Abstract
Cerebral hypoxia-ischemia results in unique patterns of injury during development owing to selective vulnerability of specific cell populations including subplate neurons. To evaluate the contribution of glutamate excitotoxicity, we studied enriched cultures of subplate neurons in comparison with cortical neurons, deriving expression profiles for glutamate receptor subunits by microarray and immunoblot. The excitotoxic potency of specific glutamate receptors was tested with selective agonists and antagonists. After one week in culture, subplate neurons are more sensitive to oxygen glucose deprivation than cortical neurons, confirming in vivo observations. Subplate and cortical neurons are equally sensitive to glutamate and insensitive to NMDA. Subplate neurons are more sensitive than cortical neurons to AMPA and express two-fold less GluR2. Subplate neurons express significantly more mGluR3, a receptor proposed to be protective. Despite this increased expression, group II mGluR agonists increase subplate neuron death and antagonists lessen glutamate excitotoxicity, suggesting a novel mechanism for subplate vulnerability.
Keywords: subplate neuron, glutamate excitotoxicity, development, cell death
Introduction
Excessive glutamate receptor activation leading to excitotoxicity is a paradigm for neural cell death resulting from energy failure in a variety of pathologic conditions including stroke and birth asphyxia(Choi and Rothman, 1990; Johnston, 2001; Rothman and Olney, 1986). Classic studies using animal models of adult stroke or mature neuronal cultures elucidated the central role of calcium entry through the N-methyl-D-aspartate (NMDA) subtype of ionotropic glutamate receptor initiating signaling cascades that result in cell death(Rothman and Olney, 1995). Despite clear and convincing evidence for this mechanism in experimental models, clinical neuroprotective trials with NMDA receptor antagonists have uniformly failed(Hoyte et al., 2004). Factors identified as contributing to these failures are myriad and include issues of trial design and translation of preclinical data(Gladstone et al., 2002). Synthesis of this data has indicated that a uniform model of NMDA overactivation is overly simplistic(Besancon et al., 2008). Recent studies have determined that excitotoxic susceptibility is cell type specific(Jiang et al., 2004; Ullian et al., 2004) and related to many factors including the exact expression profile of glutamate receptor subunits(Deng et al., 2006; Liu et al., 2007; von Engelhardt et al., 2007) and key postsynaptic signaling proteins(Forder and Tymianski, 2008).
Formulating effective treatments for the developing brain brings added complexity in that during the development of neural circuits, neurotransmission differs in many ways from the mature state. Axonal and dendritic arbors are sparse(Antonini and Stryker, 1993). Glutamatergic synapses contain a high proportion of ‘silent’ AMPA receptors and entrain unique plasticity mechanisms (Feldman et al., 1999). The typically inhibitory neurotransmitter GABA has a depolarizing effect(Ben-Ari, 2002). Neurotransmission during development affects distinct cellular processes including proliferation, migration and maturation(Komuro and Rakic, 1998; Represa and Ben-Ari, 2005). In the immature brain, regions of vulnerability change (Ferriero, 2004), along with glutamate receptor subunit expression(Monyer et al., 1994; Talos et al., 2006a; Talos et al., 2006b). Therefore, a comprehensive, cell type specific investigation of the expression and function of glutamate receptor subunits is an essential starting point for understanding mechanisms of developmental selective vulnerability.
Subplate neurons in particular are an ideal population to study developmental selective vulnerability. They are the only neocortical lamina-specific neuronal population that can be isolated into enriched, homogenous cultures(DeFreitas et al., 2001). Subplate neurons are a transient cell type, essential for many aspects of thalamocortical development (reviewed in(Allendoerfer and Shatz, 1994; McQuillen and Ferriero, 2005)). Subplate neurons are among the first cortical neurons to mature and express glutamate receptors(Catalano et al., 1997) and they receive the earliest functional synaptic connections(Friauf et al., 1990; Hanganu et al., 2002). Subplate neurons can be eliminated by injections of the glutamate agonist kainic acid(Chun and Shatz, 1988) and are among cells selectively vulnerable to very early hypoxia-ischemia(McQuillen et al., 2003). To investigate excitotoxic mechanisms of subplate neuron selective vulnerability, we studied immunoenriched cultures of subplate neurons in comparison with other cortical plate neurons after one week in defined culture conditions, a time-point intermediate between insensitivity and maximal sensitivity of cortical neurons to glutamate(Frandsen and Schousboe, 1990).
Experimental Methods
All reagents are purchased from Sigma (St. Louis, MO) unless otherwise noted.
Subplate neuron purification and culture
To increase yield and efficiency, we adapted our existing immunopanning method(DeFreitas et al., 2001) to use Magnetic-activated Cell Separation (MACS). All MACS materials and reagents are purchased from Miltenyi Biotech (Auburn, CA), and include: the MACS Neural Tissue Dissociation kit, MACS multistand, OctoMACS separation magnet, separation columns, 30 µm pre-separation filters, and rat anti-mouse IgG1 microbeads. Monoclonal mouse anti-p75NTR antibody, 192, (Chandler et al., 1984) is the generous gift of Dr. Eric Shooter (Stanford University, CA). Brains from embryonic day 17 (day of breeding = E0) Long Evans rats (Simonsen) are dissected on ice in Hank’s Balanced Saline Solution (HBSS) (Gibco). Meninges are removed and dorsolateral caudal neocortex is digested following the MACS Neural Tissue Dissociation Kit protocol. Dissociated cells are centrifuged through a 15%/60% Percoll (Pharmacia Biotech) step gradient for 5 min at 400 × g and cells are collected at the 15%/60% Percoll interface. After a 45 min recovery in HBSS with 10% fetal bovine serum (FBS) at 37C, cell separation is done using the MACS protocol for purification of positively-labeled cells. Cell counting is done using a Hausser BrightLine hemacytometer following their instructions. Starting cell number is typically between 1–4 × 108 cells depending upon the number of embryos harvested. Cells are resuspended at a concentration of 1 × 108 cells/mL with 40 µg/mL of anti-p75NTR monoclonal, 192, in MACS buffer (HBSS with 1% bovine serum albumin (BSA) and 1% FBS), and are incubated at 4C for 15 minutes. Following a 5-minute centrifuge wash and discarding the supernatant, cells are resuspended in MACS buffer at a concentration of 2.5 × 108 cells/mL with 250 µL/mL of microbeads added prior to incubation at 4C for 30 minutes. A maximum of 2 × 108 cells (1 ml of the cell suspension) are run through a MACS filter and into each MACS column and washed with MACS buffer. The negative elution contains unbound cortical plate cells, whereas the column contains the bound, positively-labeled subplate neurons. Resultant cells are washed and cultured at 37C in Neurobasal medium (Gibco) with B-27 additives (Gibco) and Penicillin/Streptomycin on tissue culture plastic (either 12- or 96-well plates) coated with 1 mg/ml poly-L-ornithine and 15 mg/ml fibronectin (Becton Dickinson) in CMF-PBS.
Immunopanning using antibodies to p75NTR yields cultures of subplate neurons enriched to approximately 90% purity based upon bromodeoxyuridine (BrdU) birthdating and immunophenotyping(DeFreitas et al., 2001). To confirm that MACS-purified subplate neurons produce equivalent cultures, we analyzed MACS purified cultures using similar methods. Subplate neurons were identified by their early birthdates (embryonic day, E10.5–12.5(Bayer and Altman, 1990)) in comparison with upper layer cortical neurons which are generated at later dates (E13.5 – P0.5). BrdU (50 mg/kg) was administered to the dam with a single intraperitoneal injection at E12.5. Quantification of BrdU positive cells was performed using the FITC BrdU Flow kit (BD Pharmingen) per manufacturers instructions. Briefly, subplate and cortical neurons were isolated as described above from time pregnant dams receiving BrdU injections at E12.5. The cells were fixed with paraformaldehyde, permeabilized with saponin and treated with DNase to expose BrdU prior to staining with FITC-conjugated anti-BrdU antibody. Stained cells were washed and resuspended in staining buffer for analysis by flow cytometry. Flow cytometry was performed on an LSRII and quantified using FACSDiva software (BD). Data were analyzed using FlowJo software (TreeStar). A single pulse of BrdU at E12.5 labels 26+/−10% (N=3) of MACS-purified subplate neurons, which is similar to that reported in the original description using immunopanning (33%)(DeFreitas et al., 2001). BrdU at E12.5 also labels a similar number of cortical neurons (13+/−2%, N=3 vs. 10%)(DeFreitas et al., 2001). Immunophenotyping MACS-purified subplate neuron cultures shows a similar enrichment for cells reacting with antibodies to the neuron-specific intermediate filament Tau to that reported for immunopanning (88+/−3%, N=3 vs. 90%)(DeFreitas et al., 2001). MACS-purified subplate neuron cultures contain relatively few cells reacting with antibodies to Nestin, a marker enriched in progenitor cells, compared with cortical cultures (7+/−3% vs. 33%).
Serum-free cell culture, Oxygen-glucose Deprivation and Survival assays
Subplate and cortical plate neurons were cultured in 96-well dishes coated with poly-L-ornithine and fibronectin in Neurobasal medium containing B-27 additives with rhBDNF (Peprotech) and forskolin. On DIV1 forskolin is removed, and the antimitotic agents uridine and fluoro-deoxy-uridine (FDU) are added to inhibit growth of non-neuronal cells. On DIV4 Neurobasal medium is changed to remove BDNF, uridine, and FDU. On DIV7 cells undergoing oxygen-glucose deprivation are transferred into Neurobasal media without glucose and B27-additive without antioxidants. Cells are placed in an airtight chamber under 95 % nitrogen/5% CO2 at 37C for 7 hours. At the end of the oxygen-glucose deprivation period, glucose-containing Neurobasal medium is replaced and the cells are returned to the incubator. 24 hours later survival is measured using the Live/Dead Assay (Molecular Probes) in conjunction with the Lactate Dehydrogenase (LDH) Assay (Roche). Each experiment is done in duplicate and repeated at least three times and results are reported as average ± SEM.
