Abstract
It is known that visual performance is better on the horizontal than the vertical meridian, and in the lower than the upper region of the vertical meridian (Vertical Meridian Asymmetry, “VMA”), and that exogenous spatial attention increases the apparent contrast of a stimulus. Here we investigate whether the VMA also leads to differences in the subjective appearance of contrast between the upper and lower vertical meridian, and how the effects of exogenous spatial attention on appearance interact with the VMA. Two Gabor stimuli were presented North and South of fixation at 4° eccentricity along the vertical meridian. Observers were asked to report the orientation of the Gabor that was higher in contrast. By assessing which stimulus observers perceived to be higher in contrast, we obtained psychometric functions and their concomitant points of subjective equality (PSE). These functions were measured both when a neutral cue was presented in the middle of the display and transient attention was deployed via a peripheral cue to the location of one of the stimuli. Observers were told that the cues were uninformative as to the stimulus contrast or its orientation. We report two novel findings. First, apparent contrast is higher on the lower vertical meridian than on the upper. Second, the attentional enhancement of apparent contrast is asymmetrical with both low and high contrast stimuli; the effect of exogenous spatial attention is greater on the lower than the upper vertical meridian. As in prior studies, we find no corresponding asymmetry in orientation discrimination. Signal detection-based models explain the asymmetrical appearance effects as a function of differential multiplicative gain factors for the North and South locations, and predict a similar but much smaller asymmetry for orientation discrimination.
Keywords: exogenous attention, appearance, vertical meridian asymmetry
Introduction
The human visual system is not able to process all the visual information available from the outside world. Consequently, our visual system incorporates both physiological (differential distribution of receptors, cortical resources, etc.) and processing characteristics (notably attention) that enable us to select and process the information that is most behaviorally relevant. The goals of this study are two-fold: first, to examine whether the uneven distribution of processing capacity across the vertical meridian affects the appearance of stimuli; second, to evaluate whether the effect of exogenous spatial attention (henceforth, “attention”) on contrast appearance differs across the vertical meridian. In addition, we concurrently measure orientation discrimination performance in one experiment as a verification of our cueing paradigm.
Visual spatial attention can be covertly dissociated from the direction of gaze in two ways: voluntarily, via a mechanism known as “endogenous” attention, or in an automatic, stimulus-driven fashion termed “exogenous” attention. Another distinguishing factor between the two is the difference in their time-courses. Whereas the effects of endogenous attention require a few hundred milliseconds to fully develop and can be maintained with effort, exogenous attention peaks within 100 to 120 ms and diminishes rapidly thereafter (Cheal & Lyon, 1991; Nakayama & Mackeben, 1989).
Human visual performance varies widely across the visual field in multiple dimensions. It has long been known that the pronounced decrease in acuity at more peripheral locations in the visual field correlates to physiological properties at many levels of the visual system. One factor affecting visual performance is the decreasing density of receptors away from the fovea (Curcio, Sloan, Packer, Hendrickson, & Kalina, 1987; Curcio & Allen, 1990; Curcio, Sloan, Kalina, & Hendrickson, 1990). This imbalance carries through to lateral geniculate nucleus (Connolly & Van Essen, 1984) and into striate and extrastriate visual cortex both in non-human primates (Maunsell &Van Essen, 1987; Tootell, Switkes, Silverman, & Hamilton, 1988; Van Essen, Newsome, & Maunsell, 1984) and other mammals (e.g. ferrets: Law, Zahs, & Stryker, 1988). There is a well-documented eccentricity effect, whereby performance decreases with eccentricity for a variety of tasks (e.g., Carrasco, Evert, Chang, & Katz, 1995). Within the visual field there are additional, well-documented asymmetries besides the effect of eccentricity. The Horizontal-Vertical Asymmetry (“HVA”) supports better contrast sensitivity, visual acuity and performance at isoeccentric spatial locations on the horizontal than on the vertical meridian (Carrasco et al., 1995; Carrasco, Talgar, & Cameron, 2001; Rijsdijk, Kroon, & van der Wildt, 1980; Rovamo, & Virsu, 1979). Several studies have reported generalized superiority for the lower versus the upper visual hemifield (Edgar & Smith, 1990; He, Cavanagh, & Intriligator, 1996; Levine & McAnany, 2005; McAnany & Levine, 2007; Previc, 1990; Rubin, Nakayama, & Shapley, 1996). Others have found this asymmetry to be restricted to the vertical meridian (the Vertical Meridian Asymmetry, “VMA”), with visual acuity and performance at locations on the lower, or “South,” vertical meridian superior to isoeccentric locations on the upper, or “North,” vertical meridian (Cameron, Tai, & Carrasco, 2002; Carrasco, Giordano, & McElree, 2004a; Carrasco et al., 2001; Carrasco, Williams, & Yeshurun, 2002; Liu, Heeger, & Carrasco, 2006b; Talgar & Carrasco, 2002). Note that some of the accounts of general hemifield asymmetry may reflect averaged results over locations that included the vertical meridian or placement of large stimuli centered directly or only above or below fixation (e.g. He et al., 1996; Rubin et al., 1996).
The degree to which the VMA affects visual perception and performance varies with eccentricity, and also with certain stimulus characteristics. The magnitude of the VMA increases with eccentricity (Carrasco et al., 2001), and is correlated with an asymmetrical decrease in retinal receptor density away from the fovea; cone density falls off more rapidly in the North than the South (Perry & Cowey, 1985). Moreover, at a given eccentricity, the VMA is small at low spatial frequencies and becomes more pronounced with increasing spatial frequency of the stimuli (Cameron et al., 2002; Carrasco et al., 2001; Liu et al., 2006b; Skrandies, 1987).
A study by Carrasco et al. (2001), the first to examine the effects of exogenous covert attention on performance in detection, discrimination, and location tasks, as a function of the location in the visual field, found that attention improves performance at both North and South locations on the vertical meridian, without changing the fundamental imbalance in performance that exists when attention is not specifically allocated to a peripheral stimulus location but is distributed throughout the display. Adjusting task difficulty to equate performance in attended and non-attended conditions yielded similar performance differences between North and South (Cameron et al., 2002). The authors concluded that the vertical meridian asymmetry stems from visual but not attentional factors.
Recently, a series of studies have examined the effects of exogenous attention on the appearance of various static and dynamic stimulus dimensions, including luminance contrast (Carrasco, Ling, & Read, 2004b; Ling & Carrasco, 2007), spatial frequency (Gobell & Carrasco, 2005), temporal flicker rate (Montagna & Carrasco, 2006), motion coherence (Liu, Fuller, & Carrasco, 2006a), color saturation (Fuller & Carrasco, 2006), perceived speed of motion (Turatto, Vescovi, & Valsecchi, 2007), and perceived size of moving patterns (Anton-Erxleben, Henrich, & Treue, 2007). These studies employed a task in which observers report the value of characteristic A (e.g., orientation right or left of vertical) for one of two stimuli based on their subjective comparison of the two on characteristic B (e.g., choose the stimulus that is higher in contrast). Although the experimenter’s main interest is in how attention affects the comparative judgment on B, by having to indicate the orientation of the stimulus of higher contrast, the observer is focused mainly on correctly reporting the value of characteristic A.
Several types of control have been used to rule out response bias as an explanation for results obtained with this paradigm. Extending the stimulus onset asynchrony (SOA) from approximately 100 ms to 500 ms will eliminate effects due to exogenous attention because of its short time-course (Nakayama & Mackeben, 1989), but response bias, if present, will remain (Carrasco et al., 2004b; Liu et al., 2006b). This timing control has been used successfully in previous appearance studies (Carrasco et al., 2004b; Gobell & Carrasco, 2005; Montagna & Carrasco, 2006; Turatto et al., 2007). Reversing the comparison judgment in the task (e.g. “report the orientation of the lower contrast stimulus” versus “report the orientation of the higher contrast stimulus”) yields the same pattern of results if they are due to a difference in appearance, but reverses the pattern if the only “effect” is response bias (Anton-Erxleben et al., 2007; Carrasco et al., 2004a, 2004b; Fuller & Carrasco, 2006; Montagna & Carrasco, 2006). The reverse instruction control has also been used successfully without the performance component of the task (size: Anton-Erxleben et al., 2007; orientation: Carrasco et al., 2004b). The polarity of the pre-cue contrast, i.e. whether it is black or white, also makes no difference in the direction of the appearance effect, ruling out a possible bias introduced by sensory interaction between the cue and the nearby stimulus as an explanation for the cueing effects in the appearance paradigm (Ling & Carrasco, 2007). Finally, one can replace the precue with a postcue, one which follows the stimulus presentation. This contral has been used in appearance tasks for spatial frequency (Gobell & Carrasco, 2005) and size (Anton-Erxleben et al., 2007). The spatial and temporal contiguity between cue and stimulus is the same for both cue configurations, but whereas the precue increased perceived spatial frequency and apparent size, the postcue did not alter appearance.
Across the range of visual characteristics studied so far, exogenous attention has been demonstrated to consistently alter subjective perception of all except hue; interestingly, although the other chromatic dimension, saturation, subjectively increases with attention, subjective hue perception remains unchanged (Fuller & Carrasco, 2006). Critically, however, for all characteristics tested, even for stimuli defined solely by hue contrast with the background (i.e., no difference in luminance and saturation) attention does enhance orientation discrimination performance. This dissociation shows that although each visual dimension manifesting an attentional effect on appearance has also demonstrated performance-based effects (e.g., higher proportion of correct responses in orientation discrimination), the performance effect is not necessarily mediated by the subjective change in appearance or vice versa. Moreover, it suggests that the effect of attention on visual perception is dependent on the nature of the visual process on which it acts.
