Abstract
The contemporaneous association between maternal salivary cortisol and fetal motor activity was examined at 32 and 36 weeks gestation. Higher maternal cortisol was positively associated with the amplitude of fetal motor activity at 32 weeks, r(48) = .39, p < .01, and 36 weeks, r(77)=.27, p < .05, and the amount of time fetuses spent moving at 32 weeks during the 50 minute observation period, r(48) = 33, p < .05. Observation of periods of unusually intense fetal motor activity were more common in fetuses of women with higher cortisol, Mann-Whitney U = 58.5. There were no sex differences in fetal motor activity, but the associations between maternal cortisol and fetal motor amplitude and overall movement were significantly stronger for male than female fetuses.
Keywords: Fetus, Pregnancy, Cortisol, Fetalmotor activity, Temperament, Maternal stress/anxiety
Fetal motor activity is a conspicuous feature of prenatal development. Spontaneous motor activity commences in the embryonic period and motor patterns become increasingly complex as gestation advances (deVries & Fong, 2006). Trajectories of motor activity during gestation depend on the metric for assessment; measures of vigor tend to increase while instances of movements tend to decline (DiPietro, Costigan, Shupe, Pressman, & Johnson, 1998; Robles de Medina, Visser, Huizink, Buitelaar, & Mulder, 2003; ten Hof et al., 2002). Observations that the human fetus moves, on average, once per minute and is motorically active up to 30% of the time have been reported from studies that use various methods of ascertaining fetal movement (DiPietro et al., 2004a; Nasello-Paterson, Natale, & Connors, 1988; Roberts, Griffin, Mooney, Cooper, & Campbell, 1980; Roodenburg, Wladimiroff, van Es, & Prechtl, 1991).
Motor activity is a core dimension of temperament, displaying evidence of individuality (Eaton & Saudino, 1992) and stability over time (Roberts & DelVecchio, 2000). Individual differences emerge before birth: both wide inter-fetal variation in motor activity and intra-fetal stability during the second half of gestation have been documented (DiPietro, Hodgson, Costigan, & Johnson, 1996; Groome et al., 1999; ten Hof et al., 2002). Moreover, there is evidence that prenatal variation is conserved postnatally. Stability has been reported from term gestation to the neonatal and early infancy periods (Almli, Ball, & Wheeler, 2001, DiPietro et al., 1996; Groome et al., 1999) and at 1 year postpartum (DiPietro et al., 2002).
Exploration of the constitutionality of activity level has focused on the genetic contribution (e.g., Ganiban, Saudino, Ulbricht, Neiderhiser, & Reiss, 2008). However, maternal cortisol levels in the third trimester have been positively linked to activity level in early infancy (deWeerth, van Hees, & Buitelaar, 2003), suggesting a non-genetic contributory role of the prenatal milieu. In the fetus, a number of anecdotal reports observe increased fetal motor activity in response to a range of stressful situations (Hepper, 1990; Ianniruberto & Tajani, 1981; Sontag, 1941). Greater motor activity has been observed in fetuses of women with higher anxiety (Van den Bergh, 1990) and higher levels of pregnancy-specific stress (DiPietro et al., in press; DiPietro, Hilton, Hawkins, Costigan, & Pressman, 2002), although not all studies have detected associations (e.g., Sjostrom, Valentin, Thelin, & Marsal, 2002).
Activation of the maternal hypothalamic-pituitary-adrenal (HPA) axis is generally considered to be the putative mechanism underlying these observations but two studies that have directly examined concurrent associations between maternal cortisol levels and motor activity in the human fetus yielded conflicting results. The first study was conducted at 15 weeks gestation on a small sample (n = 20) using a 40-minute observation period but found no association (Bartha, Martinez-del-Fresno, Romero-Carmosa, Hunter, & Comino-Delgado, 2003). In the second, maternal cortisol explained 12% of the variability in fetal motor activity observed during a 5-minute ultrasound observation between 20 and 28 weeks gestation (Field, Diego, Hernandez-Reif, Gil, & Vera, 2005).
Although there is a well-documented and robust sex difference in motor activity after birth, with boys displaying greater and more vigorous motor activity (Eaton & Enns, 1986), this has not been consistently observed in the prenatal period. Although male fetuses have been reported to make more frequent leg movements (Almli et al., 2001) and female fetuses more frequent mouthing movements (Hepper, Shannon, & Dorman, 1998), a meta-analysis of six studies concluded that there are no antenatal sex differences in motor activity level (Eaton & Enns, 1986). This has been confirmed by two more recent and larger scale studies with data generated longitudinally across the second half of gestation (DiPietro et al., 2004a; Robles de Medina et al., 2003).
