Abstract
Purpose
Whole-brain irradiation (WBI) leads to cognitive impairment months to years after radiation. Numerous studies suggest that decreased hippocampal neurogenesis and microglial activation are involved in the pathogenesis of WBI-induced brain injury. The goal of this study was to investigate whether administration of the peroxisomal proliferator-activated receptor (PPAR)α agonist, fenofibrate, would prevent the detrimental effect of WBI on hippocampal neurogenesis.
Methods and Materials
129S1/SvImJ wild-type (WT) and PPARα knock-out (KO) mice that were fed either regular or 0.2% w/w fenofibrate-containing chow received either sham irradiation or WBI (10 Gy single dose of 137Cs γ rays). Mice were injected i.p. with bromodeoxyuridine (BrdU) to label the surviving cells at 1 month post-WBI and the newborn neurons were counted at 2 months post-WBI using BrdU/NeuN double-immunofluorescence. Proliferation in the sub-granular zone (SGZ) and microglial activation were measured at 1 week and 2 months post-WBI using Ki-67 and CD68 immunohistochemistry, respectively.
Results
WBI led to a significant decrease in the number of newborn hippocampal neurons 2 months post-WBI. Fenofibrate prevented this decrease by promoting the survival of newborn cells in the dentate gyrus (DG). In addition, fenofibrate treatment was associated with decreased microglial activation in the DG following WBI. The neuroprotective effects of fenofibrate were abolished in the KO mice, indicating a PPARα-dependent mechanism(s).
Conclusion
These data highlight a novel role for PPARα ligands in improving neurogenesis following WBI, and offer the promise of improving the quality of life for brain cancer patients receiving radiotherapy.
Keywords: radiation, PPARα, microglia, neurogenesis, inflammation
INTRODUCTION
Of the ~ 1.5 million new cancer patients estimated in the U.S in 2009, up to 30% will develop brain metastases (1,2). Large-field partial or whole-brain irradiation (WBI) is the primary mode of treatment for brain metastases; approximately 170,000 patients are treated annually with cranial irradiation (3). Unfortunately, up to 50% of brain tumor patients who survive ≥ 6 months post-irradiation will develop progressive, cognitive impairment (4). This cognitive dysfunction is evident often as deficits in hippocampal-dependent learning and memory, including spatial information processing (4,5). Moreover, it has been suggested that the severity of cognitive impairment correlates with the radiation dose delivered to the medial-temporal lobe, the site of the hippocampus (6).
The exact mechanisms involved in the development and progression of radiation-induced brain injury are unknown. However, studies on both rodents and human tissue samples indicate that WBI has a detrimental effect on hippocampal neurogenesis (7,8). Active neurogenesis occurs throughout adulthood in a specialized region of the hippocampus called the dentate gyrus (DG) (9). Neural precursor cells residing in the sub-granular zone (SGZ) of the DG give rise to new neurons that functionally integrate into the granule cell layer (GCL) of the hippocampus (10). The extreme sensitivity of these neural precursor cells to irradiation has been demonstrated previously (7,11,12). Irradiation of the rodent brain leads to a marked decrease in the number of newborn mature and immature neurons in the DG, associated with impairments in hippocampal-dependent cognitive tasks (13,14). This WBI-induced decrease in neurogenesis has been linked with neuroinflammation, evidenced as a marked increase in the number of activated microglia (7,14). Specifically, inhibiting microglial activation using indomethacin and minocycline partially restores neurogenesis (15,16). Thus, anti-inflammatory strategies might be effective in preserving hippocampal neurogenesis and ameliorating radiation-induced cognitive impairment.
Peroxisomal proliferator-activated receptor (PPAR)α is a nuclear receptor belonging to the PPAR family of ligand-activated transcription factors (17). PPARα has been shown to play a major role in regulating inflammatory processes. In vitro, PPARα agonists inhibit pro-inflammatory responses in a variety of cell types, including microglia and astrocytes (18). Moreover, PPARα agonists confer neuroprotection in a variety of preclinical models, including stroke, experimental autoimmune encephalomyelitis and Parkinson’s disease (19). We previously reported that pre-treating BV-2 murine microglial cells with the PPARα agonist, fenofibrate, inhibited the radiation-induced pro-inflammatory response, via negative regulation of NF-κB and AP-1 pathways (20). We hypothesized in this study that fenofibrate would ameliorate the WBI-induced decrease in hippocampal neurogenesis, in part, by inhibiting microglial activation.
