Abstract
Background
Low-grade gliomas (LGGs) are uncommon in older patients, and long-term clinical behavior and prognostic factors are not well defined in this group.
Methods
We retrospectively searched our patient database for the records of adult patients (≥18 years) diagnosed as having nonpilocytic LGG between 1960 and 1992 at Mayo Clinic. The Kaplan-Meier method was used to estimate progression-free survival and overall survival (OS) in patients aged 55 years and older.
Results
Of 314 patients initially identified, 32 were aged at least 55 years, with a median (range) age at diagnosis of 61 (55-74) years. Median follow-up was 17.3 years for survivors. Operative pathologic diagnoses comprised astrocytoma (n=22, 69%), mixed oligoastrocytoma (n=7, 22%), and oligodendroglioma (n=3, 9%). Gross total resection was achieved in 1 patient, radical subtotal resection in 1, and subtotal resection in 14; 16 patients had biopsy only. Postoperative radiotherapy or chemotherapy was given to 23 patients (72%) and 1 patient (3%), respectively. Median OS was 2.7 years for all patients—3 years with resection and 2.2 years with biopsy only (P=.58). The 5- and 10-year OS rates were 31% and 18%, respectively. Factors adversely affecting OS on univariate analysis were enhancement on computed tomography (P<.001) and supratentorial location (P=.03).
Conclusions
This retrospective series of older patients suggests that intracranial LGG in this age group behaves aggressively. Pathologic sampling error failing to recognize higher-grade tumors does not seem to account for these poor outcomes. Aggressive management with maximally safe resection followed by adjuvant therapy should be strongly considered.
Keywords: adult, combined modality therapy, low-grade glioma, radiotherapy, surgery
Low-grade gliomas (LGGs) are uncommon primary brain tumors classified as gliomas of grade I and II by the World Health Organization (WHO) grading system (1). LGGs, more typically diagnosed in younger adults, are known to have widely varying outcomes based on histologic characteristics and other well-documented prognostic factors (2-26). Age has been recognized as an important prognostic factor by numerous authors (3,5,7,13,14,17,18,20,21,27-33). Most authors have specifically used an age of 30, 40, or 45 years as a cutpoint for analysis (5,13,18,27), describing worse outcomes for older cohorts. However, few data are available on patients aged 55 years and older. The limited data suggest that these older patients with LGGs have worse outcomes than younger patients (3,29). Because prospective trials (2-4,34) have not specifically studied older patients, controversy exists about management of LGG in this cohort, including the role for and extent of resection and the optimal timing of radiotherapy (RT), either in the immediate postoperative period or as a salvage strategy at progression. Therefore, we analyzed a cohort of consecutive patients aged at least 55 years with nonpilocytic WHO grade II LGG to assess outcomes and the effects of tumor and treatment characteristics.
Materials and Methods
This study was approved by the Mayo Clinic Institutional Review Board. Our tumor registry was used to retrospectively search for records of consecutive patients aged 18 years or older with newly diagnosed, nonpilocytic LGG who were seen at Mayo Clinic, Rochester, Minnesota, between 1960 and 1992. To be included, patients had to be aged 55 years or older at diagnosis, and a detailed operative report as well as biopsy confirmation and pathologic review from Mayo Clinic were required. Tumors located within the optic tract or lower brainstem were excluded from analysis, as were patients who did not allow their data to be used for medical research.
Data were retrieved regarding patient presentation, extent of resection, histologic type, adjuvant therapy, and other prognostic factors, as well as type of recurrence, progression-free survival (PFS), and overall survival (OS). Recurrence data including imaging, clinical signs and symptoms, pathologic information, or initiation of any additional intervention such as surgery, RT, or chemotherapy were used to determine progression.
The extent of resection—gross total resection (GTR), subtotal resection (STR), or radical subtotal resection (rSTR)—was determined by assessment of the operative report, the neurosurgeon’s impression, and imaging, as available. Resection was deemed rSTR 1) if the operative report specifically described “radical subtotal resection,” 2) if GTR was clearly the operative intent but minimal tumor was known to be left in situ, or 3) if imaging reports indicated minimal, questionable amounts of residual tumor after GTR. Tumors were classified by histologic type and grade per WHO criteria and the Kernohan grading system (23,35,36).
Statistical Analysis
Twelve possible prognostic factors were analyzed for potential association with OS and PFS outcomes by univariate analysis. These variables (age, sex, midline/bilateral involvement, size, histology, extent of resection, RT, chemotherapy, sensorimotor symptoms at presentation, location, Kernohan grade, and enhancement on computed tomography [CT]) were chosen because of their documented prognostic value (2-26).
PFS and OS curves were calculated using the Kaplan-Meier method (37), and PFS and OS were compared between groups with the log-rank test (38). For patients who died without documented recurrence or with unknown disease status, death was considered as censored for recurrence at time of death. All tests were 2-tailed. P<.05 was considered statistically significant.