The present study first examines whether there is a baseline difference in the appearance of contrast across the vertical meridian. Although there is ample evidence of performance differences, this question has not yet been addressed. Further, we are interested in whether and how the effect of exogenous attention on apparent contrast interacts with the VMA to produce an asymmetrical effect across the meridian, or whether the effects are symmetrical. We expected that attention would increase apparent contrast on the vertical meridian as it does on the horizontal meridian (Carrasco et al., 2004b). Note that the previous findings showing that exogenous attention improves discrimination performance while preserving the basic asymmetry of the VMA do not logically imply that this should be true of appearance; attention may have comparable or differential effects on apparent contrast across the vertical meridian. The dissociation between apparent hue and orientation performance noted above shows that the effects of exogenous attention on these two aspects of visual perception do not always correspond.
Experiment 1
We investigate whether the Vertical Meridian Asymmetry extends to a difference in subjective appearance between the North and South segments.
Methods
Observers
Nineteen undergraduate and graduate students in the Psychology Department at New York University participated in this experiment. They were screened for normal or corrected to normal visual acuity, and wore their glasses or contact lenses during the experiment. Ten observers participated in the main condition, and an additional ten observers participated in the control condition in which task instructions were reversed (see Procedure). One of the authors participated in both conditions.
Apparatus
The experiment was programmed using the Psychophysics Toolbox (Pelli, 1997) and MATLAB 5.2, running on an Apple G4 computer with a 22 inch Viewsonic P220f color monitor set for 1600 × 1200 pixel resolution at an 85 Hz refresh rate. The monitor signal was run through a Pelli-Zhang attenuator (Pelli & Zhang, 1991), which limited the output of the monitor to the green gun and provided finer contrast control. The monitor with attenuator in place was characterized with a Minolta luminance meter to generate a luminance lookup table. Background luminance was set at 14 cd/m2.
Stimuli and procedure
The stimuli were Gabor gratings (sinusoidal luminance gratings in a Gaussian window) of 2, 4 and 6 cpd spatial frequency, subtending 2 degrees of visual angle, which were randomly intermixed throughout the experiment. We used the same nine contrast values for 2 and 4 cpd, ranging from 15.8% to 39.8% Michelson contrast, equally spaced in increments of 0.05 log-contrast units. The contrast range for 6 cpd was higher in order to offset lower contrast sensitivity at this spatial frequency. The nine contrast values ranged from 28.2% to 70.8% Michelson contrast in increments of 0.05 log-contrast units.
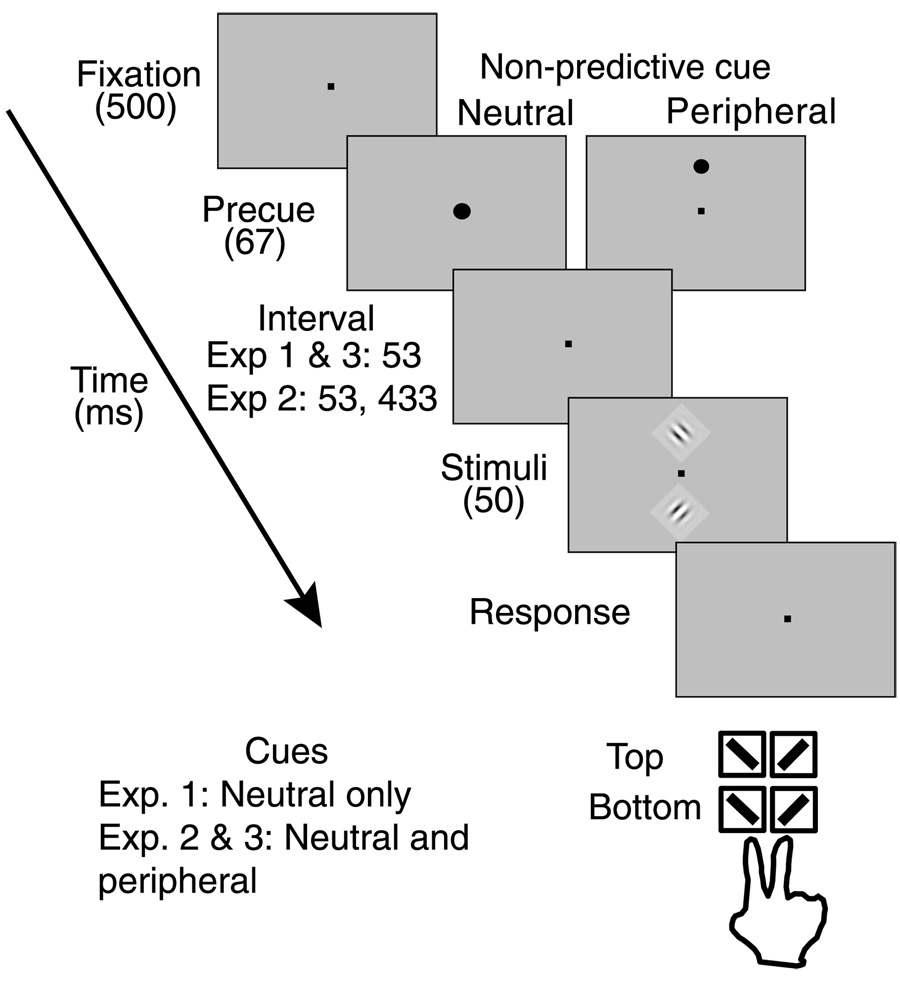
The procedure for all three experiments is illustrated in Figure 1, with notations for differences in timing and cueing specific to each experiment. Observers were seated 57 cm from the monitor in a darkened room, with head position maintained by a chinrest. A trial began with the observer fixating a small black cross at the center of the screen; observers were instructed to maintain fixation on the cross, which was onscreen throughout the experiment. After 500 ms, a small (0.35 degrees of visual angle) black cue appeared for 67 ms at fixation to signal the start of each trial in Experiment 1. Such cues have been used as neutral cues, similar to multi-element distributed cues (Carrasco, Penpeci-Talgar, & Eckstein, 2000; Carrasco et al., 2002; Phelps, Ling, & Carrasco, 2006; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1999). They have also been used in “neutral” exogenous attention conditions in prior appearance studies (Carrasco et al., 2004b; Turatto et al., 2007) and are used for the same purpose in Experiment 2 and 3 in this study. An interval of 53 ms followed the offset of the cue, after which two Gabor stimuli were presented for 50 ms, centered at 4° eccentricity on the vertical meridian, one in the North and one in the South.
Figure 1.
Trial Sequence for Experiment 1–3. Experiment 1 included only a Neutral cue at fixation, Experiment 2 and 3 used randomized Neutral and Peripheral cues. Control condition for Experiment 2 increased the blank interval between cue offset and stimulus onset to 433 ms.
In every trial, one Gabor, termed the “Standard,” had the middle value in the range (25.1% for 2 and 4 cpd stimuli, 44.7% for 6 cpd stimuli); the other, termed the “Test,” could take any one of the nine contrasts in the range, including that of the Standard. The Test contrast could be higher, lower, or equal to the Standard contrast. The stimuli were tilted 20° to the right or left of vertical. Test contrast, the locations of the Test and Standard on the North or South vertical meridian, stimulus spatial frequency (2, 4, and 6 cpd) and the independent orientations of the stimuli were fully randomized. After stimulus offset, observers reported “the orientation of the stimulus that has higher contrast” using one of four buttons from the numerical keyset at the right of the computer keyboard. The “1” or “2” keys indicated that the South stimulus had higher contrast and was oriented leftward or rightward, respectively, and the “3” or “4” keys directly above to make corresponding reports if the North stimulus had higher contrast. In a 1-hr experimental session, observers performed a practice block of 50 trials that included audio feedback for correct contrast and orientation responses, and then completed 2000 experimental trials without feedback in 20 blocks of 100.
Results
We fitted individual observer psychometric functions to the data grouped according to the position of the Test stimulus on the North or South vertical meridian. We used the Psignifit Toolbox (© J. Hill, 1995–2005) with Matlab 7.0.4.352 (R14) to fit four-parameter Weibull functions to the data, using as the dependent variable the probability that the Test stimulus was chosen as having higher contrast than the Standard, and Test stimulus log-contrast as the independent variable. Goodness of fit was determined by calculating deviance scores (Wichmann & Hill, 2001), which were evaluated against chi-square critical values for significance. Based on examination of the deviance scores, we allowed the lower asymptote to vary between 0 and 0.1 in order to achieve good fits and to ensure that the threshold estimates were stable. Data for observers who met the orientation discrimination performance requirement (>90%) and whose deviance scores were below the critical value (~16) were included in the analysis. The fitted functions were inverted to estimate the Test contrast corresponding to chance (0.5) probability of selecting the Test over the Standard. This probability is the “Point of Subjective Equality” (PSE), the Test contrast at which the observer randomly chooses the Test or the Standard because she is not able to distinguish them. The individual PSEs were then input to a within-subjects ANOVA for further analysis.