However, unexpected and unexplained findings of sex-specific disparities in prenatal motor development abound. For example, despite the absence of a main effect, a significant sex by time interaction during the second half of gestation has been reported, with significantly different trajectories of motor development in male as compared to female fetuses (Robles de Medina et al., 2003) and prenatal differences extend through the first six weeks of life (Almli et al., 2001). Unpredicted sex differences in reports of intrafetal stability are also typical. For example, more active female fetuses showed greater motor activity in newborn sleep but more active male fetuses were less active newborns (Almli et al., 2001), but the reverse was reported in another study such that more active male fetuses were observed to be more active boys at 1-year but more active female fetuses were less active girls (DiPietro et al., 2002).
The goal of the current study is to further establish whether maternal cortisol level is associated with variation in fetal motor activity and to evaluate potential sex differences in any detected associations.
Methods
Participants
Eligibility was restricted to normotensive, non-smoking women with uncomplicated pregnancies at the time of enrollment. Accurate dating of the pregnancy was required and based on early first trimester pregnancy testing or examination and confirmed by ultrasound. A total of 110 self-referred pregnant women from the local community were enrolled in the study. Of these, 18 developed pregnancy complications or delivered infants with conditions that required additional medical attention. These included preterm labor or delivery (8), gestational diabetes (2), fetal demise in utero (1), or conditions in the fetus or neonate that required additional care (7). Because preterm birth and other pregnancy complications have been independently linked to products of the HPA axis during the gestational period studied (Mulder et al., 2002; Sandman et al., 2006), analysis was restricted to uncomplicated pregnancies that resulted in full-term deliveries of infants that were discharged from the hospital per routine schedule (n = 92). The sample represents a population of mature, relatively well-educated women (M age = 31.3, SD = 4.8, range 21 to 43; M years education = 16.8 years, SD = 2.2, range 12 to 20). Most (87%) women were non-Hispanic white and the remainder was of African-American (7.6%), Hispanic or Asian (5.4%) decent. The majority were married (94%) and expecting their first child (55%). Fifty-two percent of the fetuses were female.
Design and Procedure
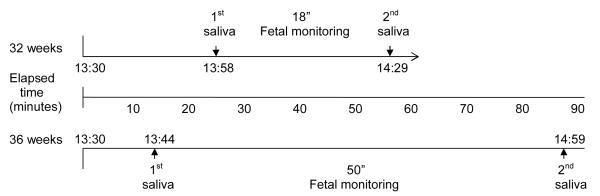
Data were collected at 32 and 36 weeks gestation; visits at both gestational ages commenced at 13:30. Data collection at each visit included two saliva collections used to measure salivary cortisol and a period of fetal monitoring to detect and quantify fetal motor activity. The 32 week visit was part of a larger protocol that evaluated the effect of maternal relaxation on fetal neurobehavior, results of which are reported elsewhere (DiPietro, Costigan, Nelson, Gurewitsch, & Laudenslager, 2008). Cortisol data at 32 weeks was collected immediately before and following an 18 minute fetal monitoring period that served as the baseline period for the relaxation protocol. The 36 week visit involved 50 minutes of undisturbed fetal monitoring; salivary cortisol was collected before and after this period. A schematic protocol for each visit is presented in Figure 1. Three women participated at 32 weeks but not 36 weeks; ten participated at 36 weeks but not at 32 weeks.
Figure 1.
Schematic description of 32 and 36 week protocols. Note that the initial saliva sample was collected after other protocol activities such as informed consent (week 32) and ultrasound. The elapsed time between saliva collections is longer than the fetal monitoring period because it includes activities such as application of monitoring equipment and preparation for sample collection.
Fetal Monitoring
Fetal data were collected from the output port of a Toitu (MT320) fetal actocardiograph. This monitor detects fetal heart rate and movement through a single wide array transabdominal Doppler transducer. Data were sampled at 1000 Hz using an external A/D board and digitized via streaming software. The actograph feature of the monitor detects fetal movements by preserving the remaining signal after band passing frequency components of the Doppler signal that are associated with fetal heart rate and maternal somatic activity. Reliability studies comparing actograph based vs. ultrasound visualized fetal movements have found the performance of the Toitu monitor to be accurate in detecting both fetal motor activity and quiescence (Besinger & Johnson, 1989; DiPietro, Costigan, & Pressman, 1999; Maeda, Tatsumura, & Utsu, 1999). The Toitu generates calibrated values in arbitrary units (a.u.s) ranging from 0 to 100, represented as a series of spikes corresponding to individual movements.