Results
Patient, Tumor, and Treatment Characteristics
The tumor registry search identified 314 consecutive adult patients with LGG during the study period. Of these, 32 were aged 55 years and older and comprise the study group. These patients were included in a previous analysis of the entire group of 314 patients (39). Patient, tumor, and treatment factors are shown in Table 1. Tumor characteristics were determined from operative and radiographic reports (CT, magnetic resonance imaging [MRI], or angiography) and electroencephalograms. Because the beginning of the study interval (1960) predated the modern neuroimaging era (1975) and the end of the study period (1992) predated routine use of MRI, CT data were available for only 18 patients (56%), and MRI data were available for only 5 patients (16%). Enhancement on imaging was seen for 8 patients (44%) with CT and 1 patient (20%) with MRI. The vast majority of patients (30/32; 94%) had supratentorial tumors.
Table 1.
Patient, Tumor, and Treatment Characteristics (N=32)
| Characteristic | Valuea |
|---|---|
| Age, y | 61 (55-74) |
| Sex | |
| Male | 19 (59) |
| Female | 13 (41) |
| Extent of resection | |
| Gross total | 1 (3) |
| Radical subtotal | 1 (3) |
| Subtotal | 14 (44) |
| Biopsy only | 16 (50) |
| Histology | |
| Astrocytoma | 22 (69) |
| Mixed oligoastrocytoma | 7 (22) |
| Oligodendroglioma | 3 (9) |
| Kernohan gradeb | |
| 1 | 3 (9) |
| 2 | 29 (91) |
| Location | |
| Supratentorial | 30 (94) |
| Infratentorial | 2 (6) |
| Size, cm | |
| ≥5 | 10 (31) |
| <5 | 12 (38) |
| Unknown | 10 (31) |
| Postoperative radiotherapy | |
| Yes | 23 (72) |
| No | 9 (28) |
| Postoperative chemotherapy | |
| Yes | 1 (3) |
| No | 31 (97) |
Values are median (range) or number (percentage).
By definition all patients had World Health Organization grade II tumors.
Surgery and Pathology
Extent of resection was GTR in 1 patient (3%), rSTR in 1 (3%), STR in 14 (44%), and biopsy only in 16 (50%). Histologic subtype was pure astrocytoma in 22 tumors (69%), mixed oligoastrocytoma in 7 (22%), and oligodendroglioma in 3 (9%). Operative pathologic analysis showed 29 (91%) of the 32 WHO grade II tumors to be Kernohan grade 2. Because pilocytic tumors were intentionally excluded from analysis, most Kernohan grade 2 tumors were pure astrocytoma (19/29 [66%]).
Postoperative RT and Chemotherapy
After surgery, 8 patients (25%) were observed and received no adjuvant RT or chemotherapy postoperatively. Postoperative RT was given to 23 patients (72%), and chemotherapy was given to 1 patient (3%). No patient received a combination of RT and chemotherapy.
Of the 23 patients who received postoperative RT, details regarding dose and field were available for review in 19 cases (83%). The median total radiation dose, calculated at midplane for opposed treatments or at isocenter for nonopposed treatments, was 54 Gy (range, 40-70.2 Gy) in a median of 30 fractions over a median 42 days. The 2 most common total doses delivered were 50 or 50.4 Gy (6 patients [32%]) and 60 Gy (4 patients [21%]). Whole-brain RT was administered to 3 patients (16%), whole-brain RT plus partial-brain boost to 2 patients (11%), and a partial-brain–only field to 14 patients (73%).
Survival Outcomes
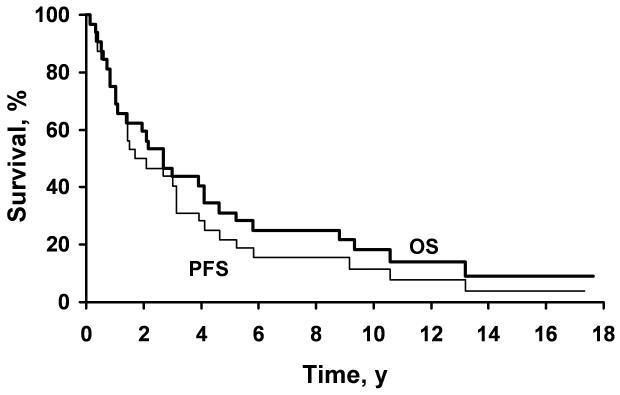
The median OS was 2.7 years with a range of 1.2 months to 17.6 years; 28 deaths were documented. The 5-year OS was 31%, and the 10-year OS was 18% (Figure 1). Nineteen patients (59%) died from or with disease, 6 (19%) died from unknown cause and/or with undocumented disease status, and 3 (9%) died without known evidence of disease. Of the 4 patients who were alive at last follow-up, 2 (6%) were alive without evidence of disease, 1 (3%) was alive with disease, and 1 (3%) was alive with disease status unknown.
Figure 1.
Kaplan-Meier curves showing estimated overall survival (OS) and progression-free survival (PFS) for all 32 patients.
