Abstract
This study examined the associations between older adults’ daily physical symptoms (e.g., chest pain or difficulty breathing) and 2-year changes in chronic health problems (e.g., cardiovascular disease or cancer) and in functional problems (e.g., difficulty dressing or moving around at home). We reasoned that these associations depend on a person’s active control processes aimed at counteracting physical health problems (i.e., health-engagement control strategies, or HECS). In particular, we hypothesized that high levels of HECS buffer the adverse effect of daily physical symptoms on increases in chronic and functional health problems. We found that daily physical symptoms were associated with declines in chronic and functional health among older adults who were not engaged in addressing their health problems, but not among their counterparts who reported high levels of HECS. These findings suggest that active control strategies play an important role in the maintenance of older adults’ physical health.
Rage, rage against the dying of the light.
—Dylan Thomas (1951, p. 208)
The increase in human life expectancy (Oeppen & Vaupel, 2002) presents a challenge to scientists and practitioners because old age is not always associated with good health. Many older adults experience chronic health problems, such as high blood pressure, cardiovascular disease, and arthritis (Centers for Disease Control and Prevention, 2007). Moreover, chronic illness and disability contribute to emotional distress and instigate biological changes that accelerate subsequent health declines (Dew, 1998; Lenze et al., 2001, 2005; Schulz, Martire, Beach, & Scheier, 2000; Wrosch, Schulz, Miller, Lupien, & Dunne, 2007). These facts imply that older adults are at risk of experiencing a downward spiral in which physical, psychological, and biological factors influence each other and compromise quality of life.
As a first step toward identifying psychological factors that could prevent adverse consequences of illness and disability on older adults’ quality of life, we proposed that older adults’ use of active control strategies may help prevent declines in their psychological and physical health. Such strategies include investing time and energy in addressing health challenges, seeking help when health problems are encountered, and building a strong commitment toward overcoming threats to physical health. The life-span theory of control identifies these three types of control strategies as core motivational processes that support the attainment of personal goals (Heckhausen & Schulz, 1995; Schulz & Heckhausen, 1996). In addition, these types of strategies are typically correlated and, among older adults, can form a single factor, which we named health-engagement control strategies (HECS; Wrosch, Schulz, & Heckhausen, 2002, 2004; Wrosch et al., 2007).1
We think that it is important to examine the relation between adaptive control strategies and physical health because overcoming health threats has implications for a variety of outcomes. Our previous work demonstrated that high levels of HECS can prevent depressive symptoms (Wrosch et al., 2002) among older adults who confront treatable physical threats. Moreover, the emotional benefits of HECS predicted adaptive levels of diurnal cortisol secretion (Wrosch et al., 2007), a biological process that is widely thought to play an important role in the health-related consequences of stressful experiences (Kiecolt-Glaser, Mc-Guire, & Robles, 2002; Miller, Chen, & Zhou, 2007; Segerstrom & Miller, 2004). Thus, HECS are adaptive control strategies that enable older adults to overcome common late-life stressors.
However, whether HECS also play a role in the prevention, development, or exacerbation of older adults’ physical health problems has not been investigated. This question is important because identifying pathways to the maintenance and improvement of physical health can lead to improved intervention strategies. We addressed this gap in the literature by examining whether high levels of HECS buffer the adverse effects of daily physical symptoms (e.g., chest pain, leg pain, or difficulty breathing) on chronic health problems and functional difficulties over time. Daily physical symptoms may be a sign of underlying or developing disease (e.g., cardiovascular disease, arthritis, or cancer), which could be actively managed through control strategies that optimize appropriate treatment and health-maintenance behaviors (Wrosch et al., 2002, 2007). In addition, failing to actively address these problems may exacerbate existing health conditions and accelerate functional declines (Williamson & Schulz, 1995).
On the basis of these considerations, we hypothesized that HECS forecast whether daily physical symptoms result in chronic and functional health declines. Although we expected that older adults who are not engaged in actively addressing their daily physical symptoms experience an increase in chronic and functional health problems, we hypothesized that high levels of HECS buffer an effect of daily physical symptoms on prospective health declines. Finally, given that low levels of HECS may be associated with emotional problems and cortisol dysregulation (Wrosch et al., 2007), we explored whether these factors partially mediate the hypothesized health effects.