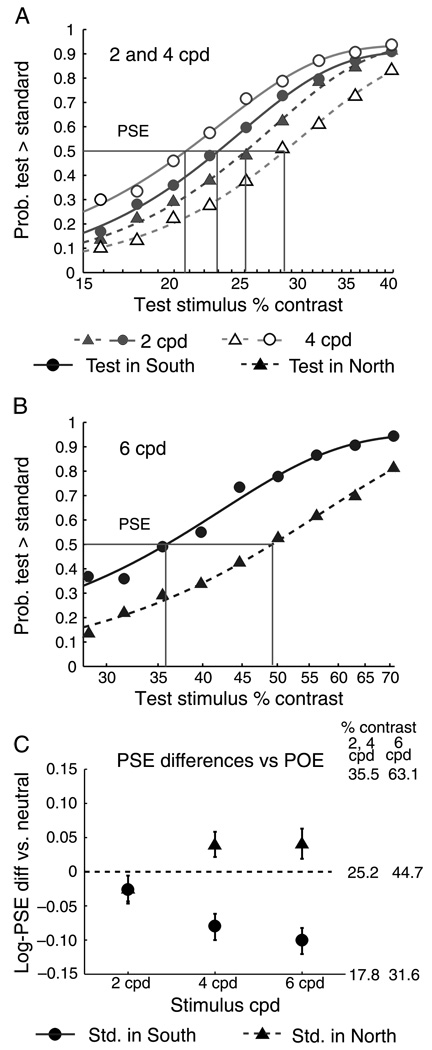
Figure 2 shows the pooled psychometric functions for all observers (Panel A: 2 and 4 cpd; Panel B: 6 cpd). For 4 cpd and 6 cpd stimuli, but not 2 cpd stimuli, the PSE is at physical Test contrasts that are lower than the Standard stimulus when the Test is in the South location, i.e., the PSE is below the “Point of Objective Equality” (POE). When the Test is in the North location, the PSE is above the POE. Stimuli presented on the South vertical meridian have higher apparent contrast than physically identical stimuli presented on the North vertical meridian.
Figure 2.
Results of Experiment 1. Panel A: pooled psychometric functions for 2 and 4 cpd stimuli. Panel B: functions for 6 cpd stimuli. Panel C: summary of mean PSEs when Test stimulus is in the South (dots) and in the North (triangles). PSEs are lower than the POE when the Test is in the South, and higher when it is in the North. Stimuli appear to have higher contrast in the South and lower contrast in the North.
We subjected the individual PSE estimates to a 3 × 2 within-subjects ANOVA (stimulus cpd × Test location). A significant main effect of cpd (F(2, 18) = 857.8, p < .001, h2 =.99) resulted from the higher contrast range used for 6 cpd stimuli than for 2 and 4 cpd stimuli. The main effect of Test location was significant (F(1, 9) = 7.74, p < .05, h2 = .46), as was the interaction of Test location and cpd (F(2, 88) = 10.06, p <. 001, h2 = .53). Figure 2C, shows the mean results for Test location by cpd. PSEs were shifted below the POE when the Test was in the South location, and higher when it was in the North, but only for the 4 cpd and 6 cpd stimuli. This pattern with regard to stimulus spatial frequency parallels prior reports that the VMA in performance-based tasks is minimal at very low spatial frequencies and becomes stronger at higher spatial frequencies (Cameron et al., 2002; Carrasco et al., 2001; Liu et al., 2006b; Skrandies, 1987).
Experiment 1 showed that the visual performance asymmetries that constitute the VMA are accompanied by a difference in apparent contrast between the North and South segments of the vertical meridian. In the following experiments we examine how exogenous spatial attention interacts with this inherent difference in appearance.
Experiment 2
Here we tested the effects of exogenous attention on apparent contrast across the vertical meridian using “low” contrast Gabor stimuli. The stimuli and procedure used are those used by Carrasco et al. (2004b), who found that attention increases apparent contrast on the horizontal meridian. We used 2 and 4 cpd stimuli for comparison to that study, because the authors reported similar attentional modulation of apparent contrast at both spatial frequencies. Given that our Experiment 1 yielded baseline appearance differences at 4 cpd but not 2 cpd in the upper and lower regions of the vertical meridian, we explored whether the magnitude of the attentional effects would differ for these spatial frequencies.
Methods
Apparatus
The apparatus was the same as described for Experiment 1. For the control condition in which we increased the interval between the cue offset and stimulus onset to 433 ms (SOA 500 ms), we monitored eye position using an ISCAN video camera, recording to a separate Dell computer.
Observers
Thirty-nine undergraduate and graduate students in the Psychology Department at New York University participated in this experiment. They were screened for normal or corrected to normal visual acuity, and wore their glasses or contact lenses during the experiment. Twenty observers participated in the main condition in which we manipulated exogenous attention, and an additional twenty observers participated in the control condition. One of the authors participated in both conditions.1
Stimuli and procedure
The stimuli were Gabor gratings of 2 and 4 cpd spatial frequency, subtending 2° of visual angle with a tilt of 45° to the left or right, which were randomly intermixed throughout the experiment. We used nine contrast values, ranging from 2.5% to 16% Michelson contrast, equally spaced in increments of 0.1 log-contrast units.
The procedure is identical to that of Experiment 1, except for the addition of peripheral cues (see Figure 1). The cue could appear at three possible locations with equal probability: on the vertical meridian 5.6° North or South of fixation, or at fixation. Cue position was randomized across trials so as to be completely uninformative with regard to the characteristics of the stimuli and the task.