Immunocytochemistry
Neuronal cultures grow in 96-well plates are fixed by applying a 95% methanol/5% acetic acid solution and incubating at −20C for 15 minutes. Cultures that are plated on Nunc LabTek II system chamber slides for synaptic and neurite staining are fixed by applying a 4% paraformaldehyde/4% sucrose PBS solution and incubating at 4C for 12 minutes, followed by application of 100% methanol at −20C for 10 minutes. They are washed with 0.1 M phosphate buffered saline (PBS), then exposed to blocking solution (PBS with 10% fetal bovine serum, 10% gelatin, and 0.1% Triton X-100) for 1hr at room temperature. Primary antibody is diluted in blocking solution and applied for one hour at the following dilutions: mouse anti-Nestin at 1:100 (Abcam), rabbit anti-Tau at 1:200 (Sigma), mouse anti-GFAP at 1:200 (Sigma), mouse anti-PSD-95 at 1:200 (Affinity Bioreagents), rabbit anti-GluR2 at 1:100 (Epitomics), rabbit anti-MAP2 at 1:200 (Millipore), and mouse anti-phosphorylated neurofilaments, SMI 31at 1:200 (Covance). Following primary incubation we incubate our cultures with Alexa-488 Goat anti-Mouse (Invitrogen) and Alexa-555 Goat anti-Rabbit (Invitrogen) at a concentration of 1:200 in serum-containing PBS at room temperature for 1hr covered in aluminum foil. Counterstained nuclei are visualized with TOPRO-3 (Molecular Probes). Each culture is immersed in 100 µL of Vectashield mounting medium (Vector Laboratories) prior to fluorescent imaging on a Nikon inverted fluorescent microscope or a Zeiss Axio Imager upright fluorescent microscope. Cell counting based on morphological staining is performed with Metamorph v. 7.0 on a PC.
mRNA isolation and Affymetrix microarray analysis
Immunopanning was performed as described(DeFreitas et al., 2001) to produce three independent replicates for isolation and analysis of mRNA. Unbound cells following immunopanning with anti-p75NTR antibody represent a mixed population containing approximately 30% neurons based upon expression of intermediate filaments(DeFreitas et al., 2001). To produce a pure neuronal population for comparison with p75NTR immunopurified subplate neurons, cortical neurons within the unbound cell population were isolated by a subsequent immunopanning step using an antibody to the axonal glycoprotein TAG-1 (3.1C12, Developmental Studies Hybridoma Bank), which is expressed by a subset of neocortical projection neurons(Furley et al., 1990). Yields of a pure neuronal population were confirmed by expression of the intermediate filament and neuronal marker Tau. Multiple immunopanning preparations were pooled to produce at least 5 million cells. Individual cell preparations were frozen at −80C in RNAlater (Ambion) and pooled (N=3–12 samples per replicate) to produce three independent biologic replicates. Cells were thawed in Trizol (Invitrogen, Carlsbad CA) and RNA was isolated using RNeasy Mini columns (Qiagen, Germantown MD). RNA, at least 5 µg per replicate, was amplified with one round of cDNA synthesis and in vitro transcription to produce biotin-labeled cRNA according to the manufacturers protocols (Affymetrix, Santa Clara CA). RNA quality was assessed on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA) before and after amplification and fragmentation. Hybridization of the labeled cRNA to the Rat Genome U34A array, washing and scanning of the chip were performed at the University of California, San Francisco affiliated J. David Gladstone Genomics Core according to manufacturers protocols (Affymetrix).
Microarray Data Analysis
Images of probe hybridization intensity on the scanned chips were quantified and scaled by Affymetrix Microarray Suite 5.0 software, using default settings per the manufacturers protocols. The software uses normalized intensities from the 11 probe pairs per sequence to report a detection confidence score (Present, Absent or Marginal) and a signal intensity value representing expression level. Data was subsequently analyzed in Microsoft Excel to calculate gene expression fold-change between cell types.
Agonist / antagonist excitotoxicity
Subplate and cortical plate neurons were cultured as described in the previous section. On DIV7 cells are transferred into Neurobasal media containing a range of concentrations from different agonists, including l-Glutamic Acid (glutamate, Sigma) from 0 to 80µM, NMDA (Tocris) from 0 to 1000µM, or (RS)-AMPA (Tocris) from 0 to 1000µM. Antagonists used to pretreat cells for 10 minutes prior to experiments include NBQX (Tocris) at 30µM, DL-AP5 (Tocris) at 200µM, LY341495 (Tocris) at 200nM and β-NAAG (Sigma) at 100µM. The mGluR group II agonist DCG-IV (Tocris) is also used in conjunction with AMPA experiments at 100µM to pretreat cells. Glutamate vesicle release is blocked by incubating the cells in 30 nM Tetanus toxin (EMD Biosciences) for 3 hours prior to glutamate agonist application. Cells are incubated with the agonists for one hour at 37C. At the end of the incubation, glucose-containing Neurobasal medium is replaced and the cells are returned to the incubator. 24 hours later survival is measured as described below.
Quantification of cell death in vitro
Digital images of live (cystein-AM) or dead (ethidium) stained cells in 96 well plates are acquired with a Nikon fluorescent microscope. Three separate images are acquired per well. Live cells and dead cells are identified using morphometry and intensity thresholding, along with the Count Nuclei application from Metamorph software. Total cell number is derived from the sum of live and dead cells, and percent live is expressed as a fraction of total (Live/(Live + Dead)). Background cell death is determined in control plates and subtracted from experimental plates. To further verify results, culture supernatants are harvested from the same plates and analyzed for lactate dehydrogenase (LDH) activity with a commercially available assay kit (Roche). The total cellular LDH activity from live cells is determined by freezing the cells in −80C for 30 min, followed by thawing them in a 37C incubator and collecting the resulting supernatants. LDH activities are determined by colorimetry as per the LDH assay kit’s instructions. Percent live from LDH activity was calculated as follows: LDH percent live = 1 − {Dead/(Dead+Live)} where Dead is the LDH activity of culture supernatants prior to freezing and Live is the cellular LDH activity resulting from supernatants after freeze-thawing. For all data, LDH results show the same trend as Live/Dead results, and are thus not reported.
Immunoblotting and densitometry
5ubplate and cortical plate neurons were cultured as described. On DIV7, proteins were extracted and harvested with appropriate volume of cell lysis buffer (1X PBS, 1% NP-40, 0.5% Sodium deoxycholate, 0.2% SDS, 1X Complete Mini Protease Cocktail [Roche]). Extracts were spun at 19,000 × g for 20 minutes at 4C, and the supernatant containing the proteins was collected. Protein content was estimated with a BCA protein assay kit (Pierce, Rockford, IL) using a BSA-based standard curve. Equal amounts of protein were separated by SDS-PAGE and transferred to a Polyvinylidene Difluoride (PVDF) membrane using rapid semidry immunoblotting techniques (Biorad, Hercules, CA). PVDF blots (Bio Rad) were blocked using 5% nonfat milk in 1X TBS with 0.05% Tween 20 (TBST) for one hour at RT, then washed 3 times for 10 minutes each in TBST. Blots were then incubated in TBST containing 5% bovine serum albumin (BSA) with appropriate dilution of primary antibody overnight at 4°C, including Rabbit anti-GluR1 (Upstate, product #07-660) at 1:1000, Mouse anti-GluR2 (Chemicon, product #MAB397) at 1:1000, Mouse anti-NR1 (BD Biosciences, product #556308) at 1:1000, Rabbit anti-NR2A monoclonal (Upstate, product #05-901) at 1:500, Mouse anti-NR2B (BD Biosciences, product #610416) at 1:1000, and Rabbit anti-mGluR2/3 (Chemicon, product #AB1553) at 1:2000. After washing 3 times 10 minutes in TBST blots were incubated for 2 hr at RT with HRP-conjugated secondary antibody, including 0.1 µg/ml Goat anti-Rabbit IgG (CalBioChem, San Diego, CA) and 0.1 µg/ml Goat anti-Mouse (CalBioChem), accordingly. Blots were visualized using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), and captured on autoradiographic films (GE Healthcare, Buckinghamshire, UK). For loading controls, all blots were incubated with anti-β-actin (Sigma) at 0.2 µg/ml overnight at 4C, followed by incubation with HRP-conjugated goat anti-mouse as described above. Films were digitized with an Epson Perfection 4490 Photo scanner, and band optical densities (OD) were measured using ImageJ version 1.40g (NIH, USA). For each sample, we calculated the ratio of protein to β-actin values, and these numbers were used to calculate the mean ± SEM. These values were presented as arbitrary OD control values, and ratio of OD where appropriate.
Statistics
Results are summarized by mean and standard error of the mean (SEM) and shown graphically by single condition histogram or by curves indicating percent live over drug concentration. To determine the effects of multiple concentrations of glutamate agonist or antagonists, analysis of variance (ANOVA) was performed considering the separate effects of drug concentration and cell type using Bonferroni correction for multiple comparisons. When a significant difference existed between cell types, the effects at specific drug concentrations were assessed using a two-tailed Student’s T-test.