METHOD
Participants
The study we report here is based on 184 adults who took part in a 2-year follow-up (range = 1.73–2.13 years) that was part of the Montreal Aging and Health Study (N at baseline, or T1 = 215, Wrosch et al., 2007). Participants were recruited through a newspaper advertisement. Because we were interested in examining a normative sample of older adults, the only inclusion criterion was that participants had to be more than 60 years old. At baseline, participants were, on average, 72.27 years old (SD = 5.79); 94 participants were female and 121 were male; 60 participants had received an undergraduate university degree. Attrition was not significantly associated with baseline measures of the study variables.
Measures
The main measures were measures of chronic health problems, difficulties with activities of daily living (ADL), daily physical symptoms, and HECS. In addition, age, sex, and socioeconomic status (SES) were used as control variables, and depressive mood and diurnal cortisol secretion were assessed to examine potential mediation effects.
Chronic health problems and ADL difficulties were measured both at baseline and at the 2-year follow-up (T2). Participants reported whether they were affected by 17 different health problems (e.g., heart disease, cancer, high blood pressure, stroke, arthritis, lung disease, and diabetes). In addition, participants reported whether they had difficulty with performing each of six ADLs (eating, dressing, showering, using the toilet, walking around at home, and getting in and out of a bed or chair). Levels of health problems were obtained by counting the number of reported chronic health problems (MT1 = 2.29, SDT1 = 1.63, rangeT1 = 0–8; MT2 = 2.49, SDT2 = 1.79, rangeT2 = 0–8) and ADL difficulties (MT1= 0.15, SDT1= 0.56, rangeT1 = 0–4; MT2= 0.21, SDT2 = 0.66, rangeT2 = 0–6). Over time, participants experienced an increase in chronic health problems, t(183) = 2.72, p <.05, but not in ADL difficulties, t(183) = 1.04, p >.05. Changes in chronic health problems and ADL difficulties were computed as difference scores to allow for interpretation of absolute change. Higher difference scores reflected a greater increase in health problems. Because difference scores may depend on initial levels, baseline levels of chronic problems or ADL difficulties were controlled in all analyses.2
Daily physical symptoms were measured at baseline. Participants were asked to report at bedtime on 3 typical days whether they had experienced each of 12 physical health symptoms during the day (e.g., chest pain, joint pain, or shortness of breath). This list of symptoms was derived from the PRIME MD screening questionnaire (Spitzer et al., 1994). We counted the total number of symptoms experienced across the 3 days (M = 2.55, SD = 3.11, range = 0–14).
To measure HECS, we administered a previously validated nine-item instrument at baseline. Items include “If I have a health problem that gets worse, I put in even more effort to get better,” “If I develop a new health problem, I immediately get help from a health professional (e.g., doctor, nurse),” and “When I decide to do something about a health problem, I am confident that I will achieve it.” Participants responded to the items on 5-point Likert-type scales (0 = almost never true, 4 = almost always true; Wrosch et al., 2002). Our measure of HECS was the mean score across the nine items (M = 3.14, SD = 0.66, α = .88).
Age, sex, and SES (averaged standardized scores for income, education, and perceived SES) were measured in the baseline questionnaire. In addition, depressive mood (i.e., sum scores of feeling sad and unhappy over 3 days—0 = very slightly or not at all, 4 = extremely; M = 1.17, SD = 2.18, α = .75) and diurnal cortisol secretion (i.e., averaged area under the curve, based on five daily samples; M = 119.04, SD = 53.94, α = .82) were assessed at baseline across 3 typical days.3 These measures are described in detail elsewhere (Wrosch et al., 2007).
RESULTS
We tested our hypotheses by predicting the difference scores for chronic health problems and ADL difficulties in separate hierarchical regression analyses. We included the centered baseline measures of the respective health problems, sociodemographic characteristics, daily physical symptoms, and HECS in the regression equations and tested the interactions between daily physical symptoms and HECS for significance (for zero-order correlations, see Table 1).
TABLE 1.
Zero-Order Correlations Among the Main Constructs
| Main construct | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Change in chronic health problems | |||||
| 2. Chronic health problems at baseline | −.21** | ||||
| 3. Change in ADL difficulties | −.09 | .02 | |||
| 4. ADL difficulties at baseline | .09 | .14 | −.47** | ||
| 5. Daily physical symptoms at baseline | −.04 | .31** | .01 | .15* | |
| 6. Health-engagement control strategies at baseline | .02 | −.01 | −.06 | −.04 | −.06 |
Note. ADL = activities of daily living.
p < .05.
p < .01.
