the remaining neurons are temporarily arrested (63). Mitochondria are transported along cell processes with variable speed, as their movement is “saltatory”, which means that they stop and go in response to physiologic events (17) and intracellular signaling (14). Neuronal mitochondrial distribution is regulated by local regulation of the stopping events of fast transport in response to changes in the local Ca2+ gradient (105, 112). The direction of mitochondrial transport has been proposed to correlate with their bioenergetic state: mitochondria with normal membrane potential tend to move toward the periphery (anterograde movement), whereas loss of membrane potential results in increased retrograde transport (72).
Mitochondrial Anterograde and Retrograde Axonal Transport
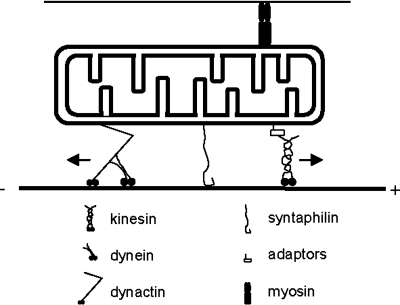
Long-distance, fast transport of mitochondria requires microtubules tracks organized with their plus ends directed toward the nerve terminals. In addition, cargo transport requires kinesin motors for anterograde transport and dyneins for retrograde transport (46). For short-range, slow movements in areas devoid of microtubules mitochondria can also use myosin motors acting along actin axes (64) (Fig. 2).
FIG. 2.
Molecular transport machinery involved in mitochondrial transport. Kinesins and dynein/dynactin complex are responsible for the anterograde and retrograde transport of mitochondria, respectively. Kinesins bind to mitochondria through specific adaptors, and dynein, through dynactin. Syntaphilin docks mitochondria to the microtubules and immobilizes them. The polarity of the microtubule (plus and minus end) is indicated. Myosin, which binds to actin filaments, also can interact with mitochondria and modulate transport.
Mitochondria are associated with members of the kinesin-1 superfamily. At least three mitochondria-binding kinesins have been identified: KIF1B (77), KIF5B (99), and KLC3 (118). The genetic ablation of kinesin heavy-chain KIF5B results in clustering of mitochondria and lysosomes in the cell body of mouse neurons (99). Kinesin-1 mutations in Drosophila motor neurons inhibits both anterograde and retrograde transport, and results in large axonal swellings filled with accumulated organelles, possibly including damaged mitochondria targeted for autophagy (80). This suggests a functional interdependence between kinesin-1 and dynein (65).
The activity of kinesins and their binding to mitochondria is dynamic and depends on the degree of phosphorylation of specific kinesin residues localized mainly at the kinesin light chain (KLC) (25, 26, 60). Kinesin-1 is phosphorylated by glycogen synthase kinase 3b (GSK3β) (74), which inhibits its activity. Furthermore, GSK3β phosphorylates KLC, which leads to release of kinesin-1 from their cargos (25). The phosphorylation of GSK3β is controlled by Akt, which inhibits GSK3β by phosphorylation (51).
The specificity of mitochondrial transport depends on the correct combination of motors and linkers, and two recently discovered cargo adaptor proteins (Miro-1 and Milton) are implicated in the specific linkage of mitochondrial to kinesin-1 (36, 38). The current view is that the mitochondrial rho-like GTPase Miro-1, which resides on the external side of the outer membrane, anchored by a carboxy-terminal transmembrane domain (41), binds to the mitochondria-specific adaptor protein Milton, which in turn is linked to the kinesin-1 heavy chain (38). Interestingly, Miro-1 possesses GTPase and Ca2+ binding domains, and mutations in these domains have been shown to cause a dominant-negative phenotype characterized by aggregation of mitochondria in the perinuclear region and increased apoptosis in cultured cells (36). Therefore, Miro-1 has the potential for being an important regulator of mitochondrial motility in neurons, working as a sensor of local concentrations of Ca2+. It was recently demonstrated that Ca2+ induces Miro-1 directly to interact with the motor domain of kinesin-1, preventing kinesin attachment to the microtubules (105).
Syntaphilin (SNPH) is an outer-membrane mitochondrial protein that can bind to microtubules and controls docking of mitochondria along the axons and near the synapses (53). Alteration of mitochondria positioning along neurites, as observed when deleting syntaphilin, caused local defects in Ca2+ homeostasis and synaptic dysfunction (53).
Mitochondrial Fusion and Fission
Mitochondria form a highly dynamic and interconnected network that undergoes continuous remodeling through rounds of organelle fusion and fission (15).
Mitochondrial fusion requires the coordinated coalescence of both the outer membrane (OM) and inner membrane (IM). In mammals, OM fusion is mediated by the GTPases mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) (18), whereas IM fusion is mediated by the dynamin-related GTPase OPA1, which is an intermembrane-space protein associated with the IM (20). Loss of Mfn1, Mfn2, and OPA1 results in fragmented mitochondria (18, 20) and embryonic lethality (18).
Fis1 regulates mitochondrial fission in mammalian cells and dynamin-related protein 1 (Drp1). Fis1 is an OM protein that is thought to recruit Drp1-discrete sites on the mitochondrial membrane (93). Drp1 is a GTPase that couples the energy liberated from GTP hydrolysis to mitochondrial membrane constriction (91). Silencing of Drp1 in cells results in a loss of mitochondrial fission, causing excessive tubulation and hyperelongated mitochondria (6).
Mitochondrial fusion and fission have been proposed to be implicated in several neurodegenerative diseases, such as Charcot-Marie-Tooth and autosomal dominant optic atrophy, which are associated with mutations in some of the proteins described earlier [reviewed in (35)]. Although deregulation of fusion and fission has not yet been studied in ALS, the presence of fragmented, smaller mitochondria in ALS motor neurons (Fig. 1) (27) predicts that fusion and fission mechanisms may be affected.
