1 Introduction
Mutations in the photoreceptor-specific protein, Rds, have been associated with a large number of inherited retinal degenerative diseases. Reports have described the incidence of retinitis pigmentosa, cone-rod dystrophy, diffuse retinal dystrophy, macular degeneration, and central areolar choroidal dystrophy in patients carrying mutations in the RDS gene (Dryja, Hahn, Kajiwara, & Berson, 1997; Ekstrom et al., 1998; Fishman et al., 1997; Fossarello et al., 1996; Jacobson, Cideciyan, Kemp, Sheffield, & Stone, 1996; Nakazawa, Wada, Chida, & Tamai, 1997; Sears, Aaberg, Daiger, & Moshfeghi, 2001; Zhang, Garibaldi, Li, Green, & Zack, 2002). As a membrane glycoprotein located in the disc rim region in the OSs of rods and cones (Connell & Molday, 1990; Molday, Hicks, & Molday, 1987; Travis, Brennan, Danielson, Kozak, & Sutcliffe, 1989), Rds is thought to be necessary for disc assembly, maintenance and renewal (Boesze-Battaglia & Goldberg, 2002; Molday et al., 1987; Wrigley, Ahmed, Nevett, & Findlay, 2000). While current beliefs propose Rds to serve a primarily structural role in OSs, alterations in Rds have strong functional as well as structural implications since this is the region where phototransduction occurs (Ridge, Abdulaev, Sousa, & Palczewski, 2003). Complete absence of the protein in the retinal degeneration slow (rds) mouse (van Nie, Ivanyi, & Demant, 1978) results in a failure of OS formation, an absence of retinal function, and the progression of photoreceptor degeneration (Cohen, 1983; Jansen & Sanyal, 1984; Sanyal, De Ruiter, & Hawkins, 1980; Sanyal & Jansen, 1981). A phenotype of haploinsufficiency has also been reported to associate with the under-expression of Rds (in rds+/− animals) and is characterized by malformed and swirl-like OSs, and an early-onset of slow rod degeneration followed by a late-onset and slow cone degeneration (Cheng et al., 1997; Nour, Ding, Stricker, Fliesler, & Naash, 2004). In addition to the rds null mutant (Ali et al., 2000; Sarra et al., 2001; Schlichtenbrede et al., 2003), two transgenic mouse models have been generated (Kedzierski, Lloyd, Birch, Bok, & Travis, 1997; Kedzierski et al., 2001), one of which, P216L, has been used to evaluate therapeutic interventions for the treatment of Rds-associated retinal disease (Bok et al., 2002; Liang et al., 2001). However, to date no study has reported both significant functional and structural rescue in any animal model carrying point mutations in the rds gene. In this study, we assessed the feasibility of Rds supplementation to resolve rod defect associated with expression of the C214S mutant of Rds. The C214S mutation causes a loss-of-function defect which primarily affects rods in humans and mice (Saga et al., 1993; Stricker, Ding, Quiambao, Fliesler, & Naash, 2005). To reduce the effect of the mutant protein by competing it out with the wild-type (WT) protein, we generated a transgenic line expressing the WT Rds in both rods and cones driven by the human interphotoreceptor retinoid binding protein (hIRBP) promoter (Nour et al., 2004). A single residue change at the C-terminus was incorporated in the WT transgene to mimic the human sequence that would facilitate specific recognition of the transgene product (Nour et al., 2004). This change is proline to glutamine at position 341 (P341Q), and here the line is named NMP for normal mouse peripherin/rds. Structural and functional studies on NMP retinas demonstrated the benign effect of the P341Q modification (Nour et al., 2004). Uniform expression of Rds in rods and cones was achieved by crossing C214S mice with NMP mice. By studying the structure of the double transgenic retinas by histology and the function by electroretinography, we have shown that genetic supplementation of WT Rds is a viable option for loss-of-function mutations in the RDS. As proof-of-principle, this finding provides a basis for the development of future therapeutic approaches. The following represents original material, not previously published or under consideration for publication elsewhere.