Sociodemographic characteristics, HECS, and daily physical symptoms did not predict difference scores for either chronic health problems or ADL difficulties, Fs(1, 177) <2.51, ps >.05. Furthermore, lower baseline levels of chronic health problems and ADL difficulties were associated with larger difference scores for these problems, Fs(1, 177) >9.07, ps <.01. This finding supports the inclusion of these predictors in the analyses. The second step of the analyses demonstrated that daily physical symptoms and HECS had significant interactive effects on difference scores for chronic health problems, F(1, 176) = 4.71, R2 = .024, p < .05, and ADL difficulties, F(1, 176) = 6.62, R2 = .027, p = .01.4
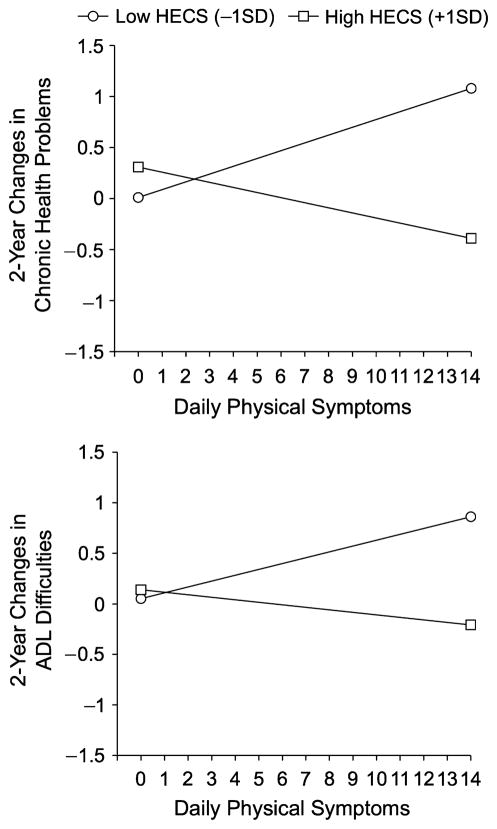
Figure 1 illustrates the interactions by plotting the associations between daily physical symptoms and difference scores for chronic health problems (upper panel) and ADL difficulties (lower panel), separately for participants who scored 1 standard deviation above and 1 standard deviation below the sample mean of HECS(Aiken & West, 1991). The figure shows that the largest difference scores for both chronic health problems and ADL difficulties were found among participants who experienced high levels of daily physical symptoms and did not engage in strategies to overcome their health problems. Analyses of the simple slopes support this interpretation: Higher levels of daily physical symptoms were significantly associated with larger difference scores among participants who scored low on the HECS scale (chronic health problems: b = 0.08, SE = 0.04, p = .05; ADL difficulties: b = 0.06, SE =0.02, p < .01), but not among participants who scored high on the HECS scale (bs =−0.02 and −0.05, SEs =0.02 and 0.04, ps > .05).
Fig. 1.
Two-year changes in chronic health problems (top panel) and difficulties in activities of daily living (ADL; bottom panel) as a function of daily physical symptoms, separately for participants with high versus low baseline levels of health-engagement control strategies (HECS).
Finally, we conducted a set of mediation analyses to explore whether depressive mood or cortisol secretion could partially explain the observed interaction effects. There was not much evidence for a statistical influence of the mediators on the outcome variables, except for a significant interactive effect of cortisol level and daily physical symptoms on chronic health problems, F(1, 176) = 4.87, R2 = .025, p < .05. The nature of this effect was such that larger difference scores for chronic health problems were found among participants with higher levels of cortisol and higher levels of daily physical symptoms.5 This variance associated with cortisol reduced the effect size of the interaction between HECS and daily physical symptoms by 34% and rendered this effect nonsignificant, R2 =.016, p =.08. In this control analysis, a higher level of daily physical symptoms no longer significantly predicted larger difference scores for chronic problems among participants with low levels of HECS (b = 0.07, SE = 0.04, p = .07).
DISCUSSION
This study demonstrated that active engagement in strategies for overcoming physical health problems (HECS) can ameliorate an adverse effect of daily physical symptoms on increases in older adults’ chronic health problems and functional difficulties. In fact, participants who were at the higher end of the physical-symptoms distribution reported approximately one additional chronic health problem and close to one additional ADL problem over 2 years if they did not report actively addressing their health problems. By contrast, no increases in chronic health problems and ADL difficulties were obtained among their counterparts who reported high levels of HECS. These findings have practical significance and should inform psychological intervention programs aimed at managing physical symptoms. Optimizing control strategies through clinical interventions (Nathan & Gorman, 1998) might contribute to improving the physical health of older adults.