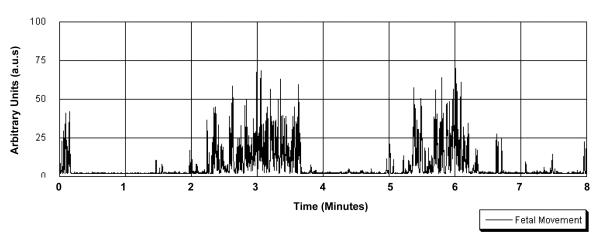
A sample of the fetal motor data is presented in Figure 2. There are a number of ways to quantify this signal; variable extraction was based on a priori definitions developed in prior reliability studies of this monitor (Besinger & Johnson, 1989; DiPietro et al., 1999). Three variables reflecting various aspects of fetal motor activity were quantified via computerized methods applied to the digitized data using software developed in our laboratory (GESTATE, James Long Company, Caroga Lake NY). Motor amplitude was quantified as the mean of all actograph values exceeding a threshold of 5 a.u.s, established to eliminate background noise and motion of the fetal diaphragm. Signal amplitude reflects the degree of excursion of the fetal body part(s) through the transducer field. Number of individual movements were counted by detecting each time the actograph attained an amplitude of 15 units and concluded with cessation of 15 unit signals for at least 10 s, based on previously established criteria. Total time spent moving was calculated by multiplying the number of movements by the durations of individual movements. In addition, the 50 min polygraphic record at 36 weeks was coded for episodes of exceptionally high intensity motor activity that were at least 3 minutes long. This procedure is based on methods developed for scoring fetal behavioral state and has been described elsewhere (DiPietro et al., 1998). The number of 3-minute segments in which this behavior, defined as continuous movement of high amplitude (i.e., > 50 a.u.s. and including period of > 75 a.u.s.) was recorded.
Figure 2.
Eight-minute sample of digitized fetal actograph data.
Salivary Cortisol
Saliva collection and cortisol assay
The first saliva sample was collected in early afternoon at 32 weeks (M = 1358 h; SD = 31 m) and again at the conclusion of the 18 minute fetal monitoring period (M = 1429 h; SD = 33 m). At 36 weeks, the initial sample occurred at near the same time of day (M = 1344 h; SD = 54 m) with the second after 50 minutes of monitoring (M = 1459 h; SD = 55 m). Participants were instructed to eat no more than 1.5 hours prior to arrival at the laboratory and to restrict fluid intake prior to collection. Saliva was collected by having the participant moisten a small filter paper (2.5 × 9.0 cm, Whatman Grade 42), as previously described in detail (Kivlighan, DiPietro, Costigan, & Laudenslager, 2008). Filters were air dried. This procedure has been previously validated and demonstrated 92% recovery of cortisol from filters compared to whole saliva (Neu, Goldstein, Gao, & Laudenslager, 2007).
Dried filters were cut and extracted in assay buffer as described (Neu et al., 2007). Salivary cortisol concentration in the extraction buffer was determined using a commercial expanded range high sensitivity EIA kit (No. 1-3002/1-3012, Salimetrics) that detects cortisol levels in the range of .003 – 3.0 μg/dl (0.083 – 82.77 nmol/L). Standard curves were fit by a weighted regression analysis using commercial software (Revelation 3.2) for the ELISA plate reader (Dynex MRX). The detection limit after accounting for the extraction dilution is 0.018 μg/dl (0.50 nmol/L). This kit shows minimal cross reactivity (4% or less) with other steroids present in the saliva. Controls run on every plate for determination of inter-assay coefficients of variation were less than 7.5% for high and low laboratory controls in the present study. Intra-assay coefficients of variation for duplicate determinations were less than 3% in the present study.
Data Management and Analysis
Variables were examined for outliers and skewness and distributions were determined to be normal and without significant outliers. Pearson correlations were used to examine associations among measures. Data analysis was based on straightforward descriptive techniques including Pearson correlations to examine associations between fetal motor activity and maternal cortisol and t-tests and repeated measures analysis of variance to evaluate sex differences and change over time. Fisher’s Z’s were used to determine significance of differences in correlations between male and female sub-samples.