2 Materials and Methods
2.1 Generation of Transgenic Mice
The WT Rds with the proline to glutamine substitution has been previously studied in transgenic mice (Nour et al., 2004), where the substitution, used for the specific recognition of the protein by mAb 3B6, caused no aberrant affects on transgenic protein expression or function. Because P341Q seems harmless to Rds structure and function, the C214S transgenic construct used in this study contained this modification. Expression of all constructed transgenes (NMP and C214S) was directed to rods and cones by a 1.3 kb fragment of the human interphotoreceptor retinoid binding protein (hIRBP) promoter. Transgenic mice were identified by polymerase chain reaction (PCR) primers specific to the hIRBP promoter (Forward: 5’-CAGTGTCTGGCATGTAGCAGG) and the coding region of RDS cDNA (Reverse: 5’-GGCTTCCACTTGGCGTACTTG). Transgene product at the protein level generated from one allele of the NMP on the rds−/− background corresponded to 30% of the WT Rds while the C214S transgene showed a transcript level comparable to WT Rds with a trace amount of the mutant protein detected by Western blot analysis (Stricker et al., 2005). Transgenic lines show a rod dominant-defect in C214S (Stricker et al., 2005) while no alteration in retinal phenotype or structure was observed in mice over-expressing Rds (NMP on WT background) (Nour et al., 2004). Both transgenic mouse lines on C57BL/6 genetic backgrounds were crossed to generate single (C214S) and double (C214S/NMP) transgenics on an rds+/− background. Mice were screened for the presence of the RDS mutation as previously described (Cheng et al., 1997).
Animals were maintained under cyclic-light conditions (12 hours dark, 12 hours light, at 20 lux). All experiments were approved by the local Institutional Animal Care and Use Committees and conformed to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Rod and Cone Electroretinography (ERG)
ERG testing was carried out as previously described (Nour, Quiambao, Peterson, Al-Ubaidi, & Naash, 2003). Briefly, for the assessment of rod photoreceptor function, a strobe flash stimulus was presented to the dark-adapted, dilated eyes in a Nicolet ganzfeld (GS-2000) with a 137 cd (sec/m2) flash intensity. The dark-adapted a-wave amplitude was measured from pre-stimulus baseline to the a-wave trough and the b-wave amplitude measured from the a-wave trough to the b-wave peak. For the evaluation of cone function, a strobe flash stimulus was presented to 5-minute light-adapted, dilated eyes in a Nicolet ganzfeld (GS-2000) with a 77 cd (sec/m2) flash intensity. The amplitude of the cone b-wave was measured from the trough of the a-wave to the peak of the b-wave. Analysis of variance (ANOVA) and post-hoc statistical tests using Bonferroni’s pairwise comparisons were used to determine the significance of differences in ERG responses (GraphPad Prism 3.02, San Diego, CA, USA).
2.3 Electron and Light Microscopy
Enucleated eyes were fixed, processed for embedment in plastic resin, and sectioned as previously described (Ding et al., 2004; Tan et al., 2001). For light microscopy, sections (0.75-µm thickness, stained with 1% Toluidine Blue) were photographed with an Olympus BH-2 photomicroscope (auto-expose mode) using 20× or 60× (oil immersion) DPlanApo objectives. For electron microscopy, ultra-thin (silver-gold) sections were post-stained with lead citrate and uranyl acetate on-grid, and viewed with a JEOL JEM-1200EX electron microscope at an accelerating voltage of 80 KeV.
2.4 Western Blot Analysis
Rabbit polyclonal antibody against residues 331–346 of murine Rds C-terminus was used for detection of Rds as described previously (Ding et al., 2004). Anti-actin antibody (1:250 dilution) (Sigma-Aldrich, St. Louis, MO, USA), was used to control for sample loading. Retinal protein extraction and blot analysis were carried out as previously described (Ding et al., 2004; Li, Ding, O’Brien, Al-Ubaidi, & Naash, 2003).
3 Results
3.1 Functional Rescue of the C214S Mutation in Rds
In order to test the ability of Rds supplementation to resolve retinal degeneration associated with expression of the C214S mutation in Rds, C214S transgenics were crossed to transgenic mice expressing NMP in both rods and cones. Single (C214S) and double transgenic (C214S/NMP) mice, heterozygous for both transgenes, were generated onto an rds+/− genetic background. The C214S+/−/rds+/− genotype mimics the situation in patients, where one correct allele of RDS is accompanied by one mutated allele (Stricker et al., 2005; Saga et al., 1993). The C214S mutation in mice renders a small amount of protein present in retinas, although an abundant amount of mutant RDS mRNA is detected (Stricker et al., 2005).