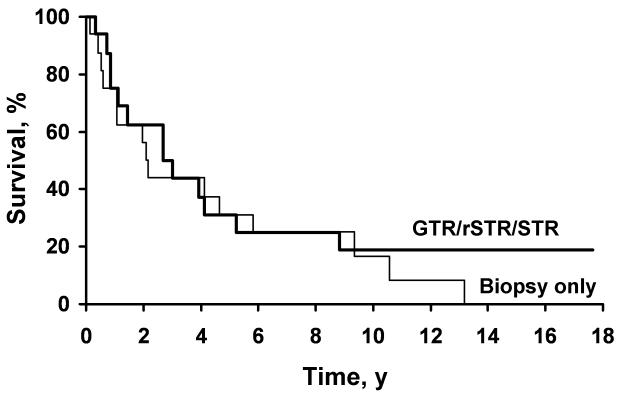
The only adverse prognostic factors for OS identified on univariate analysis were enhancement on CT (P<.001) and supratentorial location of tumor (P=.03) (Table 2). Patients with Kernohan grade 2 disease and astrocytoma histologic type had decreased median OS, but the associations were not statistically significant (P=.08 and P=.15, respectively). Neither postoperative RT nor chemotherapy affected OS (P=.25 and P=.17, respectively). Analysis of OS by extent of resection (biopsy only vs GTR/rSTR/STR) showed the 5-year survivals to be 31% and 31% and the 10-year survivals to be 17% and 19%, respectively (P=.58; Figure 2). When the enhancement data were analyzed to include either CT or MRI, any enhancement by either modality remained statistically significant (P<.001). However, because MRI information was only available for 5 patients, and 4 of these 5 patients also had CT information, this result was not unexpected. Furthermore, because MRI was infrequently available, further statistical analysis regarding MRI was not performed.
Table 2.
Univariate Analysis of Overall and Progression-Free Survival (N=32)
| Overall Survival |
Progression-Free Survival |
|||
|---|---|---|---|---|
| Risk Factor | Median, y | P | Median, y | P |
| Sex | .94 | .52 | ||
| Male | 2.1 | 2.1 | ||
| Female | 2.7 | 1.7 | ||
| Age, y | .69 | .50 | ||
| ≤61 | 2.7 | 1.7 | ||
| >61 | 2.1 | 2.1 | ||
| Sensorimotor symptoms at presentation |
.59 | .35 | ||
| No | 4.1 | 3.0 | ||
| Yes | 1.9 | 1.3 | ||
| Tumor size, cm | .82 | .52 | ||
| <5 | 2.7 | 1.5 | ||
| ≥5 | 4.1 | 3.1 | ||
| Extent of resection | .57 | .93 | ||
| GTR/rSTR/STR | 3.0 | 3.0 | ||
| Biopsy only | 2.2 | 1.5 | ||
| Histology | .15 | .17 | ||
| Astrocytoma | 2.1 | 2.1 | ||
| Other | 8.8 | 3.1 | ||
| Kernohan grade | .08 | .82 | ||
| 1 | Not met | 3.1 | ||
| 2 | 2.7 | 1.7 | ||
| Location | .03 | .54 | ||
| Infratentorial | Not met | 9.1 | ||
| Supratentorial | 2.7 | 1.7 | ||
| Midline/bilateral involvement | .22 | .91 | ||
| Yes | 4.1 | 1.7 | ||
| No | 2.7 | 3.1 | ||
| CT enhancement | <.001 | <.001 | ||
| Yes | 0.9 | 0.9 | ||
| No | 9.3 | 3.0 | ||
| Postoperative radiotherapy | .25 | .99 | ||
| Yes | 2.2 | 1.7 | ||
| No | 4.1 | 3.0 | ||
| Postoperative chemotherapy | .17 | .17 | ||
| Yes | 0.9 | 0.9 | ||
| No | 2.7 | 2.1 | ||
Abbreviations: CT, computed tomography; GTR, gross total resection; rSTR, radical subtotal resection; STR, subtotal resection.
Figure 2.
Kaplan-Meier curves showing estimated overall survival by extent of resection: biopsy only (n=16) versus gross total resection, radical subtotal resection, and subtotal resection (GTR/rSTR/STR) (n=16) (P=.58).
Because disease status at death was unknown or undocumented for 6 patients, cause-specific survival was analyzed using 2 scenarios by which these patients were assumed to have 1) died of disease or 2) died of other causes. Median cause-specific survival for these 2 scenarios was 2.7 years and 4.1 years, respectively.
Outcomes by Histology
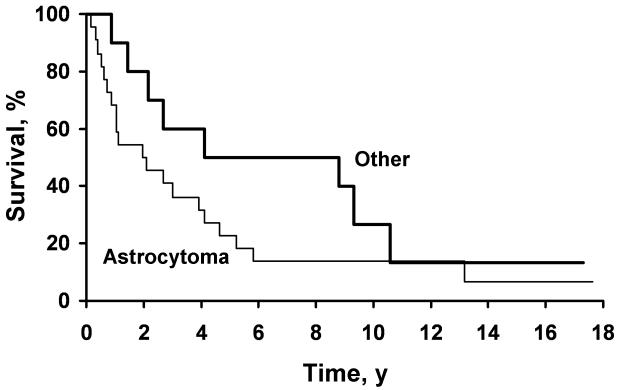
Median survival for patients with tumors of pure astrocytoma histology was 2.1 years and was 8.8 years for patients with oligodendrogliomas and mixed oligoastrocytomas. Survival at 2, 5, and 10 years was 50%, 23%, and 14% for astrocytoma histology and 80%, 50%, and 27% for other histology (P=.15; Figure 3). OS and tumor histology were analyzed using the Cox proportional hazards model to control for age (40). After accounting for age, pure astrocytoma histology still was associated with worse OS, but this difference failed to reach statistical significance (hazard ratio, 2.0; P=.13).