Results
Fetal Motor Activity
Technical difficulties resulted in missing fetal motor activity data for 3 cases at 32 weeks and 1 at 36 weeks, respectively. Because excursions of the fetal chest wall associated with hiccups creates fetal motor activity artifact, cases in which at least 25% of the recording period included hiccups were excluded (4 at 32 weeks and 2 at 36 weeks). Descriptive statistics for fetal movement at 32 (n = 73) and 36 weeks (n = 82) are presented in Table 1. Note that the time spent moving variable was converted to percent of the observation period for comparison purposes. Despite the variation in recording duration, there was within-individual stability over time; that is, fetuses that displayed relatively greater or lesser motor activity as measured by each variable at 32 weeks tended to continue to do so at 36 weeks. Correlation coefficients for fetal motor amplitude, number of movements, and time spent moving were as follows: rs (67) = .41, .34, and .31, ps < .01, respectively. Repeated measures analyses were computed to evaluate whether motor values changed from 32 to 36 weeks gestation, based on 69 cases with fetal movement data at both visits. There was a significant increase in motor amplitude from 32 to 36 weeks, F(1, 68) = 7.82, p < .01 but no change over time for the other two measures. Note that for this analysis the number of movements at 32 weeks was prorated to control for the difference in observation time. There were no main effects for fetal sex on any movement measure.
Table 1.
Fetal Motor Activity at 32 and 36 Weeks Gestation
| 32 Weeks | 36 Weeks | |||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Fetal motor activity measure: | ||||||
| Overall motor amplitude | 73 | 10.31 | 2.04 | 82 | 11.13 | 2.39 |
| Number of movements† | 73 | 21.81 | 7.82 | 82 | 57.41 | 15.44 |
| Percent of time spent moving | 73 | 28.5 | .20 | 82 | 33.1 | .20 |
Per 18 minute observation period at 32 weeks; 50 min at 36 weeks.
Maternal Cortisol
At 32 weeks, salivary cortisol assays were conducted for only a subset of participants with complete data due to budgetary constraints (n = 58). Assay selection was determined by the completeness of salivary collections in other facets of the 32 and 36 week study design. Both cortisol assays tested outside assay range for one subject and 2 others were outside range for the second collection only. Values were M =.29 μg/dl, SD = .12 and M = .25 μg/dl, SD = .11 for the first and second collections, respectively. Time 1 and 2 cortisol levels were highly correlated, r (53) = .83, p < .001, so mean values were used in all subsequent analyses (n =55, M = .27, SD = .11).
At 36 weeks, saliva cortisol assays were conducted for the full sample (n = 92). Samples from 3 participants were not sufficient for analysis, resulting in 89 cases with cortisol data. Of these, the first sample tested outside assay range for one participant and the second sample was insufficient (n = 4) or outside of assay range (n = 3) for seven cases. First and second values were M =.30 μg/dl, SD = .12 and M = .26 μg/dl, SD = .12. Again, both values were highly correlated, r (79) = .75, p < .001 and mean values (n = 81) were used in analyses (M =.28 μg/dl, SD = .11). Cortisol levels increased from 32 to 36 weeks, F(1, 51) = 5.66, p < .05 and there was within-subject stability in level between the first and second visit, r (48) = .56, p < .001. Women carrying male fetuses displayed marginally higher cortisol levels, F (1, 48) = 3.85, p = .056.
Associations Between Fetal Motor Activity and Maternal Cortisol
Unadjusted correlation coefficients showing the associations between the three fetal motor activity variables are presented in Table 2. Maternal cortisol was significantly associated with fetal motor amplitude at both 32 and 36 weeks; at 32 weeks there was also a significant association with the total time spent moving during the observation period. Five fetuses (3 male, 2 female) displayed one or more periods of high intensity fetal motor activity at 36 weeks. Maternal cortisol levels in these were significantly higher than in fetuses that displayed this type of activity than those that did not, M = .42 μg/dl, SD = .11 versus M = .27 μg/dl, SD = .11, Mann-Whitney U = 58.5, p < .01. Cortisol was unrelated to the number of discrete individual movements at either gestational age.
Table 2.