The only difference between the main and control conditions was the time between cue onset and display onset (SOA): 120 ms in the main condition and 500 ms in the control condition. This manipulation ensured that transient attention was active in the former but no longer active in the latter (Carrasco et al., 2004b; Liu et al., 2006a). The logic of this control is that any effect not due to attention, such as response bias favoring the cued location, would be present in both conditions, as they would be independent of the temporal relation between the cue and stimuli. Alternatively, any effects evident in the main condition but absent from the control condition could only be attributed to exogenous attention. The time course of exogenous attention is short, with the peak effect occurring 100–120 ms following cue onset, and decaying rapidly thereafter (Cheal & Lyon, 1991; Nakayama & Mackeben, 1989). With the short interval used in the main condition, it is not possible for an observer to saccade from the fixation point to a peripherally cued location before the stimuli are presented. However, it is possible to execute a saccade during the longer delay in the control condition, therefore we recorded eye position using an ISCAN camera throughout the experiment for all control observers, to ensure that fixation was maintained.
Results
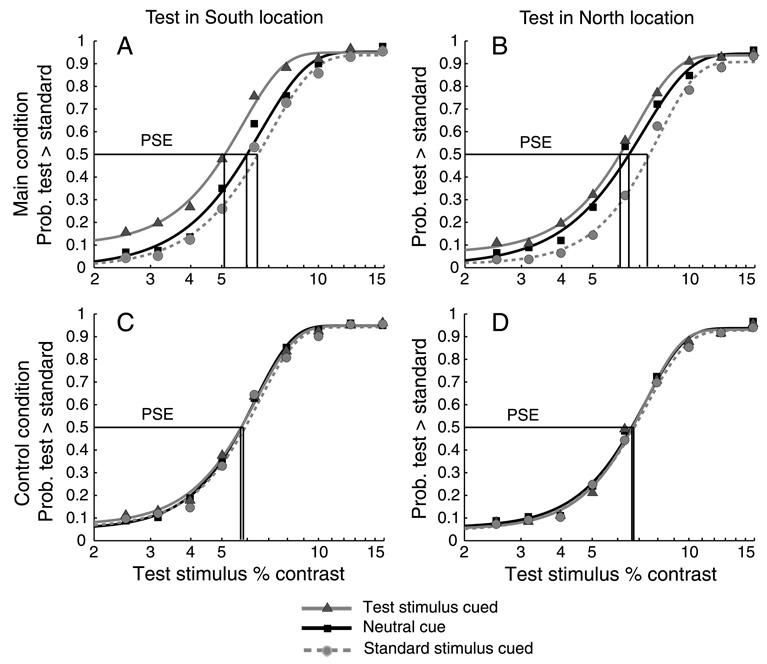
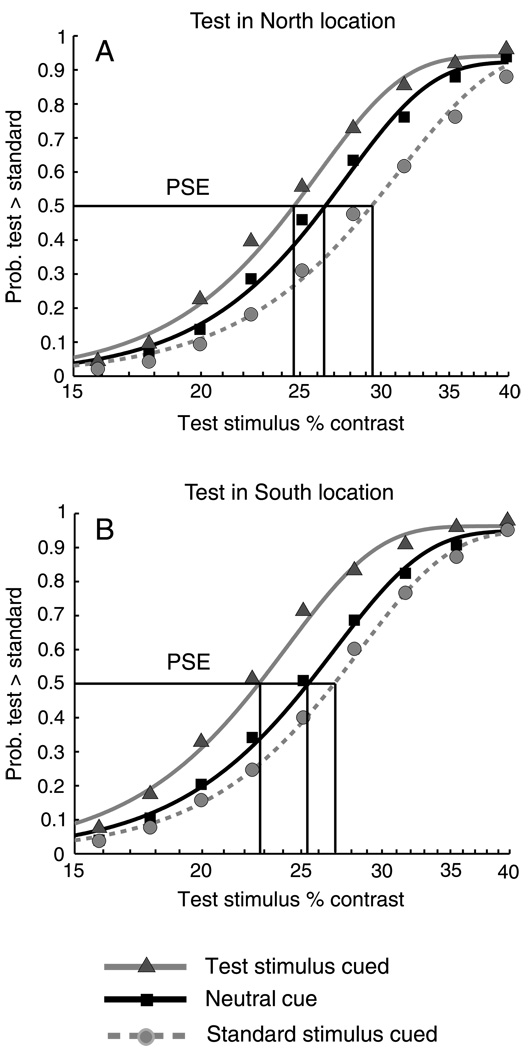
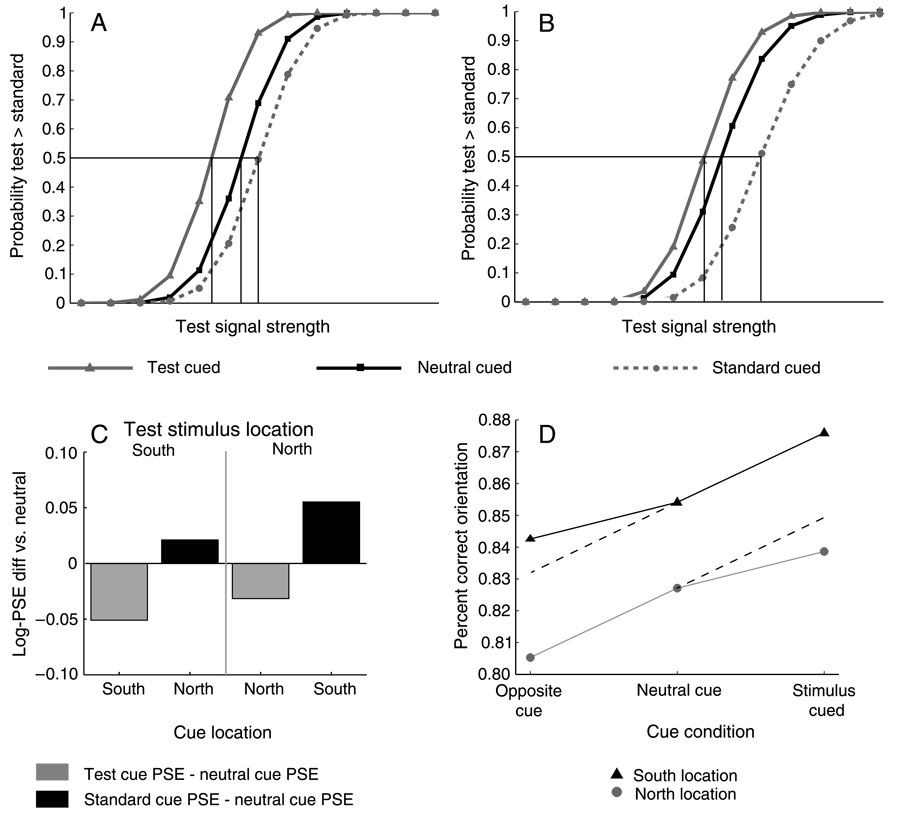
We used the procedures described in Experiment 1 to fit individual observer psychometric functions to the data grouped according to the location of the Test stimulus (North or South) and three cue conditions: a Neutral condition in which the cue appeared at the central fixation point; a Test cued condition in which the peripheral cue appeared on the same side of the vertical meridian as the Test stimulus; and a Standard cued condition in which the peripheral cue and the Standard stimulus appeared on the same side of the vertical meridian. Within-subjects 2 × 2 × 3 ANOVAs (cpd × Test location × cue condition) were conducted on the individual observer PSEs for the main and control conditions. The interaction of cpd × Test location × cue condition was not significant either for the main (short ISI) condition (F(2, 38) = 2.30, p > .1, h2 = .10) or for the control (long ISI) condition (F(2, 38) = 2.25, p > .1, h2 = .1). We therefore combined the 2 cpd and 4 cpd data for each observer, refit the psychometric functions on the collapsed data, and performed 2 × 3 within-subject ANOVAs (Test location × cue condition) on the resulting PSEs. The collapsed data, pooled across observers and fitted, are plotted in Figure 3. As can be seen in the figure, the psychometric functions differ for the three cue conditions in the main, short ISI condition, but not for the control condition, indicating an effect of exogenous attention. Moreover, the shifts of functions for the Test and Standard cue condition appear to be asymmetrical for the North and South locations on the vertical meridian. The statistical analysis of the individual observers’ data bears this out.
Figure 3.
Pooled psychometric functions for Experiment 2. Top row (panels A and B): Main condition in which the SOA (cue onset to stimulus onset) was 120 ms, showing effects of exogenous attention on apparent contrast. Bottom row (panels C and D): Control condition with SOA of 500 ms, exogenous attention has expired and there is no effect on appearance. Left column (panels A and C): Test in South location. Right column (panels B and D): Test in North location. Increase in apparent contrast with attention is greater in the South than in the North.
In the main condition, there was a significant main effect of cue (F(2, 38) = 24.04, p <. 001, h2 = .55), and of Test location (F(1, 19) = 4.30, p = .05, h2 = .18), as well as a significant interaction between cue and Test location (F(2, 38) = 4.6, p < .05, h2 = .19). The interaction indicates that the cue effect varies with the position of the Test stimulus on the North or South of the vertical meridian. The effect of a peripheral cue on apparent contrast is greater on the South vertical meridian than on the North, leading to asymmetrical shifts in the mean PSEs of the Test cued and Standard cue conditions relative to the Neutral cue.
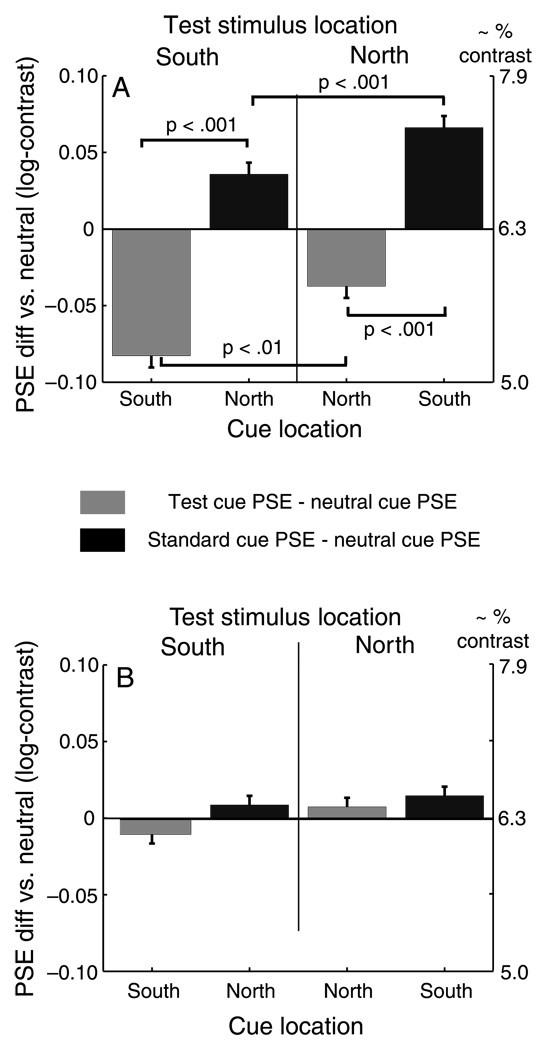
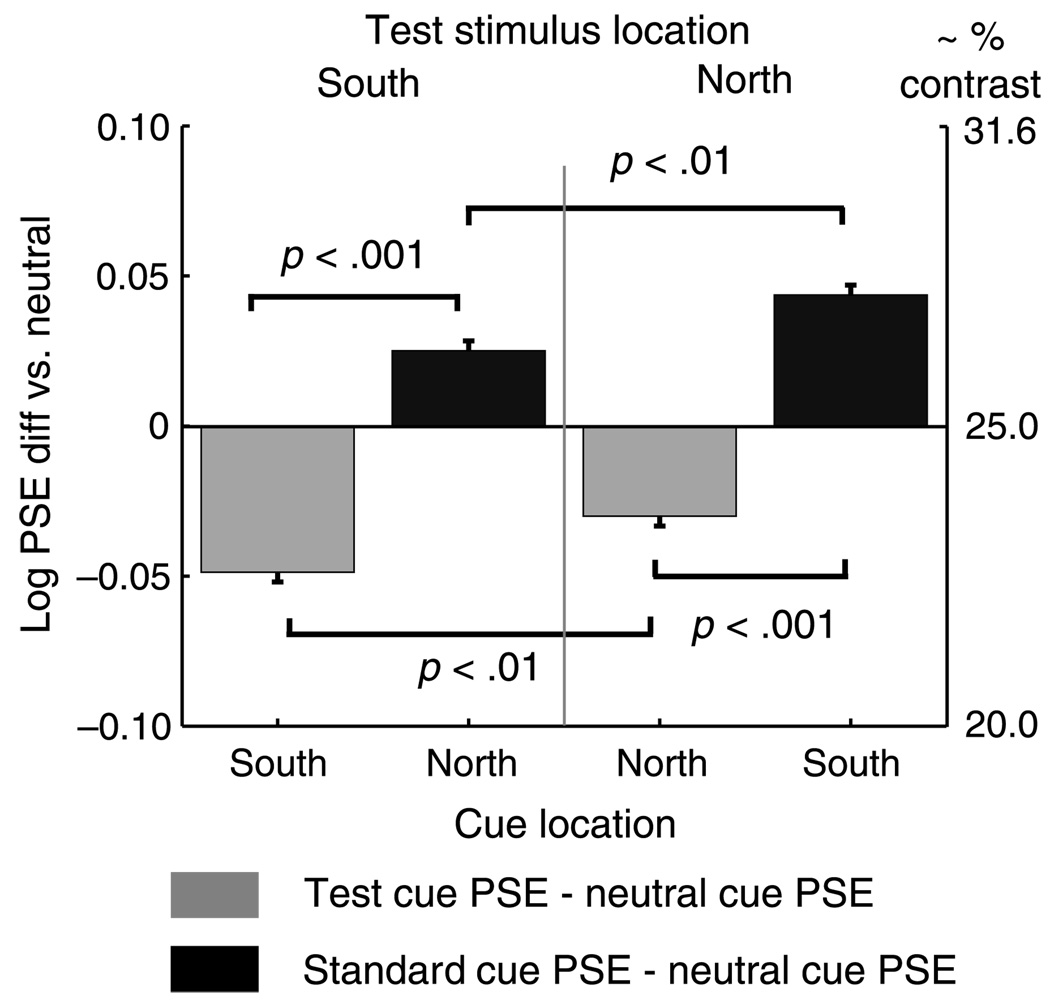
This asymmetry is visible in Figure 3, but can be more clearly demonstrated by reorganizing the data as in Figure 4, which plots the differences of mean PSEs of the Test cue and Standard cue conditions from the Neutral cue condition, separated by the two Test location conditions (i.e. Test stimulus in North, right side of the figure, and Test stimulus in South, left side of the figure) and by the South and North cue locations on the horizontal axis. The bar colors correspond to cue condition (Test, light gray; Standard, black) with values on the vertical axis indicating the difference versus the Neutral PSE in log contrast units. The negative shift in the Test cue PSE (physical Test contrast lower than physical Standard contrast) is significantly greater when the Test stimulus and cue are in the South than when both are in the North. The same is true for the Standard cue PSE when the Standard and the cue are in the South, although the polarity of the shifts is positive, toward higher Test contrast.