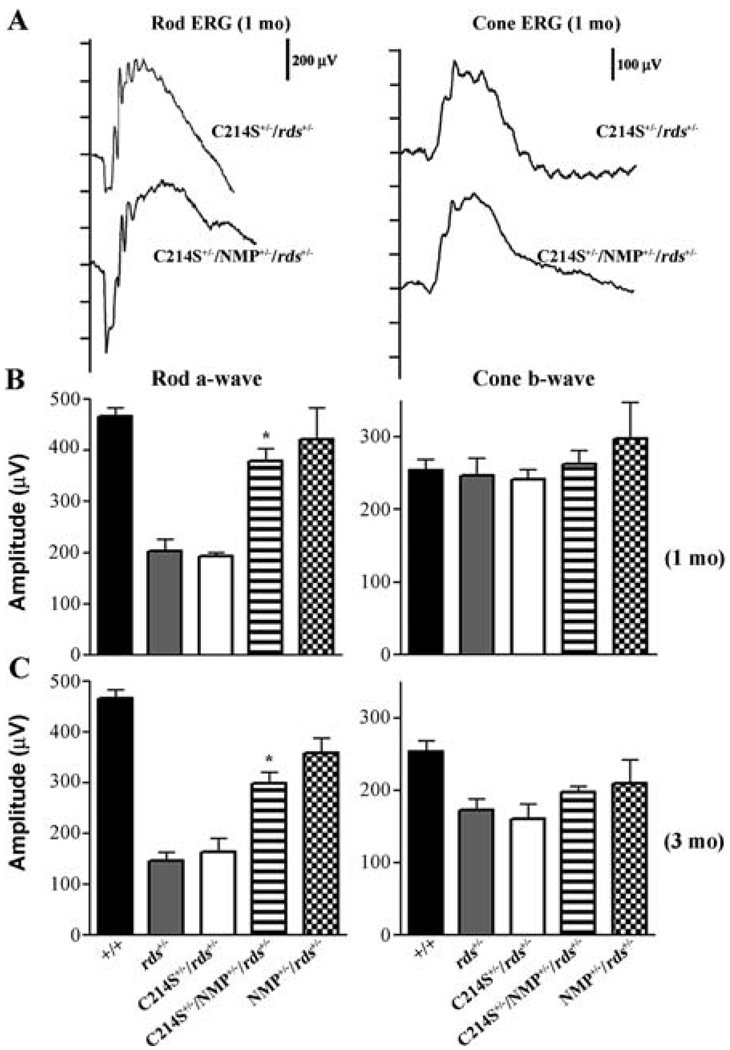
The cumulative data indicate that retinas of C214S transgenic mice display a loss-of-function defect similar to haploinsufficiency phenotype seen in rds+/− mice as well as retinitis pigmentosa patients carrying the C214S mutation (Stricker et al., 2005; Saga et al., 1993). Representative rod ERG waves demonstrate remarkable restoration in rod function afforded by the supplementation of Rds in double transgenic (C214S+/−/NMP+/−/rds+/−) when compared to single transgenic (C214S+/−/rds+/−) mice (Fig. 1 A). As early as 1 month of age, C214S+/−/rds+/− mice exhibit a reduction in rod a-wave amplitudes (Fig. 1 A & B). This reduction is comparable to the diminished rod photoreceptor function observed in non-transgenic rds+/− controls (Fig. 1 B). In sharp contrast to the reduced rod photoreceptor response generated by C214S+/−/rds+/− single transgenics, C214S+/−/NMP+/−/rds+/− double transgenic mice showed a statistically significant (P<0.001) improvement in rod a-wave amplitudes at one month and 3 months of age (Fig. 1 B & C).
Fig. 1.