Associations between fetal motor activity and maternal cortisol.
| 32 Weeks | 36 Weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 50) |
Male (n =24) |
Female (n = 26) |
Z | All (n =79) |
Male (n =37) |
Female (n =42) |
Z | |
|
Fetal Motor Activity
Measure |
||||||||
| Overall motor amplitude | .39** | .67*** | .10 | 2.35* | .27* | .16 | .38** | 1.02 |
| Number of movements | .06 | −.04 | .27 | 1.05 | .05 | .18 | −.12 | 1.20 |
| Time spent moving | .33* | .73*** | −.13 | 3.51*** | .13 | .10 | .20 | 0.39 |
p < .05
p < .01
p < .001
Stratifying by fetal sex revealed that associations between maternal cortisol and fetal motor amplitude and total time spent moving were significantly stronger for male than female fetuses at 32 weeks (Table 2). However, although there was a significant association for female, but not male fetuses, at 36 weeks the difference between these two correlations was not significant.
Discussion
Fetal motor activity measures were significantly correlated with concurrent levels of maternal salivary cortisol at both gestational periods, although associations were more consistent at the earlier time point. In addition, maternal cortisol was significantly elevated in mothers of fetuses that displayed an uncommon pattern of highly intensive bursts of fetal motor activity at 36 weeks. The degree of shared variance between fetal activity and cortisol at 32 weeks ranged from 11 to 15%, which is comparable to that observed previously on fetuses measured, on average, at 24 weeks (Field et al., 2005). Because those results were generated from data collected for a much shorter interval (i.e., 5 minutes) using ultrasound-observed motor activity as opposed to actograph-detected motor activity, it suggests that the maternal cortisol-fetal motor activity association may be fairly robust. No significant associations were observed with the number of individual movements, underscoring the importance of operationalizing motor activity constructs in several different dimensions. This may be a result of the intrinsic construct of counting individual movements without discriminating between isolated limb movements versus more complex and sustained movements in general, or with limitations in the a priori definitions we used to define when one movement ends and another begins. Others have also noted the variation in counts that can be introduced by minor variation in parameters such as inter-burst intervals (ten Hof et al., 1999). The motor amplitude variable, which was significant for the overall sample at both gestational ages, reflected the totality of the signal generated by the actograph so provides a more generalized indicator of the total motor activity during the observation period.
Glucocorticoid exposure during the fetal period exerts a variety of organizational effects on the developing brain, including modification of synaptogenesis, neurotransmitter function, and glucocorticoid receptor expression (for review, see Owen, Andrews, & Matthews, 2005) with long-term implications for structure and function. During pregnancy, increases in maternal cortisol level parallel a dramatic rise in corticotrophin releasing-hormone of placental origin (Levine, Zagoory-Sharon, Feldman, Lewis, & Weller, 2007) with the initiation of the increase above non-pregnant levels between 25 to 28 weeks gestation (Allolio, Hoffmann, Linton, Winkelmann, Kusche, & Shulte, 1990). Although the fetus is largely protected from elevated maternal cortisol through the catabolic activity of placental 11β-hydroxysteroid-dehydrogenase type 2 (11β-HSD 2; Benediktsson, Calder, Edwards, & Seckl, 1997), maternal levels still account for 33-40% of the variance in fetal cortisol (Gitau, Fisk, Teixeira, Cameron, & Glover, 2001). The stronger observed association at 32 weeks relative to 36 weeks was unexpected and we have no ready explanation for this. It may be a result of its closer temporal proximity to the initial cortisol surge in mid-gestation after which time compensatory mechanisms might begin to limit placental transfer, but this is purely speculative.
However, the tendency to assume directionality such that that maternal cortisol levels generate the observed greater fetal motor activity may be premature. Fetal motor activity has been shown to stimulate a systemic maternal sympathetic response (DiPietro et al., 2006). Thus, it is possible that an active fetus may modulate additional maternal physiologic systems, including the HPA axis, in ways that are not yet apparent.