Figure 3.
Kaplan-Meier curves showing estimated overall survival by tumor histology: astrocytoma (n=22) versus other type (n=10) (P=.15).
Recurrence and Progression Outcomes
The median length of follow-up was 17.3 years for surviving patients. Recurrences were documented in 11 patients with a median time to progression of 4.1 years and a median PFS of 2.1 years. The 2-, 5-, and 10-year PFS rates were 50%, 22%, and 12%, respectively (Figure 1). The only factor found to be associated with decreased PFS was enhancement on CT (P<.001; Table 2). No other stastically significant adverse prognostic factors for PFS were identified on univariate analysis.
Analysis by extent of resection (biopsy alone vs GTR/rSTR/STR) showed no differences in PFS. The 5-year PFS was 25% for those who underwent biopsy alone versus 19% for those with GTR/rSTR/STR (P=.93). Postoperative RT also did not affect PFS. However, by χ2 analysis, use of RT was statistically associated with factors traditionally regarded to be prognostically adverse (2-26). Specifically, RT was preferentially delivered to patients with higher Kernohan grade tumors (grade 2 vs grade 1) (P=.004), supratentorial tumor location (P=.02), and biopsy only (P=.04).
Findings at Recurrence
Presumed recurrences were documented in 11 patients. Among these, symptoms were documented at recurrence in 10 patients (91%). Eight patients (73%) had documented radiographic disease recurrence. Four underwent at least a biopsy with confirmation of recurrence. In 2 of these patients, the tumor was found to have progressed to a higher grade than was recorded at diagnosis.
Discussion
To our knowledge, this is the largest report on outcomes of older patients with LGG. Only 2 other reports have targeted this rare entity. An abstract recently reported on 16 patients aged 55 years and older with supratentorial WHO grade II gliomas (29). Nine patients underwent at least a partial resection and 7 patients underwent biopsy alone. Ten of 16 patients (63%) received adjuvant therapy consisting of RT, chemotherapy, or both. They reported a median PFS of 1.5 years. Similar to our findings, neither extent of resection nor use of adjuvant therapy was associated with OS. The authors concluded that LGG in patients 55 years of age or older behaves like a more malignant process and suggested aggressive management. Another recent report by Pouratian et al (33), described outcomes for 20 patients aged 60 years or older; after a median follow-up of 27.3 months, median survival was 2.3 years. Our findings of a median OS of 2.7 years and median PFS of 2.1 years also support the idea that LGG follows a more malignant course in older patients; this prognosis is similar for anaplastic types of primary brain tumors (Table 3).
Table 3.
Overall Survival and Progression-Free Survival Results for Intracranial Gliomas
| Histology (WHO Grade) [References] | Median OS, y | Median PFS, y |
|---|---|---|
| Glioblastoma multiforme (IV) [34,41-47] | ≤1.2 | ≤0.5 |
| Anaplastic astrocytoma (III) [43,45,46,48-50] | 2-3 | ≤1 |
| Anaplastic oligoastrocytoma (III) [43,45,46,48-52] | 2-5 | ≤1-2 |
| Anaplastic oligodendroglioma (III) [43,45,46,48-54] | 2-10 | ≤1-2.5 |
| Low-grade astrocytoma (II) [2-4,13,18,22,34,39] | 5 | 3 |
| Low-grade oligoastrocytoma (II) [3,4,13,22,34,39] | 7 | 3-5 |
| Low-grade oligodendroglioma (II) [3,4,13,22,34,39] | 10 | 3-7 |
| Low-grade glioma, age ≥55 y (II) [29] | NR | 1.5 |
| Astrocytoma | 2.1 | 2.1 |
| Oligoastrocytoma | 4.1 | 3.1 |
| Oligodendroglioma | 9.3 | 1.7 |
| Low-grade glioma, age ≥55 y (II) N=32 [present study] | 2.7 | 2.1 |
Abbreviations: NR, not reported; OS, overall survival; PFS, progression-free survival; WHO, World Health Organization.
Although the differences in OS for different histologic types did not reach statistical significance, astrocytoma was associated with decreased median OS, similar to previous descriptions (2-4,13,18,22,34,39). Median OS for pure astrocytoma was 2.1 years but was 8.8 years for oligodendrogliomas and mixed oligoastrocytomas; 10-year OS was 14% and 27%, respectively (P=.15; Figure 3). An explanation for the poor outcome in older patients may lie in the lower percentage of patients with oligodendrogliomas, a histologic type known to carry a better prognosis (3,4,13,22,34,39). Specifically, in randomized trials from the European Organisation for Research and Treatment of Cancer (EORTC) with a younger cohort, a much higher percentage of patients had oligodendroglioma tumors than in the present cohort (21%-25% vs 9%) (2,34). It is possible that this shift in histology alone may account for the poor outcomes seen with older patients. This explanation is in part supported by our data showing poorer outcomes for pure astrocytomas, even though it did not reach statistical significance (P=.15).