Methods for Studying Mitochondrial Dynamics in Neurons
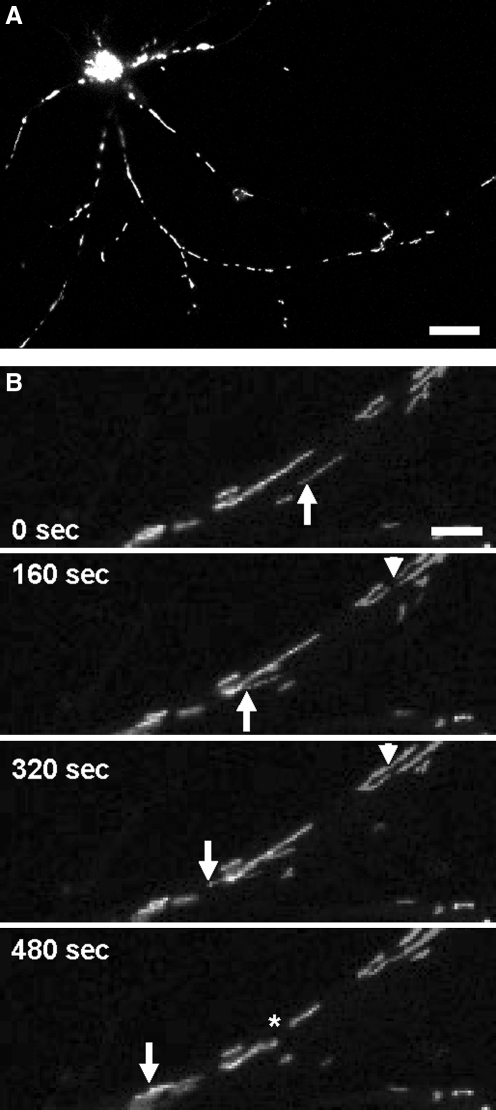
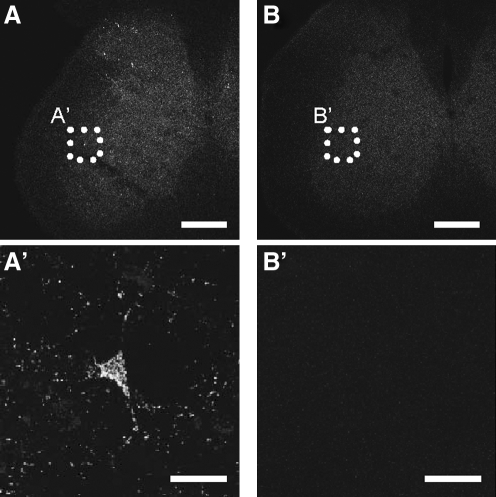
The discovery of fluorescent dyes that accumulate inside mitochondria and the use of fluorescent proteins targeted to mitochondrial compartments (Fig. 3A), together with the development of live imaging microscopy techniques, has greatly improved our knowledge of mitochondria dynamics (Fig. 3B). The generation of a transgenic mouse with fluorescent mitochondria coupled with the use of emerging in vivo imaging techniques allows a “dynamic” approach (i.e., to track mitochondria over prolonged periods, ranging from minutes to hours). A neuron-specific inducible mitoYFP mouse was recently described (16), in which transgene expression is driven by a tetracycline-regulated forebrain-specific calcium/calmodulin-dependent kinase II (CaMKII) promoter (Fig. 4). In another recent work (73), a mitoCFP mouse was generated and used to track mitochondrial movement in the peripheral nerves with time-lapse photography. These mice expressing mitochondrial fluorescent proteins that do not interfere with mitochondrial function, such as mitoCFP, can be crossed with disease models to investigate mitochondrial dynamics ex vivo, in cultured neurons, and in vivo.
FIG. 3.
Targeted expression of fluorescent proteins to mitochondria in vitro. (A) Cortical neuron transfected with mitoGFP. Fluorescent mitochondria fill the neuronal soma and neurites. Scale bar, 25 μm. (B) Live-imaging microscopy allows the study of mitochondrial dynamics. Moving mitochondria (arrow), fusion events (arrowhead), and fission events (asterisk) can be followed up over time in culture. Scale bar, 2.5 μm.
FIG. 4.
Targeted expression of fluorescent proteins to mitochondria in vivo. Expression of mitochondrial fluorescent proteins in transgenic animals allows to investigate mitochondrial dynamics either ex vivo (cultured dissociated neurons), or in vivo. (A) Spinal cord section from a mitoYFP transgenic mouse presents some labeled motor neurons. (A′) Higher magnification of a motor neuron cell body and processes filled with fluorescently labeled mitochondria. (B and B′) Spinal cord section from a non-transgenic littermate control mouse. Scale bar, 250 μm in A and B, 25 μm in A′ and B′.
Potential Mechanisms of Mutant SOD1 Interference with Mitochondrial Transport
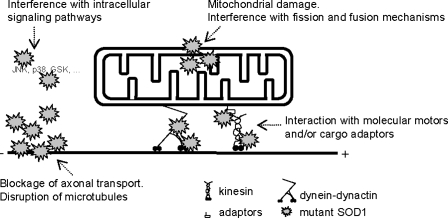
The work by De Vos and colleagues (27) and our unpublished data indicate that mitochondrial axonal transport is impaired in mutant SOD1 neurons. Mutant SOD1 can potentially affect mitochondrial transport by multiple, nonexclusive mechanisms, resulting in dysfunctional mitochondria that can no longer reach the cellular sites where they are most needed. First, abnormal accumulation of mutant SOD1 around or inside mitochondria could trigger mitochondrial damage and metabolic dysfunction. Second, aggregates of mutant SOD1 with other abundant axonal proteins, such as neurofilaments, could physically block axonal transport or disrupt the cytoskeleton, or both, thereby impairing mitochondria ability to move normally. Mutant SOD1 could also interact with molecular motors or cargo adaptors or both involved in mitochondrial axonal transport. Finally, mutant SOD1 could interfere with cell-signaling pathways that regulate cytoskeleton stability and motor activity (Fig. 5).