Conclusions concerning the association between fetal motor activity and maternal cortisol are tempered by the different patterns that emerged by sex. This was particularly true at 32 weeks, when approximately half of the variance in fetal motor amplitude and time spent moving was attributable to cortisol for boys, but was negligible for girls. We know of no other report of differences in peripheral maternal cortisol levels by fetal sex, but it is possible that the marginal elevation in male pregnancies observed here may have contributed to this finding. There is mounting evidence that antenatal exposure and sensitivity to glucocorticoids differs by fetal sex. The activity of placental 11β-HSD 2 is significantly higher in pregnancies with female as compared to males fetuses (Murphy et al., 2003), resulting in greater male exposure to cortisol. Further, a number of animal and clinical studies have suggested sex-specific differential sensitivity to the glucocorticoid environment within various components of the maternal-fetal-placental unit (Clifton, 2005; Papageorgiou, Colle, Farri-Kostopoulos, & Gelfand, 1981; Sweezey, Ghibu, Gagnon, Schotman, & Hamid, 1998). While clear understanding of sex differences in exposure or sensitivity has not yet emerged, the variegated pattern of findings in provides further support for conducting analyses by fetal sex in studies involving prenatal cortisol.
These results support the notion that motor activity level, a core dimension of temperament, may be shaped by features of the prenatal milieu. The contribution of the “womb environment” to non-heritable but constitutional individuality has been applied previously to IQ (Devlin, Daniels, & Roeder, 1997). These results also underscore the often unexpected manner in which fetal sex, which had previously been shown to affect activity level trajectory and its predictability to the postnatal period, can serve to moderate contemporaneous associations between fetal motor activity and cortisol. More systematic examination of the role of fetal sex in subsequent studies with larger samples is necessary to better understand these complex relations.
Acknowledgments
This research was supported by grant R01 HD27592, National Institute of Child Health and Human Development, awarded to the first author. The contribution by MLL was partially supported by R01 AA013973. We are grateful for the diligent and generous participation of the women in this study, without which this research would not have been possible.
Contributor Information
Kathleen A. Costigan, Johns Hopkins Medical Institutions
Mark L. Laudenslager, University of Colorado Denver School of Medicine
References
- Allolio B, Hoffmann J, Linton EA, Winkelmann W, Kusche M, Schulte HM. Diurnal salivary cortisol patterns during pregnancy and after delivery: Relationship to plasma corticotrophin-releasing-hormone. Clinical Endocrinolology. 1990;33:279–289. doi: 10.1111/j.1365-2265.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Almli CR, Ball RH, Wheeler ME. Human fetal and neonatal movement patterns: Gender differences and fetal-to-neonatal continuity. Developmental Psychobiology. 2001;38:252–273. doi: 10.1002/dev.1019. [DOI] [PubMed] [Google Scholar]
- Bartha J, Martinez-del-Fresno P, Romero-Carmosa R, Hunter A, Comino-Delgado R. Maternal anxiety and fetal behavior at 15 weeks gestation. Ultrasound in Obstetrics and Gynecology. 2003;22:57–62. doi: 10.1002/uog.138. [DOI] [PubMed] [Google Scholar]
- Benediktsson G, Calder A, Edwards C, Seckl J. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clinical Endocrinology. 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Besinger RE, Johnson TRB. Doppler recordings of fetal movement: Clinical correlation with real-time ultrasound. Obstetrics and Gynecology. 1989;74:277–280. [PubMed] [Google Scholar]
- Buitelaar J, Huizink A, Mulder E, Robles de Medina P, Visser G. Prenatal stress and cognitive development and temperament in infants. Neurobiology of Aging. 2003;24:S53–S60. doi: 10.1016/s0197-4580(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Clifton V. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell Tissue Reearch. 2005:63–71. doi: 10.1007/s00441-005-1117-5. [DOI] [PubMed] [Google Scholar]
- Devlin B, Daniels M, Roeder K. The heritability of IQ. Nature. 1997;388:468–471. doi: 10.1038/41319. [DOI] [PubMed] [Google Scholar]