MATERIALS AND METHODS
Animals and irradiation procedures
Adult (12–16 weeks old) PPARα KO mice (129S4/ScJac-Pparatm/Gonz, Jackson Laboratory, Bar Harbor, Maine, Stock # 003580) and their appropriate wild-type controls (129S1/SvImJ, Jackson Laboratory, stock # 002448) were housed in specific pathogen free conditions, five mice/cage with free access to drinking water and standard mouse chow (Harlan Teklad, Madison, WI). All animal handling and experiments were performed in strict accordance with the NIH Guide for Care and Use of Laboratory Animals as approved by the WFUSM Institutional Animal Care and Use Committee. Mice were randomly divided into 4 groups (n = 4–6 per group): 1) Sham-irradiation and control diet, 2) Sham-irradiation and fenofibrate (Fen; 0.2% w/w, Sigma-Aldrich, St.Louis, MO), 3) WBI and control diet, 4) WBI and fenofibrate. The composition of the control diet has been described previously (21). Mice were started on their respective diets 14 days prior to WBI and maintained on these until euthanized (1-week or 2-months post-irradiation). Prior to irradiation, mice were anesthetized using a ketamine/xylazine mixture injected i.p. (150/10 mg/kg body weight [BW]). WBI was performed using a 7,214 Ci, self- shielded 137Cs irradiator using lead and Cerrobend shielding devices so that the whole brain, including the brain stem, was irradiated, while the eyes and the rest of the body were shielded. Irradiated mice received a single dose of 10 Gy γ-rays at a dose rate of 3.33 Gy/min with half the dose (5 Gy) delivered to each side of the head. Sham-irradiated mice were anesthetized but were not irradiated. All mice were weighed weekly and no significant weight loss was observed in any of the groups (data not shown).
Bromodeoxyuridine (BrdU) injection
One month post-WBI, mice received 50 mg/kg BW of BrdU (labels cells in S phase) injected i.p every day for 7 days as described previously (7,12). Three weeks after the last BrdU injection, i.e. 2 months post-WBI, animals were euthanized and tissues were processed as outlined below.
Tissue processing
Animals were deeply anesthetized using a ketamine/xylazine mixture (200/10 mg/kg BW, i.p.) and perfused with phosphate-buffered 4% paraformaldehyde. Brains were isolated and cryoprotected in 10%, 20%, and 30% sucrose and then frozen in tissue embedding medium. Coronal sections containing the hippocampus (40-µm thickness) were sectioned on a freezing stage using a cryostat, collected in anti-freeze solution (1:1:2 ethylene glycol, glycerol and 0.1 M phosphate-buffered saline) and stored at −20°C.
Immunohistochemistry and immunofluorescence
For all immunostaining experiments, tissue sections were chosen based on systematically random sampling. Depending on the antibody used, a 1-in-12 series (CD68 staining, BrdU/NeuN and BrdU/Iba-1 staining) or a 1-in-8 series (Ki67) of sections representing the entire anterior-to-posterior extent of the DG were washed in Tris-buffered saline (1X TBS; pH 7.4). For immunohistochemical (IHC) staining, sections were treated with 1% H2O2 in 1X TBS to block endogenous peroxide activity. For Ki67 IHC, antigen retrieval was performed by boiling the sections in 10 mM sodium citrate pH 6 for 10 min prior to the H2O2 step. Sections were first incubated for 1 h in blocking solution (5% normal serum, 0.3% Triton X-100 in 1X TBS) and then overnight at 4° C in primary antibody diluted in blocking solution. The primary antibodies used were rabbit α-Ki67 (labels mitotic cells; 1:200; Abcam, Cambridge, MA), rat α-CD68 (clone FA-11, labels activated microglia; 1:100; AbD Serotec, Raleigh, NC). Ki67 and CD68 labeling were detected using biotinylated secondary antibodies (1:200, Vectorlabs, Burlingame, CA) and visualized using peroxidase-conjugated avidin-biotin complex (ABC Elite kit) with either SG (Ki67) or nickel-enhanced DAB (CD68) substrate (Vectorlabs, Burlingame, CA). For immunofluorescence staining, sections were treated with 2 M HCl at 37° C to denature the DNA and subsequently washed in 1X TBS pH 8.5 to neutralize the acid. The sections were then incubated for 2 h in blocking solution (10% normal serum, 0.3% Triton X-100 in 1X TBS) and then overnight at 4° C with rat α-BrdU (1:200; AbD Serotec, Raleigh, NC) and either mouse α-NeuN (1:200; Chemicon, Billerica, MA) or rabbit α-Iba-1 (1:200; Wako Pure Chemicals, Richmond, VA). The BrdU, NeuN and Iba-1 labeling was detected using Cy3, Alexa-Fluor® 488 and Cy5-conjugated secondary antibodies, respectively (1:200, Jackson ImmunoResearch, West Grove, PA). The DNA binding fluorescent dye 4’, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St.Louis, MO) was used as a counter-stain to visualize anatomical landmarks.