RESULTS
Immunopurified Subplate and Cortical Neurons Develop Equivalent Mature Morphology After One Week of Culture
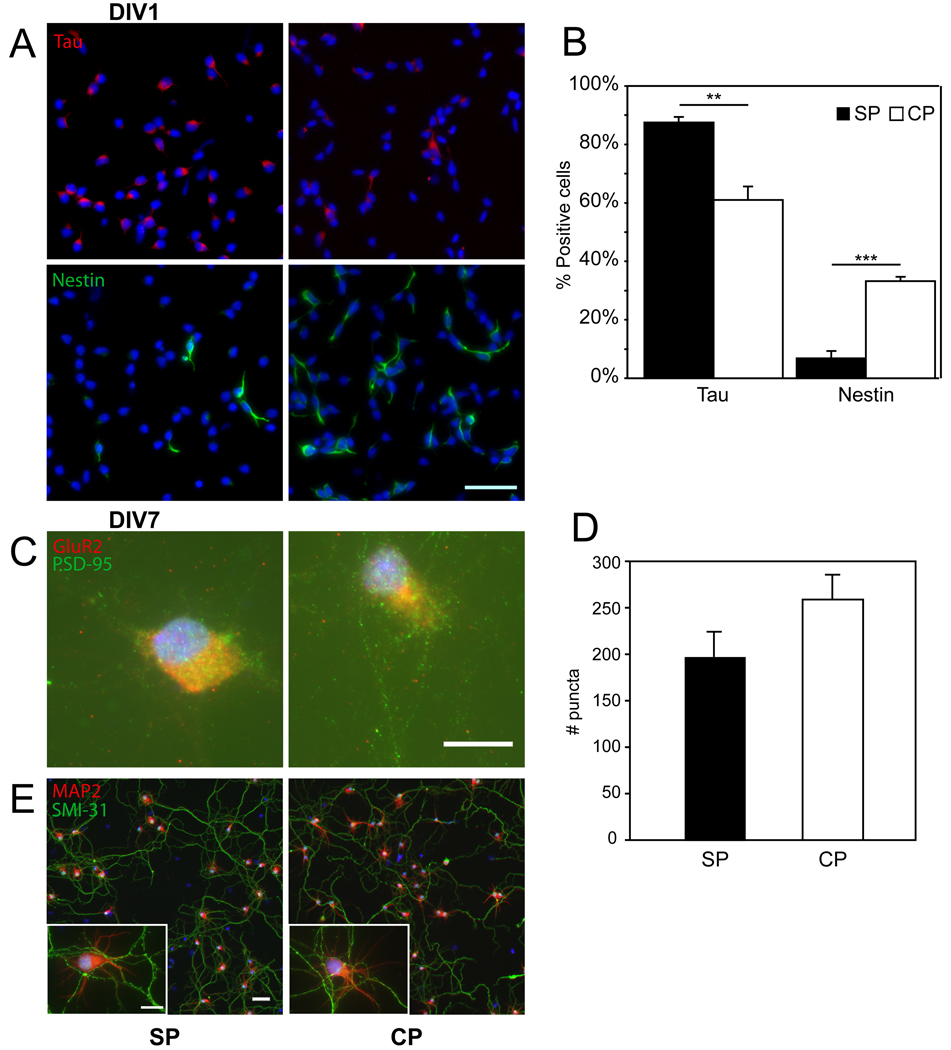
Subplate neurons can be purified into highly enriched cultures from dissociated cells prepared from posterior dorsolateral neocortex. Subplate neurons are isolated from other neocortical cells using immunoaffinity methods based upon their selective expression of the low-affinity pan-neurotrophin receptor p75NTR at early embryonic ages (DeFreitas et al., 2001). Cultures enriched for subplate neurons are almost exclusively neuronal after one day in vitro (DIV1) based upon expression of Tau, a marker enriched in mature neurons, with very few cells expressing other markers (Figure 1A, B). In contrast, cultures of unbound cortical cells are comprised of significantly less Tau-expressing neurons and more cells expressing Nestin, a marker enriched in immature neuronal progenitor cells (Figure 1A, B). Approximately 50% of subplate neurons are viable after one week in culture and supplementation with neurotrophins (BDNF or NT3) improves subplate neuron survival(DeFreitas et al., 2001). Following one week in identical defined serum-free culture conditions (Neurobasal, B27, BDNF), both cortical and subplate cultures become essentially pure neuronal cultures as assessed by abundant immunostaining for the neuronal marker Tau (mean +/− SD % Tau positive Subplate: 97+/−2%, Cortical: 96+/−1%, N=3 cultures, results not shown), with rare astrocytes based upon a paucity of cells expressing glial fibrillary acidic protein (GFAP, mean +/− SD % GFAP positive Subplate: 1+/−0.5%, Cortical: 0.4+/−0.6%, N=3 cultures, results not shown). Because the cortical cultures initially include immature progenitor cells and later-born neurons, we compared the morphological development of both cell cultures over seven days to determine if both cultures achieved similar morphological maturity. Cells in both cultures develop processes that evolve from immature forms (Banker stage 1–2 (Craig et al., 1994)) after DIV1 (Figure 1A) to mature morphology by DIV7 (Figure1E). Equivalent development of polarized axons and dendrites is demonstrated by immunostaining with specific markers (SMI 31 – axons, MAP2 – dendrites)((de Lima et al., 1997)). Neurons in both cultures exhibit mature dendritic arbors (Banker stage 5) with extensive networks of interconnected axons (Figure 1E). After DIV7, cells in both cultures have developed punctate accumulation of pre- and postsynaptic markers (Figure 1C). Quantification of postsynaptic (PSD95) puncta over the cell body reveals similar numbers in subplate and cortical cultures (Figure 1D, mean+/−SD PSD95 puncta subplate: 198+/−83 vs. cortical 259+/−84, P=0.12, N=10 cells per culture type).
Figure 1.
Immunophenotyping of subplate (SP) and cortical (CP) cells at days in vitro 1 (DIV1) and 7 (DIV7). (A) Tau (red) was used to identify neurons in DIV1 purified subplate cultures (top left) and DIV1 non-purified cortical cultures (top right). Nestin (green) was used to identify neural progenitors in DIV1 purified subplate cultures (bottom left) and DIV1 non-purified cortical cultures (bottom right). Nuclei are counterstained (blue). Scale bar, 50 µm. (B) Quantification of immunostaining from DIV1 (black bars = SP cells, white bars = CP cells, error bars = SEM; ** P < 0.01, ***P < 0.001 by Student’s T-test). N = 4 per culture type. (C) Postsynaptic PSD-95 (green) and presynaptic GluR2 (red) identify puncta in both subplate (left) and cortical plate (right) cultures. Scale bar, 10 µm. (D) Quantification of synaptic puncta from DIV7 (black bars = SP cells, white bars = CP cells, error bars = SEM) (E) Neuronal polarity is shown with dendritic staining of MAP2 (red) and axonal staining of SMI-31 (green) in DIV7 cultures. Scale bar 50 µm, insert, 10 µm.
Cultured Subplate Neurons are More Vulnerable than Cortical Plate Neurons to Oxygen Glucose Deprivation
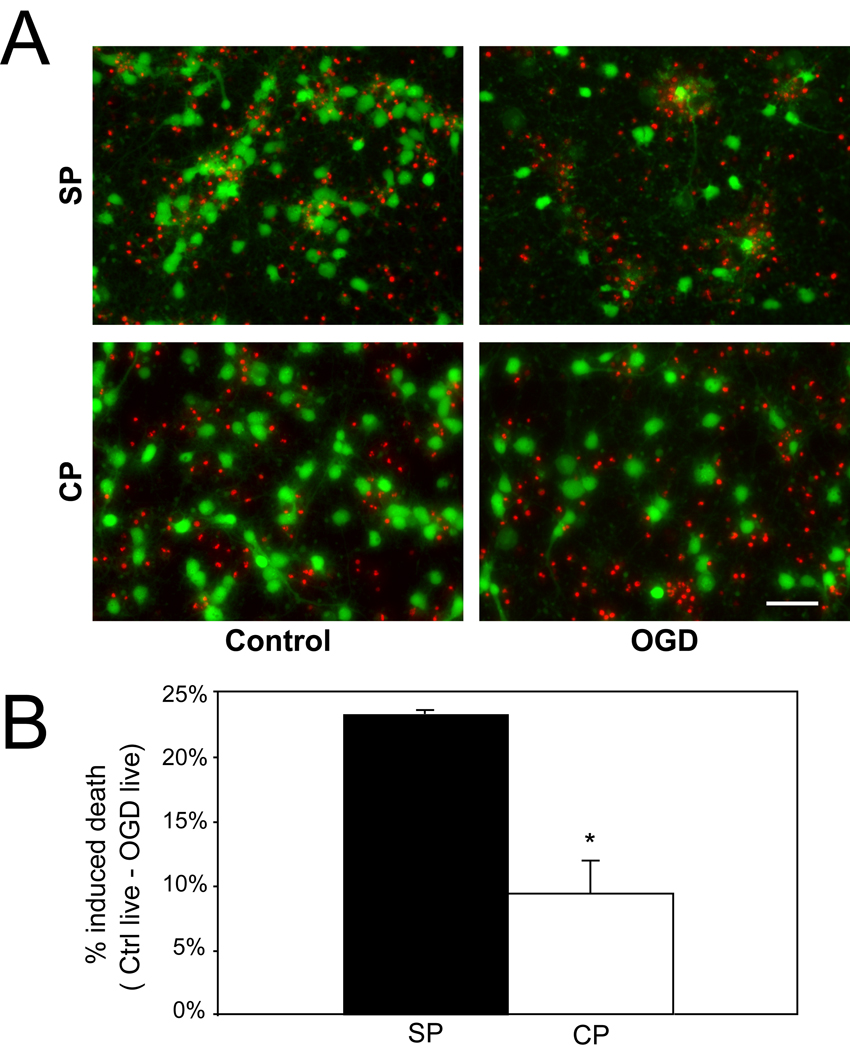
In the intact animal, subplate neurons are selectively vulnerable to hypoxia ischemia at early postnatal ages (P0.5–2.5)(McQuillen et al., 2003). To further establish equivalence of the in vitro conditions, we measured cell death in response to oxygen glucose deprivation at DIV7. Oxygen glucose deprivation is an in vitro manipulation that models hypoxia ischemia in vivo. Subplate neurons are optimally immunopurified at embryonic day 17 (E17)(DeFreitas et al., 2001). As the typical gestation of the Long-Evans rat is 21 days, DIV7 is temporally equivalent to postnatal day 2, the age at which subplate neurons are selectively vulnerable to hypoxia-ischemia in vivo(McQuillen et al., 2003). On DIV7, cultures are transferred into glucose and oxygen free media for 7 hours. Subsequently, the plates are removed and returned to basal glucose and oxygen containing media. Twenty-four hours following oxygen glucose deprivation, immunopurified subplate neurons show significantly more induced death than cortical plate neurons (mean+/−SEM subplate: 23.4 ± 0.23% vs. cortical: 9.4 ± 2.6%, P=0.002, Figure 2) confirming in vivo observations(McQuillen et al., 2003).
Figure 2.