FIG. 5.
Mutant SOD1 can perturb mitochondrial dynamics at multiple levels: (a) SOD1 accumulates inside mitochondria and induces mitochondrial damage and metabolic dysfunction. Mutant SOD1 accumulation in the outer membrane and the intermembrane space can interfere with the fission and fusion machinery; (b) mutant SOD1 can also interact with the anterograde and retrograde transport machinery; (c) intracellular signaling pathways that control mitochondrial transport can also be disrupted by SOD1; and (d) mutant SOD1 aggregates can act as physical blocks to axonal transport.
As described earlier, ample evidence indicates that mutant SOD1 affects normal mitochondria function (69). However, the molecular mechanisms underlying the mitochondrial damage remain to be identified. One possibility involves aberrant interactions of mutant SOD1 with mitochondrial proteins, resulting in disruption of their normal folding or import (29, 67). Thus, it was reported that mutant SOD1 interacts with proteins that may affect mitochondria directly or indirectly, including Hsps (78), members of the Bcl-2 family (22, 79), and components of the protein translation machinery (55, 59). We and others demonstrated that in yeast (96, 107), rats (78), and in transgenic mice expressing WT or mutant human SOD1 (44, 49, 67, 70, 79, 104), a substantial amount of SOD1, estimated at between 1 and 2% of total SOD1, is localized in various mitochondria compartments. It has been suggested that mutant SOD1 preferentially accumulates within mitochondria of neuronal tissues (67, 79, 104). The accumulation of mutant SOD1 occurred before the appearance of mitochondria vacuolization, which suggests that the leakage or translocation of mutant SOD1 into mitochondria may be the primary event triggering their further degeneration (49). SOD1 mutants associate with mitochondria isolated from spinal cord and motor neuronal cells to a much greater extent than WT SOD1 or endogenous SOD1 (33, 66), suggesting that this accumulation may represent a common toxic property of various SOD1 mutants.
In NSC-34 cells, SOD1 mutants localized into mitochondria and caused mitochondrial impairment, even when a relatively low level of mutant protein was expressed (33, 71). Mitochondrial localization of SOD1 was essential for mutant SOD1-mediated neurotoxicity in another motor neuron–like cell model of fALS, but no effects were observed when SOD1 mutants were targeted to nuclei or the endoplasmic reticulum (98). Our unpublished data from NSC34 neuronal cells stably overexpressing either cytosol-targeted or mitochondria-targeted SOD1 (WT, G93A, and G85R) suggest that SOD1 targeted to mitochondria is sufficient to cause cell toxicity similar to that of untargeted SOD1, under a variety of stress conditions (I. Hervias and G. Manfredi, unpublished observations). Furthermore, overexpression of human copper chaperone for SOD1 (CCS) in G93A SOD1 mice highly enriched the amount of mutant SOD1 within mitochondria and caused a remarkably accelerated disease phenotype accompanied by very early mitochondrial abnormalities (92). Because of the effects of mutant SOD1, mitochondria may become bad cargos for the transport machinery and deliver a reduced ATP supply to molecular motors, resulting in abnormal mitochondrial dynamics. However, a lack of ATP locally available to the motors would not be sufficient to explain the apparently selective impairment of anterograde transport in ALS motor neuron axons (27). Therefore, it is likely that factors other than bioenergetic impairment contribute to mitochondrial motility defects.
Aberrant SOD1 aggregation affects cytoskeleton integrity and may also hinder mitochondrial transport. Indeed, neurofilament proteins inclusions appear in the axons of motor neurons (115). Abnormal activation of p38 or cdk5/p35 kinases or both by mutant SOD1 can phosphorylate neurofilaments and impair their transport. Axonal transport was impaired in the ventral roots of G93A mice coincidental with the appearance of neurofilament inclusions and vacuoles in the proximal axons and soma of motor neurons (115). Perturbations of normal transport can affect mitochondria distribution along neurites and around synapses, which are highly dependent on mitochondria to maintain their structure and function.
It is likely that SOD1 also interacts with components of the mitochondrial transport machinery and that these aberrant interactions lead to dysfunctional mitochondrial dynamics. Topologically, all of the proteins involved in mitochondrial transport and dynamics hold the potential to interact with mutant SOD1, because we have demonstrated that SOD1 resides both on the cytosolic side of the outer membrane and in the intermembrane space (104).
Kinesin-1, the principal motor for mitochondrial anterograde transport, is an interesting candidate for interaction with mutant SOD1, because it has been shown to play an important role in retrograde movement of organelles (80), although mitochondrial transport was not investigated specifically in this study. In particular, the heavy-chain kinesin family-5B (KIF5B) has been implicated in mitochondrial and membrane-bound organelle transport in axons (45). Therefore, aberrant SOD1-kinesin-1 interactions may underlie both anterograde and retrograde transport defects in motor neurons in a nonmitochondria-specific manner. However, mutant SOD1 interactions with specific mitochondrial cargo adaptors would point toward a specific alteration of transport. For example, interactions of SOD1 with either Milton or Miro-1, two known mitochondrial cargo adaptors, would have an impact on mitochondrial docking to kinesin heavy chain.