Quantitative analysis
All analyses were performed blindly using coded sections. CD68 immunolabeled activated microglia (intensely stained, punctate cells) were counted in the GCL and hilus including the SGZ. The volume of the GCL and the hilus was used to normalize the CD68 counts. Ki67 immunolabeled cells were counted in the SGZ region as previously described (22) and the length of the SGZ at the GCL-hilar border was used to standardize counts. All counts were performed on an Olympus BX-60 microscope using Neurolucida software (MBF Biosciences, Colchester, VT). To avoid overestimation, the cells on the top focal plane were excluded. The BrdU+, BrdU+/NeuN+ and BrdU+/Iba-1+ positive cells were counted (in the combined GCL and SGZ) in stacks of optical sections acquired using a Leica TCS SP2 confocal microscope (Leica Microsystems, Bannockburn, IL). Depending on the antigen, counts were expressed as number of i] cells per mm3 of GCL+Hilus (CD68); ii] cells per mm3 of GCL+SGZ (BrdU/NeuN and BrdU/Iba-1); and iii] cells per mm of SGZ (Ki67)
Statistical Analysis
Analysis of variance (ANOVA) was used for determining statistical significance between more than two experimental groups. Tukey’s studentized range test was used for the pairwise comparisons.
The constant variance assumption was tested using Levene’s test for homogeneity of variance. When the assumption of constant variance was not valid, the Kruskal-Wallis test was then performed. When only two experimental groups were compared, 2-sample t-tests were used and Bonferroni correction was used to correct for multiple comparisons.
RESULTS
Fenofibrate inhibited the WBI-induced decrease in newborn neurons in the hippocampus, affecting cell survival but not proliferation
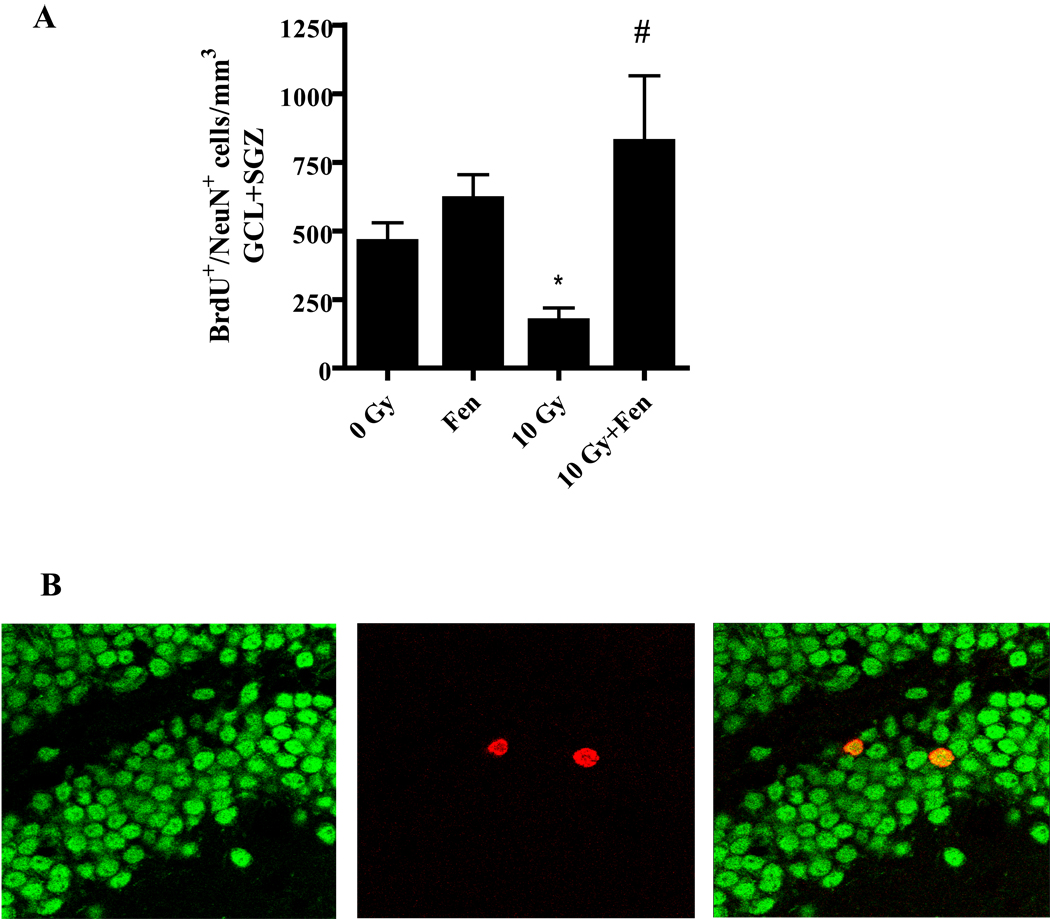
Given the neuroprotective role of PPARα ligands in other models of brain injury, we hypothesized that fenofibrate administration would prevent the deleterious effect of WBI on hippocampal neurogenesis. As shown in Figure 1A, the WT control diet mice showed a 60% reduction in the number of BrdU+/NeuN+ cells in the GCL/SGZ at 2 months post-WBI. This radiation-induced decrease in newborn neurons was prevented in the mice that received fenofibrate.