Surgery allows for tissue diagnosis as well as potential therapeutic benefit. However, no prospective trials to date have specifically compared up-front surgery with a conservative approach of delayed surgery. Therefore, when considering both retrospective and prospective data based largely on younger patients, many neurosurgeons favor maximally safe resection (2,3,6,8,12,18,24,26,27,55-57). Although not randomized, 3 prospective reports correlated aggressive surgery (GTR or near GTR) with improved prognosis (2,3,58). However, none of these studies specifically reported outcome for older patients as it relates to extent of resection.
Contrary to data based largely on younger cohorts, the current study of patients aged 55 years and older did not indicate improved outcomes for patients who underwent more aggressive resections—median OS was 3 years for patients undergoing GTR/rSTR/STR and 2.2 years for those who had biopsy only (P=.57). However, other data from our institution suggest that GTR and rSTR correlate with improved OS and PFS (39), and only 2 patients in the present analysis underwent such aggressive resection, precluding meaningful statistical analysis. This finding argues against the idea that the poor OS outcomes in these patients could be explained by pathologic sampling error.
For younger patients, retrospective data are inconsistent relative to the effects of RT in the postoperative setting. Because of perceived potential toxicities and the indolent nature of the disease in younger patients (56,59), some advocate delaying RT until progression, appearance of symptoms, or evidence of high-grade transformation (10,13,32,56,58-70). Specifically, the EORTC (EORTC 22845) evaluated the timing of RT, randomly assigning patients to immediate RT (54 Gy) or observation. Postoperative RT significantly prolonged PFS (median, 5.3 vs 3.4 years) without affecting OS (median, 7.4 vs 7.2 years) (4,34). Even though the EORTC allowed enrollment of older patients (up to 65 years of age; 20% >50 years old), they did not specifically report outcomes for older patients. Furthermore, the study by Pouratian et al (33), on multivariate analysis, showed improved outcomes for older patients receiving postoperative RT. Contrary to the EORTC data and the Pouratian et al (33) study, the current report did not show an association of the use of postoperative RT with PFS or OS outcomes. It is possible that the small sample size of our study precluded the ability to detect differences in outcomes based on prognostic and treatment factors.
Only 1 patient (3%) received chemotherapy at the time of diagnosis. In younger patients, no strong evidence exists for routine use of postoperative chemotherapy, especially in light of 2 phase 3 trials that did not show significant improvement in either OS or PFS (58,71). Temozolomide has been used in some small trials to delay RT in younger patients with newly diagnosed LGG (72,73). Ongoing trials by the EORTC, the Eastern Cooperative Oncology Group, and the Radiation Therapy Oncology Group will evaluate temozolomide’s role for patients at high risk for early progression. Although these trials do not specifically target older patients, they are intended to analyze temozolomide’s effects for patients deemed high risk. Therefore, the results of these trials may prove useful in making treatment decisions regarding older patients with LGGs. Furthermore, patients aged 40 years or older (18) or those undergoing less than GTR are considered to be at high risk for recurrence and subsequent death and are thus offered immediate RT in the Radiation Therapy Oncology Group 9802 trial (18,28). Therefore, our institution currently favors early use of RT for patients who have undergone STR or biopsy alone or who are 40 years or older.
As a retrospective report, this review has some limitations. In order to assess long-term survivorship, the analysis ended in 1992; this allowed for a long follow-up (median, 17.3 years) but predated the routine use of CT and MRI. In contrast, the more modern study of Pouratian et al (33) included CT and MRI but had a much shorter follow-up (median, 2.3 years). Our study design precluded collection of certain data such as radiographic assessment of extent of resection, status of disease at death in some patients, and molecular marker prognostics. The specific rationale for postsurgical therapies also could not be determined retrospectively. Furthermore, only 1 of our 32 study patients underwent GTR, which could lead to sampling error. However, 14 patients underwent STR, and the outcomes for those patients were no different than those for the 16 patients who underwent biopsy only; this finding argues against a significant role for sampling error. Finally, after treatment, patients were not followed up prospectively; therefore, only limited data regarding salvage therapy and neurocognitive toxicities were available.
Because of the acknowledged limitations of our dataset, we did not have the data needed to validate the EORTC prognostic factors for this population (18). Thus, we cannot know from this dataset whether these factors alone would predict outcome in these patients or if “older” age is also significant. Based on the EORTC data of Pignatti et al (18), patients with 2 risk factors (such as age and astrocytoma histology) would have had a median survival of 5.5 years. Some of our patients with astrocytoma likely would have fit into that category. Median survival in our group of patients with astrocytoma was only 2.1 years. We cannot ascertain whether survival was lower in our cohort because of other risk factors (size, tumor crossing midline, neurologic deficit) or because of some undetermined factor associated with the older age of this population.
In summary, analysis of a series of older patients with nonpilocytic WHO grade II LGG with long-term follow-up indicates that, for most patients, LGGs in this age group behave aggressively and prognosis is quite poor. We were not able to identify a subset of patients who had significantly better outcomes. Pathologic sampling error failing to recognize higher-grade tumors does not seem to account for these poor outcomes. Because of the aggressive nature of LGGs in older patients, aggressive management with maximally safe resection followed by the early use of adjuvant therapy should be strongly considered.
Acknowledgments
We thank the biostatisticians at the Mayo Clinic Center for Translational Science Activities.
The project described was supported by Grant Number 1 UL1 RR024150-01* from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overviewtranslational.asp.