Rod and cone ERG analysis in C214S transgenics. (A) Representative rod and cone ERG wave forms at 1 month of age (1mo) show a rescue in rod function in double transgenic (C214S+/−/NMP+/−/rds+/−) when compared to single transgenic (C214S+/−/rds+/−) mice. No differences are seen in cone wave response. (B) Rod a-wave and cone b-wave averages at 1 month demonstrate a statistically significant (P<0.001) rescue in rod function in double (C214S+/−/NMP+/−/rds+/−), compared to single (C214S+/−/rds+/−) transgenic mice. At this age, all mice tested generate WT levels of cone ERG response. (C) The statistically significant (P<0.001) improvement in rod function resulting from Rds supplementation in C214S transgenics (C214S+/−/NMP+/−/rds+/−) persists even at three 3 months of age (3mo)
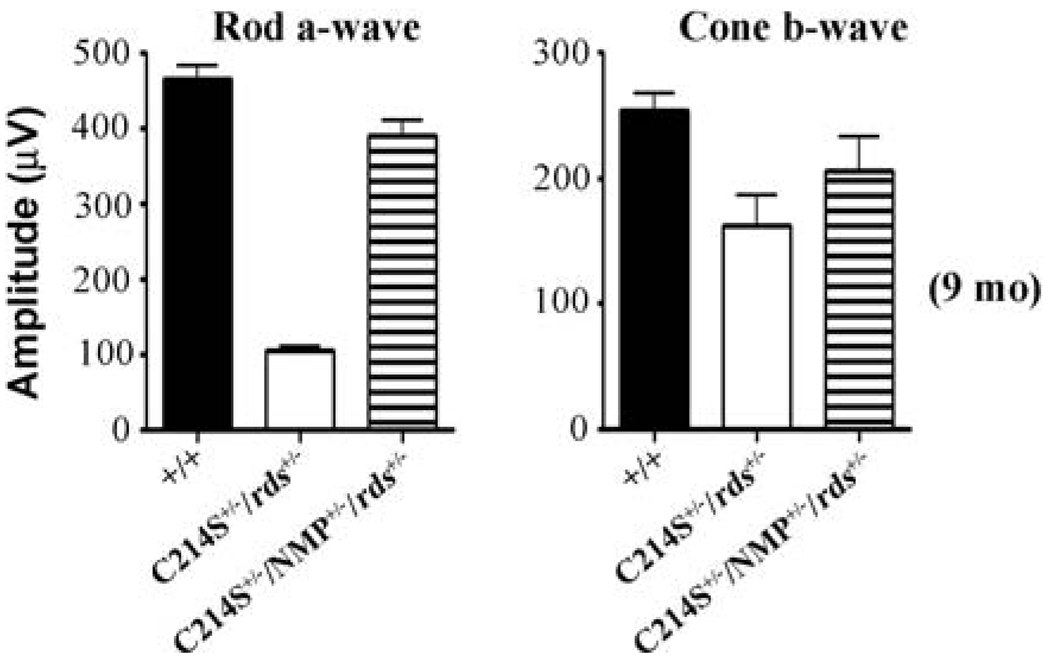
Interestingly, this functional improvement continued up to 9 months of age, the latest time point tested (Fig. 2D). In terms of cone function, at early ages, haploinsufficiency in Rds has no deleterious impact as shown by rds+/− and C214S+/−/rds+/− responses (Fig. 1A & B). The increased expression of WT Rds in double transgenics (C214S+/−/NMP+/−/rds+/−) caused no adverse effects on cone photoreceptor function (Fig. 1A & B). A comparable decline in cone function between single transgenic (C214S+/−/rds+/−) and rds+/− mice was observed at 3 and 9 months of age (Fig. 1 C & Fig. 2). Although double transgenics (C214S+/−/NMP+/−/rds+/−) showed slight protection from this age-related decline in cone function (Fig. 1C & Fig. 2, right graph), this level of cone rescue is not statistically significant when compared to WT. NMP+/−/rds+/− controls generated rod and cone ERG responses correlated with total Rds levels (Fig. 1B & C).
Fig. 2.