Figure 1. Dietary administration of fenofibrate prevents the WBI-induced decrease in number of newborn neurons in the mouse hippocampus.
A). A significant decrease in the number of newborn neurons (BrdU+/NeuN+) in the GCL/SGZ was observed 2 months post-WBI. This was prevented in the mice that received fenofibrate (0.2% w/w) in their diet. Data are presented as Mean ± SEM; n=4 mice/group; an average, 6–8 sections/animal were double-labeled fluorescently using α-BrdU (proliferation) and α-NeuN (neuron) antibodies and labeled cells counted using a confocal microscope; * p<0.05 vs. 0 Gy; # p<0.05 vs. 10 Gy. B). Representative confocal microscope images showing cells stained for NeuN (green; left) and BrdU (red; center). A merged image depicting double-positivity (yellow) is shown on the right.
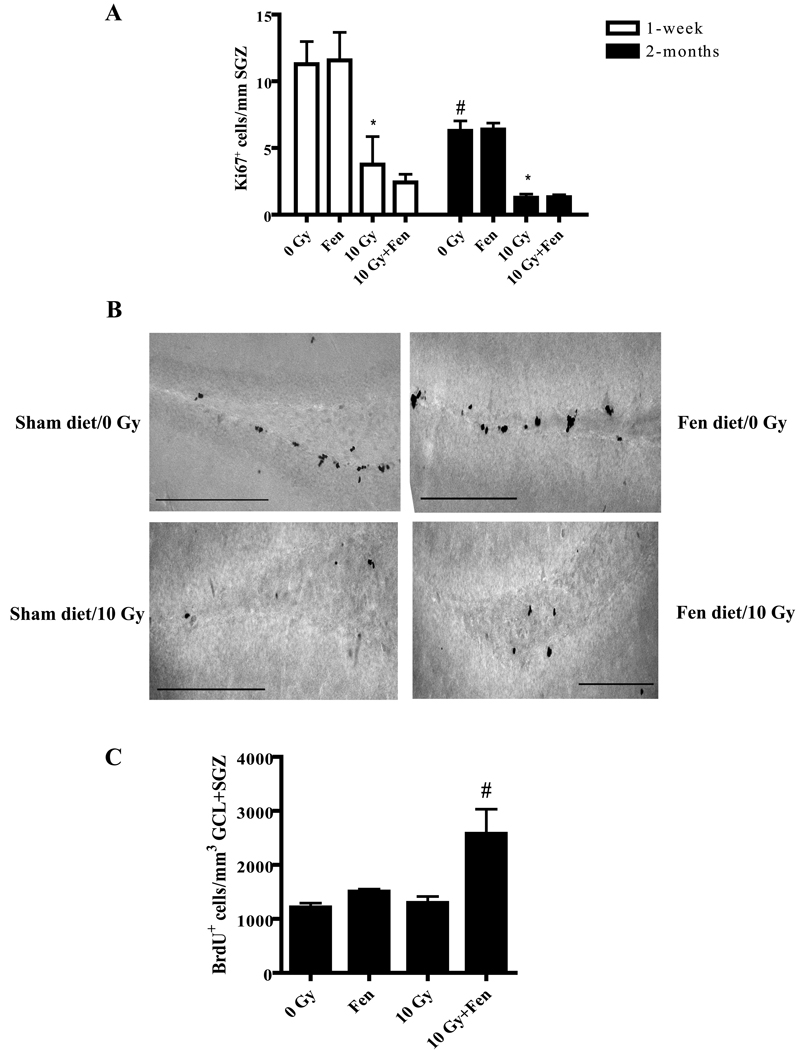
Extending previous studies (12), WBI led to a significant decrease in proliferation in the SGZ as evidenced by a reduction in the number of Ki67+ cells observed at 1-week (70%) and 2 months (85%) post-WBI (Figure 2A and B). Fenofibrate administration did not prevent this decrease in proliferation. Nevertheless, fenofibrate increased the total number of BrdU+ cell counts up to 3-fold in the GCL/SGZ of the irradiated WT mice at 2 months post-WBI (Figure 2C). These findings suggest that the protective effect of fenofibrate on the WBI-induced decrease in hippocampal neurogenesis is mediated, in part, by promoting the survival of the newborn cells following radiation.
Figure 2. Fenofibrate does not prevent the WBI-induced decrease in proliferation in the SGZ but increases the number of BrdU+ cells in the DG following WBI.
A). The number of Ki67+ cells in the SGZ was significantly decreased at 1-week and 2 months post-WBI; this was unaffected by fenofibrate. Data are presented as Mean ± SEM; n=4 mice/group; an average 6–8 sections were stained using α-Ki67 antibody and counted/animal; * p<0.05 vs. 0 Gy; # p< 0.05 vs. 1-week 0 Gy. B). Representative images showing Ki67+ cells in the SGZ of mice 1 week after sham or WBI with or without fenofibrate administration. Scale bar = 25 µM. C). The number of newly generated (BrdU+) cells significantly increased at 2 months post-WBI in the GCL/SGZ of the fenofibrate-fed WT mice compared to the control diet-fed animals. Data are presented as Mean ± SEM; n=4 mice/group; an average of 6–8 sections were stained using α-BrdU antibody (1:100) and counted/animal; # p<0.05 vs. 10 Gy.