- de Vries J, Fong B. Normal fetal motility: an overview. Ultrasound in Obstetrics and Gynecology. 2006;27:701–711. doi: 10.1002/uog.2740. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar J. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Bornstein MH, Costigan KA, Pressman E, Hahn C, Painter K, et al. What does fetal movement predict about behavior during the first two years of life? Developmental Psychobiology. 2002;40:358–371. doi: 10.1002/dev.10025. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Nelson P, Gurewitsch E, Laudenslager ML. Maternal and fetal responses to induced relaxation during pregnancy. Biological Psychology. 2008;77:11–19. doi: 10.1016/j.biopsycho.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Rubin S, Shiffler D, Henderson J, et al. Prenatal antecedents of newborn neurological maturation. (in press) [DOI] [PMC free article] [PubMed]
- DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RHN, Zavaleta N, et al. Fetal neurobehavioral development: A tale of two cities. Developmental Psychology. 2004a;40:445–456. doi: 10.1037/0012-1649.40.3.445. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield LE, Irizarry RA, Chen P, Merialdi M, Zavaleta N. Prenatal development of intrafetal and maternal-fetal synchrony. Behavioral Neuroscience. 2006;120:687–701. doi: 10.1037/0735-7044.120.3.687. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Pressman EK. Fetal movement detection: Comparison of the Toitu actograph with ultrasound from 20 weeks gestation. Journal of Maternal-Fetal Medicine. 1999;8:237–242. doi: 10.1002/(SICI)1520-6661(199911/12)8:6<237::AID-MFM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Shupe AK, Pressman EK, Johnson TRB. Fetal neurobehavioral development: Associations with socioeconomic class and fetal sex. Developmental Psychobiology. 1998;33:79–91. [PubMed] [Google Scholar]
- DiPietro JA, Hilton SC, Hawkins M, Costigan KA, Pressman EK. Maternal stress and affect influence fetal neurobehavioral development. Developmental Psychology. 2002;38:659–668. [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Johnson TRB. Fetal antecedents of infant temperament. Child Development. 1996;67:2568–2583. [PubMed] [Google Scholar]
- Eaton W, Enns L. Sex differences in human motor activity level. Psychological Bulletin. 1986;100:19–28. [PubMed] [Google Scholar]
- Eaton WO, Saudino KJ. Prenatal activity level as a temperament dimension? Individual differences and developmental functions in fetal movement. Infant Behavior and Development. 1992;15:57–70. [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Gil K, Vera Y. Prenatal maternal cortisol, fetal activity, and growth. International Journal of Neuroscience. 2005;115:423–429. doi: 10.1080/00207450590521082. [DOI] [PubMed] [Google Scholar]
- Ganiban J, Saudino K, Ulbricht J, Neiderhiser J, Reiss D. Stability and change in temperament during adolescence. Journal of Personality and Social Psychology. 2008;95:222–236. doi: 10.1037/0022-3514.95.1.222. [DOI] [PubMed] [Google Scholar]
- Gitau R, Fisk N, Teixeira J, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. Journal of Clinical Endocrinology and Metabolism. 2001;86:104–109. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- Groome L, Swiber M, Holland S, Bentz L, Atterbury J, Trimm R. Spontaneous motor activity in the perinatal infant before and after birth: Stability in individual differences. Developmental Psychobiology. 1999;35:15–24. doi: 10.1002/(sici)1098-2302(199907)35:1<15::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hepper P, Shannon E, Dorman J. Sex differences in fetal mouth movements. Lancet. 1998;350:1820. doi: 10.1016/S0140-6736(05)63635-5. [DOI] [PubMed] [Google Scholar]
- Hepper PG. Fetal response to maternal shock. The Lancet. 1990:1068. doi: 10.1016/0140-6736(90)92537-r. [DOI] [PubMed] [Google Scholar]
- Ianniruberto A, Tajani E. Ultrasonographic study of fetal movement. Seminars in Perinatology. 1981;5:175–181. [PubMed] [Google Scholar]
- Kivlighan K, DiPietro J, Costigan K, Laudenslager M. Diurnal rhythm of cortisol during late pregnancy: associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology. 2008;33:1225–1235. doi: 10.1016/j.psyneuen.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon A, Feldman R, Lewis J, Weller A. Measuring cortisol in human psychobiological studies. Physiology and Behavior. 2007;90:43–53. doi: 10.1016/j.physbeh.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Maeda K, Tatsumura M, Utsu M. Analysis of fetal movements by Doppler actocardiogram and fetal B-mode imaging. Clinics in Perinatology. 1999;26:829–851. [PubMed] [Google Scholar]