Effect of oxygen-glucose deprivation (OGD) on subplate (SP) and cortical (CP) cultures at DIV7. (A) The fluorescent live/dead assay of calcein AM (green, live cells) and ethidium homodimer-1 (red, dead cells) shows resulting cell death due to OGD in purified subplate (top row) and cortical (bottom row) cultures, 24 hr after control (left column) or OGD (right column). (B) Quantification of percent induced death of subplate and cortical plate cells (black bar = subplate cells, white bar = cortical plate cells, error bars = SEM; * P < 0.05, Student’s T-test). Subplate N = 5, Cortical N = 6; scale bar, 50 µm.
Differences in Glutamate Receptor Subunit RNA Expression Profile Between Subplate and Cortical Plate Neurons
To explore gene expression differences between subplate and cortical neuronal populations, RNA was isolated for analysis by oligonucleotide microarray immediately after preparing the cells at E17. At this stage, unpurified dissociated cell suspensions from neocortex are composed of approximately equal fractions of neurons, glia and precursors based upon intermediate filament expression(DeFreitas et al., 2001). To obtain a pure neuronal reference population for comparison with purified subplate neurons, it was necessary to isolate neocortical neurons based upon expression of the axonal glycoprotein TAG-1(Furley et al., 1990). Three separate biologic replicates for each cell type were prepared from pooled immunopanning experiments to obtain sufficient RNA for analysis without amplification and gene expression was quantified using the Affymetrix U34A microarray. The U34A microarray contains oligonucleotide probe sets representing 22 glutamate receptor subunits (Table 1). Among these glutamate receptor subunit genes, 12 are detected, with only four genes meeting the Affymetrix confidence score of ‘present’ or ‘marginal’ in at least 2 of 3 samples. NMDAR1 and GluR1 are expressed at higher levels in subplate neurons but are among the genes detected but judged absent by Affymetrix criteria (Table 1). Differences between the cell types include lower levels of the ionotropic AMPA GluR2 subunit in subplate neurons compared with cortical neurons. Subplate neurons also express 3–4 fold higher mGluR3 expression. Subplate neurons and TAG1-purified cortical neurons express similar levels of the glutamate receptor-associated proteins PSD-95 and neuronal nitric oxide synthase (nNOS).
Table 1.
| Gene Family, subunit |
Probe Set ID |
Subplate | Subplate call |
Cortical Plate |
Cortical call |
Ratio |
|---|---|---|---|---|---|---|
| Ionotropic, NMDA |
mean±SD | mean±SD | SP/CP | |||
| R1 | S39221_g_at | 89±31 | A | 69±8 | A | 1.2 |
| R2A | D13211_s_at | |||||
| R2B | U11419_at | 136±38 | P | 148±29 | P | −1.1 |
| R2C | D13212_s_at | |||||
| R2D | D13213_s_at | |||||
| R3A | AF061945_at | |||||
| Ionotropic, non-NMDA |
||||||
| AMPA1 | M36418_s_at | 43±30 | A | 24±21 | A | 1.5 |
| AMPA2 | M36419_s_at | 55±22 | P | 117±6 | P | −2.3 |
| AMPA3 | M36420_s_at | 22±17 | A | 14±3 | A | 1.3 |
| AMPA4 | M36421_s_at | |||||
| delta1 | U08255_at | |||||
| delta2 | U08256_at | 10±4 | A | 15±7 | A | −1.0 |
| ka1 | M83561_s_at | |||||
| ka2 | Z11548_at | 21±14 | A | 23±6 | A | 1.1 |
| ka3 | AF027331_at | |||||
| ka4 | U08257_at | |||||
| ka5 | Z11581_at | 314±88 | P | 411±162 | P | −1.3 |
| Metabotropic | ||||||
| R1 | M61099_at | |||||
| R2 | M92075_at | 47±18 | A | 32±4 | A | 1.4 |
| R3 | M92076_at | 271±126 | P | 86±25 | P | 3.0 |
| R4 | M90518_at | 87±32 | A | 81±22 | A | 1.0 |
| R5 | D10891_at | |||||
| R6 | D13963_at | 19±3 | A | 19±2 | A | 1.0 |
| R7 | D16817_at | |||||
| R8 | U63288_at | |||||
| Postsynaptic Density Proteins |
||||||
| PSD-95 | U67140 | 213±117 | P | 225±61 | P | −1.1 |
| nNOS | U02534_at | 59±6 | P | 61±6 | A | −1.0 |
Missing values indicate that mRNA expression was not detected for that Probe Set.
Abbreviations: A – absent, P – present. Present indicates an Affymetrix call of present or marginal in 2/3 samples.
Subplate and Cortical Plate Neurons Are Equally Sensitive To Glutamate Excitotoxicity
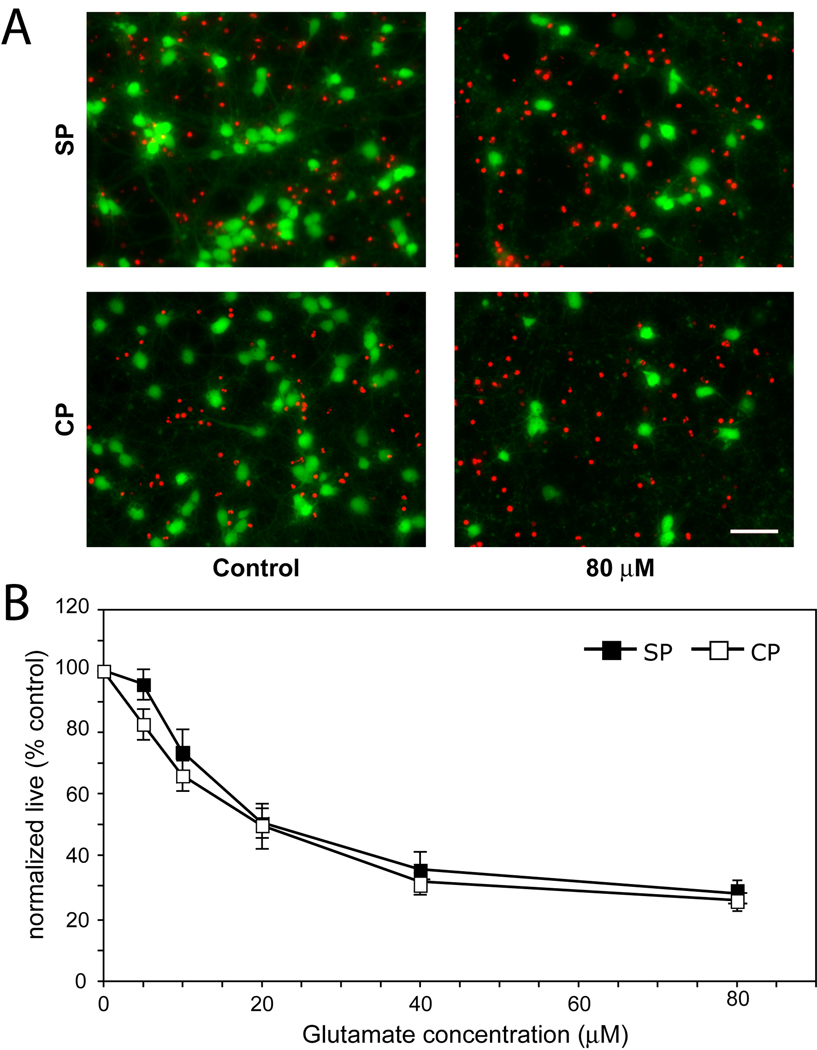
Having established that cultured subplate neurons are more vulnerable to oxygen glucose deprivation and identified differences in glutamate receptor gene expression, we sought to determine functional differences in the excitotoxic response of cortical and subplate neurons to glutamate or specific glutamate receptor agonists. Subplate and cortical neurons were isolated and cultured under identical minimal defined conditions until DIV7. On DIV7, individual subplate and cortical cultures were exposed to graded concentrations of glutamate from 5–80 µM. After one hour, glutamate was removed and the cultures were returned to basal media. Twenty-four hours later we measured the percentage of surviving cells. Cell death in response to glutamate was analyzed for effects of agonist concentration and cell type by analysis of variance (ANOVA). Glutamate application results in significant cell death (Figure 3, P<0.0001, ANOVA), with no differences observed between the cell types (P= 0.11, ANOVA). Both types of neurons are extremely sensitive to glutamate, with a significant increase in cell death starting at concentrations as low as 5 µM (P=0.02 SP, P<0.001 CP, ANOVA Bonferroni). At the highest glutamate concentration, less than 30% of subplate (mean ± SEM, 28.4 ± 4.7%) and cortical plate (26.2 ± 4.3%) cells survive (Figure 3A,B). To further characterize their excitotoxic profile, we next examined their response to specific glutamate receptor agonists.
Figure 3.
Glutamate-mediated excitotoxicity in DIV7 subplate (SP) and cortical (CP) cultures. Cultures were exposed to a range of glutamate concentrations for 1hr, then assayed for cell survival 24 hours later. (A) Fluorescent live/dead assay shows effect of the maximum glutamate concentration used (right column) on cell survival in subplate (top row) or cortical (bottom row) cells as compared to control conditions (left column). (B) Quantification of live cells after exposure to increasing glutamate concentrations (black boxes = subplate cells, white boxes = cortical cells, error bars = SEM) N = 5; scale bar, 50 µm.