Figure 4.
Mean PSE differences of Test and Standard cue conditions relative to Neutral cue condition for Experiment 2. Panel a: main condition, 120 ms SOA. PSE shifts due to exogenous attention are greater on the South vertical meridian (left-most and right-most bars), than when the cue is in the North (center pair of bars). Panel B: control condition, 500 ms SOA. When the interval exceeds the duration of exogenous attention, the peripheral cues have no effect on PSE.
The control condition paradigm was identical in all respects to the main condition except for the duration of the interval between the cue onset and stimulus onset, which was 500 ms, more than sufficient time for exogenous attention to diminish (Nakayama & Mackeben, 1989). Because it also provided adequate time for saccade to the cued location, for all observers and trials we recorded eye position with an infrared video camera. Fewer than 0.1% of trials showed any movement from fixation during the trial; these trials were excluded from the analysis.
A within-subjects ANOVA indicated a significant main effect of Test location (F(1, 19) = 5.16, p < .05, h2 = .21), but neither the main effect of cue (F(2, 38) = 1.61, p > .1, h2 = .08) nor the interaction of Test location and cue (F(2, 38) = 1.16, p > .1, h2 = .06) were significant.
The results of the main ISI condition in Experiment 2 support the idea that exogenous attention elicits a greater increase in apparent contrast on South segment than on the North segment of the vertical meridian. This asymmetry occurred even with 2 cpd stimuli, despite the lack of a baseline appearance difference for this spatial frequency in Experiment 1. When the ISI was lengthened sufficiently to allow the exogenous attention effects of the cue to expire before stimulus presentation, the cue effect disappeared. This control validates that the cueing effects in the main condition are due to exogenous attention, and that the asymmetry with regard to South or North position on the vertical meridian represents a differential effect of exogenous spatial attention on appearance: it further exaggerates the inherent difference in appearance of the VMA.
Experiment 3
In this experiment, we increased the contrasts of our stimuli in order to determine if the asymmetry in attentional effects on the lower and upper vertical meridian would be robust. Moreover, given that the vertical meridian asymmetry has been documented for a performance-based task with exogenous attention (Cameron et al., 2002; Carrasco et al., 2001, 2002; Talgar & Carrasco, 2002), here we chose to measure orientation discrimination performance concurrently with appearance as a control with which to confirm that the peripheral cues engaged exogenous spatial attention. We increased the difficulty of the orientation discrimination task to maintain performance between 70% and 80% overall for each observer, in order to avoid floor and ceiling effects on performance. This type of concurrent control was used successfully in a prior study of exogenous attention and motion coherence (Liu et al., 2006a). In addition, we doubled the number of trials for each observer from Experiment 2 in order to increase precision on the PSE estimates and to provide sufficient trials for the analysis of orientation discrimination.
Methods
Apparatus
The apparatus was the same as described in Experiment 1.
Observers
Twenty graduate and undergraduate students in the Psychology Department at NewYork University participated in this experiment. They were screened for normal or corrected to normal visual acuity. One observer was an author.
Stimuli and procedure
Three changes were made from Experiment 2. The Gabor contrasts were higher, ranging from 16% to 40% contrast in increments of .05 log-contrast units, with the Standard stimulus at 25%. This contrast range is similar to that used in previous contrast appearance studies (Carrasco et al., 2004b; Ling & Carrasco, 2007).
Stimulus tilts from vertical were adjusted so that performance for each observer would be ~75% correct orientation discrimination. Observers participated in a training session on the task with a program that incorporated PEST staircases for stimulus orientation, and provided feedback on contrast discrimination and orientation performance. The contrast difference was fixed using the Standard and the highest value Test from the actual contrasts used in the main experiment, in order to make this part of the training task relatively easy. During the main task, separate tilt adjustments were made for the 2 and 4 cpd stimuli after each run of 500 trials based on aggregate performance including both North and South location conditions. The number of trials per observer was doubled from Experiment 2 to 4000, in order to increase statistical power both for the psychometric functions and for the evaluation of orientation discrimination performance. Observers completed 8 runs, each consisting of 10 blocks of 50 trials each, generally in one-hour sessions on three separate days.
Results
The fitting procedures from Experiments 1 and 2 were used to analyze the appearance data from this experiment. There were no significant deviance scores for the fitted psychometric functions (Wichmann & Hill, 2001).
Given that the interaction of cpd, cue condition, and Test location was not significant (F(2, 38) <1), we collapsed each observer’s data across cpd before re-fitting and further analysis. The psychometric functions for the data pooled across observers are shown in Figure 5. The shifts of the PSEs for Test and Standard cue conditions are asymmetric, with the greater shift for cues on the South vertical meridian. We performed a 2 × 3 within-subjects ANOVA on the PSEs (Test location × cue condition). The main effect of Test location was significant (F(1, 19) = 6.93, p < .05, h2 = .27). The main effect of cue was significant (F(2, 38) = 95.00, p < .001, h2 = .83); cueing the Test stimulus shifted the PSE toward lower Test contrast and cueing the Standard shifted the PSE toward higher Test contrast. We found a significant interaction between Test location and cue condition (F(2, 38) = 6.14, p < .01, h2 = .24).
Figure 5.
Pooled appearance psychometric functions for Experiment 3, high contrast stimuli. Panel A: Test stimulus on the South vertical meridian. Panel B: Test stimulus on the North vertical meridian. Exogenous attention elicits a greater increase in apparent contrast when it is located on the South vertical meridian.
We subtracted the Neutral PSE from the Test and Standard PSEs within Test location condition as in Experiment 2, and conducted a within-subjects ANOVA; the mean differences are shown in Figure 6. There was a significant main effect of location (F(1, 19) = 8.10, p < .01, h2 = .30), and of Test and Standard cue differences from Neutral (F(1, 19) = 101.5, p < .01), but the interaction of Test location and cue difference was not significant (F(1, 19) <1). Consistent with the findings in Experiment 2, cues in the South elicited larger PSE shifts than cues in the North.
Figure 6.
Mean PSE differences of Test and Standard cue conditions relative to Neutral cue condition for Experiment 3. Comparisons between the two Test cue conditions (light bars) and the two Standard cue conditions (black bars) show that PSE shifts are significantly larger when the exogenous attention is cued to the South vertical meridian than when it is cued to the North.
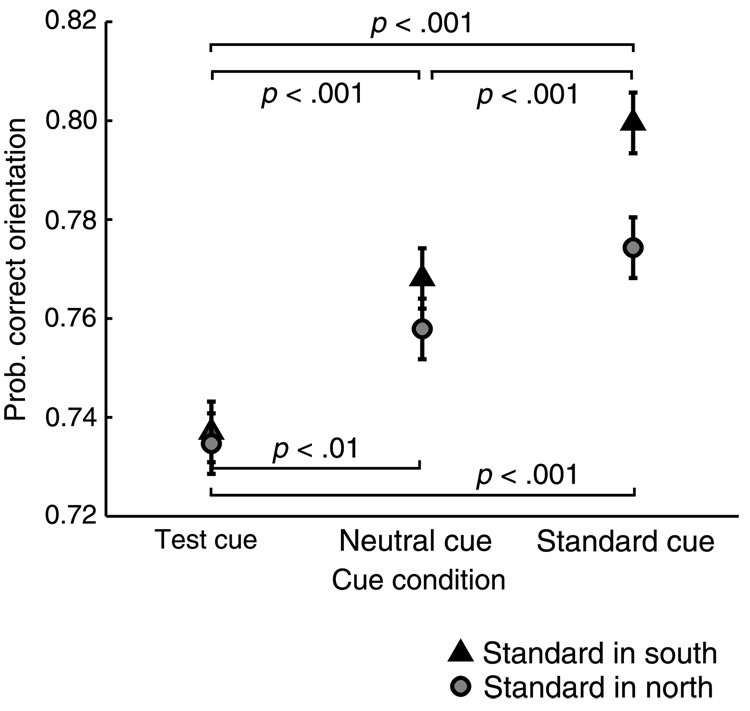
The mean orientation discrimination performance results are shown in Figure 7. We analyzed the performance data using the trials in which observers chose the Standard stimulus as having higher contrast than the Test and reported the orientation for the Standard stimulus. This ensured that all the performance data pertained to stimuli with the same physical contrast and yielded sufficient numbers of trials to make the analysis feasible (Liu et al., 2006b). We conducted a two-way within subjects ANOVA (Standard location × cue condition) on the percent correct orientation responses for these Standard responses. The main effect of cue (F(2, 38) = 40.992, p < .001, h2 = .68) indicates that in comparison to the Neutral condition, orientation discrimination improved when the Standard was cued and was impaired when the Test was cued. The main effect of Standard location was not significant (F(1, 19) = 3.99, p > .05, h2 = .17), nor was the interaction of cue and Standard location and (F(2, 38) = 2.46, p > .1, h2 = .12). These results indicate that orientation discrimination performance was generally better on the lower (South) vertical meridian than the upper (North) segment, and that the pattern of cue effects on performance was comparable in both segments.