Abbreviations
- CT
computed tomography
- EORTC
European Organisation for Research and Treatment of Cancer
- GTR
gross total resection
- LGG
low-grade glioma
- MRI
magnetic resonance imaging
- OS
overall survival
- PFS
progression-free survival
- rSTR
radical subtotal resection
- RT
radiotherapy
- STR
subtotal resection
- WHO
World Health Organization
Footnotes
Conflict of interest: None
Presented at the 13th Annual Scientific Meeting of the Society for Neuro-Oncology, Lake Las Vegas, NV, November 20-23, 2008.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007 Aug;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996 Oct 1;36(3):549–56. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 3.Shaw E, Arusell R, Scheithauer B, O’Fallon J, O’Neill B, Dinapoli R, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002 May 1;20(9):2267–76. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 4.van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005 Sep 17-23;366(9490):985–90. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 5.Bauman G, Lote K, Larson D, Stalpers L, Leighton C, Fisher B, et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999 Nov 1;45(4):923–9. doi: 10.1016/s0360-3016(99)00284-9. [DOI] [PubMed] [Google Scholar]
- 6.Berben DMMJ. Follow-up of post-op irradiation for low-grade gliomas: results [abstract] Neuro-Oncol. 2006;8(4):335–6. [Google Scholar]
- 7.Brown PD, Buckner JC, O’Fallon JR, Iturria NL, O’Neill BP, Brown CA, et al. Importance of baseline mini-mental state examination as a prognostic factor for patients with low-grade glioma. Int J Radiat Oncol Biol Phys. 2004 May 1;59(1):117–25. doi: 10.1016/j.ijrobp.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Claus EB, Horlacher A, Hsu L, Schwartz RB, Dello-Iacono D, Talos F, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005 Mar 15;103(6):1227–33. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 9.Daniels TB, Brown PD, Ballman K, Felton S, Buckner J, Arusell RM, et al. Validation of EORTC prognostic factors for adults with low grade glioma: a report utilizing intergroup 86-72-51. Int J Radiat Oncol Biol Phys. 2006;66(3 Suppl 1):S83. doi: 10.1016/j.ijrobp.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janny P, Cure H, Mohr M, Heldt N, Kwiatkowski F, Lemaire JJ, et al. Low grade supratentorial astrocytomas: management and prognostic factors. Cancer. 1994 Apr 1;73(7):1937–45. doi: 10.1002/1097-0142(19940401)73:7<1937::aid-cncr2820730727>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Karim ABM, Cornu P, Bleehen N, Afra D, Witte OD, Schraub S, et al. Immediate postoperative radiotherapy in low grade glioma improves progression free survival but not overall survival: preliminary results of an EORTC/MRC randomized phase III study. Proc Am Soc Clin Oncol. 1998;17:400a. [Google Scholar]
- 12.Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001 Nov;95(5):735–45. doi: 10.3171/jns.2001.95.5.0735. [DOI] [PubMed] [Google Scholar]
- 13.Leighton C, Fisher B, Bauman G, Depiero S, Stitt L, MacDonald D, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997 Apr;15(4):1294–301. doi: 10.1200/JCO.1997.15.4.1294. [DOI] [PubMed] [Google Scholar]
- 14.Lote K, Egeland T, Hager B, Stenwig B, Skullerud K, Berg-Johnsen J, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997 Sep;15(9):3129–40. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- 15.Marsa GW, Goffinet DR, Rubinstein LJ, Bagshaw MA. Megavoltage irradiation in the treatment of gliomas of the brain and spinal cord. Cancer. 1975 Nov;36(5):1681–9. doi: 10.1002/1097-0142(197511)36:5<1681::aid-cncr2820360523>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Medbery CA, 3rd, Straus KL, Steinberg SM, Cotelingam JD, Fisher WS. Low-grade astrocytomas: treatment results and prognostic variables. Int J Radiat Oncol Biol Phys. 1988 Oct;15(4):837–41. doi: 10.1016/0360-3016(88)90115-0. [DOI] [PubMed] [Google Scholar]
- 17.Nicolato A, Gerosa MA, Fina P, Iuzzolino P, Giorgiutti F, Bricolo A. Prognostic factors in low-grade supratentorial astrocytomas: a uni-multivariate statistical analysis in 76 surgically treated adult patients. Surg Neurol. 1995 Sep;44(3):208–21. doi: 10.1016/0090-3019(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 18.Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002 Apr 15;20(8):2076–84. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 19.Shafqat S, Hedley-Whyte ET, Henson JW. Age-dependent rate of anaplastic transformation in low-grade astrocytoma. Neurology. 1999 Mar 10;52(4):867–9. doi: 10.1212/wnl.52.4.867. [DOI] [PubMed] [Google Scholar]
- 20.Shaw EG, Daumas-Duport C, Scheithauer BW, Gilbertson DT, O’Fallon JR, Earle JD, et al. Radiation therapy in the management of low-grade supratentorial astrocytomas. J Neurosurg. 1989 Jun;70(6):853–61. doi: 10.3171/jns.1989.70.6.0853. [DOI] [PubMed] [Google Scholar]
- 21.Shaw EG, Scheithauer BW, Gilbertson DT, Nichols DA, Laws ER, Earle JD, et al. Postoperative radiotherapy of supratentorial low-grade gliomas. Int J Radiat Oncol Biol Phys. 1989 Mar;16(3):663–8. doi: 10.1016/0360-3016(89)90482-3. [DOI] [PubMed] [Google Scholar]