Subplate Neurons Are More Vulnerable To AMPA Than Cortical Neurons and Express Less AMPA GluR2 Receptor Subunits
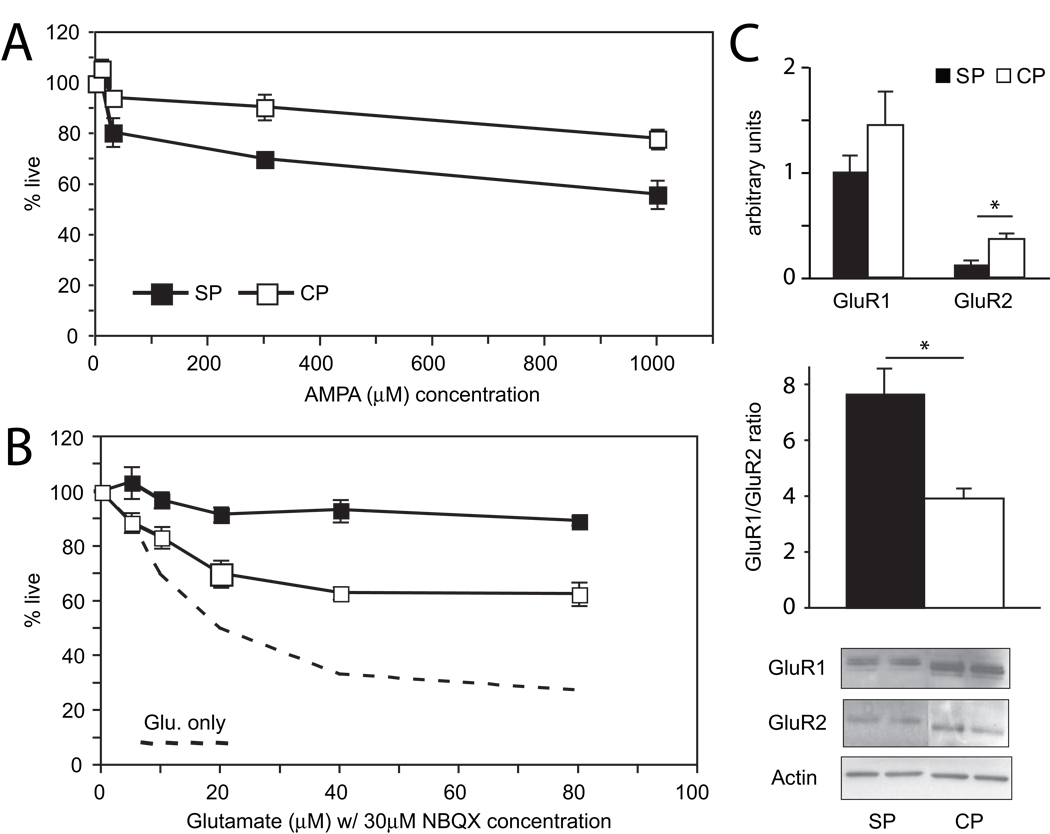
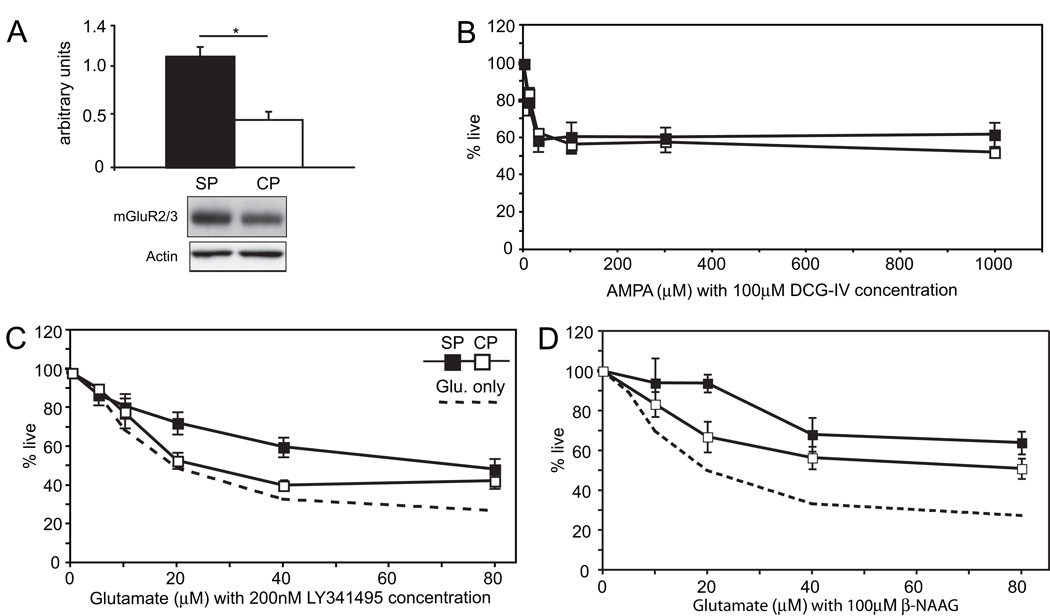
Gene expression profiles at DIV1 suggest that subplate neurons may express less of the GluR2 AMPA receptor subunit (Table 1). To explore AMPA receptor contribution to glutamate excitotoxicity, we exposed subplate and cortical plate neurons to graded concentrations of AMPA at DIV7 for one hour. Twenty-four hours later, we find significant cell death in both cell types (Figure 4A P<0.0001, ANOVA). In contrast to glutamate however, there is significant difference in cell death between the two cell types (P= 0.0007, ANOVA) with less live subplate neurons, especially at AMPA concentrations of 300 µM (70.1 ± 1.9% in subplate, 90.7 ± 5.1% in cortical plate, P = 0.005, T-test) and at 1000 µM (56.0 ± 5.6% in subplate, 78.2 ± 3.6% in cortical plate, P = 0.016, T-test).
Figure 4.
Effect of AMPA receptor agonist and antagonist, and characterization of AMPA subunits expression (GluR1 and GluR2) in DIV7 cultures. (A) Quantification of live cells after exposure to increasing AMPA concentrations in DIV7 subplate and cortical cells. N = 7. (B) Summary graph showing effect of AMPA receptor antagonist, NBQX on glutamate-mediated excitotoxicity in subplate and cortical cultures. N = 3. (C) Quantification and representative western blots of GluR1 and GluR2 AMPA-subunit expression and ratio of GluR1 to GluR2 in subplate and cortical cultures. N = 3. (black boxes/bars = subplate cells, white boxes/bars = cortical cells, dashed line = average of cell death with glutamate alone; error bars = SEM; * P < 0.05, Student’s T-test).
To confirm the differential effect of AMPA, cultured neurons were exposed to glutamate in the presence of the AMPA-selective antagonist NBQX. NBQX confers neuroprotection from glutamate excitotoxicity almost completely in subplate neurons, and partially rescues cortical plate neurons (Figure 4B, P=0.001, ANOVA). At the highest concentration of glutamate, 89.3 ± 1.6% of SP cells survive, which is more than the 62.7 ± 4.3% of CP cells that survive (P = 0.03, T-test). Again, this neuroprotective mechanism is dependent on cell type (P < 0.0001, ANOVA), favoring SP cell survival over CP cell survival.
To explore the mechanism of subplate neuron sensitivity to AMPA and to confirm the suggestion on microarray analysis that subplate neurons express lower levels of the ionotropic AMPA receptor GluR2 subunit (Table 1), we analyzed levels of protein expression of the GluR1 and GluR2 subunits after 7 days in culture. Immunoblots reveal that in general, expression of both subunits in subplate cultures is lower than cortical cultures. In particular, GluR2 is expressed significantly less in subplate neurons compared with cortical plate neurons (Figure 4C, P = 0.02, T-test); furthermore, the ratio of GluR1 to GluR2 is significantly higher in subplate neurons (Figure 4C, P = 0.007). Although AMPA significantly affects both subplate and cortical plate neurons, the maximal percentage of cell death did not reach the level seen in glutamate experiments, suggesting the involvement of other glutamatergic receptors in subplate and cortical neuron excitotoxicity.
Evoked Glutamate Release Does Not Account For Differential Vulnerability To AMPA
Cortical and subplate neuron cultures develop into networks of interconnected cells with equivalent, abundant synapses by DIV 7 (Figure 1C, E). One possibility that might explain the differences in the AMPA sensitivity by cell type is that action potentials resulting from exogenous AMPA might induce comparatively larger amounts of evoked glutamate release from subplate neurons. To test this possibility, we blocked neurotransmitter vesicle release by culturing cells in the presence of Tetanus toxin(Wu et al., 2008) prior to application of AMPA. Tetanus toxin contains a zinc endoprotease that blocks neurotransmitter vesicle release by proteolyzing synaptobrevin, a key component of the synaptic vesicle fusion machinery(Grumelli et al., 2005). Culturing cortical and subplate neurons with Tetanus toxin alone did not increase cell death at baseline (%live subplate + Tetanus toxin = 97.3+/−1.4 %, P = 0.9; %live cortical + Tetanus toxin = 105.9+/−3.5%, P = 0.5). Exposing the cultures to increasing concentrations of AMPA in the presence of tetanus toxin resulted in significant cell death in both cell types (P=0.001, ANOVA), but did not change the heightened sensitivity of subplate neurons to AMPA compared with cortical neurons (Supplemental Figure 1, P=0.008, ANOVA). Pretreament with Tetanus toxin was not without effect, tending to increase death in both cell types especially at low AMPA concentrations (Supplemental Figure 1).