Rod a-wave and cone b-wave averages at 9 months of age shows a long term rescue in double (C214S+/−/NMP+/−/rds+/−) transgenic mice. ERG wave amplitudes for B and C represent an average of 12–16 eyes for each genotype, and ERG wave amplitudes for D represent 8 eyes for each genotype
3.2 Histological Rescue of the C214S Mutation in Rds
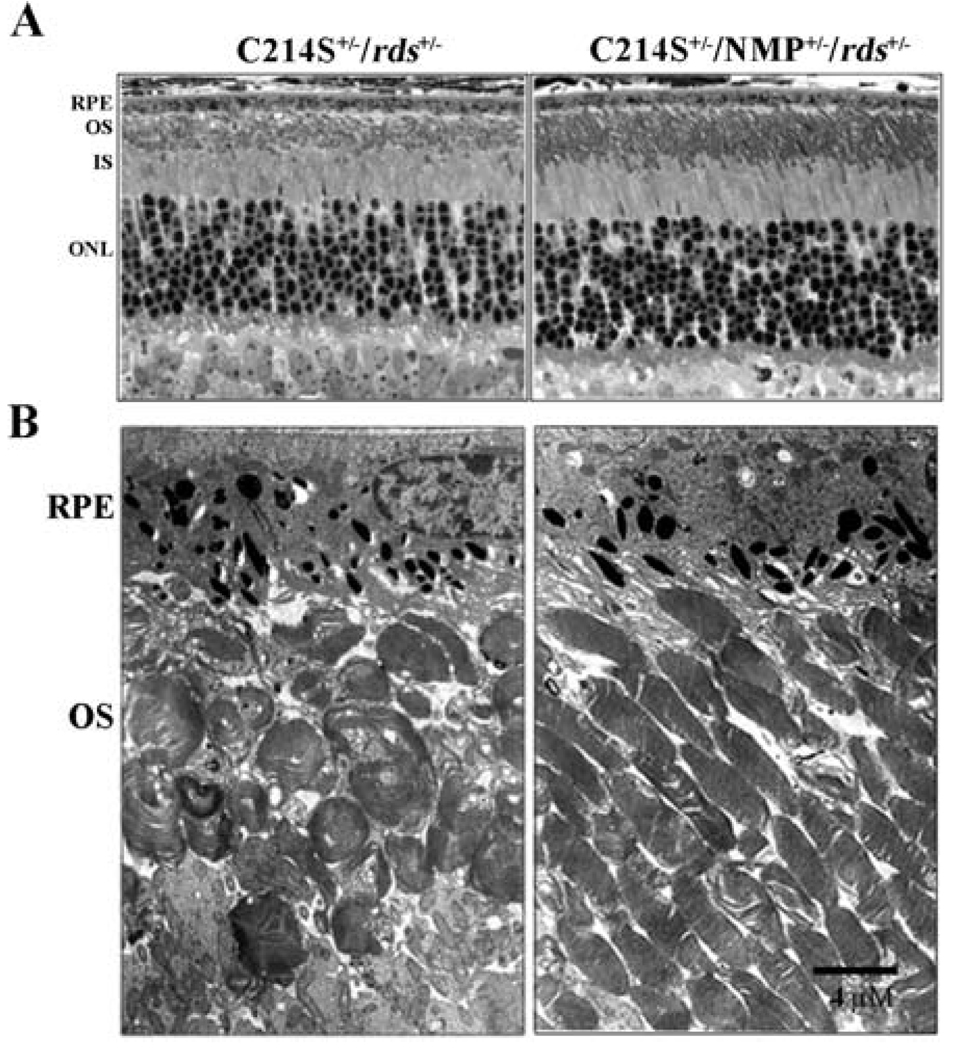
A reduction in Rds level has been shown to cause a disruption in OS alignment and integrity (Cheng et al., 1997; Nour et al., 2004). This haploinsufficiency phenotype has also been seen in C214S+/−/rds+/− retinas at 1 month of age with shorter OSs detected at the light microscopy (Fig. 3A), and typical swirl-like OSs seen at the electron microscopy (Fig. 3B). Genetic supplementation of Rds in C214S+/−/NMP+/−/rds+/− retinas results in a remarkable improvement in both OS length (Fig. 3A) and integrity (Fig. 3B). This improvement in structural integrity (Fig. 3A & B) correlates with the increase in rod photoreceptor function afforded by the expression of the NMP transgene (Fig. 1A–C).
Fig. 3.