- 22.Shaw EG, Scheithauer BW, O’Fallon JR. Supratentorial gliomas: a comparative study by grade and histologic type. J Neurooncol. 1997 Feb;31(3):273–8. doi: 10.1023/a:1005715703598. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblum MK. The 2007 WHO Classification of Nervous System Tumors: newly recognized members of the mixed glioneuronal group. Brain Pathol. 2007 Jul;17(3):308–13. doi: 10.1111/j.1750-3639.2007.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JS, Chang EF, Lamborn KR, Chang S, Prados MD, Cha S, et al. The role of extent of resection in the long-term outcome of low-grade hemispheric gliomas [abstract] Neuro-oncol. 2007;9(4):599–600. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 25.Westergaard L, Gjerris F, Klinken L. Prognostic parameters in benign astrocytomas. Acta Neurochir (Wien) 1993;123(1-2):1–7. doi: 10.1007/BF01476278. [DOI] [PubMed] [Google Scholar]
- 26.Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults: the prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989 Oct;71(4):487–93. doi: 10.3171/jns.1989.71.4.0487. [DOI] [PubMed] [Google Scholar]
- 27.Hanzely Z, Polgar C, Fodor J, Brucher JM, Vitanovics D, Mangel LC, et al. Role of early radiotherapy in the treatment of supratentorial WHO Grade II astrocytomas: long-term results of 97 patients. J Neurooncol. 2003 Jul;63(3):305–12. doi: 10.1023/a:1024376719067. [DOI] [PubMed] [Google Scholar]
- 28.Shaw EG, Won M, Brachman DG, Schultz CJ, Suh JH, Buckner JC, et al. Preliminary results of RTOG Protocol 9802: a phase II study of observation in completely resected adult low-grade glioma. Neuro-oncol. 2005;7:284. [Google Scholar]
- 29.Chi A, Batchelor TT. Low-grade gliomas in older patients: a malignant tumor? Neuro oncol. 2007;9(4):545. [Google Scholar]
- 30.Laws ER, Jr, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984 Oct;61(4):665–73. doi: 10.3171/jns.1984.61.4.0665. [DOI] [PubMed] [Google Scholar]
- 31.Shaw E, Arusell R, Scheithauer B, O’Fallon J, O’Neill B, Dinapoli R, et al. A prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a NCCTG-RTOG-ECOG study. Proc Am Soc Clin Oncol. 1998;17:401a. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 32.van Veelen ML, Avezaat CJ, Kros JM, van Putten W, Vecht C. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998 May;64(5):581–7. doi: 10.1136/jnnp.64.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pouratian N, Mut M, Jagannathan J, Lopes MB, Shaffrey ME, Schiff D. Low-grade gliomas in older patients: a retrospective analysis of prognostic factors. J Neurooncol. 2008 Dec;90(3):341–50. doi: 10.1007/s11060-008-9669-3. Epub 2008 Aug 6. [DOI] [PubMed] [Google Scholar]
- 34.Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, De Witte O, et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys. 2002 Feb 1;52(2):316–24. doi: 10.1016/s0360-3016(01)02692-x. [DOI] [PubMed] [Google Scholar]
- 35.Kernohan JW, Sayre GP. Tumors of the central nervous system. Armed Forces Institute of Pathology; Washington DC: c1952. pp. 17–42. [Google Scholar]
- 36.Zulch KJ. Histological Typing of Tumours of the Central Nervous System. World Health Organization; Geneva: c1979. World Health Organization Classification of Tumours. [Google Scholar]
- 37.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 38.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schomas DA, Laack NN, Rao RD, Meyer FB, Shaw EG, O’Neill BP, et al. Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol. 2008 Nov 18; doi: 10.1215/15228517-2008-102. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox DR. Statistical significance tests. Br J Clin Pharmacol. 1982 Sep;14(3):325–31. doi: 10.1111/j.1365-2125.1982.tb01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993 May 20;26(2):239–44. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 42.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979 Oct;5(10):1725–31. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 43.Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J Neurosurg. 1978 Sep;49(3):333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 44.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001 Aug;95(2):190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 45.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993 May 5;85(9):704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 46.Kristiansen K, Hagen S, Kollevold T, Torvik A, Holme I, Nesbakken R, et al. Combined modality therapy of operated astrocytomas grade III and IV: confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer. 1981 Feb 15;47(4):649–52. doi: 10.1002/1097-0142(19810215)47:4<649::aid-cncr2820470405>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 47.Athanassiou H, Synodinou M, Maragoudakis E, Paraskevaidis M, Verigos C, Misailidou D, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005 Apr 1;23(10):2372–7. doi: 10.1200/JCO.2005.00.331. [DOI] [PubMed] [Google Scholar]
- 48.Laramore GE, Martz KL, Nelson JS, Griffin TW, Chang CH, Horton J. Radiation Therapy Oncology Group (RTOG) survival data on anaplastic astrocytomas of the brain: does a more aggressive form of treatment adversely impact survival? Int J Radiat Oncol Biol Phys. 1989 Dec;17(6):1351–6. doi: 10.1016/0360-3016(89)90549-x. [DOI] [PubMed] [Google Scholar]