NMDA Does Not Cause Excitotoxicity In Either Subplate Or Cortical Plate Neurons
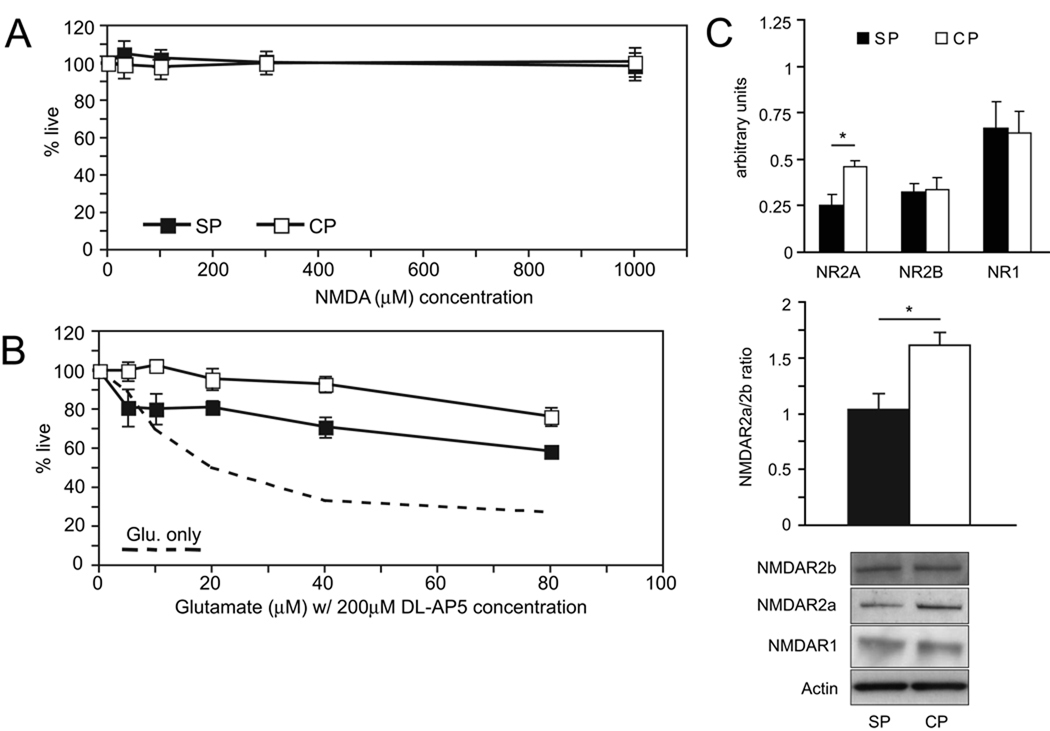
NMDA is well known to cause excitotoxicity in a wide variety of mature neuronal cultures (hippocampal, cortical, cerebellar granule)(Frandsen and Schousboe, 1990; Mizuta et al., 1998; Peterson et al., 1989; Xia et al., 1995). To investigate a contribution of the NMDA receptor to glutamate excitotoxicity of cortical and subplate neuron cultures at DIV7, we exposed the cultures to graded concentrations of NMDA for 1 hour and analyzed cell death twenty-four hours later. Unlike application of glutamate and AMPA, there are no effects of NMDA at any concentration ranging from 0 to 1000 µM on cell survival for either type of culture (Figure 5A, P = 1.0, ANOVA,).
Figure 5.
Effect of NMDA receptor agonist and antagonist, and characterization of NMDA subunits (NR1, NR2A, NR2B) in DIV7 cultures. (A) Summary graph showing lack of excitotoxic effect of NMDA at all concentrations in DIV7 subplate and cortical cells. N = 4 (B) Quantification of the effect of NMDA antagonist DL-AP5 on glutamate-mediated excitotoxicity for subplate and cortical cultures. N = 3 (C) Quantification and representative western blots for NR2A, NR2B, and NR1 NMDA-subunit expression, as well as the ratio of NR2A to NR2B, in subplate and cortical plate cultures (black boxes/bars = subplate cells, white boxes/bars = cortical plate cells, dashed line = average of cell death with glutamate only; error bars = SEM; * P < 0.05, Student’s T-test. N = 3).
Application of NMDA to mature cortical or hippocampal cultures is extremely toxic(Cheng et al., 1999; Frandsen and Schousboe, 1990; Peterson et al., 1989). However, in the absence of depolarization the NMDA receptor channel pore is blocked by magnesium(Nowak et al., 1984). To examine NMDA channel function in the setting of depolarization through AMPA receptors, we applied graded concentrations of glutamate in the presence of the NMDA receptor blocker DL-AP5 for one hour. Twenty-four hours later, we determined cell viability. Despite absence of effect of NMDA alone, blocking the NMDA receptor in the presence of glutamate partially rescues both cell types (Figure 5B, P<0.0001, ANOVA). The rescue is more significant for cortical plate neurons than subplate neurons (P < 0.0001, ANOVA).
NMDA receptors are heterotetramers composed of two NR1 subunits and two NR2A or NR2B subunits(Schoepfer et al., 1994). During development, forebrain neurons undergo gradual upregulation of the NR2A subunit(Monyer et al., 1994). Receptors containing the NR2B subunit show different kinetics than NR2A containing receptors, with longer open times and consequently more calcium flux and are thus thought to be more injurious(Flint et al., 1997; von Engelhardt et al., 2007). Microarray analysis suggests a trend towards higher cortical neuron NR2B expression, which might explain heightened protection of DL-AP5 for cortical neurons and would be consistent with their relative immaturity compared to subplate neurons. To determine expression levels of NMDAR subunits, we examined protein extracts from both cultures at DIV7 by Western blotting with subunit specific antibodies for NR1, NR2A and NR2B. All subunits were detected, but in contrast to the microarray results only NR2A expression was significantly lower in subplate neurons compared with cortical neurons (Figure 5C, P = 0.03,). Moreover, the ratio of NR2A to NR2B in our cultures was significantly higher (P = 0.02) in cortical cells.
Group II Metabotropic Glutamate Receptors Are Injurious, Not Protective, In Purified Subplate Cultures
The largest difference in glutamate receptor expression noted at DIV1 on microarray analysis is a 3–4 fold increased expression of the Group II metabotropic receptor, mGluR3, in subplate neurons (Table 1). Immunoblotting protein extracts prepared at DIV7 confirms this result, with SP expression approximately 2-fold higher than cortical plate (Figure 6A, representative blot, actin-normalized mean+/−SEM Subplate mGluR2/3 expression: 1.1+/−0.1 vs. Cortical mGluR2/3 expression: 0.47+/−0.1, P = 0.006, Student’s T-test). The metabotropic glutamate receptors (mGluRs) comprise a group of at least eight different glutamatergic receptors that signal through GTP-binding proteins, and allow glutamate to modulate or fine-tune activity at the same synapses. Group II receptors bind Gi type GTP binding proteins and inhibit cyclic-AMP formation, as well as inhibiting N-type Ca2+-channels, and have been proposed to be neuroprotective(Bruno et al., 2001).
Figure 6.
Characterization of mGluR3 signaling-induced excitotoxicity in DIV7 cultures. (A) Quantification and representative western blot showing two-fold difference in mGluR2/3 protein expression between subplate and cortical plate cells at DIV7 (P = 0.006, N = 3). (B) The mGluR2/3 agonist DCG-IV, when applied at 100µM, increases AMPA excitotoxicity. Both types of cultures show similar responses under these conditions, N = 4. (C) The non-selective mGluR group II antagonist LY341495 at 200nM attenuates glutamate excitotoxicity in both types of cultures (dashed line = average cell death of subplate and cortical plate cultures exposed to glutamate alone) N = 6. (D) The mGluR3-specific antagonist β-NAAG at 100 µM confirms injurious effect in both subplate and cortical cultures, N = 4. (black boxes = subplate cells, white boxes = cortical plate cells; error bars = SEM; * P < 0.05, Student’s T-test).
To determine the effects of glutamate signaling through mGluR3, we exposed subplate and cortical neurons to the group II agonist DCG-IV. Application of increasing concentrations of DCG-IV up to 300 µM had no effect on subplate or cortical plate viability when compared to basal conditions (P=0.9, T-test, data not shown). To test whether DCG-IV could protect subplate or cortical plate neurons from glutamate excitotoxicity, we applied DCG-IV along with graded concentrations of AMPA. Surprisingly, DCG-IV does not protect against neurotoxicity induced by exposure to AMPA (Figure 6B). Rather, DCG-IV increases cell death, especially in cortical cultures. Here, at 1000 µM, cortical cell survival dropped from 78. ± 2 3.6% (without agonist) to 52.8 ± 2.4% (with agonist), whereas subplate survival changed insignificantly from 56.0 ± 5.6% (without agonist) to 62. ±3 6.3% (with agonist). Application of DCG-IV in the presence of AMPA resulted in equal toxicity of AMPA for both cell types (Figure 6B, P = 0.6, ANOVA). To further explore the potentially toxic effect of signaling through the mGluR3 receptor we applied the mGluR group II antagonist LY341495, a potent nanomolar inhibitor. If glutamate signaling through the mGlur3 receptor is injurious, blocking the receptor should provide protection. Indeed we find that application of LY341495 along with graded concentrations of glutamate results in small but significant neuroprotection for both cell types (Figure 6C, P<0.0001, ANOVA) with a greater effect in subplate cultures (P = 0.008, ANOVA).
The reagents used to assess mGluR expression and function, including the antibody used to detect protein expression, as well as agonist (DCG-IV) and antagonist (LY341495), do not distinguish between group II metabotropic receptors mGluR2 and mGluR3. However, mGluR3 is preferentially expressed early in development and decreases with age(Catania et al., 1994). The opposite pattern is observed for mGluR2. Microarray data also suggests that mGluR3 is the preferentially expressed group II receptor in both subplate and cortical neurons (Table 1). To confirm a specific role for mGluR3, we repeated experiments exposing both cell types to increasing concentrations of glutamate in the presence of the mGluR3-specific antagonist β-NAAG. Consistent with the results using the non-specific group II antagonist (LY341495), β-NAAG results in small but significant neuroprotection for both cell types (Figure 6D, P < 0.0001, ANOVA) with a greater effect in subplate cultures (P = 0.003, ANOVA).
DISCUSSION
Subplate neurons are a transient population of neocortical neurons that are selectively vulnerable to early hypoxia ischemia, both in vivo (McQuillen et al., 2003) and in the present results, in vitro. Subplate neurons are involved in critical aspects of early cortical development and circuit formation including thalamocortical pathfinding, target selection and refinement of ocular dominance columns in the visual system. Subplate neurons are the only layer-specific population of neocortical neurons that can be purified. Taken together, these observations emphasize the significance of this model for understanding developmental selective vulnerability. Results presented here demonstrate that subplate neurons have a unique excitotoxic profile that emerges from differences in both mRNA and protein expression of specific glutamate receptor subunits. Consistent with prior reports(Talos et al., 2006a; Talos et al., 2006b), our results show that subplate neurons express less GluR2 subunits and are more sensitive to AMPA than cortical plate neurons. Both subplate and cortical plate neurons are extremely sensitive to application of glutamate, with no difference between cell types. Neither neuronal population is sensitive to NMDA, although cortical neurons are more protected by an NMDA receptor antagonist in the presence of glutamate. Finally, we observe a novel candidate mechanism for subplate neuron selective vulnerability based upon 4-fold higher expression by subplate neurons of the group II metabotropic glutamate receptor mGluR3. In contrast to prior reports that signaling through this receptor is protective(Battaglia et al., 1998; Berent-Spillson et al., 2004; Bond et al., 1998; Bond et al., 1999; Bruno et al., 1998; Cai et al., 1999), we find that mGluR3 signaling increases glutamate excitotoxicity and antagonists lessen injury.