The only mutant SOD1 interaction with the axonal transport machinery demonstrated so far is with the dynein complex, the motor responsible for retrograde transport (116). These aberrant interactions between dynein and several SOD1 mutants, but not WT SOD1, which were also found in ALS transgenic mice, add one more piece to the puzzle and suggest that the axonal transport machinery is a target of SOD1 toxicity (95).
Our finding of shorter, hyperfragmented mitochondria in the processes of mutant SOD1 neurons (Fig. 1) could be the result of either defective transport, because mitochondria cannot establish proper contacts with transport-based machinery, or impairment of the fusion/fission machinery. It has been shown that SOD1 aggregates with Bcl-2 in spinal cord mitochondria (79), and that members of the Bcl-2 family control mitochondrial fusion (90). Moreover, mutant SOD1 interacts with the dynein complex, and dynein may also mediate the recruitment of the fission machinery to mitochondria (102).
Another possibility is that SOD1 may target or activate signaling pathways that affect anterograde or retrograde motors, mitochondrial transport, and cytoskeleton stability. Inflammatory signals from neighboring glial cells (such as TNF-α) can activate p38 stress-activated protein kinase in mutant SOD1 transgenic mice (1, 85). Furthermore, glutamate levels are increased in mutant SOD1 mice (11) and can activate JNK, p38, and cdk5/p35 kinases (1). Activated p38 not only can phosphorylate and inhibit kinesin-1 activity (26), but also can phosphorylate neurofilaments (1). Activated JNK can phosphorylate and damage kinesin-1 (75), and cdk5/p35 can phosphorylate neurofilaments (97) and promote neurofilament accumulation, a hallmark of ALS pathology.
Mitochondrial Dynamics Impairment Causes Distal Axonopathy in Motor Neurons
Although the mechanisms leading to impaired transport in SOD1-fALS are still unclear, one of the potential consequences of transport defects is the failure of mitochondria originating in the cell body to reach the synaptic terminals and of damaged mitochondria in the periphery to reach the cell body for degradation. In line with this hypothesis, abnormal accumulations of mitochondria are found in the somas and proximal axons of motor neurons in both sALS and fALS (88, 89) and at the presynaptic end of the NMJ in G93A mice (39, 101).
If healthy mitochondria do not reach the synaptic terminals, energy starvation, disruption of Ca2+ homeostasis, and degeneration are likely consequences occurring at these cellular sites. As outlined earlier, mitochondria and synapses are functionally linked: defective synaptic transmission is associated with a loss of mitochondria from axon terminals (94, 103). Reciprocally, synaptic activity modulates the dynamics and distribution of mitochondria in dendrites (61).
A recent study in Drosophila showed NMJ degeneration and muscle atrophy occurring when mitochondria accumulate in the cell body because of mutations that inactivate the mitochondrial cargo adaptor Miro-1 (41). In ALS, the presence of abnormal, vacuolated, mitochondria at the NMJ of mutant SOD1 mice, early in the course of the disease, correlates with the beginning of denervation (39) and suggests that defective maintenance of mitochondria in the periphery of the neuron may be a primary pathogenic event. This view is consistent with the notion that ALS is a dying-back type of neuropathy that initiates and progresses from the distal to the proximal portions of the motor neurons (34) and causes paralysis even in the absence of cell body degeneration in the spinal cord (39).
Another potential effect of impaired mitochondrial transport is the loss of fusion and fission activity, because both of these processes depend on mitochondrial motility. Thus, defective transport could determine not only mislocalization of mitochondria along the axon and the synaptic terminals, but also an alteration of the normal balance between fusion and fission, which is crucial for maintaining healthy mitochondria (15).
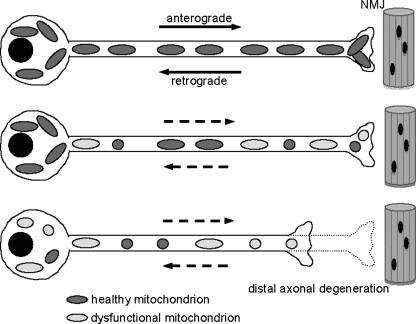
The effects of SOD1 mutations on mitochondrial transport must be further investigated, but the data described so far suggest the following model, schematized in Fig. 6. Defects in anterograde transport preclude the arrival of healthy mitochondria to distal regions of the axon (27), whereas impaired retrograde transport of aged and dysfunctional mitochondria fails to move them toward the cell body, either to fuse with upcoming healthy mitochondria or to be degraded by autophagy (116). The ultimate consequence of this scenario is the accumulation of damaged mitochondria in motor neuron terminals.
FIG. 6.
Model of mitochondrial dynamics impairment in SOD1-fALS. Mutant SOD1 causes impairment of mitochondrial dynamics, by affecting fusion and fission and transport. In ALS motor neurons, mitochondria become smaller and dysfunctional, and therefore, ATP supply is reduced, and Ca2+ buffering impaired at synapses. As a consequence, synapses are lost, and a dying-back process in the axon is initiated, which leads to a progressive distal axonopathy. NMJ, neuromuscular junction.
Concluding Remarks
As outlined earlier, numerous lines of evidence suggest that mitochondria are affected in the course of motor neuron degeneration and that mitochondrial dysfunction may actively participate to the demise of motor neurons. Impaired mitochondrial energy production can have potentially catastrophic consequences, especially in neurons with long processes that rely heavily on axonal transport. Mitochondrial dysfunction may also have consequences that go well beyond the energy defect, involving mishandling of Ca2+ and the activation of apoptotic pathways. Emerging evidence indicates that mitochondrial dysfunction correlates with impaired mitochondrial dynamics and suggests that failure to position healthy mitochondria in critical sites of energy utilization may play a significant role in the pathogenesis of motor neuron diseases. Therefore, a better comprehension of the molecular basis of mitochondrial dynamics impairment in ALS will likely have an important impact on the development of therapeutic strategies.