- 49.Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990 Feb;18(2):321–4. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- 50.Prados MD, Scott C, Curran WJ, Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: A retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999 Nov;17(11):3389–95. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]
- 51.Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006 Jun 20;24(18):2707–14. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 52.van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006 Jun 20;24(18):2715–22. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 53.Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res. 2001 Apr;7(4):839–45. [PubMed] [Google Scholar]
- 54.Ino Y, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO, Jhung S, et al. Long survival and therapeutic responses in patients with histologically disparate high-grade gliomas demonstrating chromosome 1p loss. J Neurosurg. 2000 Jun;92(6):983–90. doi: 10.3171/jns.2000.92.6.0983. [DOI] [PubMed] [Google Scholar]
- 55.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008 Mar 10;26(8):1338–45. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 56.Mulhern RK, Ochs J, Kun LE. Changes in intellect associated with cranial radiation therapy. In: Gutin PH, Leibel SA, Sheline GE, editors. Radiation injury to the nervous system. Raven Press; New York: c1991. pp. 325–40. [Google Scholar]
- 57.McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008 Oct;63(4):700–7. doi: 10.1227/01.NEU.0000325729.41085.73. [DOI] [PubMed] [Google Scholar]
- 58.Shaw EG, Berkey B, Coons SW, Brachman D, Buckner JC, Stelzer KJ, et al. Initial report of Radiation Therapy Oncology Group (RTOC) 9802: prospective studies in adult low-grade glioma (LGG) [abstract] J Clin Oncol. 2006;24(18S):1500. [Google Scholar]
- 59.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995 Mar 30;31(5):1093–112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 60.Bahary JP, Villemure JG, Choi S, Leblanc R, Olivier A, Bertrand G, et al. Low-grade pure and mixed cerebral astrocytomas treated in the CT scan era. J Neurooncol. 1996 Feb;27(2):173–7. doi: 10.1007/BF00177481. [DOI] [PubMed] [Google Scholar]
- 61.Cairncross JG, Laperriere NJ. Low-grade glioma. To treat or not to treat? Arch Neurol. 1989 Nov;46(11):1238–9. doi: 10.1001/archneur.1989.00520470106035. [DOI] [PubMed] [Google Scholar]
- 62.Grabenbauer GG, Roedel CM, Paulus W, Ganslandt O, Schuchardt U, Buchfelder M, et al. Supratentorial low-grade glioma: results and prognostic factors following postoperative radiotherapy. Strahlenther Onkol. 2000 Jun;176(6):259–64. doi: 10.1007/s000660050007. [DOI] [PubMed] [Google Scholar]
- 63.Jubelirer SJ, Rubin M, Shim C. An analysis of 38 cases of low-grade cerebral astrocytoma in adults. W V Med J. 1993 Mar;89(3):102–5. [PubMed] [Google Scholar]
- 64.Morantz RA. Radiation therapy in the treatment of cerebral astrocytoma. Neurosurgery. 1987 Jun;20(6):975–82. doi: 10.1227/00006123-198706000-00028. [DOI] [PubMed] [Google Scholar]
- 65.Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000 Apr 11;54(7):1442–8. doi: 10.1212/wnl.54.7.1442. [DOI] [PubMed] [Google Scholar]
- 66.Philippon JH, Clemenceau SH, Fauchon FH, Foncin JF. Supratentorial low-grade astrocytomas in adults. Neurosurgery. 1993 Apr;32(4):554–9. doi: 10.1227/00006123-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Piepmeier J, Christopher S, Spencer D, Byrne T, Kim J, Knisel JP, et al. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery. 1996 May;38(5):872–8. doi: 10.1097/00006123-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Recht LD, Lew R, Smith TW. Suspected low-grade glioma: is deferring treatment safe? Ann Neurol. 1992 Apr;31(4):431–6. doi: 10.1002/ana.410310413. [DOI] [PubMed] [Google Scholar]
- 69.Reijneveld JC, Sitskoorn MM, Klein M, Nuyen J, Taphoorn MJ. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001 Mar 13;56(5):618–23. doi: 10.1212/wnl.56.5.618. [DOI] [PubMed] [Google Scholar]
- 70.Soffietti R, Chio A, Giordana MT, Vasario E, Schiffer D. Prognostic factors in well-differentiated cerebral astrocytomas in the adult. Neurosurgery. 1989 May;24(5):686–92. doi: 10.1227/00006123-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Eyre HJ, Crowley JJ, Townsend JJ, Eltringham JR, Morantz RA, Schulman SF, et al. A randomized trial of radiotherapy versus radiotherapy plus CCNU for incompletely resected low-grade gliomas: a Southwest Oncology Group study. J Neurosurg. 1993 Jun;78(6):909–14. doi: 10.3171/jns.1993.78.6.0909. [DOI] [PubMed] [Google Scholar]
- 72.Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004 Aug 1;22(15):3133–8. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 73.Kaloshi G, Benouaich-Amiel A, Diakite F, Taillibert S, Lejeune J, Laigle-Donadey F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007 May 22;68(21):1831–6. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]