Fenofibrate administration prevented the WBI-induced increase in activated microglia
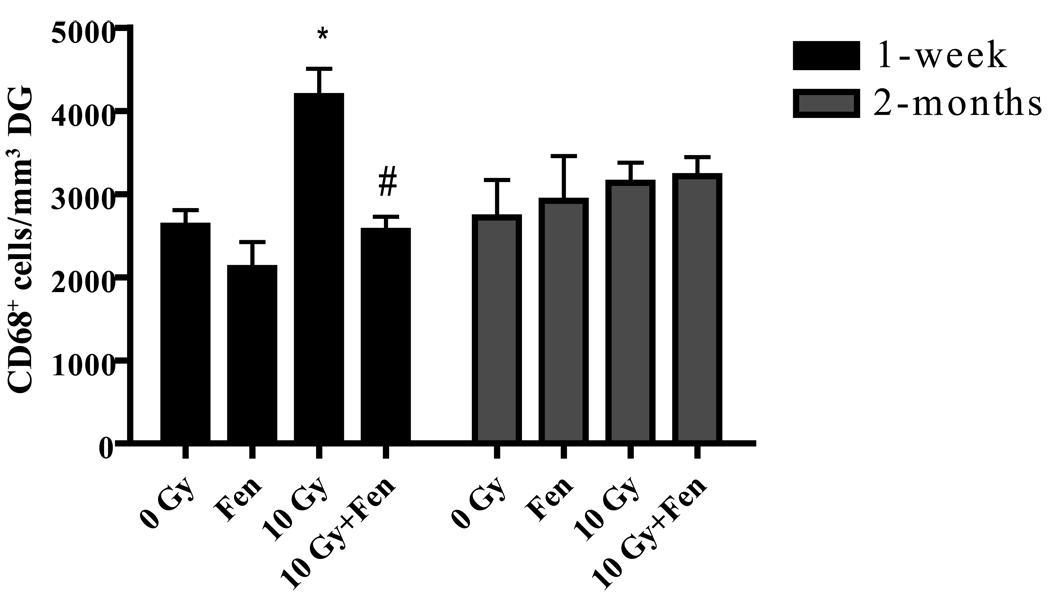
In vitro studies from our lab suggest that fenofibrate significantly inhibits radiation-induced proinflammatory responses in microglia (20). Since inflammation in the brain has been shown to be detrimental to neurogenesis, we hypothesized that the effect of fenofibrate on the newborn neurons involves inhibition of microglial activation. WBI of WT mice receiving control diet led to an ~ 70% increase in the number of activated microglia (CD68+) in the DG at 1 week post-radiation. As hypothesized, this was inhibited in the irradiated mice that received fenofibrate (Figure 3). The number of activated microglia was unaltered 2 months post-WBI in the WT mice (Figure 3). Thus, administering fenofibrate is associated with an inhibition of microglial activation seen 1 week post-WBI.
Figure 3. Fenofibrate prevents the WBI-induced increase in number of activated microglia.
A significant increase in CD68+ (activated microglia) cells was observed at 1 week post-WBI in the dentate gyrus; this increase was prevented in the fenofibrate-fed mice. At 2 months post-WBI, the CD68 numbers were similar in all four treatment groups. Data are presented as Mean ± SEM; n=4 mice/group and an average of 6–8 sections were stained using α-CD68 antibody (1:100) and counted/animal; * p<0.05 vs. 0 Gy; # p<0.05 vs. 10 Gy.