Acknowledgments
We thank the Robert Packard Center for ALS Research “The New York Community Trust”; NIH/NINDS (R01-NS051419 to G.M.); and the Muscular Dystrophy Association (to J.M. and G.M.).
Abbreviations
ALS, amyotrophic lateral sclerosis; ATP, adenosine triphosphate; CaMKII, calcium/calmodulin-dependent kinase II; CFP, cyan fluorescent protein; CMT, Charcot-Marie-Tooth; Drp, dynamin-related protein; fALS, familial ALS; Fis1, fission 1; GSK3β, glycogen synthase kinase; Hsp, heat-shock protein; HSP, hereditary spastic paraplegia; IM, inner membrane; JNK, c-Jun N-terminal kinase; KIF, kinesin superfamily; KLC, kinesin light chain; MAP, microtubule-associated protein; Mfn, mitofusin; NMJ, neuromuscular junction; NSC, neuroblastoma–spinal cord cell line; OM, outer membrane; OPA1, optic atrophy type 1; sALS, sporadic ALS; SNPH, syntaphilin; SOD1, Cu,Zn-superoxide dismutase; TNF, tumor necrosis factor; WT, wild-type; YFP, yellow fluorescent protein.
References
- 1.Ackerley S. Grierson AJ. Banner S. Perkinton MS. Brownlees J. Byers HL. Ward M. Thornhill P. Hussain K. Waby JS. Anderton BH. Cooper JD. Dingwall C. Leigh PN. Shaw CE. Miller CC. p38alpha stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2004;26:354–364. doi: 10.1016/j.mcn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Ackerley S. James PA. Kalli A. French S. Davies KE. Talbot K. A mutation in the small heat-shock protein HspB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet. 2006;15:347–354. doi: 10.1093/hmg/ddi452. [DOI] [PubMed] [Google Scholar]
- 3.Adams RD. Victor M. Ropper AH. Principles of neurology. New York: McGraw-Hill; 1997. [Google Scholar]
- 4.Baloh RH. Schmidt RE. Pestronk A. Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman JS. Carson M. Smith CD. Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 6.Benard G. Bellance N. James D. Parrone P. Fernandez H. Letellier T. Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 7.Bendotti C. Calvaresi N. Chiveri L. Prelle A. Moggio M. Braga M. Silani V. De Biasi S. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J Neurol Sci. 2001;191:25–33. doi: 10.1016/s0022-510x(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi K. Rimessi A. Prandini A. Szabadkai G. Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Borthwick GM. Johnson MA. Ince PG. Shaw PJ. Turnbull DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Ann Neurol. 1999;46:787–790. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Bowling AC. Schulz JB. Brown RH., Jr Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993;61:2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 11.Bruijn LI. Beal MF. Becher MW. Schulz JB. Wong PC. Price DL. Cleveland DW. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci U S A. 1997;94:7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carri MT. Ferri A. Battistoni A. Famhy L. Gabbianelli R. Poccia F. Rotilio G. Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Lett. 1997;414:365–368. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- 13.Cashman NR. Durham HD. Blusztajn JK. Oda K. Tabira T. Shaw IT. Dahrouge S. Antel JP. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- 14.Chada SR. Hollenbeck PJ. Mitochondrial movement and positioning in axons: the role of growth factor signaling. J Exp Biol. 2003;206:1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- 15.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekaran K. Hazelton JL. Wang Y. Fiskum G. Kristian T. Neuron-specific conditional expression of a mitochondrially targeted fluorescent protein in mice. J Neurosci. 2006;26:13123–13127. doi: 10.1523/JNEUROSCI.4191-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang DT. Honick AS. Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H. Detmer SA. Ewald AJ. Griffin EE. Fraser SE. Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XJ. Levedakou EN. Millen KJ. Wollmann RL. Soliven B. Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cipolat S. Martins de Brito O. Dal Zilio B. Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collard JF. Cote F. Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 22.Crosio C. Casciati A. Iaccarino C. Rotilio G. Carri MT. Bcl2a1 serves as a switch in death of motor neurons in amyotrophic lateral sclerosis. Cell Death Differ. 2006;13:2150–2153. doi: 10.1038/sj.cdd.4401943. [DOI] [PubMed] [Google Scholar]
- 23.Dal Canto MC. Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 24.Damiano M. Starkov AA. Petri S. Kipiani K. Kiaei M. Mattiazzi M. Flint Beal M. Manfredi G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- 25.De Vos K. Goossens V. Boone E. Vercammen D. Vancompernolle K. Vandenabeele P. Haegeman G. Fiers W. Grooten J. The 55-kDa tumor necrosis factor receptor induces clustering of mitochondria through its membrane-proximal region. J Biol Chem. 1998;273:9673–9680. doi: 10.1074/jbc.273.16.9673. [DOI] [PubMed] [Google Scholar]