Maturation of Glutamate Receptor Signaling In Vitro and In Vivo
Many studies have observed that glutamate excitotoxicity emerges over the first week in cultured hippocampal(Peterson et al., 1989), cortical(Cheng et al., 1999; Choi et al., 1987; Mizuta et al., 1998) and cerebellar granule cells(Frandsen and Schousboe, 1990; Xia et al., 1995). Development of toxicity has been linked to NMDA receptor expression(Mizuta et al., 1998), glutamate-stimulated calcium entry(Cheng et al., 1999) and secondary glutamate release(Fogal et al., 2005). Similar observations have been made in vivo (Liu et al., 1996; McDonald et al., 1992; Nitecka et al., 1984). In the present study, our goal was not to examine the development of glutamate excitotoxicity, but to compare relative toxicity between two cell types at a specific developmental stage in order to understand mechanisms of subplate neuron selective vulnerability. Thus, an important consideration of the experimental design was selection of the time point in vitro for comparison of the two cell types and the extent to which this replicates the developmental stage in vivo at which subplate neurons exhibit selective vulnerability. Development of peak sensitivity to glutamate excitotoxicity in vitro varies by study and for specific glutamate agonists (glutamate vs. kainate vs. NMDA), a difference that is likely due to effects of culture conditions, including cell density, presence of astrocytes, substrate and trophic substances on neuronal maturation(de Lima et al., 1997). In most reports, maximal sensitivity is reached by DIV10-14. Onset of sensitivity begins by DIV 5–7 days. Our choice of DIV 7 for the present study represents an intermediate time point between insensitivity and maximal sensitivity to excitotoxicity. This time point also coincides temporally with the age at which subplate neurons are selectively vulnerable in an animal model (e.g. E17- P2 = 7 days). At DIV 7 both cell cultures exhibit equivalent, mature morphological development manifested by complex dendritic arbors and axonal networks, as well as punctate immunohistochemical staining for pre- and postsynaptic markers. By seven days in culture, both subplate and cortical neurons express the glutamate receptor subunits necessary to form functional NMDA (R1, R2A, R2B) and AMPA (GluR1, GluR2) receptors. The patterns of expression of these receptor subunits are consistent with those described in immature animals, with relatively less NMDAR2A compared with R2B subunits(Cull-Candy and Leszkiewicz, 2004; Monyer et al., 1994), and low levels of GluR2 in subplate neurons(Talos et al., 2006a; Talos et al., 2006b).
Cell type-specific Excitotoxic Profiles (NMDA invulnerability)
Studies examining mechanisms of glutamate excitotoxicity have been focused largely on signaling through the NMDA receptor complex (reviewed in(Rothman and Olney, 1995)), with excitotoxic potency linked to receptor subunit composition (NR2A vs. NR2B(Liu et al., 2007)) and localization (synaptic vs. extrasynaptic(Hardingham et al., 2002) and cell body vs. dendritic(Sinor et al., 2000)). NR2B receptor subunits are expressed earlier in development than NR2A subunits, both in vitro(Li et al., 1998; Mizuta et al., 1998) and in vivo(Monyer et al., 1994). In contrast, NR2A subunits increase gradually and remain at high levels. Subplate neurons are thought to mature earlier than more superficial cortical neurons based upon their earlier birthdates and expression of mature neuronal markers(Chun and Shatz, 1989) and glutamate receptors including NR1(Catalano et al., 1997). Surprisingly, in culture subplate neurons express less NR2A and identical levels of the NR1 and NR2B NMDA subunits. NMDA receptors containing the NR2B subunit are characterized by slower deactivation time and consequently larger calcium conductance(Cull-Candy and Leszkiewicz, 2004). Signaling through NR2B receptors increases programmed cell death, while NR2A receptor signaling promotes cell survival (Liu et al., 2007). Prior studies suggesting that receptor localization controls the toxicity of NMDA receptors(Hardingham et al., 2002; Leveille et al., 2008; Sinor et al., 2000) can be understood based upon preferential subunit composition for each location, with more NR2A-containing receptors at synapses. Based upon this data, one would predict that NMDA would be more toxic to subplate neurons with their lower expression levels of NR2A in comparison with cortical neurons. However, we find that neither neuronal population is directly vulnerable to NMDA application. This may be a function of relative immaturity of the cultures as sensitivity to NMDA develops late in culture(Frandsen and Schousboe, 1990; Peterson et al., 1989). However, not all neurons develop sensitivity to NMDA mediated excitotoxicity. In fact, retinal ganglion neurons are resistant to NMDA excitotoxicity, despite having functional glutamate receptors(Ullian et al., 2004).
GluR2 Lacking AMPA Receptors
Besides the effects of glutamate itself, the most potent excitotoxic action was that of AMPA for subplate neurons; 60% of subplate neurons died in response to the highest AMPA concentration (1mM). The relative importance of the AMPA receptor for glutamate’s effect on subplate neurons is shown by almost complete protection by the AMPA-specific blocker, NBQX. In contrast, NBQX only partially protects cortical neurons from glutamate excitotoxicity. This sensitivity is intrinsic to AMPA receptor signaling and not the result of differential evoked glutamate release between the two cell types, as demonstrated by blocking neurotransmitter vesicle release with Tetanus toxin. In fact, Tetanus toxin tended to increase cell death in both cell types suggesting a protective effect of endogenous glutamate signaling. This ‘excitoprotective’ effect has been linked to activity-dependent release of brain-derived neurotrophic factor (BDNF)(Jiang et al., 2005). The potent activity of AMPA on subplate neurons is consistent with observations of GluR2-lacking AMPA receptors on subplate neurons in rats(Talos et al., 2006a) and human(Talos et al., 2006a). Presence(Hollmann et al., 1991) and mRNA editing(Burnashev et al., 1992; Jonas et al., 1994) of the GluR2 subunit renders AMPA receptors impermeable to calcium. Subplate neurons in culture express ~1/3 the level of GluR2 expressed by cortical neurons and the ratio of GluR1 to GluR2 twice as high in subplate neurons compared with cortical neurons. The subplate neuron GluR1/GluR2 ratio of 8 is consistent with similar measurements made in vivo at P3(Talos et al., 2006a). AMPA is highly toxic to subplate neurons despite an overall lower level of GluR1 expression, indicating that other factors including receptor composition and localization are more important for determining excitotoxicity than overall receptor expression. An important limitation of the present study is that GluR2 mRNA editing was not determined and the toxicity of unedited GluR2 AMPA receptor subunits may exceed that of other calcium-permeable AMPA receptors(Mahajan and Ziff, 2007). The influence of calcium entry through voltage gated calcium channels and other mechanisms of ionic imbalance were also not evaluated(Besancon et al., 2008).
Metabotropic Glutamate Receptors
The largest difference in glutamate receptor expression between subplate and cortical plate neurons was found in the group II metabotropic glutamate receptor mGluR3. Although the antibody used for quantifying protein expression cannot distinguish between mGluR2 and mGluR3, microarray analysis indicates that the changes are specific to the mGluR3 subunit and this was supported by experiments using a mGluR3-specific antagonist. Furthermore a role for mGluR3 is supported by known preferential expression of mGluR3 at high levels in neocortex early, declining to adult levels by the third postnatal week in rat(Catania et al., 1994). On the other hand, mGluR2 is not expressed at high levels at early postnatal ages and only in limited amount in neocortex(Catania et al., 1994). mGluR3 is expressed by neurons and astrocytes, but not microglia(Ohishi et al., 1993). The receptor is localized to the perisynaptic regions of dendrites and preterminal regions of axons(Tamaru et al., 2001). mGluR3 is protective against various forms of neurotoxicity including hyperglycemia(Berent-Spillson et al., 2004) and NMDA-mediated excitotoxicity, although the latter effect is reported to be mediated by astrocytic secretion of TGF-beta(Bruno et al., 1998). The present culture conditions produce pure neuronal cultures, excluding a role for astrocytic mGluR3 expression in the present results. mGlur2/3 is expression levels are correlated with anoxia tolerance in fish(Poli et al., 2003) and mGluR2/3 agonists are protective against adult global ischemia(Bond et al., 1999) as well as neonatal hypoxia ischemia(Cai et al., 1999). In subplate and cortical cultures, mGluR2/3 agonists or blockers have no effect in isolation. In the presence of AMPA, the mGluR2/3 agonist (DCG-IV) worsened survival in for both cell types. In the presence of glutamate, blocking mGluR3 signaling improved subplate neuron survival at all glutamate concentrations, while the same agonist did not effect cortical plate neuron survival except at the highest glutamate concentration. Taken together, the effects of stimulating or blocking the mGluR3 receptor suggest a neurotoxic role, augmenting glutamate excitotoxicity. The only data suggesting a possible detrimental mechanism for mGluR3 signaling are reports that activation of group II mGluRs can lead to increase in glutamate release through an arachidonic acid-mediated mechanism in cortical synaptosomes(Herrero et al., 1992). The net effect of this novel injurious in vitro activity will need to be assessed in vivo in the setting of potential protection mediated by astrocyte mGluR3-stimulated TGF-beta secretion.