PPARα is required for the protective effects of fenofibrate following WBI
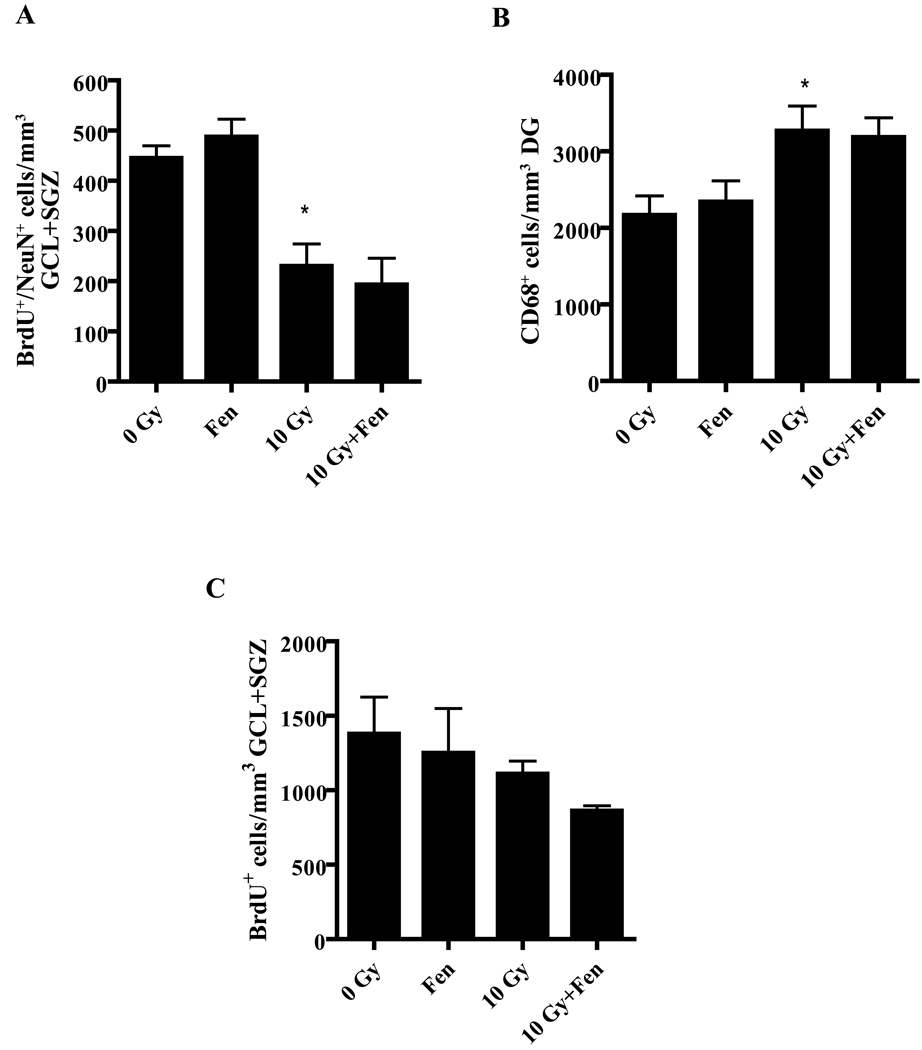
To test whether the effect of fenofibrate on hippocampal neurogenesis and microglial activation requires a functional PPARα, the effect of WBI on hippocampal neurogenesis and neuroinflammation was examined in PPARα KO mice receiving either the control or fenofibrate diet. The number of BrdU+/NeuN+ cells was significantly decreased in KO mice on the control diet at 2 months post-WBI (Figure 4A). Moreover, in contrast to the WT mice, these mice showed a significant increase in the number of activated microglia at both 1 week (data not shown) and 2 months post-WBI (Figure 4B). Administering fenofibrate to the KO mice failed to prevent either the decrease in the number of newborn neurons following radiation or the increase in activated microglia (Figure 4A and B). Similarly, fenofibrate did not increase the total number of BrdU+ cells in the GCL/SGZ of the KO mice following WBI (Figure 4C). These data suggest that PPARα is required for the neuroprotective and anti-inflammatory effects of fenofibrate.
Figure 4. The anti-inflammatory and neuroprotective actions of fenofibrate are PPARα dependent.
WBI leads to both a significant decrease in the number of BrdU+/NeuN+ cells in the GCL/SGZ (A) and a significant increase in the number of CD68+ cells in the DG of PPARα KO mice (B). Dietary administration of fenofibrate to the KO mice failed to prevent either the decrease in the newborn neurons or the increase in CD68+ cells following WBI; * p<0.05 vs. 0 Gy. Data are presented as Mean ± SEM; n=4 mice/group; an average of 6–8 sections/animal were stained and counted. C). The number of newly generated (BrdU+) cells was unchanged at 2 months post-WBI in the GCL/SGZ of the fenobrate-fed KO mice compared to the control diet-fed animals. Data are presented as Mean ± SEM; n=4 mice/group; an average of 6–8 sections were stained using α-BrdU antibody (1:100) and counted/animal.
DISCUSSION
The central finding of this study is that dietary administration of the PPARα ligand, fenofibrate, preserves the number of newborn hippocampal neurons and decreases microglial activation following WBI in WT mice. These protective effects are not observed in PPARα KO mice, highlighting the importance of this nuclear receptor in mediating the action of fenofibrate. To our knowledge, this is the first demonstration of a role for PPARα in modulating adult hippocampal neurogenesis following WBI.