- 26.De Vos K. Severin F. Van Herreweghe F. Vancompernolle K. Goossens V. Hyman A. Grooten J. Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J Cell Biol. 2000;149:1207–1214. doi: 10.1083/jcb.149.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vos KJ. Chapman AL. Tennant ME. Manser C. Tudor EL. Lau KF. Brownlees J. Ackerley S. Shaw PJ. McLoughlin DM. Shaw CE. Leigh PN. Miller CC. Grierson AJ. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vos KJ. Grierson AJ. Ackerley S. Miller CC. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 29.Deng HX. Shi Y. Furukawa Y. Zhai H. Fu R. Liu E. Gorrie GH. Khan MS. Hung WY. Bigio EH. Lukas T. Dal Canto MC. O'Halloran TV. Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan JE. Goldstein LS. The genetics of axonal transport and axonal transport disorders. PLoS Genet. 2006;2:e124. doi: 10.1371/journal.pgen.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupuis L. Fergani A. Braunstein KE. Eschbach J. Holl N. Rene F. Gonzalez De Aguilar JL. Zoerner B. Schwalenstocker B. Ludolph AC. Loeffler JP. Mice with a mutation in the dynein heavy chain 1 gene display sensory neuropathy but lack motor neuron disease. Exp Neurol. 2009;215:146–152. doi: 10.1016/j.expneurol.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Evgrafov OV. Mersiyanova I. Irobi J. Van Den Bosch L. Dierick I. Leung CL. Schagina O. Verpoorten N. Van Impe K. Fedotov V. Dadali E. Auer-Grumbach M. Windpassinger C. Wagner K. Mitrovic Z. Hilton-Jones D. Talbot K. Martin JJ. Vasserman N. Tverskaya S. Polyakov A. Liem RK. Gettemans J. Robberecht W. De Jonghe P. Timmerman V. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- 33.Ferri A. Cozzolino M. Crosio C. Nencini M. Casciati A. Gralla EB. Rotilio G. Valentine JS. Carri MT. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc Natl Acad Sci U S A. 2006;103:13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer LR. Culver DG. Tennant P. Davis AA. Wang M. Castellano-Sanchez A. Khan J. Polak MA. Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Frank S. Dysregulation of mitochondrial fusion and fission: an emerging concept in neurodegeneration. Acta Neuropathol. 2006;111:93–100. doi: 10.1007/s00401-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 36.Fransson S. Ruusala A. Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 37.Fujita K. Yamauchi M. Shibayama K. Ando M. Honda M. Nagata Y. Decreased cytochrome c oxidase activity but unchanged superoxide dismutase and glutathione peroxidase activities in the spinal cords of patients with amyotrophic lateral sclerosis. J Neurosci Res. 1996;45:276–281. doi: 10.1002/(SICI)1097-4547(19960801)45:3<276::AID-JNR9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Glater EE. Megeath LJ. Stowers RS. Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould TW. Buss RR. Vinsant S. Prevette D. Sun W. Knudson CM. Milligan CE. Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green DR. Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 41.Guo X. Macleod GT. Wellington A. Hu F. Panchumarthi S. Schoenfield M. Marin L. Charlton MP. Atwood HL. Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Hafezparast M. Klocke R. Ruhrberg C. Marquardt A. Ahmad-Annuar A. Bowen S. Lalli G. Witherden AS. Hummerich H. Nicholson S. Morgan PJ. Oozageer R. Priestley JV. Averill S. King VR. Ball S. Peters J. Toda T. Yamamoto A. Hiraoka Y. Augustin M. Korthaus D. Wattler S. Wabnitz P. Dickneite C. Lampel S. Boehme F. Peraus G. Popp A. Rudelius M. Schlegel J. Fuchs H. Hrabe de Angelis M. Schiavo G. Shima DT. Russ AP. Stumm G. Martin JE. Fisher EM. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 43.Hervias I. Beal MF. Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve. 2006;33:598–608. doi: 10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- 44.Higgins CM. Jung C. Ding H. Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci. 2002;22:RC215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirokawa N. Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 46.Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:d91–d102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- 47.Ilieva HS. Yamanaka K. Malkmus S. Kakinohana O. Yaksh T. Marsala M. Cleveland DW. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proc Natl Acad Sci U S A. 2008;105:12599–12604. doi: 10.1073/pnas.0805422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irobi J. Van Impe K. Seeman P. Jordanova A. Dierick I. Verpoorten N. Michalik A. De Vriendt E. Jacobs A. Van Gerwen V. Vennekens K. Mazanec R. Tournev I. Hilton-Jones D. Talbot K. Kremensky I. Van Den Bosch L. Robberecht W. Van Vandekerckhove J. Van Broeckhoven C. Gettemans J. De Jonghe P. Timmerman V. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- 49.Jaarsma D. Rognoni F. van Duijn W. Verspaget HW. Haasdijk ED. Holstege JC. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol (Berl) 2001;102:293–305. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- 50.Jiang YM. Yamamoto M. Tanaka F. Ishigaki S. Katsuno M. Adachi H. Niwa J. Doyu M. Yoshida M. Hashizume Y. Sobue G. Gene expressions specifically detected in motor neurons (dynactin 1, early growth response 3, acetyl-CoA transporter, death receptor 5, and cyclin C) differentially correlate to pathologic markers in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2007;66:617–627. doi: 10.1097/nen.0b013e318093ece3. [DOI] [PubMed] [Google Scholar]
- 51.Jope RS. Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Jung C. Higgins CMJ. Xu Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J Neurochem. 2002;83:535–545. doi: 10.1046/j.1471-4159.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 53.Kang JS. Tian JH. Pan PY. Zald P. Li C. Deng C. Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsuno M. Adachi H. Minamiyama M. Waza M. Tokui K. Banno H. Suzuki K. Onoda Y. Tanaka F. Doyu M. Sobue G. Reversible disruption of dynactin 1-mediated retrograde axonal transport in polyglutamine-induced motor neuron degeneration. J Neurosci. 2006;26:12106–12117. doi: 10.1523/JNEUROSCI.3032-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamata H. Magrane J. Kunst C. King MP. Manfredi G. Lysyl-tRNA synthetase is a target for mutant SOD1 toxicity in mitochondria. J Biol Chem. 2008;283:28321–28328. doi: 10.1074/jbc.M805599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kieran D. Hafezparast M. Bohnert S. Dick JR. Martin J. Schiavo G. Fisher EM. Greensmith L. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol. 2005;169:561–567. doi: 10.1083/jcb.200501085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirkinezos IG. Bacman SR. Hernandez D. Oca-Cossio J. Arias LJ. Perez-Pinzon MA. Bradley WG. Moraes CT. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kong J. Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunst CB. Mezey E. Brownstein MJ. Patterson D. Mutations in SOD1 associated with amyotrophic lateral sclerosis cause novel protein interactions. Nat Genet. 1997;15:91–94. doi: 10.1038/ng0197-91. [DOI] [PubMed] [Google Scholar]