The present experiments represent a developmentally relevant in vitro model, studying pure neuronal cultures of subplate and cortical neurons. Here we find differences in gene expression for glutamate receptors as early as E17/DIV0 with decreased expression in subplate neurons of AMPA receptor GluR2 subunit and increased expression of the metabotropic receptor mGluR3 subunit. Validity of these differences in mRNA expression is supported by finding concordant changes in protein expression at DIV7. Significant insights into excitotoxicity mechanisms have been made with similar in vitro cultures, which offer the ability to precisely define glutamate receptor expression and function in isolation. However, this reductionist approach simultaneously produces significant limitations, including the possibility that in vitro development is not equivalent to the intact animal. Recent observations for instance, suggest that subplate neurons in isolated culture form fewer synapses without exposure to ‘feeder-layers’ of cortical neurons or conditioned media(McKellar and Shatz, 2008). Furthermore, we have only probed the effects of glutamate receptors themselves, while associated downstream signaling molecules are known to have potent effects on excitotoxicity(Besancon et al., 2008; Forder and Tymianski, 2008; Sattler and Tymianski, 2001). Nevertheless the present results are significant in that they confirm the selective vulnerability of subplate neurons to in vitro oxygen-glucose deprivation and glutamate excitotoxicity, particularly mediated by GluR2-lacking AMPA receptors. Finally, we identify a novel injurious effect of mGluR3 signaling that requires further characterization in animal models that are carefully designed to replicate clinically relevant human disease(1999).
Supplementary Material
Contribution of evoked glutamate release to AMPA excitotoxicity. Quantification of live cells following exposure to increasing AMPA concentrations with tetanus toxin pretreatment to block endogenous glutamate release in DIV7 subplate and cortical cells, (black boxes = subplate cells, white boxes = cortical cells, dashed line = subplate response to AMPA only, dotted line = cortical response to AMPA only, N = 3).
Acknowledgments
The authors would like to acknowledge the contributions of Michael DeFreitas, Merritt Evans and Jacqueline Kamrath to preliminary experiments and assay development, Benjamin Braun for assistance with cell sorting for quantification of BrdU staining and finally, Donna Ferriero for critical reading of the manuscript.
This work was supported by an NIH NINDS Independent Scientist Award (K02 NS047098) and March of Dimes Research Grant (6-FY2008) to P.S.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annual Review of Neuroscience. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J Neurosci. 1993;13:3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, et al. Selective activation of group-II metabotropic glutamate receptors is protective against excitotoxic neuronal death. Eur J Pharmacol. 1998;356:271–274. doi: 10.1016/s0014-2999(98)00551-2. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Experimental Neurology. 1990;107:48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berent-Spillson A, et al. Protection against glucose-induced neuronal death by NAAG and GCP II inhibition is regulated by mGluR3. J Neurochem. 2004;89:90–99. doi: 10.1111/j.1471-4159.2003.02321.x. [DOI] [PubMed] [Google Scholar]
- Besancon E, et al. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29:268–275. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Bond A, et al. Neuroprotective effects of a systemically active group II metabotropic glutamate receptor agonist LY354740 in a gerbil model of global ischaemia. Neuroreport. 1998;9:1191–1193. doi: 10.1097/00001756-199804200-00042. [DOI] [PubMed] [Google Scholar]
- Bond A, et al. LY379268, a potent and selective Group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci Lett. 1999;273:191–194. doi: 10.1016/s0304-3940(99)00663-1. [DOI] [PubMed] [Google Scholar]
- Bruno V, et al. Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta. J Neurosci. 1998;18:9594–9600. doi: 10.1523/JNEUROSCI.18-23-09594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno V, et al. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Burnashev N, et al. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Cai Z, et al. Protection of neonatal rat brain from hypoxic-ischemic injury by LY379268, a Group II metabotropic glutamate receptor agonist. Neuroreport. 1999;10:3927–3931. doi: 10.1097/00001756-199912160-00037. [DOI] [PubMed] [Google Scholar]
- Catalano SM, et al. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. J Neurosci. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania MV, et al. Metabotropic glutamate receptors are differentially regulated during development. Neuroscience. 1994;61:481–495. doi: 10.1016/0306-4522(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Chandler CE, et al. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259:6882–6889. [PubMed] [Google Scholar]
- Cheng C, et al. Emergence of excitotoxicity in cultured forebrain neurons coincides with larger glutamate-stimulated [Ca(2+)](i) increases and NMDA receptor mRNA levels. Brain Res. 1999;849:97–108. doi: 10.1016/s0006-8993(99)01995-2. [DOI] [PubMed] [Google Scholar]
- Choi DW, et al. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Chun JJ, Shatz CJ. A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons. J Cell Biol. 1988;106:857–872. doi: 10.1083/jcb.106.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJ, Shatz CJ. The earliest-generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter immunohistochemistry during fetal life. J Neurosci. 1989;9:1648–1667. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, et al. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc Natl Acad Sci U S A. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- de Lima AD, et al. Neuritic differentiation and synaptogenesis in serum-free neuronal cultures of the rat cerebral cortex. J Comp Neurol. 1997;382:230–246. doi: 10.1002/(sici)1096-9861(19970602)382:2<230::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- DeFreitas MF, et al. A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons. J Neurosci. 2001;21:5121–5129. doi: 10.1523/JNEUROSCI.21-14-05121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, et al. Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit composition and cAMP-response element-binding protein regulate oligodendrocyte excitotoxicity. J Biol Chem. 2006;281:36004–36011. doi: 10.1074/jbc.M606459200. [DOI] [PubMed] [Google Scholar]
- Feldman DE, et al. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Flint AC, et al. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogal B, et al. Changes in secondary glutamate release underlie the developmental regulation of excitotoxic neuronal cell death. Neuroscience. 2005;132:929–942. doi: 10.1016/j.neuroscience.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Forder JP, Tymianski M. Postsynaptic mechanisms of excitotoxicity: Involvement of postsynaptic density proteins, radicals, and oxidant molecules. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Frandsen A, Schousboe A. Development of excitatory amino acid induced cytotoxicity in cultured neurons. Int J Dev Neurosci. 1990;8:209–216. doi: 10.1016/0736-5748(90)90013-r. [DOI] [PubMed] [Google Scholar]
- Friauf E, et al. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley AJ, et al. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, et al. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Grumelli C, et al. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology. 2005;26:761–767. doi: 10.1016/j.neuro.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, et al. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 2002;22:7165–7176. doi: 10.1523/JNEUROSCI.22-16-07165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, et al. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Herrero I, et al. Positive feedback of glutamate exocytosis by metabotropic presynaptic receptor stimulation. Nature. 1992;360:163–166. doi: 10.1038/360163a0. [DOI] [PubMed] [Google Scholar]
- Hollmann M, et al. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hoyte L, et al. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4:131–136. doi: 10.2174/1566524043479248. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. Differential vulnerability of immature murine neurons to oxygen-glucose deprivation. Exp Neurol. 2004;190:224–232. doi: 10.1016/j.expneurol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in neonatal hypoxia. Ment Retard Dev Disabil Res Rev. 2001;7:229–234. doi: 10.1002/mrdd.1032. [DOI] [PubMed] [Google Scholar]
- Jonas P, et al. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998;37:110–130. [PubMed] [Google Scholar]
- Leveille F, et al. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Li JH, et al. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci. 1998;10:1704–1715. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. Age-dependent effects of glutamate toxicity in the hippocampus. Brain Res Dev Brain Res. 1996;97:178–184. doi: 10.1016/s0165-3806(96)00141-1. [DOI] [PubMed] [Google Scholar]
- Mahajan SS, Ziff EB. Novel toxicity of the unedited GluR2 AMPA receptor subunit dependent on surface trafficking and increased Ca2+-permeability. Mol Cell Neurosci. 2007;35:470–481. doi: 10.1016/j.mcn.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, et al. Susceptibility of brain to AMPA induced excitotoxicity transiently peaks during early postnatal development. Brain Res. 1992;583:54–70. doi: 10.1016/s0006-8993(10)80009-5. [DOI] [PubMed] [Google Scholar]
- McKellar CE, Shatz CJ. Synaptogenesis in Purified Cortical Subplate Neurons. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol. 2005;15:250–260. doi: 10.1111/j.1750-3639.2005.tb00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, et al. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta I, et al. Developmental expression of NMDA receptor subunits and the emergence of glutamate neurotoxicity in primary cultures of murine cerebral cortical neurons. Cell Mol Life Sci. 1998;54:721–725. doi: 10.1007/s000180050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, et al. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nitecka L, et al. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience. 1984;13:1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- Nowak L, et al. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ohishi H, et al. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Peterson C, et al. Development of N-methyl-D-aspartate excitotoxicity in cultured hippocampal neurons. Brain Res Dev Brain Res. 1989;48:187–195. doi: 10.1016/0165-3806(89)90075-8. [DOI] [PubMed] [Google Scholar]
- Poli A, et al. Group II metabotropic glutamate receptors regulate the vulnerability to hypoxic brain damage. J Neurosci. 2003;23:6023–6029. doi: 10.1523/JNEUROSCI.23-14-06023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor--still lethal after eight years. Trends Neurosci. 1995;18:57–58. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Schoepfer R, et al. Molecular biology of glutamate receptors. Prog Neurobiol. 1994;42:353–357. doi: 10.1016/0301-0082(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Sinor JD, et al. NMDA and glutamate evoke excitotoxicity at distinct cellular locations in rat cortical neurons in vitro. J Neurosci. 2000;20:8831–8837. doi: 10.1523/JNEUROSCI.20-23-08831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J Comp Neurol. 2006a;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006b;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru Y, et al. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Ullian EM, et al. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004;26:544–557. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, et al. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Wu MP, et al. Reverse mode Na+/Ca2+ exchangers trigger the release of Ca2+ from intracellular Ca2+ stores in cultured rat embryonic cortical neurons. Brain Res. 2008;1201:41–51. doi: 10.1016/j.brainres.2008.01.042. [DOI] [PubMed] [Google Scholar]
- Xia Y, et al. Developmental expression of N-methyl-D-aspartate (NMDA)-induced neurotoxicity, NMDA receptor function, and the NMDAR1 and glutamate-binding protein subunits in cerebellar granule cells in primary cultures. Neurochem Res. 1995;20:617–629. doi: 10.1007/BF01694545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contribution of evoked glutamate release to AMPA excitotoxicity. Quantification of live cells following exposure to increasing AMPA concentrations with tetanus toxin pretreatment to block endogenous glutamate release in DIV7 subplate and cortical cells, (black boxes = subplate cells, white boxes = cortical cells, dashed line = subplate response to AMPA only, dotted line = cortical response to AMPA only, N = 3).