Retinal structure in C214S transgenics. (A) Histological evaluation (light microscopy) at 1 month of age reveals an improvement in OS length in C214S+/−/NMP+/−/rds+/− when compared to C214S+/−/rds+/− retinas.(B) Electron microscopy shows amelioration of defects in the alignment and integrity of photoreceptor OSs in double transgenic retinas (C214S+/−/NMP+/−/rds+/−). All tissue sections were evaluated from the superior central region of the retina. Scale bar, 4 µm, (RPE: retinal pigment epithelium; OS: outer segment; IS: inner segment; ONL: outer nuclear layer)
Under reducing conditions, Western blot analysis with a polyclonal antibody raised against the murine Rds-C-terminus 22 shows a decrease of Rds level in C214S+/−/rds+/− retina relative to WT and confirms an increase of total Rds levels in C214S+/−/NMP+/−/rds+/− retina (Fig. 4). Dimers and higher order oligomers represent partially reduced samples. Actin was used as an internal control and showed comparable levels in all samples examined (Fig. 4).
Fig. 4.
Rds expression levels in C214S transgenic retinas. Western blot analysis reacted with polyclonal antibody against the murine Rds-C-terminus demonstrates the increase in Rds protein levels in C214S+/−/NMP+/−/rds+/− relative to C214S+/−/rds+/− retinas. Actin immunoblotting shows equal loading in examined samples
4 Discussion
The design of widely applicable therapeutic interventions for Rds-associated retinal diseases has been hindered by multiple factors. These factors include the large number of variable point mutations, the diversity in severity and patterns of disease expression, and the haploinsufficiency phenotype, which dictates a well-regulated level of Rds required for OS morphogenesis. Since over-expression of Rds in the retina is well-tolerated (Nour et al., 2004), gene transfer (or Rds supplementation) logically presents itself as a viable option for resolving defects associated with loss-of-function mutations. However, therapeutic strategies for gain-of-function, dominant mutations have been complicated by the fact that elimination of the mutant protein requires simultaneous transfer of WT Rds in order to prevent haploinsufficiency-related defects. In this study, we provide the first experimental evidence that supports the use of supplementation or gene transfer strategy as a short term and generalized approach for prospective treatment of loss-of-function mutations in Rds. Furthermore, we provide evidence of long-term rescue of photoreceptor structure and function with loss-of-function mutation that is achieved by genetic supplementation of WT Rds.
Patients carrying the C214S mutation in Rds present with retinitis pigmentosa, a rod-dominant functional defect (Saga et al., 1993). In the mouse model, the haploinsufficiency-like phenotype caused by the C214S (loss-of-function) mutation also results in a reduction in rod ERG function stemming from an abnormality in OS structure formation (Stricker et al., 2005). We show that expression of Rds in double transgenics (C214S+/−/NMP+/−/rds+/−) results in long-term resolution of rod functional defects as well as restoration of OS ultrastructural organization.
The replacement of Rds in mice has been demonstrated by two other labs with limited success. First, the Rds transgene expressed exclusively in rods (Opsin-rds) was able to rescue the structure of the rds null retina, but only if the transgene was expressed at high levels (Travis, Groshan, Lloyd, & Bok, 1992). This study did not assess any functional rescue of rods afforded by the transgene, nor did the study allow for the investigation of cone structure or function. However, the Opsin-rds transgene established that more than 50% of a WT gene dosage is needed for optimal OS formation. Second, the use of an adeno-associated virus vector containing a rds construct driven by the rhodopsin promoter (AAV-Opsin-rds) was capable of developing tiny rod OS structures and a trace of rod function in cells expressing the viral vector (Ali et al., 2000). Furthermore, the AAV method of delivery (subretinal injection) was not sufficient for expression throughout the entire retina (Sarra et al., 2001). The authors suggest that the unequal distribution of the Rds supplementation coupled with possible apoptotic signals from the untreated cells may have lead to the continued degeneration seen in their treated models. When looking at WT retinas treated with the AAV-Opsin-rds, the authors noted marked photoreceptor loss that was attributed to the delivery procedure, Rds over-expression, or the viral vector (Sarra et al., 2001). In our recent studies involving genetic supplementation in transgenic mice, we did not find over-expression of Rds to be deleterious (Nour et al., 2004), suggesting that the method of delivery of the gene product is crucial. Several therapeutic delivery systems will need to be tested in order to establish optimal expression of the RDS replacement gene as well as minimize possible damaging effects caused by the delivery. The future of gene therapy for the retina is promising with the development of new expression systems such as nanoparticles. When taken together our data provide proof-of-principle for genetic supplementation as a promising treatment for both loss-of-function mutations in Rds. Our use of transgene supplementation is widely applicable and simplifies strategies for therapeutic intervention by providing the fundamental basis for virally- or otherwise-mediated transfer of Rds to the diseased retina.