Figure 7.
Orientation discrimination performance results for Experiment 3. All data are for trials in which observers chose the Standard stimulus as higher in contrast such that physical stimulus contrast is constant. The cue improved orientation discrimination when the Standard stimulus was cued, and impaired it when the cue appeared near the Test stimulus location. These performance effects confirm that the peripheral cues engaged exogenous spatial attention, and rule out response bias as an explanation for the appearance effects in Experiment 3.
Modeling
Our results show an interaction of exogenous spatial attention with North and South locations on the vertical meridian for the subjective appearance comparison task. One explanation for this interaction could be that the magnitude of attentional modulation depends on whether its spatial locus is on the South or North vertical meridian. However, prior studies testing the effects of attention on visual performance (Cameron et al., 2002; Talgar & Carrasco, 2002), and the present results for orientation discrimination performance, showed no such interaction. How to explain these apparently contradictory results?
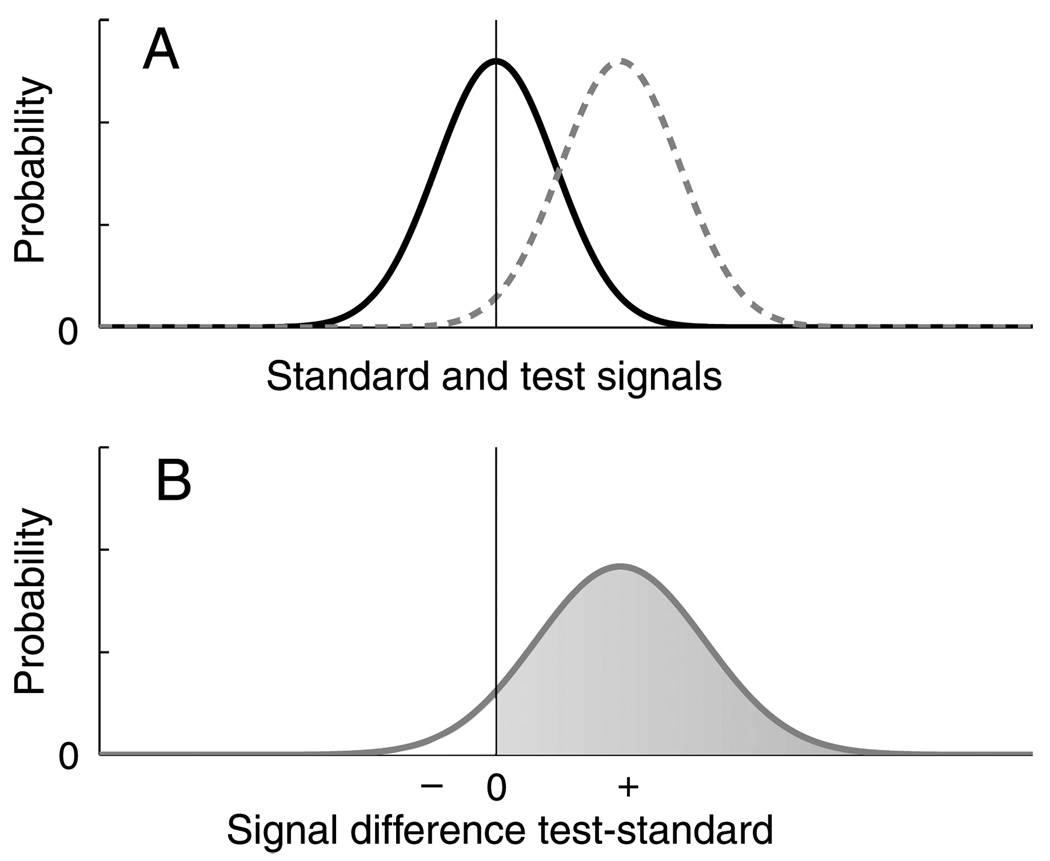
To answer this question, we implemented simple signal detection theory (SDT) models of the appearance and orientation discrimination tasks. A schematic representation of the appearance model is shown in Figure 8A. The horizontal axis represents strength of the visual signal generated by the stimuli. The solid line represents signal probability distribution for a hypothetical Standard stimulus as in our experiments; because the system responding to the stimulus is noisy, the visual response to this stimulus varies from trial to trial. One of the Test contrasts used in the experiment, higher than the Standard, is represented by the distribution with the dashed line in Figure 8A. The model computes the probability distribution for the difference between Test and Standard signals (Figure 8B), which has mean µdiff = µtest − µstandard and variance equal to the sum of the variances of the two signals, (Wickens, 2002).2 The probability that the Test signal is higher than the Standard is the area under this difference distribution for values greater than zero. When the Standard and Test distributions are identical, this probability is 0.5, or chance.
Figure 8.
Appearance Signal Detection Model Calculation. For a given combination of Standard and Test contrasts (panel A), the model calculates the probability distribution of the difference between Standard and Test signals and evaluates the cumulative probability that it is greater than zero (panel B). This corresponds to the probability that an ideal observer would respond that the Test stimulus has higher contrast.
Differences in contrast sensitivities for the two stimulus locations are implemented by introducing scaling factors that shift the sets of distributions. We assumed greater sensitivity in the South and assigned a scaling factor >1, whereas the North location has a scaling factor of 1. This shifts the distribution for the stimulus in the South to the right (higher signal strength) in Figure 8A. If the Test is in the South, its probability distribution is shifted to the right, resulting in a higher proportion of “Choose Test” responses. When the Standard is in the South, the result is fewer “Choose Test” responses.
Similar multiplicative scaling factors are used to implement the effects of attention, based on the multiplicative model (e.g., (Cook & Maunsell, 2004; McAdams & Maunsell, 1999; Martinez-Trujillo & Treue, 2004). We assume, as noted above, that attention has a greater effect in the South than the North; hence, we apply a larger factor to that location. Attention increases the signal at the attended location, so both factors are >1. Exogenous spatial attention provides a benefit at the attended location with a cost at unattended locations (e.g., (Pestilli & Carrasco, 2005; Pestilli, Viera, & Carrasco, 2007). Our model reduces signal strength at the unattended location by applying a factor <1 for that stimulus. We further assume that these costs are directly proportional to the magnitude of the benefit at the attended spatial location. For example, if attention is deployed to the South and increases the signal strength there by a factor of 1.1, then the signal from the North stimulus is modulated by 1/1.1 or ~0.91. As a consequence, a smaller benefit of attention in the North, say 1.05, carries with it smaller cost in the South, which has a signal reduced by a factor of 1/1.05 or ~0.95.
For the discrimination model we adopted a simple mechanism in which the ideal observer monitors two channels, tuned to right and left orientation. Whichever channel has the higher signal on a given trial determines the orientation response. The percentage of correct orientation responses is calculated by computing the probability distribution for the difference between the signals in the two channels. The essential difference between this model and the appearance model is that both channels respond to a stimulus at the same spatial location. If a right tilted stimulus is presented at that location, then the right-preferring channel will respond more strongly than the left-preferring channel. When, as in our Experiment 3, the right and left tilts from vertical are small, the difference in channel responses will be relatively small, the probability distributions of the signals will overlap, and the proportion of correct discriminations will be between chance (here, 0.5 for a 2AFC discrimination) and 1. Because both channels operate on the same spatial location, both will experience the same upward multiplicative attentional modulation when that spatial location is cued and the same downward modulation when another spatial location is cued. In the attended condition, although the signals from both channels are increased, the overlap between the distributions is smaller, resulting in a higher proportion of correct responses. The reverse is true for the unattended condition; the signals from both channels are decreased and the overlap between the distributions is larger, resulting in a lower proportion of correct responses. This change in the spacing and overlap of the two distributions results from the multiplicative attentional modulation in the model. (An additive factor, in contrast, would move the signal distributions by equal amounts, causing attention to have no effect on discriminability). The differences in contrast sensitivity and attentional modulation for North and South locations are implemented in this model using the same multiplicative scaling factors as in the appearance model.
Model results are shown in Figure 9. The appearance psychometric functions closely resemble those from our actual data (panels A, B, and C). We find the appearance asymmetry, the rightward and leftward shifts of the Neutral cue conditions from Experiment 1 (compare Neutral conditions in panels A and B), and the same asymmetry in attentional effects with Test location that we found in Experiment 2 and 3 (see Figure 3 and Figure 5). Panel C depicts the PSE differences between the Test Cued and Standard Cued conditions and the Neutral Cued condition in the same format as Figure 4 and Figure 6, making the similarity of the attentional asymmetry more apparent. We achieved this particular asymmetry by setting the attentional benefit in the South at twice that of the North.
Figure 9.
Appearance Model Results (panels A, B, and C): Model-generated psychometric functions when the Test stimulus is in the South (panel A) and North (panel B) have asymmetrical shifts in peripherally cued conditions as the data from Experiment 2 and 3 (compare to Figure 3 and Figure 5). Panel C plots the model PSE shifts for peripherally cued conditions relative to Neutral in the same format as Figure 4 and Figure 6. Discrimination Model Results (panel D): Assumptions of differential attentional modulation in South and North locations produces asymmetrical performance effects. Dotted lines represent symmetrical effects when North and South attention factors in the model are equal.