- 60.Lee KD. Hollenbeck PJ. Phosphorylation of kinesin in vivo correlates with organelle association and neurite outgrowth. J Biol Chem. 1995;270:5600–5605. doi: 10.1074/jbc.270.10.5600. [DOI] [PubMed] [Google Scholar]
- 61.Li Z. Okamoto K. Hayashi Y. Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Ligon LA. LaMonte BH. Wallace KE. Weber N. Kalb RG. Holzbaur EL. Mutant superoxide dismutase disrupts cytoplasmic dynein in motor neurons. Neuroreport. 2005;16:533–536. doi: 10.1097/00001756-200504250-00002. [DOI] [PubMed] [Google Scholar]
- 63.Ligon LA. Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–350. doi: 10.1002/1096-9861(20001120)427:3<340::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 64.Ligon LA. Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 65.Ligon LA. Tokito M. Finklestein JM. Grossman FE. Holzbaur EL. A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J Biol Chem. 2004;279:19201–19208. doi: 10.1074/jbc.M313472200. [DOI] [PubMed] [Google Scholar]
- 66.Liu D. Wen J. Liu J. Li L. The roles of free radicals in amyotrophic lateral sclerosis: reactive oxygen species and elevated oxidation of protein, DNA, and membrane phospholipids. FASEB J. 1999;13:2318–2328. doi: 10.1096/fasebj.13.15.2318. [DOI] [PubMed] [Google Scholar]
- 67.Liu J. Lillo C. Jonsson PA. Velde CV. Ward CM. Miller TM. Subramaniam JR. Rothstein JD. Marklund S. Andersen PM. Brannstrom T. Gredal O. Wong PC. Williams DS. Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Lo Giudice M. Neri M. Falco M. Sturnio M. Calzolari E. Di Benedetto D. Fichera M. A missense mutation in the coiled-coil domain of the KIF5A gene and late-onset hereditary spastic paraplegia. Arch Neurol. 2006;63:284–287. doi: 10.1001/archneur.63.2.284. [DOI] [PubMed] [Google Scholar]
- 69.Manfredi G. Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5:77–87. doi: 10.1016/j.mito.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Mattiazzi M. D'Aurelio M. Gajewski CD. Martushova K. Kiaei M. Beal MF. Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 71.Menzies FM. Cookson MR. Taylor RW. Turnbull DM. Chrzanowska-Lightowlers ZM. Dong L. Figlewicz DA. Shaw PJ. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain. 2002;125:1522–1533. doi: 10.1093/brain/awf167. [DOI] [PubMed] [Google Scholar]
- 72.Miller KE. Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 73.Misgeld T. Kerschensteiner M. Bareyre FM. Burgess RW. Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nat Methods. 2007;4:559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- 74.Morfini G. Pigino G. Beffert U. Busciglio J. Brady ST. Fast axonal transport misregulation and Alzheimer's disease. Neuromol Med. 2002;2:89–99. doi: 10.1385/NMM:2:2:089. [DOI] [PubMed] [Google Scholar]
- 75.Morfini G. Pigino G. Szebenyi G. You Y. Pollema S. Brady ST. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- 76.Mozdy AD. Shaw JM. A fuzzy mitochondrial fusion apparatus comes into focus. Nat Rev Mol Cell Biol. 2003;4:468–478. doi: 10.1038/nrm1125. [DOI] [PubMed] [Google Scholar]
- 77.Nangaku M. Sato-Yoshitake R. Okada Y. Noda Y. Takemura R. Yamazaki H. Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 78.Okado-Matsumoto A. Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 79.Pasinelli P. Belford ME. Lennon N. Bacskai BJ. Hyman BT. Trotti D. Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Pilling AD. Horiuchi D. Lively CM. Saxton WM. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puls I. Jonnakuty C. LaMonte BH. Holzbaur EL. Tokito M. Mann E. Floeter MK. Bidus K. Drayna D. Oh SJ. Brown RH., Jr Ludlow CL. Fischbeck KH. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 82.Radi R. Rodriguez M. Castro L. Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 83.Radi R. Rubbo H. Bush K. Freeman BA. Xanthine oxidase binding to glycosaminoglycans: kinetics and superoxide dismutase interactions of immobilized xanthine oxidase-heparin complexes. Arch Biochem Biophys. 1997;339:125–135. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 84.Raimondi A. Mangolini A. Rizzardini M. Tartari S. Massari S. Bendotti C. Francolini M. Borgese N. Cantoni L. Pietrini G. Cell culture models to investigate the selective vulnerability of motoneuronal mitochondria to familial ALS-linked G93ASOD1. Eur J Neurosci. 2006;24:387–399. doi: 10.1111/j.1460-9568.2006.04922.x. [DOI] [PubMed] [Google Scholar]
- 85.Raoul C. Estevez AG. Nishimune H. Cleveland DW. deLapeyriere O. Henderson CE. Haase G. Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas: potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 86.Rizzardini M. Mangolini A. Lupi M. Ubezio P. Bendotti C. Cantoni L. Low levels of ALS-linked Cu/Zn superoxide dismutase increase the production of reactive oxygen species and cause mitochondrial damage and death in motor neuron-like cells. J Neurol Sci. 2005;232:95–103. doi: 10.1016/j.jns.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Rothstein JD. Excitotoxicity hypothesis. Neurology. 1996;47:S19–25; discussion S26. doi: 10.1212/wnl.47.4_suppl_2.19s. [DOI] [PubMed] [Google Scholar]