Hippocampal neurogenesis is a complex process involving proliferation of progenitor cells in the SGZ, survival of the newborn cells, and differentiation as well as maturation of these cells into functional neurons in the GCL (23). Measuring proliferation in the hippocampal neurogenic region showed that WBI significantly decreased SGZ proliferation at both 1-week and 2-months post-irradiation. Of note, we observed a significant decrease in the basal level of proliferation in the sham-irradiated mice between the 1-week and 2-month cohorts (Figure 2A). These results demonstrate an age-associated decrease in SGZ proliferation as reported previously (10,11). Although administering fenofibrate had no effect on the radiation-induced decrease in proliferation in the SGZ, it increased survival of the newborn cells in the SGZ/GCL following WBI. Our results are consistent with previous reports in which environmental enrichment was demonstrated to preserve the newborn hippocampal number following WBI without altering cell proliferation (24). One possible explanation for such a preferential protection might be the very process of hippocampal neurogenesis. Although there is active proliferation occurring in the adult rodent brain, only a fraction of these cells will survive, differentiate and give rise to functional neurons (23,25). The survival of the progenitor cells is largely dependent on the presence of growth factors such as brain-derived neuronal factor (BDNF) and expression of anti-apoptotic signaling molecules such as Bcl-2 (26). Therefore, the neuroprotective effect of fenofibrate could have, in part, been through modulation of signaling events occurring downstream of progenitor cell proliferation.
A possibility arises as to whether the BrdU labeled cells in our model could be cells undergoing DNA damage repair following WBI and that fenofibrate treatment could have enhanced this repair process. However, the infusion of high doses of BrdU directly into the brain following irradiation does not significantly label DNA undergoing repair, and that labeling is not detected in vulnerable or dying post-mitotic neurons (27). Thus, the BrdU+ cells observed in the current study are primarily dividing cells in the SGZ+GCL and the neuroprotective effect of fenofibrate appears to most likely reflect preservation of the capacity of a sub-population of the labeled cells to give rise to GCL neurons following WBI.
Although WBI led to a significant decrease in BrdU+/NeuN+ cells at 2 months in the WT mice, it did not alter the total number of BrdU+ cells. Previous reports have demonstrated that the brain microenvironment following irradiation, although detrimental to neurogenesis, favors gliogenesis (7,12). However, measurement of proliferating microglia using BrdU/Iba-1 immunolabeling revealed no significant difference between sham-irradiated and WBI WT mice (Figure e1). Given that the newborn cells in the DG are predisposed to glial differentiation in the 129Sv/J mice (28), it is likely that WBI, apart from its detrimental effect on the generation of newborn neurons, increases the total number of non-microglial cells such as astrocytes.
Microglia represent one of the main cell-types that mediate inflammation in the brain. Although required for normal functioning of the CNS, microglia can become activated by a variety of stimuli including radiation. The transformation from a resting to an activated state results in a number of physiological changes, including upregulation of the lysosomal antigen macrosialin (CD68) and release of pro-inflammatory molecules (29). Several studies have drawn a negative correlation between activated microglia and neurogenesis (12,16). Given the potent anti-inflammatory effect of PPARα ligands in mitigating the radiation-induced pro-inflammatory responses in microglial cells in vitro (20), we hypothesized that the mechanism of neuroprotection by fenofibrate involves, in part, inhibition of the WBI-induced increase in activated microglia. Indeed, fenofibrate administration prevented the increase in the number of CD68+ cells in the DG at 1 week post-irradiation. Moreover, the number of activated microglia returned to control levels by 2 months post-WBI, the time at which we observed a significant decrease in the number of newborn neurons. These data suggest that in the WT mice, increased microglial activation is an early event and may be a key component driving the detrimental effects of radiation on hippocampal neurogenesis. It should be noted, however, that these data do not support a mechanistic link between the fenofibrate-mediated preservation of hippocampal neurogenesis and the reduced microglial activation. The regulation of adult neuro- and glial genesis - involving control of proliferation (of stem cells and more restricted progenitor cells), of commitment (to neuronal or glial lineages), of differentiation, of maturation, and of survival – includes a multitude of points of possible regulation ( see 23 for recent review). Even the specific effects of microglia and inflammatory processes on neurogenesis remain incompletely understood (25,30). Thus, it is possible that these beneficial effects of fenofibrate treatment on neurogenesis and neuroinflammation represent PPARα-mediated modulation of independent pathways.
Some studies have documented that fenofibrate can act independently of PPARα (31,32). Thus, we investigated whether PPARα is required for the neuroprotective and anti-inflammatory properties of fenofibrate. In our model, the genetic ablation of PPARα prevented the protective effect of fenofibrate following WBI. These results are consistent with previous findings (33) and more importantly highlight the critical role played by PPARα in modulating radiation-induced brain injury and provide mechanistic insight into the action of fenofibrate.
We observed a marked difference in the response of the microglial cells to WBI between the WT and PPARα KO mice. While the number of activated microglia returned to control levels by 2 months post-WBI in the WT mice, they remained significantly elevated in the KO mice. This suggests that the KO mice show a sustained neuroinflammatory response following WBI. These findings are not surprising; PPARα KO mice exhibit a prolonged response to inflammatory stimuli such as lipopolysacharide and leukotriene B4 (34,35). Thus, it is possible that the lack of PPARα enhances the basal level of inflammation and thereby leads to a protracted microglial response to radiation injury.