We ran the orientation discrimination model with the same assumptions about the relative strength of attentional modulation and reciprocity of costs at unattended locations. As with the appearance model, we tested it with both constant signal variance and Poisson signal variance and found no difference in the pattern of results. As shown in Figure 9D, the model predicts that there is indeed an asymmetry in discrimination performance. Cueing a stimulus in the South produces a slightly larger performance improvement in the South (top line) than cueing a stimulus in the North (bottom line), with corresponding differences in performance impairments at the uncued locations. When we increase the North attentional factor to make it equal with the South, the performance improvements and impairments are now exactly proportional for both locations (dashed lines in Figure 9D).
We used the models to simulate what 10 ideal observers with 500 repetitions of the appearance and orientation discrimination experiments might yield in terms of statistical significance of the main and interaction effects of attention and location. Within-subjects ANOVAs were performed on the results, and histograms of the 500 p-values are shown in Figure 10. The effects are quite strong for the appearance experiment: all p-values for main effects of attention (panel A) and location (panel B), and 98% for the interaction between attention and location (panel C) are <.05. The results for orientation discrimination are shown in panels D, E, and F. Each simulation of this experiment included 6000 trials per observer (1000 per each of the six attention × location conditions), approximately 5 times the number of “Choose Standard” trials available for this analysis in Experiment 3, to evaluate the simulations under conditions of higher statistical power. The main effects of attention and location are virtually always significant, however, even with the large number of trials and the use of 10 “ideal” observers the interaction effect reaches significance (p < .05) for only about 25% of the simulations. Although the model does produce an attentional asymmetry for discrimination performance, it is difficult to detect statistically in repeated simulations and we expect it would be highly unlikely to register using human (i.e. “non-ideal”) observers, regardless of the number of trials or observers employed. The issue is one of small effect size, not of low statistical power.
Figure 10.
Simulation Statistics for Appearance Model (panels A, B, and C). Histograms of p-values for 500 simulations of the appearance task using 10 ideal observers. All simulations yielded significant main effects of attention (panel A) and location (panel B); the interaction effect was significant (p < .05) for 98% of the simulations. Horizontal axes in panels a & b scaled 0 to 0.2; horizontal axis in panel C scaled 0 to 1.0. Simulation Statistics for Discrimination Model (panels D, E, and F): Histograms of p-values for 500 simulations of the appearance task using 10 ideal observers. All simulations yielded significant main effects of attention (panel A) and location (panel B); the interaction effect was significant (p < .05) for only 25% of the simulations. Horizontal axes in panels A and B scaled 0 to 0.2; horizontal axis in panel C scaled 0 to 1.0 for direct comparison to panel F.
Discussion
This is the first study to show that the subjective appearance of contrast is greater on the lower vertical meridian than on the upper vertical meridian. In addition, consistent with earlier results showing that attention increases apparent contrast on the horizontal meridian (Carrasco et al., 2004b; Ling & Carrasco, 2007), in the present study we found increases in apparent contrast with attention on the vertical meridian. All three studies show that the effect of exogenous attention on appearance is comparable for contrast ranges centered at low (6%) and high (22%) contrasts. Moreover, by separately testing on the North and South segments, we also found that the increase is greater on the lower vertical meridian. The magnitudes of the attentional shifts in PSE were nearly twice as large on the lower vertical meridian. In the lower contrast range (Test 2.5% to 16% contrast, Standard 6.3%) the PSE shifts on the lower vertical meridian were approximately equivalent to a 1% difference in contrast, while on the upper meridian they amounted to a 0.5% difference. With higher contrast stimuli (Test 15.9% to 39.8% contrast, Standard 25.1%) the corresponding figures were 2.7% and 1.5%.
To support the conclusion that attention affects subjective appearance, response bias to the cue must be ruled out as an explanation of our results. In this study, we used two different approaches as controls, both of which clearly distinguish between appearance effects and response bias. In Experiment 2, we used a temporal control based on the short duration of exogenous attention. It is well established that exogenous attention peaks in the range of 100 to 120 ms from cue onset and diminishes shortly there-after. At subsequent times around 300 ms, performance is briefly impaired at the attended location (inhibition of return (e.g. Klein, 2000). Our stimuli in the main condition were displayed for only 50 ms, starting 120 ms after cue onset, well within the effective period of exogenous attention. In the control condition, stimulus presentation began 500 ms after cue onset. Because response bias would not have the limited time duration of exogenous attention, similar results in both main and control conditions would be predicted if response bias drives our results. We found that the effects present in the main condition disappeared in the control condition, eliminating response bias as the explanation. This control has been successfully used in previous experiments (Carrasco et al., 2004b; Gobell & Carrasco, 2005; Montagna & Carrasco, 2006; Turatto et al., 2007).
In Experiment 3, we concurrently assessed appearance and measured orientation discrimination performance. Improvements in such performance-based tasks are well documented and a persuasive indication that exogenous attention has been engaged to a peripheral location. We found concurrent improvements at the cued stimulus locations and impairments at the uncued locations. This finding further shows that the appearance results are not due to response bias. Response bias to the cue would have biased reporting of which stimulus was higher in contrast, but would have had no effect on correct orientation discrimination because cue location was uncorrelated with stimulus orientation. Liu et al. (2006a) and Anton-Erxleben et al. (2007) used this type of concurrent performance measurement to show simultaneous cueing effects on apparent motion coherence and motion direction discrimination, validating that the change in appearance resulted from exogenous attention rather than cue bias. Fuller and Carrasco (2006) used non-concurrent discrimination performance to verify that their cueing paradigm engaged exogenous attention, and increased performance, whereas the same cueing procedure had no effect on the appearance of hue.
We propose that the baseline difference in apparent contrast from Experiment 1 is due to divergences between the contrast sensitivity functions for isoeccentric North and South locations, and thus indicates underlying differences in the visual system. Physically identical contrast stimuli generate visual signals of different strength at North and South locations, with the one in the North being weaker. In the comparison task, this makes the South stimulus more likely to be judged higher in contrast. Indeed, the North vertical meridian is disadvantaged relative to the South on a variety of visual dimensions (e.g., spatial resolution, contrast sensitivity) and tasks (detection, discrimination, and localization), with the basis of these disadvantages traceable to visual factors (Carrasco et al., 2001, 2002; Liu et al., 2006a; Talgar & Carrasco, 2002).
An alternative explanation invokes the fact that observers must simultaneously monitor both stimulus locations, and proposes that the allocation of endogenous attention is uneven between them, favoring and differentially affecting perception of the South stimulus. This is supported by a visual search study reporting such a differential effect for the entire lower visual field (Rezec & Dobkins, 2004), but see (Carrasco et al., 1995), a visual search study that found no hemifield asymmetries). The bias toward the lower hemi-field was absent when endogenous attention was cued to a specific stimulus location rather than divided. We cannot a priori rule out this explanation because our Experiment 1 did not explicitly manipulate or control for endogenous attention allocation. However, we point out that difference in appearance between South and North varies with the spatial frequency of the stimuli, the effect being greatly diminished for stimuli at 2 cpd (see Figure 2). If the cause were divided endogenous attention, then the bias should have been present for all three of the spatial frequencies tested. Moreover, the vertical meridian asymmetry is not only present, but exacerbated, as set size increases in visual search (Carrasco et al., 2001, Experiment 3).
The results of the present study also suggest that in addition to the visual system differences, attentional modulation is weaker in the North than in the South, which we have modeled at the behavioral level as asymmetrical multiplicative factors. We believe that the reason these attentional differences manifest in the appearance task, but not in an orientation discrimination task, rests on differences between the tasks, and possibly the neural mechanisms underlying them. The critical difference between the two is that the appearance paradigm involves comparing visual signals from two separate spatial locations that are attentionally modulated in opposite directions by a single peripheral cue (i.e. benefits and costs). The orientation task, however, requires discrimination between two alternatives at a single location, evaluating relative responses of multiple channels that experience the same attentional modulation depending on cue condition.
The Vertical Meridian Asymmetry consists of a peculiar set of disadvantages in perceiving objects that are directly above the center of our gaze. Even the application of exogenous attention does little to mitigate these disadvantages, with the exception that it engenders a greater increase in processing speed in the North than in the South (Carrasco et al., 2004a). One can only speculate on why this might be at all advantageous. For example, it could represent evolutionary allocation of physiological visual resources away from a less useful part of the visual field (for example, the upper vertical meridian) and toward areas in which better processing has conveyed more survival value (e.g., the lower visual hemifield: Previc, 1990) - opportunities or dangers closer to the ground may be more relevant. On the other hand, it may be a consequence of the dynamic response of the brain to the environment, in which case it could be expected to vary with height or develop as we mature (Kothari, Mahon, & Carrasco, 2005).
Conclusions
To summarize, we have found that the VMA extends to a difference in apparent contrast between the lower and upper segments. At equal eccentricity, a stimulus in the South appears to be higher in contrast than a physically identical stimulus in the North. Moreover, exogenous spatial attention, which has been shown to increase apparent contrast at the attended location, exerts a greater increase in apparent contrast in the South. This attentional asymmetry was absent in our orientation discrimination data, as it has been in prior studies investigating orientation discrimination on the vertical meridian. Modeling suggests that the effects of differential attentional modulation between North and South manifest more strongly in the appearance task, and therefore are more readily detected than in an orientation discrimination task. We conclude that exogenous attention has a lesser effect on the upper vertical meridian, compounding the perceptual weakness due to visual factors.