Our sample was relatively healthy, and the analyses therefore reveal the processes that take place in the early stages of physical decline. The fact that we found stronger increases in chronic health problems than in functional declines supports this conclusion (Kelley-Moore & Ferraro, 2005). This finding suggests that daily physical symptoms may be a sign of developing illness and can translate into serious health problems if they are not addressed. However, HECS may not have the same beneficial effects in later stages of physical decline, given that these control processes may not be effective in the case of intractable health problems (Wrosch, Dunne, Scheier, & Schulz, 2006; Wrosch et al., 2007). Thus, there may be a small window of opportunity during which long-term health declines, and ultimately mortality (Gitlin, Hauck, Winter, Dennis, & Schulz, 2006), may be postponed through the use of active control strategies.
We also examined whether depressive mood or cortisol dysregulation could explain the observed effects. Although there was not much evidence for mediation overall, the analyses demonstrated a particularly large increase in chronic health problems among participants who experienced elevated levels of cortisol and daily physical symptoms. Moreover, this effect rendered the buffering effect of HECS on increases in chronic health problems nonsignificant. Although these findings suggest that the beneficial effects of HECS may be partially mediated through low levels of cortisol secretion, we note that cortisol was not strongly associated with HECS and explained only 34% of the buffering effect of HECS on chronic health problems. Nonetheless, scientists have struggled with documenting a link between biological intermediaries and physical health (Cohen et al., 1998; Miller et al., 2007), and evidence consistent with partial mediation may inform future research. In particular, there could be additional factors, such as duration of stressors or health behaviors, that influence the effects of cortisol secretion on health (Miller et al., 2007; Wrosch, Miller, Lupien, & Pruessner, in press). In addition, HECS might benefit older adults’ physical health through other pathways, such as increased use of public-health services, earlier diagnosis and treatment, greater adherence to difficult treatment regimes, and greater use of preventive health practices.
Finally, it will be important to examine the control processes involved in managing chronic and functional declines. Adaptive management of such circumstances may require individuals to gradually adjust their health goals (e.g., walking for 10 min instead of 1 hr), and active pursuit of realistically adjusted health goals may have beneficial effects on subsequent outcomes (cf. Carver & Scheier, 2000). Illuminating how older adults can manage chronic declines requires examining the interplay between different categories of control processes (e.g., goal adjustment and goal attainment; Wrosch et al., 2006) and may greatly contribute to improving older adults’ quality of life.
Acknowledgments
Preparation of this manuscript was supported by grants and awards from the Canadian Institutes of Health Research; the National Institute on Aging (AG024827, AG13305, AG015321, AG20677, and AG19180); the National Institute of Nursing Research (NR08272); the National Institute of Mental Health (MH071944); the National Center on Minority Health and Disparities (MD000207); the National Heart, Lung, and Blood Institute (HL076852 and HL076858); and the National Science Foundation (EEEC-0540865).
Footnotes
Control strategies overlap conceptually with other self-regulation constructs (e.g., self-efficacy or coping; Bandura, 1997; Scheier et al., 1989). However, control strategies are activated not only in response to problems, but also to safeguard the attainment of developmental goals (Heckhausen & Schulz, 1995). Moreover, they reflect individuals’ intentional behavioral processes to a larger extent than do constructs such as perceived control (Skinner, 1996, 2007).
This approach accounts for the possibility that difference scores can be correlated with baseline levels, and the results were identical with those of analyses that predicted T2 levels after controlling for baseline levels.
Four subjects did not provide cortisol data, and the sample mean was used for these subjects in the analyses.
R2 values represent the unique proportion of variance explained in each step of the analyses.