- Mulder E, Robles de Medina P, Huizink A, Van den Bergh B, Buitelaar J, Visser G. Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Human Development. 2002;70:3–14. doi: 10.1016/s0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- Murphy V, Gibson P, Giles W, Zakar T, Smith R, Bisits A, et al. Maternal asthma is associated with reduced female fetal growth. American Journal of Respiratory and Critical Care Medicine. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- Nasello-Paterson C, Natale R, Connors G. Ultrasonic evaluation of fetal body movements over twenty-four hours in the human fetus at twenty-four to twenty-eight weeks’ gestation. American Journal of Obstetrics and Gynecology. 1988;158:312–316. doi: 10.1016/0002-9378(88)90145-7. [DOI] [PubMed] [Google Scholar]
- Neu M, Goldstein M, Gao D, Laudenslager ML. Salivary cortisol in preterm infants: Validation of a simple method for collecting saliva for cortisol determination. Early Human Development. 2007;83:47–56. doi: 10.1016/j.earlhumdev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Obel C, Hedegaard M, Henriksen T, Secher N, Olsen J, Levine S. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology. 2005;30:647–656. doi: 10.1016/j.psyneuen.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews M, Matthews S. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behavior. Neuroscience and Biobehavioral Reviews. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Papageorgiou A, Colle E, Farri-Kostopoulos E, Gelfand M. Incidence of respiratory distress syndrome following antenatal betamethasone: role of sex, type of delivery, and prolonged rupture of membranes. Pediatrics. 1981;67:614–617. [PubMed] [Google Scholar]
- Petraglia F, Hatch M, Lapinski R, Stomati M, Reis F, Cobellis L, et al. Lack of effect of psychosocial stress on maternal corticotropin-releasing factor and catecholamine levels at 28 weeks gestation. Journal of the Society for Gynecologic Investigation. 2001;8:83–88. [PubMed] [Google Scholar]
- Roberts AB, Griffin D, Mooney R, Cooper DJ, Campbell S. Fetal activity in 100 normal third trimester pregnancies. British Journal of Obstetrics and Gynaecology. 1980;87:480–484. doi: 10.1111/j.1471-0528.1980.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Roberts B, DelVecchio W. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Robles de Medina P, Visser G, Huizink A, Buitelaar J, Mulder E. Fetal behaviour does not differ between boys and girls. Early Human Development. 2003;73:17–26. doi: 10.1016/s0378-3782(03)00047-1. [DOI] [PubMed] [Google Scholar]
- Roodenburg PJ, Wladimiroff JW, van Es A, Prechtl HFR. Classification and quantitative aspects of fetal movements during the second half of normal pregnancy. Early Human Development. 1991;25:19–35. doi: 10.1016/0378-3782(91)90203-f. [DOI] [PubMed] [Google Scholar]
- Sandman C, Glynn L, Schetter C, Wadwha P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnany predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sjostrom K, Valentin L, Thelin T, Marsal K. Maternal anxiety in late pregnancy: effect on fetal movements and fetal heart rate. Early Human Development. 2002;67:87–100. doi: 10.1016/s0378-3782(01)00256-0. [DOI] [PubMed] [Google Scholar]
- Sontag LW. The significance of fetal envrionmental differences. American Journal of Obstetrics and Gynecology. 1941;42:996–1003. [Google Scholar]
- Sweezey N, Ghibu F, Gagnon S, Schotman E, Hamid Q. Glucocorticoid receptor mRNA and protein in fetal rat lung in vivo: modulation by glucocorticoid and androgen. American Journal of Physiology. 1998;275:L103–L109. doi: 10.1152/ajplung.1998.275.1.L103. [DOI] [PubMed] [Google Scholar]
- ten Hof J, Nijhuis IJM, Mulder EJH, Nijhuis JG, Narayan H, Taylor DJ, et al. Longitudinal study of fetal body movements: nomograms, intrafetal consistency and relationship with episodes of heart rate patterns A and B. Pediatric Research. 2002;52:568–575. doi: 10.1203/00006450-200210000-00017. [DOI] [PubMed] [Google Scholar]
- ten Hof J, Nijhuis IJ, Nijhuis JG, Narayan H, Taylor DJ, Visser GH, et al. Quantitative analysis of fetal general movements: methodological considerations. Early Human Development. 1999;56:57–73. doi: 10.1016/s0378-3782(99)00035-3. [DOI] [PubMed] [Google Scholar]
- Urizar GG, Milazzon M, Le HN, Delucchi K, Sotelo R, Munoz RF. Impact of stress reduction instructions on stress and cortisol levels during pregnancy. Biological Psychology. 2004;67:275–282. doi: 10.1016/j.biopsycho.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B. The influence of maternal emotions during pregnancy on fetal and neonatal behavior. Pre- and Peri-natal Psychology. 1990;5:119–130. [Google Scholar]
- Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosomatic Medicine. 1996;58:432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]