Acknowledgments
The authors thank Barbara Nagel for her technical assistance and Dr. Muayyad R. Al-Ubaidi for his helpful comments on the manuscript. Funding: NEI EY10609 & EY016201 (MIN), EY007361 (SJF), and Core Grant for Vision Research (EY12190); Foundation Fighting Blindness (MIN); an unrestricted departmental grant from Research to Prevent Blindness (SJF); the Norman J. Stupp Charitable Trust (SJF); and the Oklahoma Center for the Advancement of Science and Technology (OCAST) (MIN).
Contributor Information
Steven J. Fliesler, Ophthalmology and Pharmacological & Physiological Science, Saint Louis University School of Medicine, St. Louis, MO, 63104, USA
Muna I. Naash, Cell Biology, University of Oklahoma Health Sciences Center, 940 Stanton L. Young Blvd., Oklahoma City, OK 73104.
References
- Ali RR, Sarra GM, Stephens C, Alwis MD, Bainbridge JW, Munro PM, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Goldberg AF. Photoreceptor renewal: a role for peripherin/rds. Int Rev Cytol. 2002;217:183–225. doi: 10.1016/s0074-7696(02)17015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D, Yasumura D, Matthes MT, Ruiz A, Duncan JL, Chappelow AV, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74(6):719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- Cheng T, Peachey NS, Li S, Goto Y, Cao Y, Naash MI. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J Neurosci. 1997;17:8118–8128. doi: 10.1523/JNEUROSCI.17-21-08118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI. Some cytological and initial biochemical observations on photoreceptors in retinas of rds mice. Invest Ophthalmol Vis Sci. 1983;24:832–843. [PubMed] [Google Scholar]
- Connell GJ, Molday RS. Molecular cloning, primary structure, and orientation of the vertebrate photoreceptor cell protein peripherin in the rod outer segment disk membrane. Biochemistry. 1990;29:4691–4698. doi: 10.1021/bi00471a025. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Nour M, Ritter LM, Goldberg AF, Fliesler SJ, Naash MI. The R172W mutation in peripherin/rds causes a cone-rod dystrophy in transgenic mice. Hum Mol Genet. 2004;13:2075–2087. doi: 10.1093/hmg/ddh211. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Hahn LB, Kajiwara K, Berson EL. Dominant and digenic mutations in the peripherin/RDS and ROM1 genes in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1997;38:1972–1982. [PubMed] [Google Scholar]
- Ekstrom U, Ponjavic V, Abrahamson M, Nilsson-Ehle P, Andreasson S, Stenstrom I, et al. Phenotypic expression of autosomal dominant retinitis pigmentosa in a Swedish family expressing a Phe-211-Leu variant of peripherin/RDS. Ophthalmic Genet. 1998;19:27–37. doi: 10.1076/opge.19.1.27.2179. [DOI] [PubMed] [Google Scholar]
- Fishman GA, Stone EM, Alexander KR, Gilbert LD, Derlacki DJ, Butler NS. Serine-27-phenylalanine mutation within the peripherin/RDS gene in a family with cone dystrophy. Ophthalmology. 1997;104:299–306. doi: 10.1016/s0161-6420(97)30320-0. [DOI] [PubMed] [Google Scholar]
- Fossarello M, Bertini C, Galantuomo MS, Cao A, Serra A, Pirastu M. Deletion in the peripherin/RDS gene in two unrelated Sardinian families with autosomal dominant butterfly-shaped macular dystrophy. Arch Ophthalmol. 1996;114:448–456. doi: 10.1001/archopht.1996.01100130444016. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Kemp CM, Sheffield VC, Stone EM. Photoreceptor function in heterozygotes with insertion or deletion mutations in the RDS gene. Invest Ophthalmol Vis Sci. 1996;37:1662–1674. [PubMed] [Google Scholar]
- Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol. 1984;224:71–84. doi: 10.1002/cne.902240107. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci. 1997;38:498–509. [PubMed] [Google Scholar]