Clinically, radiation-induced brain injury is characterized by a progressive, cognitive impairment (4,5). One caveat to our studies is our inability to perform behavioral analyses in the WT and PPARα KO mice. These mice have a 129Sv background and perform poorly in cognitive function tasks due to defects in their corpus callosum (36). Nevertheless, we demonstrate that fenofibrate, acting via the PPARα receptor, prevented the deleterious effect of WBI on hippocampal neurogenesis and inhibited microglial activation.
In addition to their role in controlling inflammation in various normal tissues, PPARα ligands are increasingly recognized as potent anti-tumor agents. PPARα expression and activity has been documented in several cancer cell lines including glioblastoma, colon, breast and prostate cancer (39). In melanoma cells, fenofibrate inhibited cell migration and colony formation in soft agar by downregulating Akt phosphorylation in vitro and prevented the establishment of lung metastases in vivo (40). In a recent study, fenofibrate potently inhibited primary tumor formation in mice by regulating pro-angiogenic and pro-inflammatory pathways (37). Therefore, it is hypothesized that in primary/metastatic brain cancer patients, fenofibrate could enhance the radiation-induced tumor kill in addition to preventing the detrimental effects on hippocampal neurogenesis.
In summary, we have demonstrated that fenofibrate prevents the deleterious effect of WBI on hippocampal neurogenesis and microglial activation in a PPARα-dependent manner. Fenofibrate is FDA-approved for the treatment of hypercholesterolemia and hypertriglyceridemia (17), can cross the blood brain barrier (33) and is well tolerated in humans. Therefore, fenofibrate may be a promising therapy to prevent radiation-induced brain injury and improve the quality of life of cancer patients receiving brain irradiation.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grants CA112593 (MER) and AG11370 (DRR). We thank Dr. Kelly Conner, Dr. Matthew Schindler, Liz Forbes and Dr. Kun Hua (Department of Neurobiology and Anatomy, WFUSM) for providing technical assistance with immunohistochemistry and microscopy
LIST OF ABBREVIATIONS
- WBI
Whole-brain irradiation
- PPARα
peroxisomal proliferator-activated receptor alpha
- DG
dentate gyrus
- GCL
granule cell layer
- SGZ
sub-granular zone
- WT
wild-type
- KO
knock-out
- BrdU
bromodeoxyuridine
- Iba-1
ionized Ca2+ calcium binding adaptor protein-1
- NeuN
neuronal nuclei
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST NOTIFICATION
None.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Black PM, Loeffler JS. Metastatic Brain Cancer. In: DeVita V, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2001. pp. 2655–2670. [Google Scholar]
- 3.Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 4.Crossen JR, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 5.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 6.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 7.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 8.Monje ML, Vogel H, Masek M, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 10.Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- 11.Andres-Mach M, Rola R, Fike JR. Radiation effects on neural precursor cells in the dentate gyrus. Cell Tissue Res. 2008;331:251–262. doi: 10.1007/s00441-007-0480-9. [DOI] [PubMed] [Google Scholar]
- 12.Mizumatsu S, Monje ML, Morhardt DR, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 13.Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 14.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 16.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willson TM, Brown PJ, Sternbach DD, et al. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 18.Drew PD, Xu J, Storer PD, et al. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49:183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34:1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- 20.Ramanan S, Kooshki M, Zhao W, et al. PPARα ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-κB and AP-1 pathways. Free Radic Biol Med. 2008;45:1695–1704. doi: 10.1016/j.freeradbiomed.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao W, Payne V, Tommasi E, et al. Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2007;67:6–9. doi: 10.1016/j.ijrobp.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70:826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Fan Y, Liu Z, Weinstein PR, et al. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 25.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn HG, Biebl M, Wilhelm D, et al. Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- 27.Bauer S, Patterson PH. The cell cycle-apoptosis connection revisited in the adult brain. J Cell Biol. 2005;171:641–650. doi: 10.1083/jcb.200505072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 30.Whitney NP, Eldem TM, Peng H, et al. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Ahn JH, Kim JH, et al. Fenofibrate regulates retinal endothelial cell survival through the AMPK signal transduction pathway. Exp Eye Res. 2007;84:886–893. doi: 10.1016/j.exer.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita M. Peroxisome proliferator-activated receptor alpha-independent effects of peroxisome proliferators on cysteinyl leukotriene production in mast cells. Eur J Pharmacol. 2007;556:172–180. doi: 10.1016/j.ejphar.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Deplanque D, Gele P, Petrault O, et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devchand PR, Keller H, Peters JM, et al. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 35.Delerive P, Gervois P, Fruchart JC, et al. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 36.Crawley JN, Belknap JK, Collins A, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 37.Panigrahy D, Kaipainen A, Huang S, et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci U S A. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grabacka M, Placha W, Plonka PM, et al. Inhibition of melanoma metastases by fenofibrate. Arch Dermatol Res. 2004;296:54–58. doi: 10.1007/s00403-004-0479-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.