- 88.Sasaki S. Iwata M. Impairment of fast axonal transport in the proximal axons of anterior horn neurons in amyotrophic lateral sclerosis. Neurology. 1996;47:535–540. doi: 10.1212/wnl.47.2.535. [DOI] [PubMed] [Google Scholar]
- 89.Sasaki S. Iwata M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2007;66:10–16. doi: 10.1097/nen.0b013e31802c396b. [DOI] [PubMed] [Google Scholar]
- 90.Sheridan C. Delivani P. Cullen SP. Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome c release. Mol Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 91.Smirnova E. Griparic L. Shurland DL. van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Son M. Puttaparthi K. Kawamata H. Rajendran B. Boyer PJ. Manfredi G. Elliott JL. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci U S A. 2007;104:6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stojanovski D. Koutsopoulos OS. Okamoto K. Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- 94.Stowers RS. Megeath LJ. Gorska-Andrzejak J. Meinertzhagen IA. Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 95.Strom AL. Gal J. Shi P. Kasarskis EJ. Hayward LJ. Zhu H. Retrograde axonal transport and motor neuron disease. J Neurochem. 2008;106:495–505. doi: 10.1111/j.1471-4159.2008.05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sturtz LA. Diekert K. Jensen LT. Lill R. Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, Ccs, localize to the intermembrane space of mitochondria: a physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 97.Sun D. Leung CL. Liem RK. Phosphorylation of the high molecular weight neurofilament protein (NF-H) by Cdk5 and p35. J Biol Chem. 1996;271:14245–14251. doi: 10.1074/jbc.271.24.14245. [DOI] [PubMed] [Google Scholar]
- 98.Takeuchi H. Kobayashi Y. Ishigaki S. Doyu M. Sobue G. Mitochondrial localization of mutant superoxide dismutase 1 triggers caspase-dependent cell death in a cellular model of familial amyotrophic lateral sclerosis. J Biol Chem. 2002;277:50966–50972. doi: 10.1074/jbc.M209356200. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka Y. Kanai Y. Okada Y. Nonaka S. Takeda S. Harada A. Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 100.Van Den Bosch L. Van Damme P. Bogaert E. Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Vande Velde C. Garcia ML. Yin X. Trapp BD. Cleveland DW. The neuroprotective factor Wlds does not attenuate mutant SOD1-mediated motor neuron disease. Neuromol Med. 2004;5:193–203. doi: 10.1385/NMM:5:3:193. [DOI] [PubMed] [Google Scholar]
- 102.Varadi A. Johnson-Cadwell LI. Cirulli V. Yoon Y. Allan VJ. Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 103.Verstreken P. Ly CV. Venken KJ. Koh TW. Zhou Y. Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Vijayvergiya C. Beal MF. Buck J. Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X. Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Warita H. Itoyama Y. Abe K. Selective impairment of fast anterograde axonal transport in the peripheral nerves of asymptomatic transgenic mice with a G93A mutant SOD1 gene. Brain Res. 1999;819:120–131. doi: 10.1016/s0006-8993(98)01351-1. [DOI] [PubMed] [Google Scholar]
- 107.Weisiger RA. Fridovich I. Superoxide dismutase: organelle specificity. J Biol Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- 108.Wiedau-Pazos M. Goto JJ. Rabizadeh S. Gralla EB. Roe JA. Lee MK. Valentine JS. Bredesen DE. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996;271:515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- 109.Williamson TL. Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 110.Wong PC. Pardo CA. Borchelt DR. Lee MK. Copeland NG. Jenkins NA. Sisodia SS. Cleveland DW. Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 111.Yang Y. Allen E. Ding J. Wang W. Giant axonal neuropathy. Cell Mol Life Sci. 2007;64:601–609. doi: 10.1007/s00018-007-6396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yi M. Weaver D. Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yim HS. Kang JH. Chock PB. Stadtman ER. Yim MB. A familial amyotrophic lateral sclerosis-associated A4V Cu, Zn-superoxide dismutase mutant has a lower Km for hydrogen peroxide: correlation between clinical severity and the Km value. J Biol Chem. 1997;272:8861–8863. doi: 10.1074/jbc.272.14.8861. [DOI] [PubMed] [Google Scholar]
- 114.Yoshihara T. Ishigaki S. Yamamoto M. Liang Y. Niwa J. Takeuchi H. Doyu M. Sobue G. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 2002;80:158–167. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhang B. Tu P. Abtahian F. Trojanowski JQ. Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang F. Strom AL. Fukada K. Lee S. Hayward LJ. Zhu H. Interaction between familial ALS-linked SOD1 mutants and the dynein complex: implications of retrograde axonal transport in ALS. J Biol Chem. 2007;282:16691–16699. doi: 10.1074/jbc.M609743200. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y. Marcillat O. Giulivi C. Ernster L. Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]
- 118.Zhang Y. Oko R. van der Hoorn FA. Rat kinesin light chain 3 associates with spermatid mitochondria. Dev Biol. 2004;275:23–33. doi: 10.1016/j.ydbio.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao C. Takita J. Tanaka Y. Setou M. Nakagawa T. Takeda S. Yang HW. Terada S. Nakata T. Takei Y. Saito M. Tsuji S. Hayashi Y. Hirokawa N. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]