- Kedzierski W, Nusinowitz S, Birch D, Clarke G, McInnes RR, Bok D, et al. Deficiency of rds/peripherin causes photoreceptor death in mouse models of digenic and dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 2001;98:7718–7723. doi: 10.1073/pnas.141124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ding XQ, O’Brien J, Al-Ubaidi MR, Naash MI. Molecular characterization of the skate peripherin/rds gene: relationship to its orthologues and paralogues. Invest Ophthalmol Vis Sci. 2003;44:2433–2441. doi: 10.1167/iovs.02-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Dejneka NS, Dudus L, Fisher KJ, Maguire AM, et al. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- Nakazawa M, Wada Y, Chida Y, Tamai M. A correlation between computer-predicted changes in secondary structure and the phenotype of retinal degeneration associated with mutations in peripherin/RDS. Curr Eye Res. 1997;16:1134–1141. doi: 10.1076/ceyr.16.11.1134.5099. [DOI] [PubMed] [Google Scholar]
- Nour M, Ding XQ, Stricker H, Fliesler SJ, Naash MI. Modulating expression of peripherin/rds in transgenic mice: critical levels and the effect of overexpression. Invest Ophthalmol Vis Sci. 2004;45:2514–2521. doi: 10.1167/iovs.04-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Quiambao AB, Peterson WM, Al-Ubaidi MR, Naash MI. P2Y(2) receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest Ophthalmol Vis Sci. 2003;44:4505–4514. doi: 10.1167/iovs.03-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge KD, Abdulaev NG, Sousa M, Palczewski K. Phototransduction: crystal clear. Trends Biochem Sci. 2003;28:479–487. doi: 10.1016/S0968-0004(03)00172-5. [DOI] [PubMed] [Google Scholar]
- Saga M, Mashima Y, Akeo K, Oguchi Y, Kudoh J, Shimizu N. A novel Cys-214-Ser mutation in the peripherin/RDS gene in a Japanese family with autosomal dominant retinitis pigmentosa. Hum Genet. 1993;92:519–521. doi: 10.1007/BF00216463. [DOI] [PubMed] [Google Scholar]
- Sanyal S, De Ruiter A, Hawkins RK. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neurol. 1980;194:193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981;21:23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Sarra GM, Stephens C, de Alwis M, Bainbridge JW, Smith AJ, Thrasher AJ, et al. Gene replacement therapy in the retinal degeneration slow (rds) mouse: the effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet. 2001;10:2353–2361. doi: 10.1093/hmg/10.21.2353. [DOI] [PubMed] [Google Scholar]
- Schlichtenbrede FC, MacNeil A, Bainbridge JW, Tschernutter M, Thrasher AJ, Smith AJ, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- Sears JE, Aaberg TA, Sr., Daiger SP, Moshfeghi DM. Splice site mutation in the peripherin/RDS gene associated with pattern dystrophy of the retina. Am J Ophthalmol. 2001;132:693–699. doi: 10.1016/s0002-9394(01)01179-5. [DOI] [PubMed] [Google Scholar]
- Stricker HM, Ding XQ, Quiambao A, Fliesler SJ, Naash MI. The Cys214->Ser mutation in peripherin/rds causes a loss-of-function phenotype in transgenic mice. Biochem J. 2005;388:605–613. doi: 10.1042/BJ20041960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, Peachey NS, et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- Travis GH, Brennan MB, Danielson PE, Kozak CA, Sutcliffe JG. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds) Nature. 1989;338:70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- Travis GH, Groshan KR, Lloyd M, Bok D. Complete rescue of photoreceptor dysplasia and degeneration in transgenic retinal degeneration slow (rds) mice. Neuron. 1992;9:113–119. doi: 10.1016/0896-6273(92)90226-4. [DOI] [PubMed] [Google Scholar]
- van Nie R, Ivanyi D, Demant P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens. 1978;12:106–108. doi: 10.1111/j.1399-0039.1978.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Wrigley JD, Ahmed T, Nevett CL, Findlay JB. Peripherin/rds influences membrane vesicle morphology. Implications for retinopathies. J Biol Chem. 2000;275:13191–13194. doi: 10.1074/jbc.c900853199. [DOI] [PubMed] [Google Scholar]
- Zhang K, Garibaldi DC, Li Y, Green WR, Zack DJ. Butterfly-shaped pattern dystrophy: a genetic, clinical, and histopathological report. Arch Ophthalmol. 2002;120:485–490. doi: 10.1001/archopht.120.4.485. [DOI] [PubMed] [Google Scholar]