Acknowledgments
The authors thank Taosheng Liu for his advice in conceptualizing aspects of the modeling included in this paper, and the members of the Carrasco lab for their helpful comments on the manuscript. This research was supported by NIH(R01-EY016200-01A2 to MC).
Footnotes
Commecial relationships: none.
Six observers’ data in the main condition were excluded from the analysis based on their inability to perform the orientation discrimination component of the task at a level of 90% or better. Given the large difference between the two orientations, an error rate >10% raises doubt about the validity of the observer’s response for the subjective comparison element of the task
For simplicity, we keep the variance of the probability distributions fixed for appearance and discrimination models; we have run simulations both with constant variances and variances proportional to signal strength (i.e. Poisson variance) and found that the outcome is essentially the same
Contributor Information
Stuart Fuller, Email: stuart.fuller@nyu.edu, Department of Psychology, New York University, New York, NY, USA.
Ruby Z. Rodriguez, Department of Psychology, New York University, New York, NY, USA
Marisa Carrasco, Email: marisa.carrasco@nyu.edu, Department of Psychology and Center for Neural Science, New York University, New York, NY, USA.
References
- Anton-Erxleben K, Henrich C, Treue S. Attention changes perceived size of moving visual patterns. Journal of Vision. 2007;7(115):1–9. doi: 10.1167/7.11.5. http:// journalofvision.org/7/11/5/ [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Cameron EL, Tai JC, Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vision Research. 2002;42:949–967. doi: 10.1016/s0042-6989(02)00039-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- Carrasco M, Evert DL, Chang I, Katz SM. The eccentricity effect: Target eccentricity affects performance on conjunction searches. Perception & Psychophysics. 1995;57:1241–1261. doi: 10.3758/bf03208380. [PubMed] [DOI] [PubMed] [Google Scholar]
- Carrasco M, Giordano AM, McElree B. Temporal performance fields: Visual and attentional factors. Vision Research. 2004a;44:1351–1365. doi: 10.1016/j.visres.2003.11.026. [PubMed] [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004b;7:308–313. doi: 10.1038/nn1194. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein M. Spatial covert attention increases contrast sensitivity across the CSF: Support for signal enhancement. Vision Research. 2000;40:1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Talgar CP, Cameron EL. Characterizing visual performance fields: Effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spatial Vision. 2001;15:61–75. doi: 10.1163/15685680152692015. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Williams PE, Yeshurun Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision. 2002;2(64):467–479. doi: 10.1167/2.6.4. http://journalofvision.org/2/6/4/ [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Cheal M, Lyon DR. Central and peripheral precuing of forced-choice discrimination. Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1991;43:859–880. doi: 10.1080/14640749108400960. [PubMed] [DOI] [PubMed] [Google Scholar]
- Connolly M, Van Essen D. The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. Journal of Comparative Neurology. 1984;226:544–564. doi: 10.1002/cne.902260408. [PubMed] [DOI] [PubMed] [Google Scholar]
- Cook EP, Maunsell JH. Attentional modulation of motion integration of individual neurons in the middle temporal visual area. Journal of Neuroscience. 2004;24:7964–7977. doi: 10.1523/JNEUROSCI.5102-03.2004. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. Topography of ganglion cells in human retina. Journal of Comparative Neurology. 1990;300:5–25. doi: 10.1002/cne.903000103. [PubMed] [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. Journal of Comparative Neurology. 1990;292:497–523. doi: 10.1002/cne.902920402. [PubMed] [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Jr, Packer O, Hendrickson AE, Kalina RE. Distribution of cones in human and monkey retina: Individual variability and radial asymmetry. Science. 1987;236:579–582. doi: 10.1126/science.3576186. [PubMed] [DOI] [PubMed] [Google Scholar]
- Edgar GK, Smith AT. Hemifield differences in perceived spatial frequency. Perception. 1990;19:759–766. doi: 10.1068/p190759. [PubMed] [DOI] [PubMed] [Google Scholar]
- Fuller S, Carrasco M. Exogenous attention and color perception: Performance and appearance of saturation and hue. Vision Research. 2006;46:4032–4047. doi: 10.1016/j.visres.2006.07.014. [PubMed] [DOI] [PubMed] [Google Scholar]
- Gobell J, Carrasco M. Attention alters the appearance of spatial frequency and gap size. Psychological Science. 2005;16:644–651. doi: 10.1111/j.1467-9280.2005.01588.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kothari R, Mahon K, Carrasco M. Comparing performance fields of children and adults. Sarasota, FL: Vision Sciences Society; 2005. [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. Journal of Comparative Neurology. 1988;278:157–180. doi: 10.1002/cne.902780202. [PubMed] [DOI] [PubMed] [Google Scholar]
- Levine MW, McAnany JJ. The relative capabilities of the upper and lower visual hemifields. Vision Research. 2005;45:2820–2830. doi: 10.1016/j.visres.2005.04.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ling S, Carrasco M. Transient covert attention does alter appearance: A reply to Scheider (2006) Perception & Psychophysics. 2007;69:1051–1058. doi: 10.3758/bf03193943. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Fuller S, Carrasco M. Attention alters the appearance of motion coherence. Psychonomic Bulletin & Review. 2006a;13:1091–1096. doi: 10.3758/bf03213931. [PubMed] [DOI] [PubMed] [Google Scholar]
- Liu T, Heeger DJ, Carrasco M. Neural correlates of the visual vertical meridian asymmetry. Journal of Vision. 2006b;6(1112):1294–1306. doi: 10.1167/5.1.1. http://journalofvision.org/6/11/12/ [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Current Biology. 2004;14:744–751. doi: 10.1016/j.cub.2004.04.028. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. Topographic organization of the middle temporal visual area in the macaque monkey: Representational biases and the relationship to callosal connections and myeloarchi-tectonic boundaries. Journal of Comparative Neurology. 1987;266:535–555. doi: 10.1002/cne.902660407. [PubMed] [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. Journal of Neuroscience. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnany JJ, Levine MW. Magnocellular and parvocellular visual pathway contributions to visual field anisotropies. Vision Research. 2007;47:2327–2336. doi: 10.1016/j.visres.2007.05.013. [PubMed] [DOI] [PubMed] [Google Scholar]
- Montagna B, Carrasco M. Transient covert attention and the perceived rate of flicker. Journal of Vision. 2006;6(98):955–965. doi: 10.1167/6.9.8. http://journalofvision.org/6/9/8/ [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Research. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [PubMed] [Google Scholar]
- Pelli DG, Zhang L. Accurate control of contrast on microcomputer displays. Vision Research. 1991;31:1337–1350. doi: 10.1016/0042-6989(91)90055-a. [PubMed] [DOI] [PubMed] [Google Scholar]
- Perry VH, Cowey A. The ganglion cell and cone distributions in the monkey’s retina: Implications for central magnification factors. Vision Research. 1985;25:1795–1810. doi: 10.1016/0042-6989(85)90004-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Research. 2005;45:1867–1875. doi: 10.1016/j.visres.2005.01.019. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pestilli F, Viera G, Carrasco M. How do attention and adaptation affect contrast sensitivity? Journal of Vision. 2007;7(79):1–12. doi: 10.1167/7.7.9. http://journalofvision.org/7/7/9/ [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previc FH. Functional specialisation in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behavioral and Brain Sciences. 1990;13:519–575. [Google Scholar]
- Rezec AA, Dobkins KR. Attentional weighting: A possible account of visual field asymmetries in visual search? Spatial Vision. 2004;17:269–293. doi: 10.1163/1568568041920203. [PubMed] [DOI] [PubMed] [Google Scholar]
- Rijsdijk JP, Kroon JN, van der Wildt GJ. Contrast sensitivity as a function of position on the retina. Vision Research. 1980;20:235–241. doi: 10.1016/0042-6989(80)90108-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Experimental Brain Research. 1979;37:495–510. doi: 10.1007/BF00236819. [PubMed] [DOI] [PubMed] [Google Scholar]
- Rubin N, Nakayama K, Shapley R. Enhanced perception of illusory contours in the lower versus upper visual hemifields. Science. 1996;271:651–653. doi: 10.1126/science.271.5249.651. [PubMed] [DOI] [PubMed] [Google Scholar]
- Skrandies W. The upper and lower visual field of man: Electrophysiological and functional differences. In: Ottoson D, editor. Progress in sensory physiology. Berlin: Springer; 1987. pp. 1–93. [Google Scholar]
- Talgar CP, Carrasco M. Vertical meridian asymmetry in spatial resolution: Visual and attentional factors. Psychonomic Bulletin & Review. 2002;9:714–722. doi: 10.3758/bf03196326. [PubMed] [DOI] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, Hamilton SL. Functional anatomy of macaque striate cortex. II. Retinotopic organization. Journal of Neuroscience. 1988;8:1531–1568. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turatto M, Vescovi M, Valsecchi M. Attention makes moving objects be perceived to move faster. Vision Research. 2007;47:166–178. doi: 10.1016/j.visres.2006.10.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: Asymmetries, anisotropies, and individual variability. Vision Research. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception & Psychophysics. 2001;63:1293–1313. doi: 10.3758/bf03194544. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Wickens TD. xiii. New York: Oxford University Press; 2002. Elementary signal detection theory; p. 262. [Google Scholar]
- Yeshurun Y, Carrasco M. Spatial attention improves performance in spatial resolution tasks. Vision Research. 1999;39:293–306. doi: 10.1016/s0042-6989(98)00114-x. [PubMed] [DOI] [PubMed] [Google Scholar]