Physical symptoms predicted increases in chronic health problems among participants who secreted high levels of cortisol, b = 0.09, SE = 0.04, p < .05, but not among participants who secreted low levels of cortisol, b =−0.04, SE = 0.04, p > .05. In addition, the association between high levels of HECS and low levels of cortisol secretion approached significance (controlling for sociodemographics), F(1, 179) = 2.84, R2 = .015, b = −9.97, SE = 5.95, p < .10.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- Carver CS, Scheier MF. Scaling back goals and recalibration of the affect system are processes in normal adaptive self-regulation: Understanding “response shift” phenomena. Social Science & Medicine. 2000;50:1715–1722. doi: 10.1016/s0277-9536(99)00412-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. The state of aging and health in America 2007. Whithouse Station, NJ: The Merck Company Foundation; 2007. [Google Scholar]
- Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychology. 1998;17:214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- Dew MA. Psychiatric disorder in the context of physical illness. In: Dohrenwend B, editor. Adversity, stress, and psychopathology. New York: Oxford University Press; 1998. pp. 177–218. [Google Scholar]
- Gitlin LN, Hauck WW, Winter L, Dennis MP, Schulz R. Effect of an in-home occupational and physical therapy intervention on reducing mortality in functionally vulnerable older people: Preliminary findings. Journal of the American Geriatrics Society. 2006;54:950–955. doi: 10.1111/j.1532-5415.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- Heckhausen J, Schulz R. A life-span theory of control. Psychological Review. 1995;102:284–304. doi: 10.1037/0033-295x.102.2.284. [DOI] [PubMed] [Google Scholar]
- Kelley-Moore JA, Ferraro KF. A 3-D model of health decline: Disease, disability, and depression among Black and White older adults. Journal of Health and Social Behavior. 2005;46:376–391. doi: 10.1177/002214650504600405. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF. Emotions, morbidity, and mortality: New perspectives from psychoneuro-immunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Rogers JC, Martire LM, Mulsant BH, Rollman BL, Dew MA, et al. The association of late-life depression and anxiety with physical disability: A review of the literature and prospectus for future research. American Journal of Geriatric Psychiatry. 2001;9:113–135. [PubMed] [Google Scholar]
- Lenze EJ, Schulz R, Martire LM, Zdaniuk B, Glass T, Kop WJ, et al. The course of functional decline in persistently depressed elderly: Longitudinal findings from the Cardiovascular Health Study. Journal of the American Geriatrics Society. 2005;53:569–575. doi: 10.1111/j.1532-5415.2005.53202.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adreno-cortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nathan PE, Gorman JM, editors. A guide to treatments that work. New York: Oxford University Press; 1998. [Google Scholar]
- Oeppen J, Vaupel JW. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Matthews KA, Owens JF, Magovern GJ, Lefebvre RC, Abbott RA, Carver CS. Dispositional optimism and recovery from coronary artery bypass surgery: The beneficial effects on physical and psychological well-being. Journal of Personality and Social Psychology. 1989;57:1024–1040. doi: 10.1037//0022-3514.57.6.1024. [DOI] [PubMed] [Google Scholar]
- Schulz R, Heckhausen J. A life span model of successful aging. American Psychologist. 1996;51:702–714. doi: 10.1037//0003-066x.51.7.702. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire LM, Beach SR, Scheier MF. Depression and mortality in the elderly. Current Directions in Psychological Science. 2000;9:204–208. [Google Scholar]
- Segerstrom SC, Miller GE. Stress and the human immune system: A meta-analytic review of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner EA. A guide to constructs of control. Journal of Personality and Social Psychology. 1996;71:549–570. doi: 10.1037//0022-3514.71.3.549. [DOI] [PubMed] [Google Scholar]
- Skinner EA. Secondary control critiqued: Is it secondary? Is it control? Comment on Morling and Evered (2006) Psychological Bulletin. 2007;133:911–916. doi: 10.1037/0033-2909.133.6.911. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, III, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care: The PRIME-MD 1000 study. Journal of the American Medical Association. 1994;272:1749–1756. [PubMed] [Google Scholar]
- Thomas D. Do not go gentle into that good night. Botteghe Oscure. 1951;VIII:208. [Google Scholar]
- Williamson GM, Schulz R. Activity restriction mediates the association between pain and depressed affect: A study of younger and older adult cancer patients. Psychology and Aging. 1995;10:369–379. doi: 10.1037//0882-7974.10.3.369. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Dunne E, Scheier MF, Schulz R. Self-regulation of common age-related challenges: Benefits for older adults’ psychological and physical health. Journal of Behavioral Medicine. 2006;29:299–306. doi: 10.1007/s10865-006-9051-x. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Miller GE, Lupien S, Pruessner JC. Diurnal cortisol secretion and 2-year changes in older adults’ physical symptoms: The moderating roles of negative affect and sleep. Health Psychology. doi: 10.1037/0278-6133.27.6.685. (in press) [DOI] [PubMed] [Google Scholar]
- Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: The importance of health engagement control strategies. Health Psychology. 2002;21:340–348. doi: 10.1037//0278-6133.21.4.340. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: A control-process approach. Current Directions in Psychological Science. 2004;13:17–20. [Google Scholar]
- Wrosch C, Schulz R, Miller GE, Lupien S, Dunne E. Physical health problems, depressive mood, and cortisol secretion in old age: Buffer effects of health engagement control strategies. Health Psychology. 2007;26:341–349. doi: 10.1037/0278-6133.26.3.341. [DOI] [PubMed] [Google Scholar]